Sustainable Synthesis, Antiproliferative and Acetylcholinesterase Inhibition of 1,4- and 1,2-Naphthoquinone Derivatives
Abstract
:1. Introduction
2. Results and Discussion
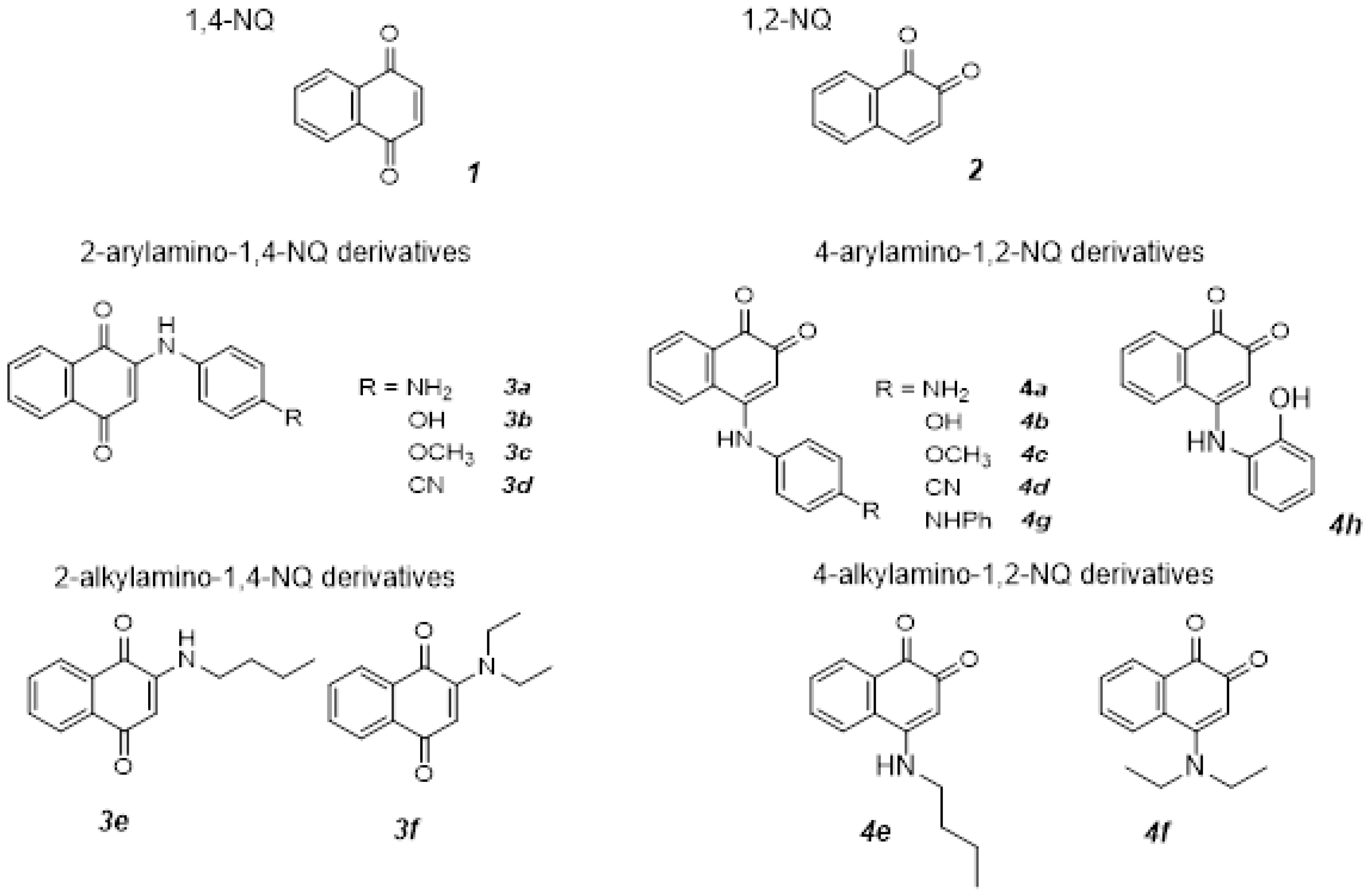
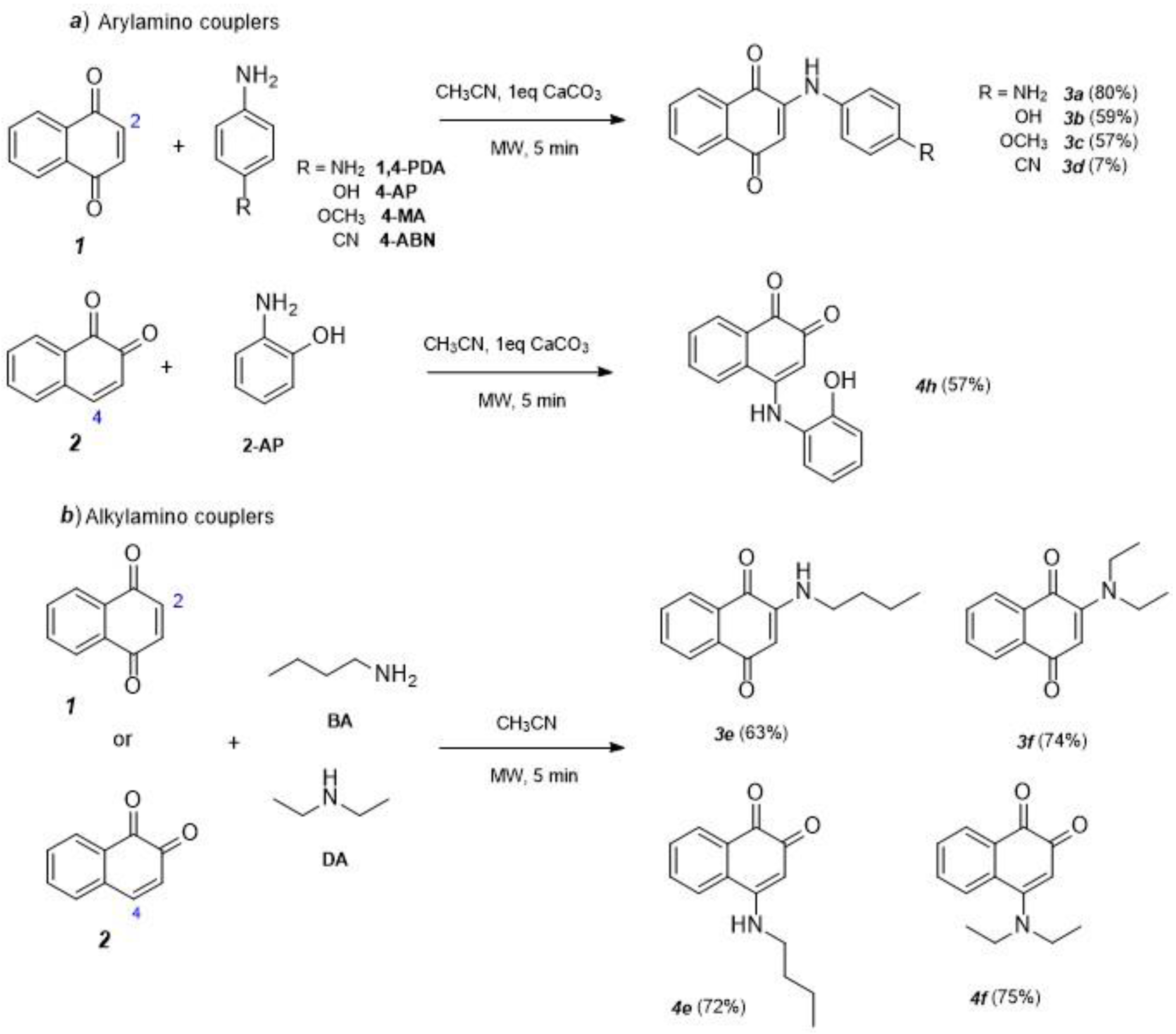
2.1. Synthesis and Characterization
2.2. Electrochemistry Studies
2.3. In Silico Studies
2.4. Biological Assays
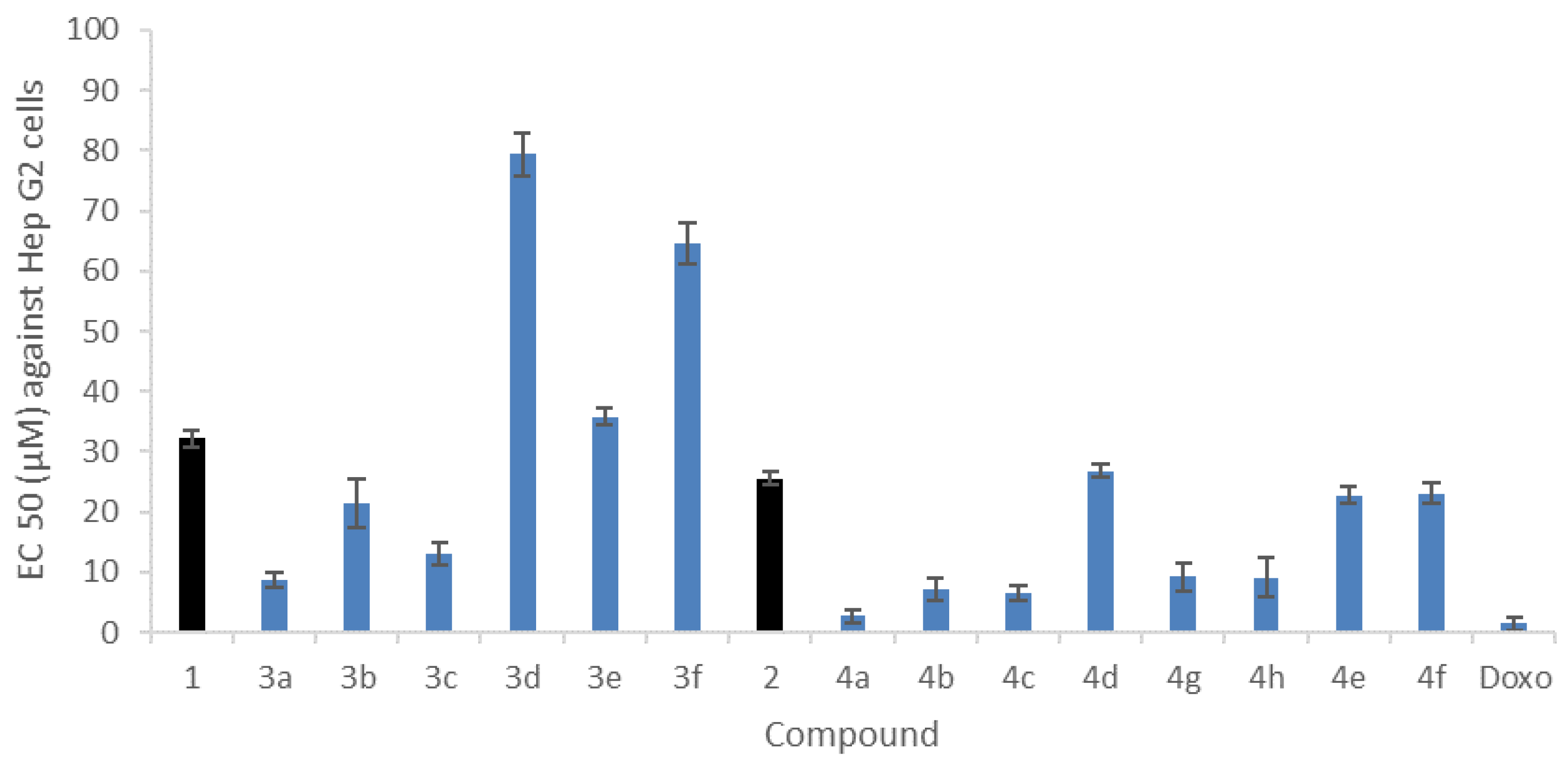
2.4.1. In Vitro Cytotoxicity against Human Hepatocarcinoma Cell Line HepG2
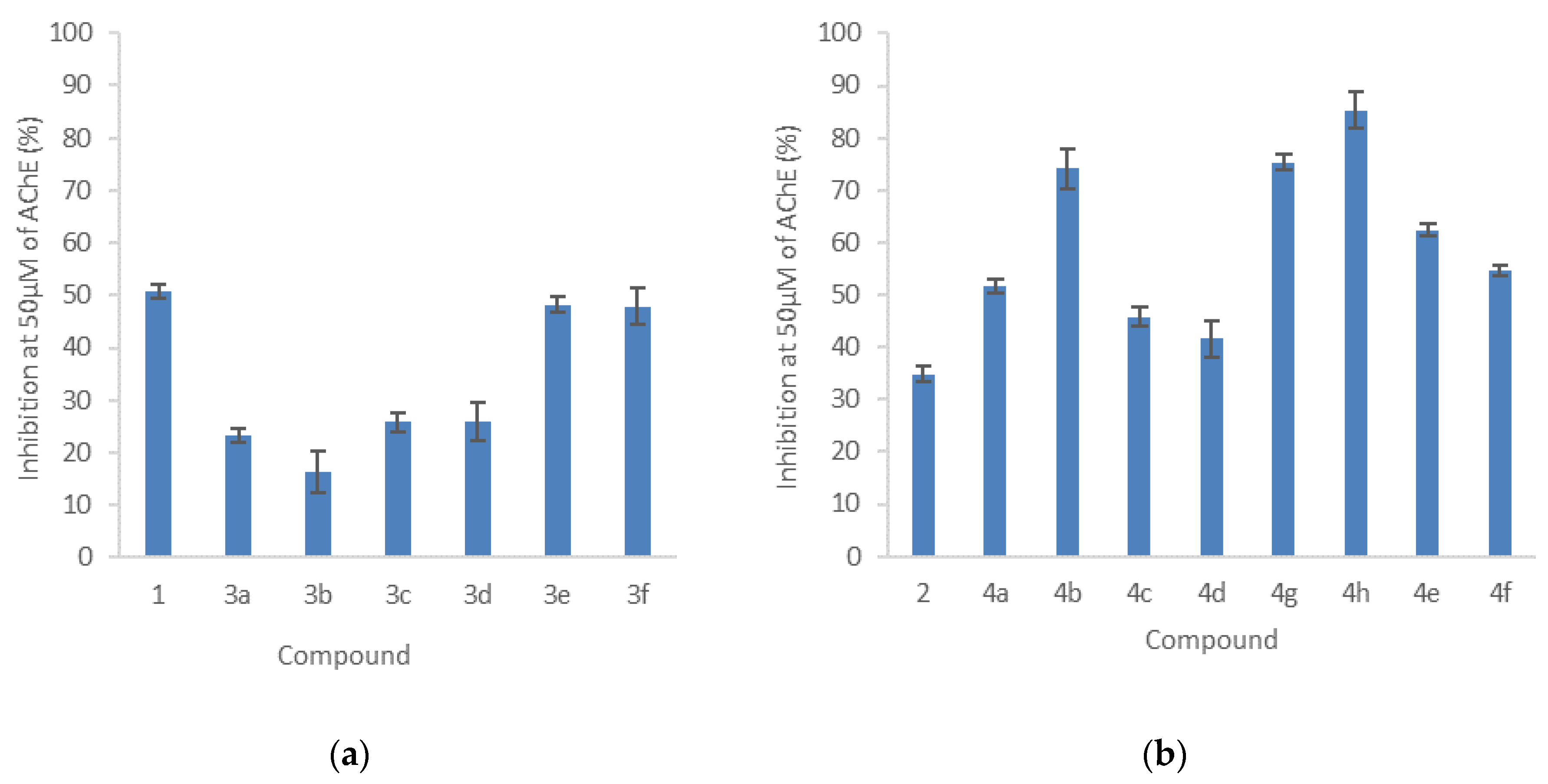
2.4.2. Inhibition of Acetylcholinesterase Activity
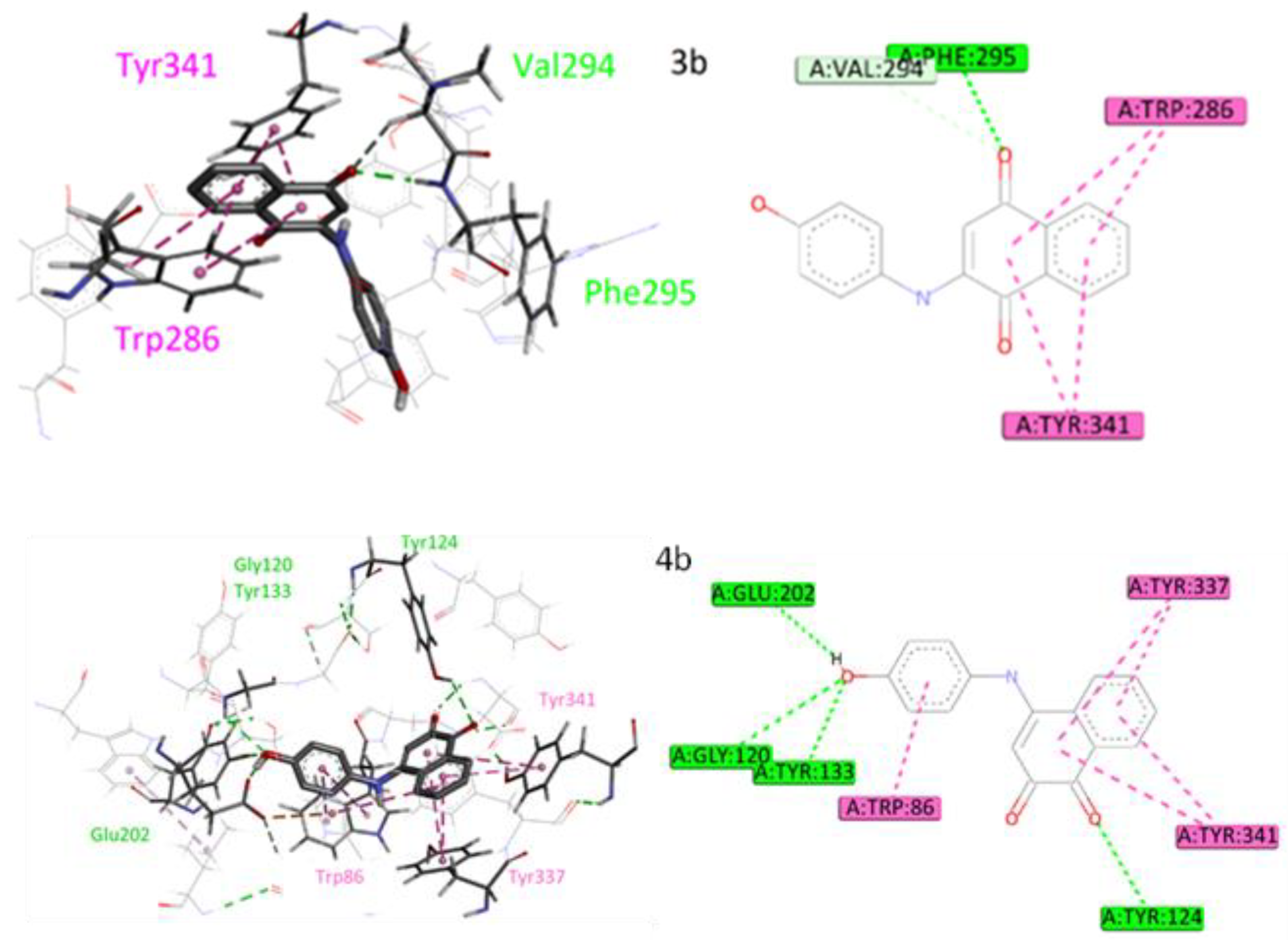
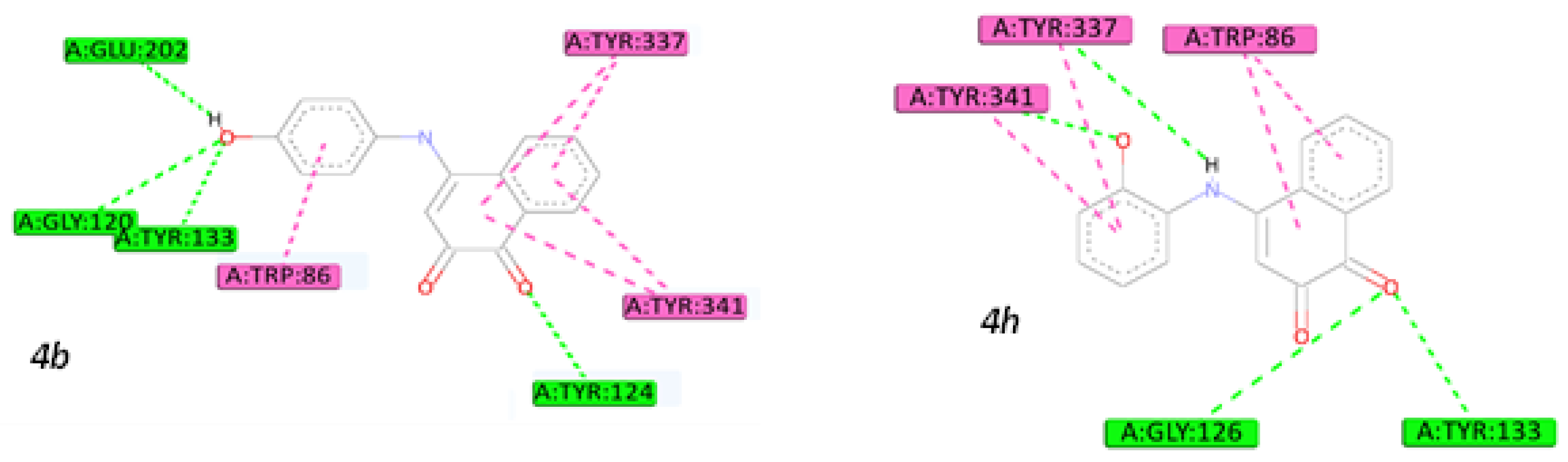
2.5. Molecular Docking Studies
3. Materials and Methods
3.1. Optimized Conditions for Microwave-Assisted Synthesis of 1,4-NQ Derivatives (3a–d)
- 2-(1,4-Phenylenediamine)-1,4-naphthoquinone (3a): Purple solid (80%): 1H NMR (400 MHz, MeOD-d4): δ(ppm) = 8.11 (d, 1H, J = 7.2 Hz, H5), 8.02 (d, 1H, J = 7.5 Hz, H8), 7.80 (t, 1H, J = 7.8 Hz, H7), 7.72 (t, 1H, J = 7.4 Hz, H6), 7.09 (d, 2H, J = 8.6 Hz, H12, H16), 6.81 (d, 2H, J = 8.6 Hz, H13, H15) and 6.01 (s, 1H, H3). 13C NMR (100 MHz, MeOD-d4): δ(ppm) = 185.5 (C4), 183.0 (C1), 147.8 (C2), 135.9 (C7), 134.8 (C10), 133.5 (C6), 132.1 (C9), 127.5 (C5), 126.8 (C8), 126.5 (C12, C16), 116.9 (C13, C15) and 101.4 (C3). ESI-MS positive mode: 287 [M+Na]+; 265 [M+H]+, MS2 (m/z): 237 [M+H-CO]+, MS3 (m/z): 209 [M+H-CO-CO]+; ESI-MS negative mode: 263 [M−H]−. ESI-HRMS: m/z calcd. for [C16H13N2O2+H]+: 265.0972; found 265.0980.
- 2-(4-aminophenol)-1,4-naphthoquinone (3b): orange solid (59%): 1H NMR (400 MHz, MeOD-d4): δ(ppm) = 8.11 (d, 1H, J = 7.7 Hz, H5), 8.02 (d, 1H, J = 7.6 Hz, H8), 7.80 (t, 1H, J = 7.5 Hz, H7), 7.70 (t, 1H, J = 7.5 Hz, H6), 7.17 (d, 2H, J = 8.8 Hz, H12, H16), 6.87 (d, 2H, J = 8.8 Hz, H13, H15) and 6.0 (s, 1H, H3). 13C NMR (100 MHz, MeOD-d4): δ(ppm) = 185.6 (C4), 182.9 (C1), 157.2 (C14), 135.9 (C7), 133.6 (C6), 132.1 (C10), 130.4 (C9), 127.4 (C5), 126.9 (C12, C16), 126.8 (C8), 117.1 (C13, C15) and 101.7 (C3). ESI-MS positive mode: 266 [M+H]+, MS2 (m/z): 238 [M+H-CO]+. ESI-HRMS: m/z calcd. for [C16H11NO3+H]+: 266.0812; found 266.0810.
- 2-(4-methoxyaniline)-1,4-naphthoquinone (3c): brown solid (57%): 1H NMR (400 MHz, MeOD-d4): δ(ppm) = 8.09 (d, 1H, J = 7.6 Hz, H5), 8.00 (d, 1H, J = 7.7 Hz, H8), 7.78 (t, 1H, J = 7.5 Hz, H7), 7.70 (t, 1H, J = 7.5 Hz, H6), 7.25 (d, 2H, J = 8.8 Hz, H12, H16), 7.00 (d, 2H, J = 8.8 Hz, H13, H15), 6.02 (s, 1H, H3) and 3.82 (s, 3H, CH3). 13C NMR (100 MHz, MeOD-d4): δ(ppm) = 185.6 (C4), 182.8 (C1), 159.4 (C14), 135.8 (C7), 133.6 (C6), 133.2 (C10), 132.1 (C9), 129.4 (C11), 127.4 (C5), 126.8 (C8), 126.7 (C12, C16), 115.8 (C13, C15), 101.9 (C3) and 56.0 (CH3). ESI-MS positive mode: 280 [M+H]+, MS2 (m/z): 252 [M+H-CO]+. ESI-HRMS: m/z calcd. for [C17H13NO3+H]+: 280.0968; found 280.0976.
- 2-(4-aminobenzonitrile)-1,4-naphthoquinone (3d): orange solid (7%): 1H NMR (400 MHz, MeOD-d4): δ(ppm) = 8.31 (d, 1H, J = 7.8 Hz, H5), 8.05 (d, 1H, J = 7.8 Hz, H8), 7.82 (t, 1H, J = 7.2 Hz, H7), 7.65 (t, 1H, J = 7.2 Hz, H6), 7.48 (d, 2H, J = 9.0 Hz, H12, H16), 7.35 (d, 2H, J = 9.0 Hz, H13, H15), 6.22 (s, 1H, H3). ESI-MS positive mode: 275 [M+H]+, MS2 (m/z): 247 [M+H-CO]+. ESI-HRMS: m/z calcd. for [C17H10N2O2+H]+: 275.0815; found 275.0819.
3.2. Optimized Conditions for Microwave Synthesis of 1,4-NQ Derivatives (3e,f) and 1,2-NQ Derivatives (4e,f)
- 2-(butan-1-amine)-1,4-naphthoquinone (3e): orange solid (63%): 1H NMR (400 MHz, MeOD-d4): δ(ppm) = 8.01 (dd, 2H, J = 6.9 Hz, H5, H8), 7.75 (t, 1H, J = 7.5 Hz, H7), 7.66 (t, 1H, J = 7.5 Hz, H6), 5.68 (s, 1H, H3), 3.23 (t, 2H, J = 7.2 Hz, H11), 1.66 (d, 2H, J = 7.4 Hz, H12), 1.44 (d, 2H, J = 7.4 Hz, H13) and 0.98 (t, 3H, J = 7.3 Hz, H14). 13C NMR (100 MHz, MeOD-d4): δ(ppm) = 184.7 (C4), 182.6 (C1), 150.8 (C2), 135.8 (C7), 135.0 (C9), 133.3 (C6), 132.1 (C10), 127.3 (C8), 126.8 (C5), 99.9 (C3), 43.2 (C11), 31.1 (C12), 21.3 (C13) 14.1 (C14). ESI-MS positive mode: 230 [M+H]+. ESI-HRMS: m/z calcd. for [C14H15NO2+H]+: 230.1176; found 230.1180.
- 2-(diethylamine)-1,4-naphthoquinone (3f): red solid (74%): 1H NMR (400 MHz, MeOD-d4): δ(ppm) = 7.98 (dd, 2H, J = 7.9 Hz, H5, H8), 7.76 (t, 1H, J = 7.4 Hz, H7), 7.68 (t, 1H, J = 7.4 Hz, H6), 5.86 (s, 1H, H3), 3.62 (m, 2H, H11) and 1.32 (t, 2H, J = 7.0 Hz, H12). 13C NMR (100 MHz, MeOD-d4): δ(ppm) = 184.7 (C4), 184.4 (C1), 153.3 (C2), 135.0 (C7), 134.3 (C9), 134.1 (C10), 133.3 (C6), 125.9 (C5, C8), 105.0 (C3), 48.0 (C11, C11′) and 12.9 (C12, C12′). ESI-MS positive mode: 230 [M+H]+. ESI-HRMS: m/z calcd. for [C14H15NO2+H]+: 230.1176; found 230.1177.
- 4-(butan-1-amine)-1,2-naphthoquinone (4e): red solid (72%): 1H NMR (400 MHz, MeOD-d4): δ(ppm) = 8.12 (d, 1H, J = 8.0 Hz, H8), 8.05 (d, 1H, J = 8.0 Hz, H5), 7.80 (t, 1H, J = 8.0 Hz, H6), 7.70 (t, 1H, J = 8.0 Hz, H7), 5.90 (s, 1H, H3), 3.52 (t, 2H, J = 8.0 Hz, H11), 1.76 (d, 2H, J = 7.4 Hz, H12), 1.48 (d, 2H, J = 8.0 Hz, H13) and 1.00 (t, 3H, J = 8.0 Hz, H14). 13C NMR (100 MHz, MeOD-d4): δ(ppm) = 180.9 (C1), 175.3 (C2), 159.4 (C4), 135.8 (C7), 135.6 (C6), 132.9 (C7), 132.6 (C10), 131.9 (C9), 129.3 (C8), 124.6 (C5), 98.9 (C3), 44.9 (C11), 31.5 (C12), 21.3 (C13) 14.1 (C14). ESI-MS positive mode: 256 [M+Na]+ and 230 [M+H]+. ESI-HRMS: m/z calcd. for [C14H15NO2+H]+: 230.1176; found 230.1183.
- 4-(diethylamine)-1,2-naphthoquinone (4f): red solid (75%): 1H NMR (400 MHz, MeOD-d4): δ(ppm) = 8.05 (dd, 2H, J = 7.9 Hz, H5, H8), 7.75 (t, 2H, J = 7.4 Hz, H6, H7), 6.02 (s, 1H, H3), 3.68 (q, 4H, H11, H11′) and 1.41 (t, 6H, J = 8.0 Hz, H12, H12′). 13C NMR (400 MHz, MeOD-d4): δ(ppm) = 183.7 (C1), 177.6 (C2), 165.6 (C4), 135.0 (C6), 133.3 (C9), 132.4 (C5), 130.2 (C10), 129.9 (C9), 129.9 (C8), 128.7 (C7), 105.4 (C3), 47.9 (C11, C11′) and 13.0 (C12, C12′). ESI-MS positive mode: ESI-MS positive mode: 256 [M+Na]+ and 230 [M+H]+. ESI-HRMS: m/z calcd. for [C14H15NO2+H]+: 230.1176; found 230.1176.
3.3. Bioactivity Score Prediction
3.4. Biological Activities
3.4.1. Cytotoxicity Assay against HepG2 Human Cell Line
3.4.2. AChE Inhibition Assay
3.4.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han-Yue, Q.; Peng-Fei, W.; Hong-Yan, L.; Cheg-Yi, T.; Hai-Liang, Z.; Yong-Hua, Y. Naphthoquinones: A continuing source for discovery of therapeutic antineoplastic agents. Chem. Biol. Drug Des. 2018, 91, 681–690. [Google Scholar]
- Fiorito, S.; Genovese, S.; Taddeo, V.A.; Mathiey, V.; Kiss, R.; Epifano, F. Novel juglone and plumbagin 5-O derivatives and their in vitro growth inhibitory activity against apoptosis-resistant cancer cells. Bioorg. Med. Chem. Lett. 2016, 26, 334–337. [Google Scholar] [CrossRef] [PubMed]
- López, J.; de la Cruz, F.; Alcaraz, Y.; Delgado, F.; Vázquez, M.A. Quinoid systems in chemistry and pharmacology. Med. Chem. Res. 2015, 24, 3599–3620. [Google Scholar] [CrossRef]
- Louvis, A.R.; Silva, N.A.A.; Semaan, F.S.; da Silva, F.C.; Saramago, G.; de Souza, L.C.S.V.; Ferreira, B.L.A.; Castro, H.C.; Salles, J.P.; Souza, A.L.A.; et al. Synthesis, characterization and biological activities of 3-aryl-1,4-naphthoquinones—Green palladium-catalysed Suzuki cross coupling. New J. Chem. 2016, 40, 7643–7656. [Google Scholar] [CrossRef]
- Aminin, D.; Polonik, S. 1,4-Naphthoquinones: Some Biological Properties and Application. Chem. Pharm. Bull. 2020, 68, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, T.L.; de Azevedo, M.L.S.G.; Ferreira, F.R.; Santos, D.C.; Amatore, C.; Goulart, M.O.F. Quinone-based molecular electrochemistry and their contributions to medicinal chemistry: A look at the present and future. Curr. Opin. Electrochem. 2020, 24, 79–87. [Google Scholar] [CrossRef]
- da Paiva, T.G.; Silva, T.L.; Xavier, A.F.A.; Cardoso, M.F.C.; da Silva, F.C.; Silva, M.F.S.; Pinheiro, D.P.; Pessoa, C.; Ferreira, V.F.; Goulart, M.O.F. Relationship between Electrochemical Parameters, Cytotoxicity Data against Cancer Cells of 3-Thio-Substituted Nor-Beta-Lapachone Derivatives. Implications for Cancer Therapy. J. Braz. Chem. Soc. 2019, 30, 658–672. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos-Brozio, M.; Zaborski, M. Characteristics of juglone (5-hydroxy-1,4-naphthoquinone) using voltammetry and spectrophotometric methods. Food Chem. 2019, 301, 125279–125286. [Google Scholar] [CrossRef]
- Janeczko, M.; Demchuk, O.M.; Strzelecka, D.; Kubiński, K.; Masłyk, M. New family of antimicrobial agents derived from 1,4-naphthoquinone. Eur. J. Med. Chem. 2016, 124, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.C.R.; Coulidiati, T.H.; Monteiro, A.L.; de Carvalho-Gonçalves, L.C.T.; Valença, W.O.; de Oliveira, R.N.; Câmara, C.A.; de Araújo, D.A.M. Antitumoral activity of novel 1,4-naphthoquinone derivative involves L-type calcium channel activation in human colorectal cancer cell line. J. Appl. Biomed. 2016, 14, 229–234. [Google Scholar] [CrossRef]
- Kubanik, M.; Kandioller, W.; Kim, K.; Anderson, R.F.; Klapproth, E.; Jakupec, M.A.; Roller, A.; Söhnel, T.; Keppler, B.K.; Hartinger, C.G. Towards targeting anticancer drugs: Ruthenium(ii)–arene complexes with biologically active naphthoquinone-derived ligand systems. Dalton Trans. 2016, 45, 13091–13103. [Google Scholar] [CrossRef]
- Cardoso, S.H.; de Oliveira, C.R.; Guimarães, A.S.; Nascimento, J.; Carmo, J.O.S.; Ferro, J.N.S.; Correia, A.C.C.; Barreto, E. Synthesis of newly functionalized 1,4-naphthoquinone derivatives and their effects on wound healing in alloxan-induced diabetic mice. Chem. Biol. Interact. 2018, 291, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wu, L. Synthesis and biological evaluation of novel 1,2-naphthoquinones possessing tetrazolo[1,5-a]pyrimidine scaffolds as potent antitumor agents. RSC Adv. 2015, 5, 24960–24965. [Google Scholar] [CrossRef]
- Shukla, S.; Srivastava, R.S.; Shrivastava, S.K.; Sodhi, A.; Kumar, P. Synthesis, characterization and antiproliferative activity of 1,2-naphthoquinone and its derivatives. Appl. Biochem. Biotechnol. 2012, 167, 1430–1445. [Google Scholar] [CrossRef]
- Hartfield, M.J.; Chen, J.; Fratt, E.M.; Chi, L.; Bollinger, J.C.; Binder, R.J.; Bowling, J.; Hyatt, J.L.; Scarborough, J.; Jeffries, C.; et al. Selective Inhibitors of Human Liver Carboxylesterase Based on a β-Lapachone Scaffold: Novel Reagents for Reaction Profiling. J. Med. Chem. 2017, 60, 1568–1579. [Google Scholar] [CrossRef] [Green Version]
- Shin, N.N.; Jeon, H.; Jung, Y.; Baek, S.; Lee, S.; Yoo, H.C.; Bae, G.H.; Park, K.; Yang, S.H.; Han, J.M.; et al. Fluorescent 1,4-Naphthoquinones To Visualize Diffuse and Dense-Core Amyloid Plaques in APP/PS1 Transgenic Mouse Brains. ACS Chem. Neurosci. 2019, 10, 3031–3044. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, Y.H.; Shen, G.N.; Piao, X.J.; Xu, W.T.; Zhang, Y.; Wang, H.X.; Wang, C.Y.; Jin, C.H. Two novel 1,4-naphthoquinone derivatives induce human gastric cancer cell apoptosis and cell cycle arrest by regulating reactive oxygen species-mediated MAPK/Akt/STAT3 signaling pathways. Mol. Med. Rep. 2019, 20, 2571–2582. [Google Scholar] [CrossRef] [Green Version]
- Campora, M.; Francesconi, V.; Schenone, S.; Tasso, B.; Tonelli, M. Journey on Naphthoquinone and Anthraquinone Derivatives: New Insights in Alzheimer’s Disease. Pharmaceuticals 2021, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Boonyaketgoson, S.; Rukachaisirikul, V.; Phongpaichit, S.; Trisuwa, K. Naphthoquinones from the leaves of Rhinacanthus nasutus having acetylcholinesterase inhibitory and cytotoxic activities. Fitoterapia 2018, 124, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, E.S.; Tajbakhsh, A.; Iranshahy, M.; Asili, J.; Kretschmer, N.; Shakeri, A.; Sahebkar, A. Naphthoquinone Derivatives Isolated from Plants: Recent Advances in Biological Activity. Mini-Rev. Med. Chem. 2020, 20, 2019–2035. [Google Scholar] [CrossRef] [PubMed]
- Estolano-Cobián, A.; Noriega-Iribe, E.; Díaz-Rubio, L.; Padrón, J.M.; Brito-Perea, M.; Cornejo-Bravo, J.M.; Chávez, D.; Rivera, R.R.; Quintana-Melgoza, J.M.; Cruz-Reyes, J.; et al. Antioxidant, antiproliferative, and acetylcholinesterase inhibition activity of amino alcohol derivatives from 1,4-naphthoquinone. Med. Chem. Res. 2020, 29, 1986–1999. [Google Scholar] [CrossRef]
- Nepovimosa, E.; Uliassi, E.; Korabecny, J.; Peña-Altamira, L.E.; Samez, S.; Pesaresi, A.; Garcia, G.E.; Bartolini, M.; Andrisano, V.; Bergamini, C.; et al. Multitarget Drug Design Strategy: Quinone–Tacrine Hybrids Designed To Block Amyloid-β Aggregation and To Exert Anticholinesterase and Antioxidant Effects. J. Med. Chem. 2014, 57, 8576–8589. [Google Scholar] [CrossRef]
- Johnson, G.; Moore, S.W. The peripheral anionic site of acetylcholinesterase: Structure, functions and potential role in rational drug design. Curr. Pharm. Des. 2006, 12, 217–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, R.A.; Gartlehner, G.; Webb, A.P.; Morgan, L.C.; Moore, D.E.J. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. Clin. Interv. Aging 2008, 3, 211–225. [Google Scholar] [PubMed]
- Jordão, A.K.; Novais, J.; Leal, B.; Escobar, A.C.; Santos Jr, H.M.; Castro, H.C.; Ferreira, V.F. Synthesis using microwave irradiation and antibacterial evaluation of new N,O-acetals and N,S-acetals derived from 2-amino-1,4-naphthoquinones. Eur. J. Med. Chem. 2013, 63, 196–201. [Google Scholar] [CrossRef]
- Kittakoop, P.; Mahidol, C.; Ruchirawat, S. Alkaloids as important scaffolds in therapeutic drugs for the treatments of cancer, tuberculosis, and smoking cessation. Curr. Top. Med. Chem. 2014, 14, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ge, H. Site-selective C–H arylation of primary aliphatic amines enabled by a catalytic transient directing group. Nat. Chem. 2016, 9, 26–32. [Google Scholar] [CrossRef]
- Leyva, E.; Cárdenas-Chaparro, A.; Loredo-Carrillo, S.E.; López, L.I.; Méndez-Sánchez, F.; Martínez-Richa, A. Ultrasound-assisted reaction of 1,4-naphthoquinone with anilines through an EDA complex. Mol. Divers. 2018, 22, 281–290. [Google Scholar] [CrossRef]
- López-López, L.I.; Garcia, J.J.V.; Sáenz-Galindo, A.; Silva-Belmares, S.Y. Ultrasonic and Microwave Assisted Synthesis of Nitrogen-Containing Derivatives of Juglone as Potential Antibacterial Agents. Lett. Org. Chem. 2014, 11, 573–582. [Google Scholar] [CrossRef]
- Berrino, E.; Supuran, C.T. Advances in microwave-assisted synthesis and the impact of novel drug discovery. Expert Opin. Drug Discov. 2018, 13, 861–873. [Google Scholar] [CrossRef]
- Rathi, A.K.; Gawande, M.B.; Zboril, R.; Varma, R.S. Microwave-assisted synthesis—Catalytic applications in aqueous media. Coord. Chem. Rev. 2015, 291, 68–94. [Google Scholar] [CrossRef]
- Sousa, A.C.; Santos, I.; Piedade, M.F.M.M.; Martins, L.O.; Robalo, M.P. Synthesis of Substituted 4-Arylamino-1,2-naphthoquinones in One-Pot Reactions Using CotA-Laccase as Biocatalyst. Adv. Synth. Catal. 2020, 362, 3380–3387. [Google Scholar] [CrossRef]
- Amatore, C.; Labbé, E.; Buriez, O. Molecular electrochemistry: A central method to understand the metabolic activation of therapeutic agents. The example of metallocifen anti-cancer drug candidates. Curr. Opin. Electrochem. 2017, 2, 7–12. [Google Scholar] [CrossRef]
- De Abreu, F.C.; Ferraz, P.A.L.; Coulart, M.O.F.; Some Applications of Electrochemistry in Biomedical Chemistry. Emphasis on the Correlation of Electrochemical and Bioactive Properties. J. Braz. Chem. Soc. 2002, 13, 19–35. [Google Scholar] [CrossRef]
- Kovacic, P.; Becvar, L.E. Mode of action of anti-infective agents: Focus on oxidative stress and electron transfer. Curr. Pharm. Des. 2000, 6, 143–167. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration Cheminformatics. Available online: molinspiration.com (accessed on 5 July 2022).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Abraham, M.H.; Le, J.; Hersey, A.; Luscombe, C.N.; Beck, G.; Sherborne, B.; Cooper, I. Rate-limited steps of human oral absorption and QSAR studies. Pharm. Res. 2002, 19, 1446–1457. [Google Scholar] [CrossRef]
- Mahmoud, I.S.; Hatmal, M.M.; Abuarqoub, D.; Esawi, E.; Zalloum, H.; Wehaibi, S.; Nsairat, H.; Alshaer, W. 1,4-Naphthoquinone is a Potent Inhibitor of IRAK1 Kinases and the Production of Inflammatory Cytokines in THP-1 Differentiated Macrophages. ACS Omega 2021, 6, 25299–25310. [Google Scholar] [CrossRef]
- Brien, Z.O.; Moghaddam, M.F. A systematic analysis of physicochemical and ADME properties of all small molecule kinase inhibitors approved by US FDA from January 2001 to October 2015. Curr. Med. Chem. 2017, 24, 3159–3184. [Google Scholar]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The curious case of HepG2 cell line: 40 years of expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef]
- Copeland, R.A.; Harpel, M.R.; Tummino, P.J. Targeting enzyme inhibitors in drug discovery. Expert Opin. Ther. Targets 2007, 11, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Jacobs, N.; Lang, S.; Panisch, R.; Wittstock, G.; Growth, U.; Nasiri, H.R. Investigation on the electrochemistry and cytotoxicity of the natural product marcanine A and its synthetic derivatives. RSC Adv. 2015, 5, 58561–58565. [Google Scholar] [CrossRef] [Green Version]
- Powis, G. Free radical formation by antitumor quinones. Free Radic. Biol. Med. 1989, 6, 63–101. [Google Scholar] [CrossRef]
- Bai, D.L.; Tang, X.C.; He, X.C. Huperzine A, a potential therapeutic agent for treatment of Alzheimer’s disease. Curr. Med. Chem. 2000, 7, 355–374. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; He, S.; Chen, Y.; Feng, F.; Qu, W.; Sun, H. Donepezil based multi-functional cholinesterase inhibitors for treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018, 158, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Khoobi, M.; Foroumadi, A.; Nadri, H.; Moradi, A.; Sakhteman, A.; Ghandi, M.; Shafiee, A. Novel coumarin derivatives bearing N-benzyl pyridinium moiety: Potent and dual binding site acetylcholinesterase inhibitors. Bioorg. Med. Chem. 2012, 20, 7214–7222. [Google Scholar] [CrossRef]
- Gaspar, H.; Bronze, S.; Oliveira, C.; Victor, B.L.; Machuqueiro, M.; Pacheco, R.; Caldeira, M.J.; Santos, S. Proactive Response to Tackle the Threat of Emerging Drugs: Synthesis and Toxicity Evaluation of New Cathinones. Forensic Sci. Int. 2018, 290, 146–156. [Google Scholar] [CrossRef]
- Silva, A.F.C.; Haris, P.I.; Serralheiro, M.L.; Pacheco, R. Mechanism of action and the biological activities of Nigella sativa oil components. Food Biosci. 2020, 38, 100783. [Google Scholar] [CrossRef]

| Entry | Time (Min) | 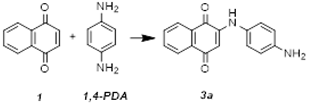 | ||
|---|---|---|---|---|
| Solvent | Catalyst | Yield (%) 2 | ||
| 1 | 5 | - | - | 30 |
| 2 | 10 | - | - | 60 |
| 3 | 10 | - | CaCO3 | 56 |
| 4 | 5 | ethanol | - | 26 |
| 5 | 10 | ethanol | - | 4 |
| 6 | 5 | acetonitrile | - | 14 |
| 7 | 10 | acetonitrile | - | 20 |
| 8 | 10 | acetone | - | 0 |
| 9 | 5 | ethanol | CaCO3 | 68 |
| 10 | 10 | ethanol | CaCO3 | 73 |
| 11 | 5 | acetone | CaCO3 | 17 |
| 12 | 10 | acetone | CaCO3 | 13 |
| 13 | 5 | acetonitrile | CaCO3 | 76 |
| 14 | 10 | acetonitrile | CaCO3 | 72 |
| 15 | 5 | acetonitrile:water (1:1) | CaCO3 | 80 |
| Compound | Coupler | Yield (%) 1 | ||
|---|---|---|---|---|
| MA2 + CaCO3 (1 Eq) | MA 2 | Conventional Heating 3 | ||
| 3a | 1,4-PDA | 80 | - - | 42 (2 h) 61 (24 h) |
| 3b | 4-AP | 59 | - - | 55 (2 h) 64 (4 h) 67 (24 h) |
| 3c | 4-MA | 57 | - | - |
| 3d | 4-ABN | 7 | - | 0 (24 h) |
| 3e | BA | 54 | 63 | 62 (2 h) 82 (4 h) 80 (24 h) |
| 3f | DA | 15 | 74 | - |
| Compound | Epc (I) (V) (E1/2; ∆E (mV)) | Epc (II) (V) (E1/2; ∆E (mV)) |
|---|---|---|
| 1,4-NQ (1) | −0.70 (−0.66; 80) | −1.36 |
| 3a | −0.86 (−0.82; 80) | −1.42 |
| 3b | −0.84 (−0.81; 70) | - |
| 3c | −0.86 (−0.82; 70) | −1.34 |
| 3d | −0.74 (−0.70; 80) | - |
| 3e | −0.98 (−0.95; 70) | −1.47 (−1.42; 100) |
| 3f | −0.97 (−0.94; 70) | - |
| 1,2-NQ (2) | −0.55 (−0.50; 110) | −0.98 (−0.91; 140) |
| 4a | −0.67 | - |
| 4b | −0.61 | - |
| 4c | −0.67 | - |
| 4d | −0.47 | −0.84 |
| 4g | −0.63 | −1.43 |
| 4h | −0.57 | −0.98 |
| 4e | −0.78 | - |
| 4f | −0.52 | −0.69 (−0.63, 120) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral, R.G.; Viegas, G.; Pacheco, R.; Sousa, A.C.; Robalo, M.P. Sustainable Synthesis, Antiproliferative and Acetylcholinesterase Inhibition of 1,4- and 1,2-Naphthoquinone Derivatives. Molecules 2023, 28, 1232. https://doi.org/10.3390/molecules28031232
Cabral RG, Viegas G, Pacheco R, Sousa AC, Robalo MP. Sustainable Synthesis, Antiproliferative and Acetylcholinesterase Inhibition of 1,4- and 1,2-Naphthoquinone Derivatives. Molecules. 2023; 28(3):1232. https://doi.org/10.3390/molecules28031232
Chicago/Turabian StyleCabral, Rafaela G., Gonçalo Viegas, Rita Pacheco, Ana Catarina Sousa, and Maria Paula Robalo. 2023. "Sustainable Synthesis, Antiproliferative and Acetylcholinesterase Inhibition of 1,4- and 1,2-Naphthoquinone Derivatives" Molecules 28, no. 3: 1232. https://doi.org/10.3390/molecules28031232






