Alkaloid Profile in Wild Autumn-Flowering Daffodils and Their Acetylcholinesterase Inhibitory Activity
Abstract
1. Introduction
2. Results and Discussion
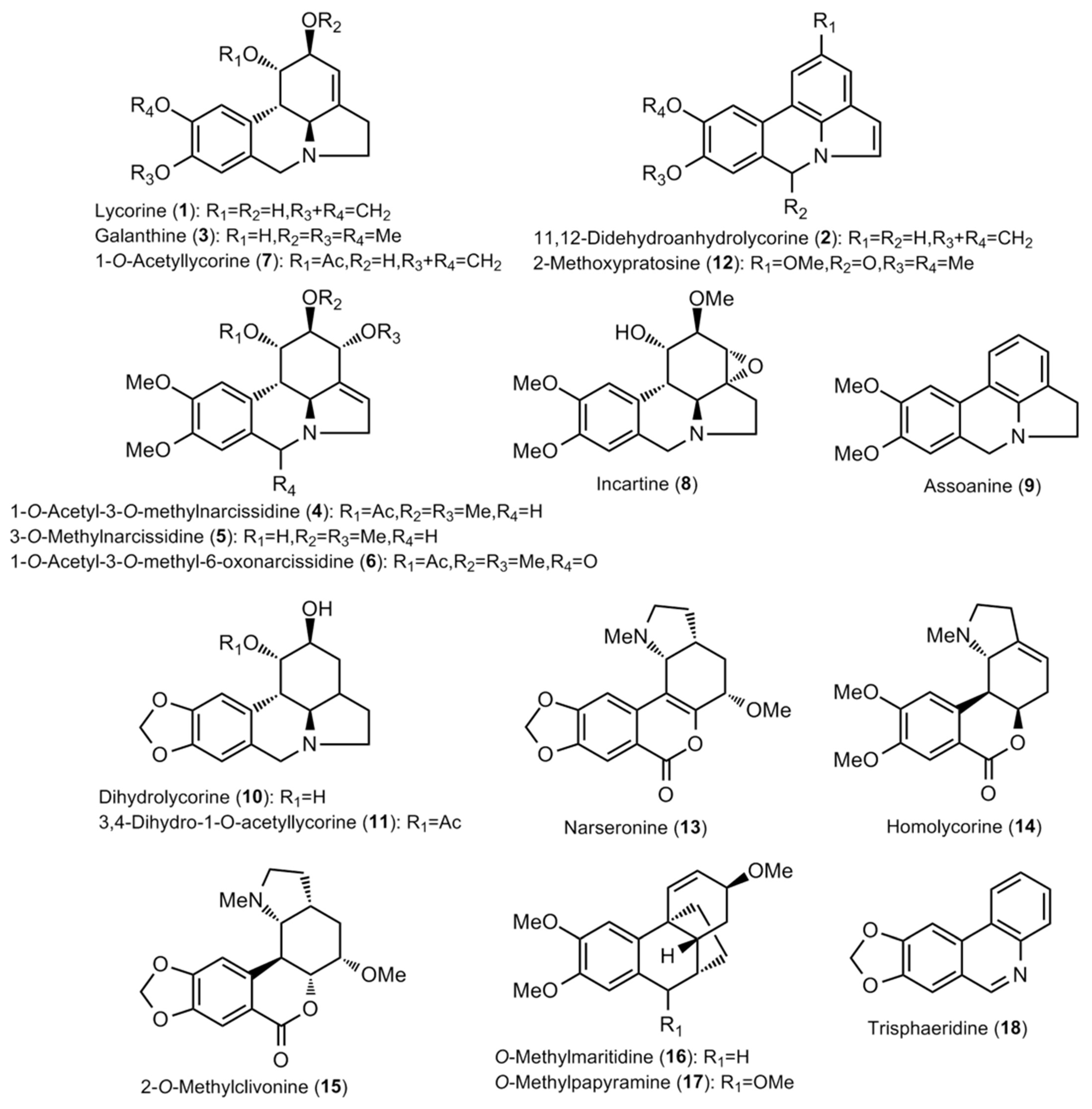
2.1. Alkaloid Profiling
2.2. Acetylcholinesterase Inhibition Activity
3. Materials and Methods
3.1. Plant Material
3.2. Alkaloid Extraction
3.3. GC–MS Analysis
3.4. Alkaloid Identification and Quantification
3.5. AChE Inhibitory Activity
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A

References
- World Alzheimer Report 2021: Journey through the Diagnosis of Dementia. Available online: https://www.alzint.org/resource/world-alzheimer-report-2021/#:~:text=The%20World%20Alzheimer%20Report%202021,national%20Alzheimer%20and%20dementia%20associations (accessed on 12 June 2022).
- World Health Organization: Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 12 June 2022).
- Tran, T.-H.; Vo, T.-T.-H.; Vo, T.-Q.-N.; Cao, T.-C.-N.; Tran, T.-S. Synthesis and Evaluation of the Acetylcholinesterase Inhibitory Activities of Some Flavonoids Derived from Naringenin. Sci. World J. 2021, 2021, 4817900. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Bastida, J.; Lavilla, R.; Viladomat, F. Chemical and biological aspects of Narcissus alkaloids. In The Alkaloids: Chemistry and Physiology; Cordell, G.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 63, pp. 87–179. [Google Scholar] [CrossRef]
- Jin, Z.; Xu, X.H. Amaryllidaceae Alkaloids. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin, Germany, 2013; pp. 479–522. [Google Scholar] [CrossRef]
- Berkov, S.; Osorio, E.; Viladomat, F.; Bastida, J. Chemodiversity, chemotaxonomy and chemoecology of Amaryllidaceae alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 83, pp. 113–185. [Google Scholar] [CrossRef]
- Fraser, M.D.; Vallin, H.E.; Davies, J.R.T.; Rowlands, G.E.; Chang, X. Integrating Narcissus-derived galanthamine production into traditional upland farming systems. Sci. Rep. 2021, 11, 1389. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Bastida, J.; Codina, C.; de Andrade, J.P.; Berbee, R.L.M. Extract of Hippeastrum papilio Rich in Galanthamine. European Patent EP2999480B1, 2013. Available online: https://patents.google.com/patent/EP2999480B1/en (accessed on 15 June 2022).
- Torras-Claveria, L.; Tallini, L.; Viladomat, F.; Bastida, J. Research in natural products: Amaryllidaceae ornamental plants as sources of bioactive compounds. In Recent Advances in Pharmaceutical Sciences VII; Muñoz-Torrero, D., Riu, M., Feliu, C., Eds.; Research Signpost Trivandrum: Kerala, India, 2017; pp. 69–82. ISBN 978-81-308-0573-3. [Google Scholar]
- Bastida, J.; Viladomat, F. Alkaloids of Narcissus. In Narcissus and Daffodil. The Genus Narcissus; Hanks, G.R., Ed.; Taylor & Francis: London, UK, 2002; pp. 141–214. ISBN 0-415-27344-7. [Google Scholar]
- Ureña-Plaza, F Fenología de la antesis en poblaciones silvestres de narcisos—Registros fotográficos en el grupo Narcissus. Available online: https://www.facebook.com/groups/158509101289275/ (accessed on 12 June 2021).
- Kornienko, A.; Evidente, A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef]
- Pigni, N.B.; Berkov, S.; Elamrani, A.; Benaissa, M.; Viladomat, F.; Codina, C.; Bastida, J. Two New Alkaloids from Narcissus serotinus L. Molecules 2010, 15, 7083–7089. [Google Scholar] [CrossRef]
- Tarakemeh, A.; Azizi, M.; Rowshan, V.; Salehi, H.; Spina, R.; Dupire, F.; Arouie, H.; Laurain-Mattar, D. Screening of Amaryllidaceae alkaloids in bulbs and tissue cultures of Narcissus papyraceus and four varieties of N. tazetta. J. Pharmaceut. Biomed. 2019, 172, 230–237. [Google Scholar] [CrossRef]
- Suau, R.; Rico, R.; García, A.I.; Gómez, A.I. New Amaryllidaceae Alkaloids from Narcissus papyraceus Ker-Gawler. Heterocycles 1990, 31, 517. [Google Scholar] [CrossRef]
- Elgorashi, E.; Malan, S.; Stafford, G.; van Staden, J. Quantitative structure–activity relationship studies on acetylcholinesterase enzyme inhibitory effects of Amaryllidaceae alkaloids. S. Afr. J. Bot. 2006, 72, 224–231. [Google Scholar] [CrossRef]
- Nair, J.J.; van Staden, J. Acetylcholinesterase Inhibition within the Lycorine Series of Amaryllidaceae Alkaloids. Nat. Prod. Commun. 2012, 7, 959–962. [Google Scholar] [CrossRef]
- Tallini, R.L.; Osorio, E.H.; Berkov, S.; Torras-Claveria, L.; Rodríguez-Escobar, M.L.; Viladomat, F.; Bastida, J. Chemical survey of three species of the genus Rauhia Traub. (Amaryllidaceae). Plants 2022, 11, 3549. [Google Scholar] [CrossRef]
- Ka, S.; Masi, M.; Merindol, N.; Di Lecce, R.; Plourde, M.B.; Seck, M.; Górecki, M.; Pescitelli, G.; Desgagne-Penix, I.; Evidente, A. Gigantelline, gigantellinine and gigancrinine, cherylline- and crinine-type alkaloids isolated from Crinum jagus with anti-acetylcholinesterase activity. Phytochemistry 2020, 175, 112390. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Sener, B. Bioactivity-directed fractionation of alkaloids from some Amaryllidaceae plants and their anticholinesterase activity. Chem. Nat. Compd. 2003, 39, 383–386. [Google Scholar] [CrossRef]
- Torras-Claveria, L.; Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. Metabolomic análisis of bioactive Amaryllidaceae alkaloids of ornamental varieties of Narcissus by GC-MS combined with k-means cluster analysis. Ind. Crops Prod. 2014, 56, 211–222. [Google Scholar] [CrossRef]
- Berkov, S.; Viladomat, F.; Codina, C.; Suárez, S.; Ravelo, A.; Bastida, J. GC-MS of amaryllidaceous galanthamine-type alkaloids. J. Mass Spectrom. 2012, 47, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Torras-Claveria, L.; Viladomat, F.; Suárez, S.; Bastida, J. GC-MS of some lycorine-type Amaryllidaceae alkaloids. J. Mass Spectrom. 2021, 56, e4704. [Google Scholar] [CrossRef] [PubMed]
- Christov, R.; Bankova, I.; Elenkov, N.; Hanjieva, S.; Spassov, S.; Popov, S. Stereo effects in the chemical ionization mass spectrometry of galanthamine epimers. Eur. Mass Spectrom. 1999, 5, 77–80. [Google Scholar] [CrossRef]
- Duffield, A.M.; Aplin, R.T.; Budzikiewicz, H.; Djerassi, C.; Murphy, C.F.; Wildman, W.C. Mass spectrometry in structural and stereochemical problems. LXXXII.1 A study of the fragmentation of some Amaryllidaceae alkaloids. J. Am.Chem. Soc. 1965, 21, 4902–4912. [Google Scholar] [CrossRef] [PubMed]
- Longevialle, P.; Smith, D.H.; Burlingame, A.L.; Fales, H.M.; Highet, R.J. High resolution mass spectrometry in molecular structure studies—V: The fragmentation of Amaryllis alkaloids in the crinine series. Org. Mass Spectrom. 1973, 7, 401–415. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef] [PubMed]




| Anthesis Months | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAT * | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | |
| N. obsoletus | 09/10 | ||||||||||||
| N. deficiens | 21/10 | ||||||||||||
| N. serotinus | 23/10 | ||||||||||||
| N. cavanillesii | 27/10 | ||||||||||||
| N. viridiflorus | 28/10 | ||||||||||||
| N. elegans ** | 19/10 | ||||||||||||
| N. papyraceus | 22/01 | ||||||||||||
| N. bulbocodium | 11/02 | ||||||||||||
| N. blancoi | 17/01 | ||||||||||||
| SAF | FAF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alkaloids | N. viridiflorus | N. serotinus | N. cavanillesii | N. obsoletus | N. elegans (M) | N. elegans (A) | N. deficiens | N. blancoi | N. bulbocodium | N. papyraceus |
| Lycorine type: | - | 108.66 | 6.58 | 8.37 | - | 12.72 | 30.74 | 9.96 | - | 23.02 |
| Lycorine (1) | - | - | 3.30 | 4.95 | - | - | - | - | - | 19.51 |
| 11,12-Didehydroanhydrolycorine (2) | - | - | - | 3.42 | - | - | - | - | - | 3.51 |
| Galanthine (3) | - | 3.48 | - | - | - | - | 3.39 | - | - | - |
| 1-O-Acetyl-3-O-methylnarcissidine (4) | - | 64.29 | - | - | - | 12.72 | 15.01 | - | - | - |
| 3-O-Methylnarcissidine (5) | - | 27.10 | - | - | - | - | 9.04 | - | - | - |
| 1-O-Acetyl-3-O-methyl-6-oxonarcissidine (6) | - | 3.69 | - | - | - | - | 3.30 | - | - | - |
| 1-O-Acetyllycorine (7) | - | 3.37 | - | - | - | - | - | - | - | - |
| Incartine (8) | - | 3.31 | - | - | - | - | - | - | - | - |
| Assoanine (9) | - | - | 3.28 | - | - | - | - | - | - | - |
| Dihydrolycorine (10) | - | - | - | - | - | - | - | 3.29 | - | - |
| 3,4-Dihydro-1-acetyllycorine (11) | - | - | - | - | - | - | - | 6.67 | - | - |
| 2-Methoxypratosine (12) | - | 3.42 | - | - | - | - | - | - | - | - |
| Homolycorine type: | 3.40 | 16.29 | 3.28 | 36.39 | - | - | 305.90 | - | - | - |
| Narseronine (13) | 3.40 | 10.13 | 3.28 | 3.30 | - | - | 293.43 | - | - | - |
| Homolycorine (14) | - | - | - | 33.09 | - | - | - | - | - | - |
| 2-O-Methylclivonine (15) | - | 6.16 | - | - | - | - | 12.47 | - | - | - |
| Haemanthamine type: | - | - | - | - | - | - | - | - | - | 12.07 |
| O-Methylmaritidine (16) | - | - | - | - | - | - | - | - | - | 7.95 |
| O-Methylpapyramine (17) | - | - | - | - | - | - | - | - | - | 4.12 |
| Narciclasine type: | - | - | - | - | - | - | - | 3.28 | - | - |
| Trisphaeridine (18) | - | - | - | - | - | - | - | 3.28 | - | - |
| Tazettine type: | - | - | - | - | - | - | - | 11.67 | 8.36 | 11.80 |
| Tazettine (19) | - | - | - | - | - | - | - | 8.19 | 5.08 | 8.46 |
| 3-O-Demethyltazettine (20) | - | - | - | - | - | - | - | 3.48 | - | - |
| 3-epi-Macronine (21) | - | - | - | - | - | - | - | - | 3.28 | 3.34 |
| Galanthamine type: | - | - | - | 52.03 | - | - | - | 3.29 | - | - |
| Galanthamine (22) | - | - | - | 32.62 | - | - | - | 3.29 | - | - |
| 3-O-Acetylgalanthamine (23) | - | - | - | 5.39 | - | - | - | - | - | - |
| 3-O-Acetylsanguinine (24) | - | - | - | 3.53 | - | - | - | - | - | - |
| Narwedine (25) | - | - | - | 10.49 | - | - | - | - | - | - |
| Miscellaneous type/Unknown: | - | - | 9.62 | 3.39 | 11.90 | 10.67 | 4.22 | 6.65 | - | - |
| Ismine (26) | - | - | - | - | - | - | - | 6.65 | - | - |
| Hordenine (27) | - | - | 4.85 | - | 11.90 | 10.67 | - | - | - | - |
| N-Methyltyramine (28) | - | - | 4.77 | - | - | - | - | - | - | - |
| Unknown I | - | - | - | 3.39 | - | - | - | - | - | - |
| Unknown II (homolycorine type) | - | - | - | - | - | - | 4.22 | - | - | - |
| ALKALOIDS | RI | [M]+ | m/z |
|---|---|---|---|
| Lycorine type: | |||
| Lycorine (1) | 2790.9 | 287 (31) | 286 (19), 268 (24), 250 (15), 227 (79), 226 (100), 211 (7), 147 (15) |
| 11,12-Didehydroanhydrolycorine (2) | 2655.4 | 249 (60) | 248 (100), 191 (10), 190 (24), 189 (7), 163 (7), 95 (17) |
| Galanthine (3) | 2731.6 | 317 (26) | 284 (22), 266 (8), 252 (4), 243 (79), 242 (100) |
| 1-O-Acetyl-3-O-methylnarcissidine (4) | 2768.5 | 389 (3) | 388 (5), 357 (50), 326 (98), 314 (3), 298 (35), 294 (20), 284 (9), 272 (19), 266 (100), 258 (31) |
| 3-O-Methylnarcissidine (5) | 2843.8 | 347 (8) | 348 (2), 346 (16), 315 (47), 298 (6), 284 (100), 266 (35), 258 (22), 242 (8), 230 (38), 228 (30) |
| 1-O-Acetyl-3-O-methyl-6-oxonarcissidine (6) | 3079.5 | 403 (1) | 371 (10), 340 (19), 312 (4), 298 (17), 280 (100), 272 (8), 255 (10) |
| 1-O-Acetyllycorine (7) | 2750.7 | 329 (31) | 268 (31), 250 (20), 226 (100), 211 (6), 192 (3), 167 (3), 147 (6) |
| Incartine (8) | 2778.9 | 333 (30) | 332 (77), 259 (72), 258 (100), 244 (18) |
| Assoanine (9) | 2648.4 | 267 (52) | 266 (100), 250 (31), 222 (14), 207 (14), 193 (12), 180 (15) |
| Dihydrolycorine (10) | 2805.5 | 288 (100) | 272 (7), 254 (3), 214 (6), 200 (2), 187 (6), 162 (6), 147 (15) |
| 3,4-Dihydro-1-acetyllycorine (11) | 2880.0 | 331 (41) | 330 (100), 270 (28), 254 (30), 226 (10), 147 (12), 119 (7), 89 (5) |
| 2—Methoxypratosine (12) | 3059.0 | 309 (100) | 294 (16), 278 (2), 266 (22), 251 (12), 236 (7), 222 (5), 208 (7), 193 (4), 164 (4), 125 (2) |
| Homolycorine type: | |||
| Narseronine (13) | 2957.5 | 329 (20) | 299 (28), 272 (38), 256 (46), 254 (30), 242 (34), 241 (98), 240 (100), 59 (42), 57 (60), 44 (37) |
| Homolycorine (14) | 2787.3 | 315 (<1) | 206 (<1), 178 (2), 109 (100), 150 (1), 108 (22), 94 (3), 82 (3) |
| 2-O-Methylclivonine (15) | 2927.1 | 331 (13) | 316 (6), 162 (3), 134 (2), 126 (2), 115 (2), 96 (39), 83 (100) |
| Haemanthamine type: | |||
| O-Methylmaritidine (16) | 2506.3 | 301 (42) | 286 (24), 270 (22), 231 (100), 203 (26) |
| O-Methylpapyramine (17) | 2544.3 | 331 (50) | 300 (40), 276 (100), 245 (60), 214 (20), 201 (10) |
| Narciclasine type: | |||
| Trisphaeridine (18) | 2356.6 | 223 (100) | 222 (38), 167 (8), 165 (9), 164 (14), 138 (20), 137 (9), 111 (13) |
| Tazettine type: | |||
| Tazettine (19) | 2682.9 | 331 (31) | 316 (15), 298 (23), 247 (100), 230 (12), 201 (15), 181 (11), 152 (7) |
| 3-O-Demethyltazettine (20) | 2748.8 | 317 (24) | 298 (2), 247 (100), 230 (12), 71 (42), 70 (52) |
| 3-epi-Macronine (21) | 2832.3 | 329 (27) | 314 (23), 245 (100), 225 (14), 201 (83), 139 (16), 70 (18) |
| Galanthamine type: | |||
| Galanthamine (22) | 2442.8 | 287 (83) | 286 (100), 270 (13), 244 (24), 230 (12), 216 (33), 174 (27), 115 (12) |
| 3-O-Acetylgalanthamine (23) | 2573.5 | 329 (25) | 270 (100), 216 (20), 165 (15),115 (10) |
| 3-O-Acetylsanguinine (24) | 2623.1 | 315 (37) | 256 (100), 255 (42), 254 (37), 212 (26), 165 (33), 152 (23), 115 (30), 96 (67) |
| Narwedine (25) | 2517.3 | 285 (91) | 284 (100), 242 (18), 216 (20), 199 (18), 174 (31), 128 (16), 115 (16) |
| Miscellaneous type/Unknown: | |||
| Ismine (26) | 2273.0 | 257 (28) | 238 (100), 211 (6), 196 (8), 168 (6), 154 (3), 106 (4), 77 (3) |
| Hordenine (27) | 1559.7 | 165 (<1) | 121 (4), 107 (16), 91 (5), 77 (14), 58 (100) |
| N-Methyltyramine (28) | 1550.6 | 151 (40) | 120 (15), 108 (42), 107 (100), 91 (26), 77 (90), 65 (25) |
| Unknown I | 2872.5 | - | 331 (10), 302 (100), 270 (10), 241 (10), 229 (10), 211 (10), 128 (10), 115 (10) |
| Unknown II (homolycorine type) | 2724.3 | - | 109 (100) |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Between groups | 704.018 | 1 | 704.018 | 0.75 | 0.393 |
| Intra groups | 26185.3 | 28 | 935.189 | ||
| Total | 26889.3 | 29 |
| Plant species | Origin | Date |
|---|---|---|
| Narcissus cavanillesii | Morón de la Frontera (Sevilla) | 18 October 2021 |
| Narcissus obsoletus | Villanueva de Cauche (Málaga) | 18 October 2021 |
| Narcissus deficiens | Morón de la Frontera (Sevilla) | 18 October 2021 |
| Narcissus serotinus | Morón de la Frontera (Sevilla) | 18 October 2021 |
| Narcissus bulbocodium | Los Barrios (Cádiz) | 13 January 2022 |
| Narcissus blancoi | Vilches (Jaén) | 17 March 2022 |
| Narcissus papyraceus | Los Barrios (Cádiz) | 13 January 2022 |
| Narcissus viridiflorus | Facinas (Cádiz) | 8 November 2021 |
| Narcissus elegans | Mallorca (Balearic Islands) | 2 September 2022 |
| Narcissus elegans | Tlemcen (Algeria) | 3 October 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisa-Molina, J.; Gómez-Murillo, P.; Arellano-Martín, I.; Jiménez, C.; Rodríguez-Escobar, M.L.; Tallini, L.R.; Viladomat, F.; Torras-Claveria, L.; Bastida, J. Alkaloid Profile in Wild Autumn-Flowering Daffodils and Their Acetylcholinesterase Inhibitory Activity. Molecules 2023, 28, 1239. https://doi.org/10.3390/molecules28031239
Lisa-Molina J, Gómez-Murillo P, Arellano-Martín I, Jiménez C, Rodríguez-Escobar ML, Tallini LR, Viladomat F, Torras-Claveria L, Bastida J. Alkaloid Profile in Wild Autumn-Flowering Daffodils and Their Acetylcholinesterase Inhibitory Activity. Molecules. 2023; 28(3):1239. https://doi.org/10.3390/molecules28031239
Chicago/Turabian StyleLisa-Molina, Julia, Pedro Gómez-Murillo, Irene Arellano-Martín, Carles Jiménez, María L. Rodríguez-Escobar, Luciana R. Tallini, Francesc Viladomat, Laura Torras-Claveria, and Jaume Bastida. 2023. "Alkaloid Profile in Wild Autumn-Flowering Daffodils and Their Acetylcholinesterase Inhibitory Activity" Molecules 28, no. 3: 1239. https://doi.org/10.3390/molecules28031239
APA StyleLisa-Molina, J., Gómez-Murillo, P., Arellano-Martín, I., Jiménez, C., Rodríguez-Escobar, M. L., Tallini, L. R., Viladomat, F., Torras-Claveria, L., & Bastida, J. (2023). Alkaloid Profile in Wild Autumn-Flowering Daffodils and Their Acetylcholinesterase Inhibitory Activity. Molecules, 28(3), 1239. https://doi.org/10.3390/molecules28031239












