Recent Advances in Supramolecular-Macrocycle-Based Nanomaterials in Cancer Treatment
Abstract
:1. Introduction
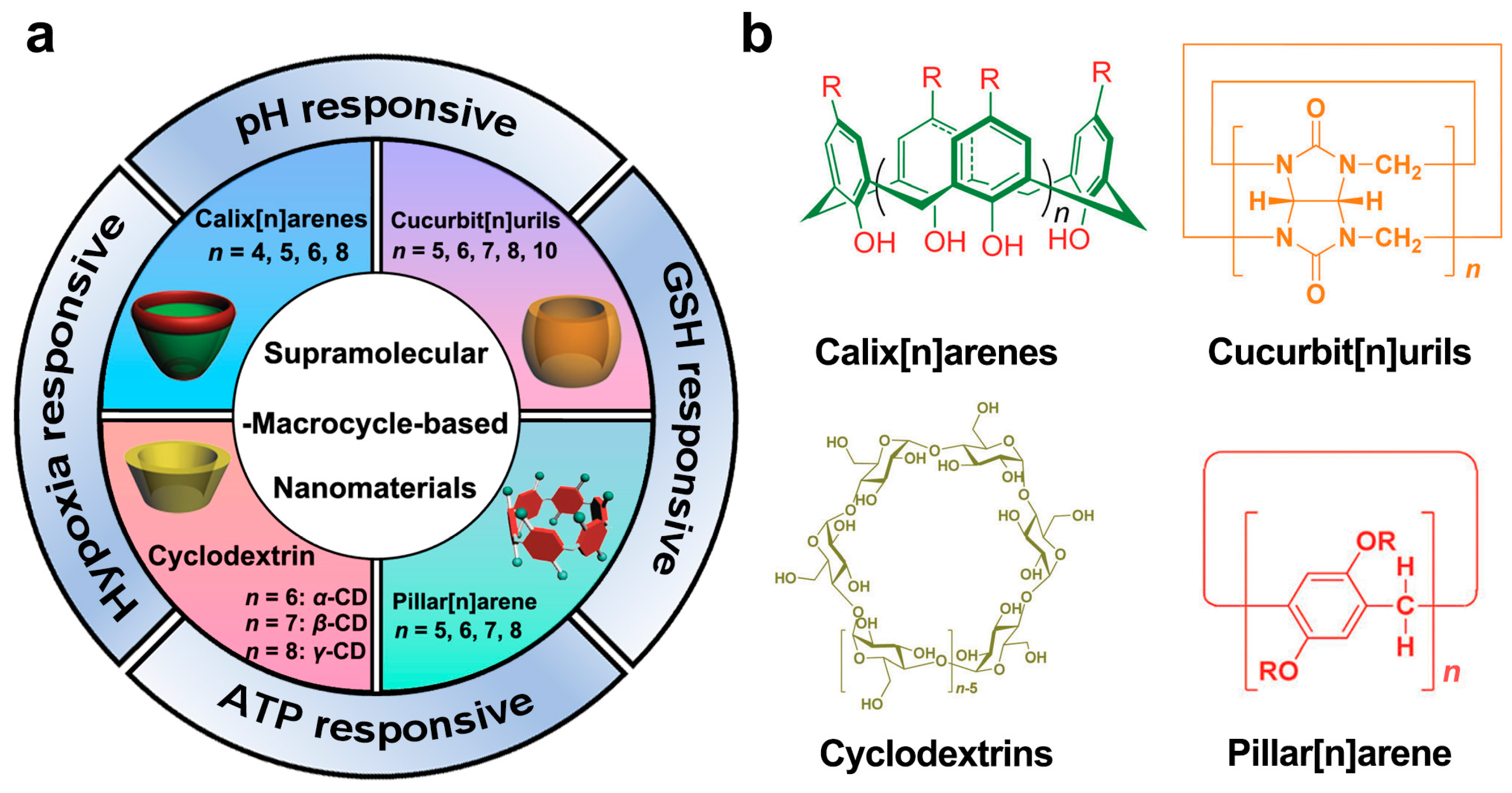
2. Macrocyclic Host Molecules
2.1. Cyclodextrin
2.2. Calix(n)arenes
2.3. Cucurbit(n)urils
2.4. Pillar(n)arene
3. Strategies for Controlled Drug Release
3.1. pH-Responsive Supramolecular-Macrocycle-Based Nanomaterials
3.2. GSH-Responsive Supramolecular-Macrocycle-Based Nanomaterials
3.3. ATP-Responsive Supramolecular-Macrocycle-Based Nanomaterials
3.4. Hypoxia-Responsive Supramolecular-Macrocycle-Based Nanomaterials
4. Supramolecular-Macrocycle-Based Nanomaterials for Enhanced Chemotherapy
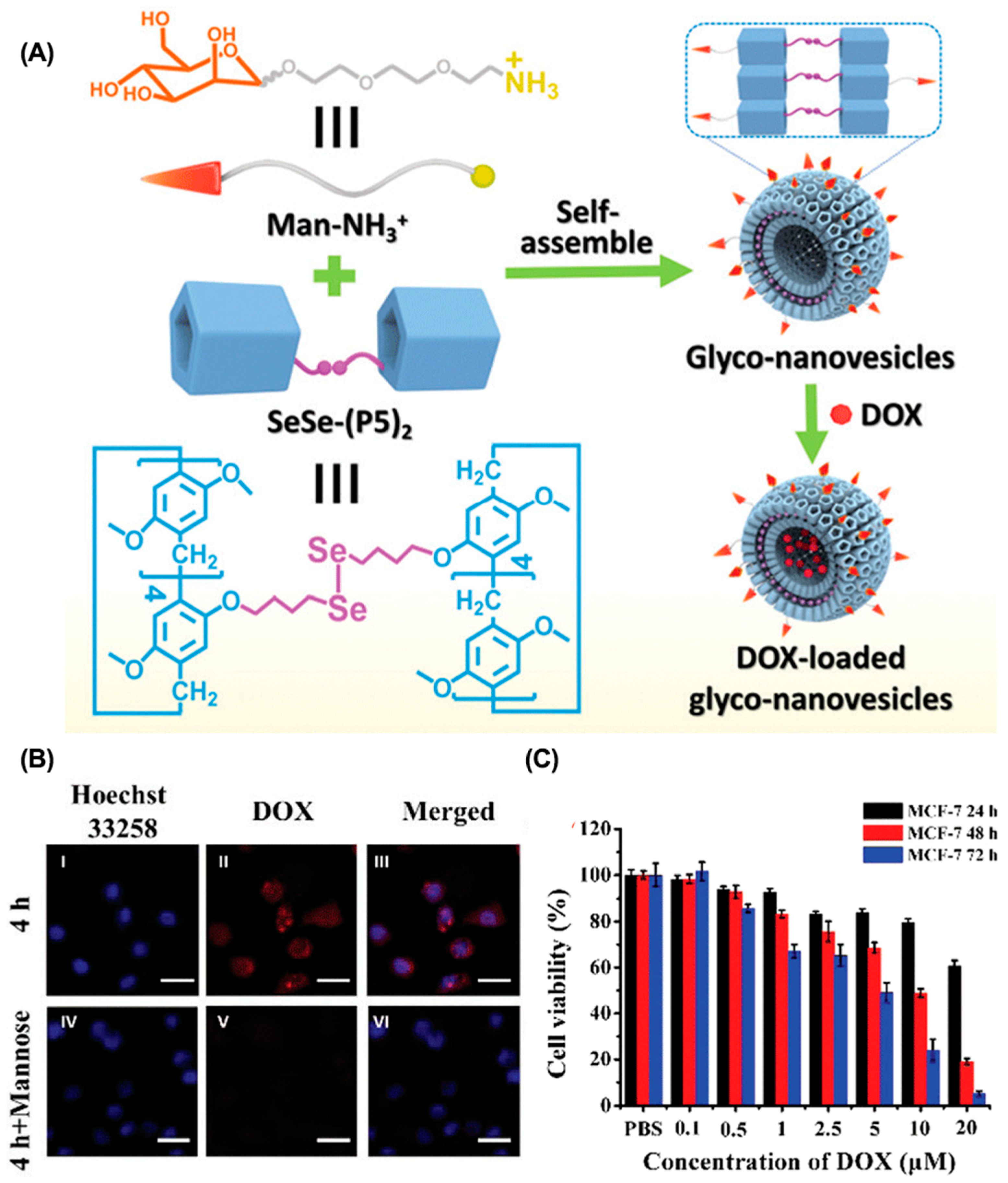
4.1. Enhancing Tumor Accumulation
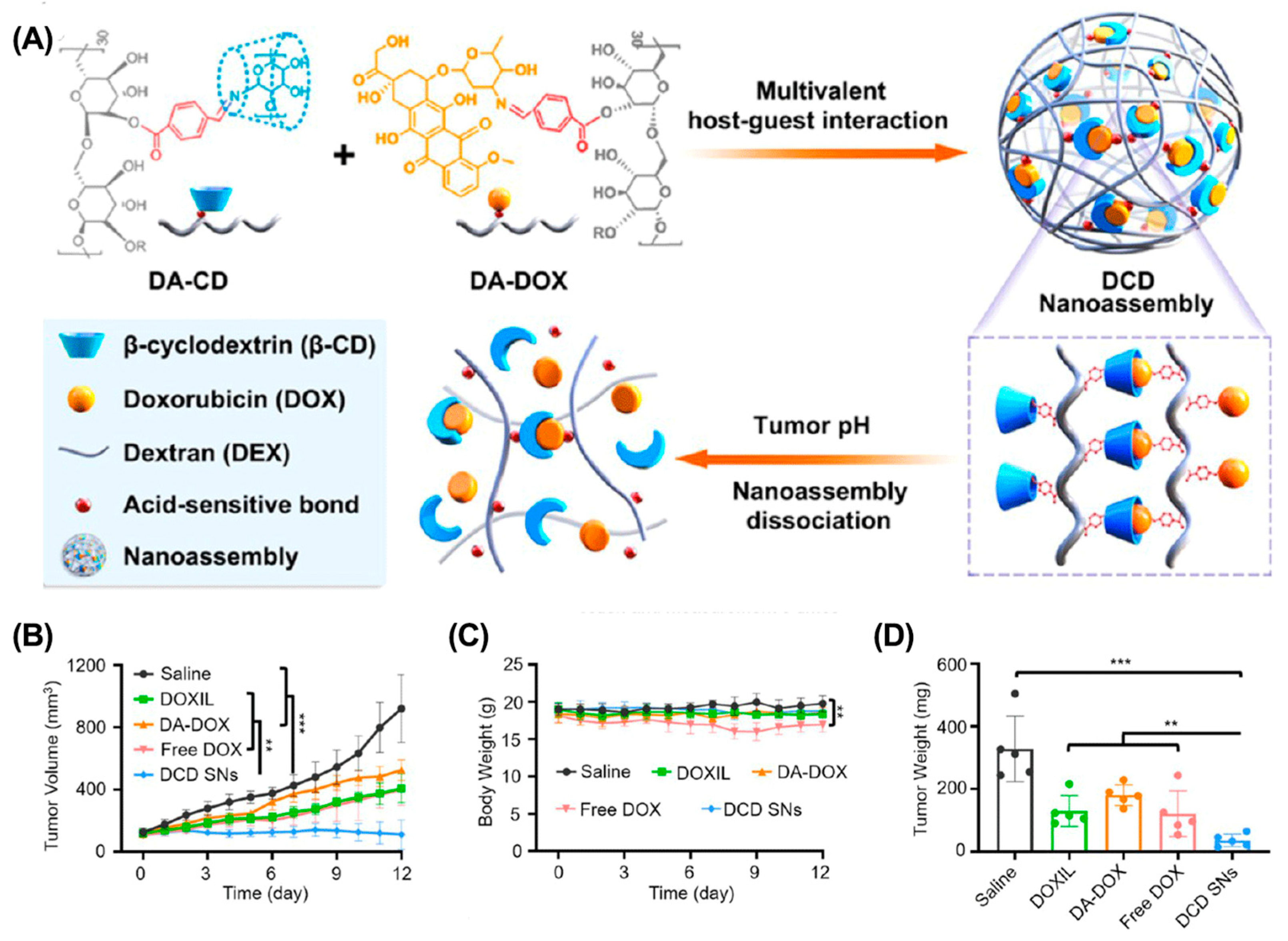
4.2. Enhancing Tumor Penetration
5. Supramolecular-Macrocycle-Based Nanomaterials for Enhanced Photodynamic Therapy
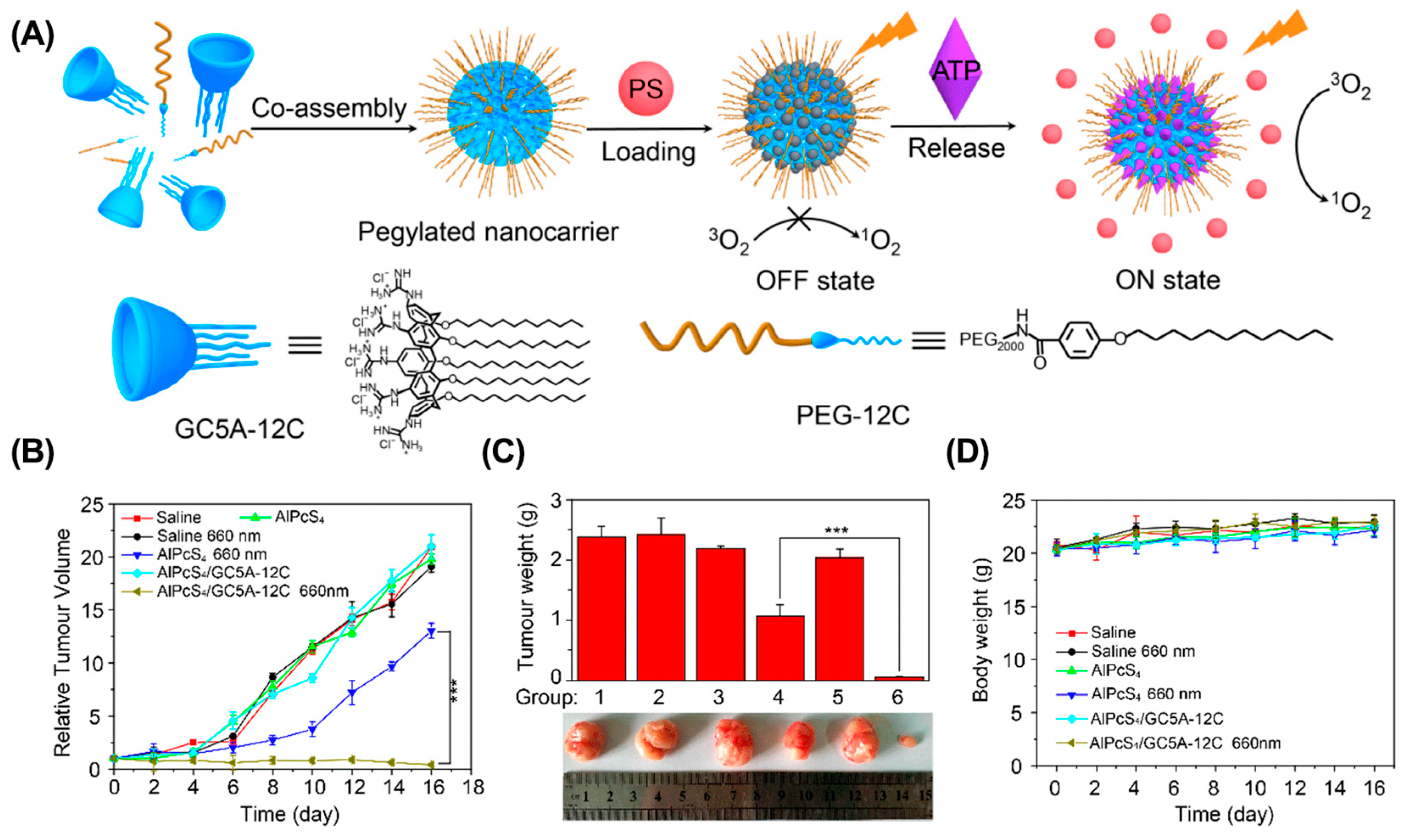
5.1. Overcome the Inherent Defects of PS
5.2. Alleviating Tumor Hypoxia
6. Supramolecular-Macrocycle-Based Nanomaterials for Enhanced Combination Therapy
6.1. Rationales for Combination Therapy
6.2. Supramolecular-Macrocycle-Based Combination Therapy
6.3. Supramolecular-Macrocycle-Based Combination Therapy in Overcoming Drug Resistance
| Types | Macrocycles | Formulations | Payloads | Tumor | Refs |
| Chemotherapy | WP5 | Supramolecular vesicles | DOX | MCF-7 | [86] |
| Chemotherapy | WP5 | Supramolecular vesicles | DOX | HpeG2 | [87] |
| Chemotherapy | WP5 | Vesicles or micelles | DOX | HpeG2 | [88] |
| Chemotherapy | β-CD | LbL films | DOX | A549 | [89] |
| Chemotherapy | β-CD | Supramolecular nanoparticles | DOX | 4T1 | [90] |
| Chemotherapy | WP5 | Supramolecular nanoparticles | DOX | CT26 | [96] |
| PDT | β-CD | Supramolecular nanoparticles | TPP | 4T1 | [107] |
| PDT | β-CD | Supramolecular organic framework | TPP | 4T1 | [108] |
| PDT | GC5A-12C | Supramolecular nanoparticles | AlPcS4 | 4T1 | [111] |
| PDT | SC4A | Supramolecular nanoparticles | TPE-PHO | 4T1 | [112] |
| PDT | WP6 | Supramolecular photosensitizer system | MB | HepG2 | [116] |
| PDT | WP5 | Supramolecular metallodrug micelles | Curcumin | B16 | [118] |
| PDT | β-CD | Supramolecular nanoparticles | Ce6 | 4T1 | [119] |
| PDT | β-CD | Supramolecular vesicles | Ce6 | 4T1 | [121] |
| Chemo-chemotherapy | SAC4A | Calixarene-modified albumin | DOX and MMC | 4T1 | [133] |
| Chemo-chemotherapy | QAAC4A-12C | Supramolecular nanoparticles | PTX and NLG919 | 4T1 | [77] |
| Chemo-photodynamic therapy | CB(7) | Supramolecular vesicles | Ce6 and AQ4N | MCF-7 | [135] |
| Chemo-photodynamic therapy | β-CD | Supramolecular micelles | DOX and P18 | 4T1 | [136] |
| Chemo-photodynamic therapy | WP6 | Supramolecular polypeptide nanomedicine | DOX and ICG | MCF-7/ADR | [137] |
| Chemo-chemotherapy | β-CD | Supramolecular micelles | CPT and Cur | B16 | [149] |
| Combination therapy | AWBpP6 | Supramolecular polypeptide nanomedicine | DOX and SNO | MCF-7/ADR | [137] |
| Chemo-chemotherapy | β-CD | Supramolecular nanoparticles | DOX and ADD | MCF-7/ADR | [151] |
| Photothermal immunotherapy | γ-CD | Gold nanorod-based supramolecular nanomaterial | MA | RM-1 | [142] |
| Photothermal immunotherapy | β-CD | Gold nanorod-based supramolecular nanomaterial | HSP-Cas9 plasmid | B16F10 | [143] |
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, T.; Zimmerman, S.C.; Nakashima, S. A highly stable quadruply hydrogen-bonded heterocomplex useful for supramolecular polymer blends. J. Am. Chem. Soc. 2005, 127, 6520–6521. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybtchinski, B. Adaptive supramolecular nanomaterials based on strong noncovalent interactions. ACS Nano 2011, 5, 6791–6818. [Google Scholar] [CrossRef]
- Liu, K.; Kang, Y.; Wang, Z.; Zhang, X. 25th anniversary article: Reversible and adaptive functional supramolecular materials: “noncovalent interaction” matters. Adv. Mater. 2013, 25, 5530–5548. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.D.; Barooah, N.; Aswal, V.K.; Pal, H.; Bhasikuttan, A.C.; Mohanty, J. Stimuli-responsive supramolecular micellar assemblies of cetylpyridinium chloride with cucurbit[5/7]urils. Soft Matter 2014, 10, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host-guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef]
- Lee, S.F.; Zhu, X.M.; Wang, Y.X.; Xuan, S.H.; You, Q.; Chan, W.H.; Wong, C.H.; Wang, F.; Yu, J.C.; Cheng, C.H.; et al. Ultrasound, pH, and magnetically responsive crown-ether-coated core/shell nanoparticles as drug encapsulation and release systems. ACS Appl. Mater. Interfaces 2013, 5, 1566–1574. [Google Scholar] [CrossRef]
- Xu, X.; Jerca, V.V.; Hoogenboom, R. Structural diversification of pillar[n]arene macrocycles. Angew. Chem. Int. Ed. 2020, 59, 6314–6316. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lin, W.; Liu, Y. Macrocyclic supramolecular assemblies based on hyaluronic acid and their biological applications. Acc. Chem. Res. 2022, 55, 3417–3429. [Google Scholar] [CrossRef]
- Hettiarachchi, G.; Nguyen, D.; Wu, J.; Lucas, D.; Ma, D.; Isaacs, L.; Briken, V. Toxicology and drug delivery by cucurbit[n]uril type molecular containers. PLoS ONE 2010, 5, e10514. [Google Scholar] [CrossRef] [Green Version]
- Shangguan, L.; Chen, Q.; Shi, B.; Huang, F. Enhancing the solubility and bioactivity of anticancer drug tamoxifen by water-soluble pillar[6]arene-based host-guest complexation. Chem. Commun. 2017, 53, 9749–9752. [Google Scholar] [CrossRef] [PubMed]
- Shetty, D.; Skorjanc, T.; Olson, M.A.; Trabolsi, A. Self-assembly of stimuli-responsive imine-linked calix[4]arene nanocapsules for targeted camptothecin delivery. Chem. Commun. 2019, 55, 8876–8879. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Chen, Y.; Huang, Z.; Xu, J.F.; Sun, Z.; Zhang, X. Supramolecular chemotherapy: Carboxylated pillar[6]arene for decreasing cytotoxicity of oxaliplatin to normal cells and improving its anticancer bioactivity against colorectal cancer. ACS Appl. Mater. Interfaces 2018, 10, 5365–5372. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, J.W.; Liu, J.D.; Zhao, Z.M.; Song, Y.J. Design, preparation, and characterization of novel calix[4]arene bioactive carrier for antitumor drug delivery. Front. Chem. 2019, 7, 732. [Google Scholar] [CrossRef]
- Yu, G.; Zhou, X.; Zhang, Z.; Han, C.; Mao, Z.; Gao, C.; Huang, F. Pillar[6]arene/paraquat molecular recognition in water: High binding strength, pH-responsiveness, and application in controllable self-assembly, controlled release, and treatment of paraquat poisoning. J. Am. Chem. Soc. 2012, 134, 19489–19497. [Google Scholar] [CrossRef]
- Petronella, F.; De Biase, D.; Zaccagnini, F.; Verrina, V.; Lim, S.I.; Jeong, K.U.; Miglietta, S.; Petrozza, V.; Scognamiglio, V.; Godman, N.P.; et al. Label-free and reusable antibody-functionalized gold nanorod arrays for the rapid detection of Escherichia coli cells in a water dispersion. Environ. Sci. Nano 2022, 9, 3343–3360. [Google Scholar] [CrossRef]
- Montanarella, F.; Kovalenko, M.V. Three millennia of nanocrystals. ACS Nano 2022, 16, 5085–5102. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y. Cyclodextrin-based bioactive supramolecular assemblies. Chem. Soc. Rev. 2010, 39, 495–505. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Villalonga, R.; Cao, R.; Fragoso, A. Supramolecular chemistry of cyclodextrins in enzyme technology. Chem. Rev. 2007, 107, 3088–3116. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug. Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.D.; Tang, G.P.; Chu, P.K. Cyclodextrin-based host-guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Engel, S.; Möller, N.; Ravoo, B.J. Stimulus-responsive assembly of nanoparticles using host-guest interactions of cyclodextrins. Chem. Eur. J. 2018, 24, 4741–4748. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Host-guest interactions mediated nano-assemblies using cyclodextrin-containing hydrophilic polymers and their biomedical applications. Nano Today 2010, 5, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Mejia-Ariza, R.; Graña-Suárez, L.; Verboom, W.; Huskens, J. Cyclodextrin-based supramolecular nanoparticles for biomedical applications. J. Mater. Chem. B 2017, 5, 36–52. [Google Scholar] [CrossRef]
- Wang, J.; Ding, X.; Guo, X. Assembly behaviors of calixarene-based amphiphile and supra-amphiphile and the applications in drug delivery and protein recognition. Adv. Colloid Interface Sci. 2019, 269, 187–202. [Google Scholar] [CrossRef]
- Pan, Y.C.; Hu, X.Y.; Guo, D.S. Biomedical applications of calixarenes: State of the art and perspectives. Angew. Chem. Int. Ed. 2021, 60, 2768–2794. [Google Scholar] [CrossRef]
- Giuliani, M.; Morbioli, I.; Sansone, F.; Casnati, A. Correction: Moulding calixarenes for biomacromolecule targeting. Chem. Commun. 2015, 51, 15208. [Google Scholar] [CrossRef] [Green Version]
- Naseer, M.M.; Ahmed, M.; Hameed, S. Functionalized calix[4]arenes as potential therapeutic agents. Chem. Biol. Drug Des. 2017, 89, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Perret, F.; Lazar, A.N.; Coleman, A.W. Biochemistry of the para-sulfonato-calix[n]arenes. Chem. Commun. 2006, 21, 2425–2438. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.H.; Zhao, Y. Efficient synthesis of water-soluble calixarenes using click chemistry. Org. Lett. 2005, 7, 1035–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, D.S.; Liu, Y. Calixarene-based supramolecular polymerization in solution. Chem. Soc. Rev. 2012, 41, 5907–5921. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Kong, Y.; Zheng, Z.; Geng, W.C.; Zhao, Z.Y.; Sun, H.; Guo, D.S. Complexation of a guanidinium-modified calixarene with diverse dyes and investigation of the corresponding photophysical response. Beilstein J. Org. Chem. 2019, 15, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Kim, T. Biological applications of functionalized calixarenes. Chem. Soc. Rev. 2013, 42, 366–386. [Google Scholar] [CrossRef]
- Day, A.; Arnold, A.P.; Blanch, R.J.; Snushall, B. Controlling factors in the synthesis of cucurbituril and its homologues. J. Org. Chem. 2001, 66, 8094–8100. [Google Scholar] [CrossRef]
- Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. The cucurbit[n]uril family. Angew. Chem. Int. Ed. 2005, 44, 4844–4870. [Google Scholar] [CrossRef]
- Liu, S.; Zavalij, P.Y.; Isaacs, L. Cucurbit[10]uril. J. Am. Chem. Soc. 2005, 127, 16798–16799. [Google Scholar] [CrossRef]
- Moghaddam, S.; Yang, C.; Rekharsky, M.; Ko, Y.H.; Kim, K.; Inoue, Y.; Gilson, M.K. New ultrahigh affinity host-guest complexes of cucurbit[7]uril with bicyclo[2.2.2]octane and adamantane guests: Thermodynamic analysis and evaluation of M2 affinity calculations. J. Am. Chem. Soc. 2011, 133, 3570–3581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Samal, S.; Selvapalam, N.; Kim, H.J.; Kim, K. Cucurbituril homologues and derivatives: New opportunities in supramolecular chemistry. Acc. Chem. Res. 2003, 36, 621–630. [Google Scholar] [CrossRef]
- Jon, S.Y.; Selvapalam, N.; Oh, D.H.; Kang, J.K.; Kim, S.Y.; Jeon, Y.J.; Lee, J.W.; Kim, K. Facile synthesis of cucurbit[n]uril derivatives via direct functionalization: Expanding utilization of cucurbit[n]uril. J. Am. Chem. Soc. 2003, 125, 10186–10187. [Google Scholar] [CrossRef]
- Sasmal, S.; Sinha, M.K.; Keinan, E. Facile purification of rare cucurbiturils by affinity chromatography. Org. Lett. 2004, 6, 1225–1228. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.A.; Nakamoto, Y. para-Bridged symmetrical pillar[5]arenes: Their lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Li, C.; Shu, X.; Li, J.; Chen, S.; Han, K.; Xu, M.; Hu, B.; Yu, Y.; Jia, X. Complexation of 1,4-bis(pyridinium)butanes by negatively charged carboxylatopillar[5]arene. J. Org. Chem. 2011, 76, 8458–8465. [Google Scholar] [CrossRef]
- Yu, G.; Xue, M.; Zhang, Z.; Li, J.; Han, C.; Huang, F. A water-soluble pillar[6]arene: Synthesis, host-guest chemistry, and its application in dispersion of multiwalled carbon nanotubes in water. J. Am. Chem. Soc. 2012, 134, 13248–13251. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Gao, Z.; Wang, H.; Shi, B.; Wu, Y.; Shangguan, L.; Hong, X.; Wang, F.; Huang, F. Pillararene host-guest complexation induced chirality amplification: A new way to detect cryptochiral compounds. Angew. Chem. Int. Ed. 2020, 59, 10868–10872. [Google Scholar] [CrossRef]
- Xia, W.; Hu, X.Y.; Chen, Y.; Lin, C.; Wang, L. A novel redox-responsive pillar[6]arene-based inclusion complex with a ferrocenium guest. Chem. Commun. 2013, 49, 5085–5087. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, X.Y.; Li, Y.; Zou, X.; Xiong, S.; Lin, C.; Shen, Y.Z.; Wang, L. Multistimuli-responsive supramolecular vesicles based on water-soluble pillar[6]arene and SAINT complexation for controllable drug release. J. Am. Chem. Soc. 2014, 136, 10762–10769. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, X.; Chen, D.; Yan, H.; Li, X.; Du, X. Supramolecular vesicles coassembled from disulfide-linked benzimidazolium amphiphiles and carboxylate-substituted pillar[6]arenes that are responsive to five stimuli. Angew. Chem. Int. Ed. 2017, 56, 2655–2659. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Yang, Y.; Zhang, Y.H.; Liu, Y. Polysaccharide nanoparticles for efficient siRNA targeting in cancer cells by supramolecular pKa shift. Sci. Rep. 2016, 6, 28848. [Google Scholar] [CrossRef] [Green Version]
- Kaizerman-Kane, D.; Hadar, M.; Tal, N.; Dobrovetsky, R.; Zafrani, Y.; Cohen, Y. pH-Responsive pillar[6]arene-based water-soluble supramolecular hexagonal boxes. Angew. Chem. Int. Ed. 2019, 58, 5302–5306. [Google Scholar] [CrossRef]
- Dan, Z.; Cao, H.; He, X.; Zhang, Z.; Zou, L.; Zeng, L.; Xu, Y.; Yin, Q.; Xu, M.; Zhong, D.; et al. A pH-responsive host-guest nanosystem loading succinobucol suppresses lung metastasis of breast cancer. Theranostics 2016, 6, 435–445. [Google Scholar] [CrossRef]
- Li, B.; Meng, Z.; Li, Q.; Huang, X.; Kang, Z.; Dong, H.; Chen, J.; Sun, J.; Dong, Y.; Li, J.; et al. A pH responsive complexation-based drug delivery system for oxaliplatin. Chem. Sci. 2017, 8, 4458–4464. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Jia, X.; Zhang, Y.M.; Li, N.; Liu, Y. Supramolecular nanoparticles based on β-CD modified hyaluronic acid for DNA encapsulation and controlled release. Chem. Commun. 2018, 54, 8713–8716. [Google Scholar] [CrossRef]
- Cheng, Y.; Ji, Y. Mitochondria-targeting nanomedicine self-assembled from GSH-responsive paclitaxel-ss-berberine conjugate for synergetic cancer treatment with enhanced cytotoxicity. J. Control. Release 2020, 318, 38–49. [Google Scholar] [CrossRef]
- Kang, Y.; Ju, X.; Ding, L.S.; Zhang, S.; Li, B.J. Reactive oxygen species and glutathione dual redox-responsive supramolecular assemblies with controllable release capability. ACS Appl. Mater. Interfaces 2017, 9, 4475–4484. [Google Scholar] [CrossRef]
- Liu, X.; Shao, W.; Zheng, Y.; Yao, C.; Peng, L.; Zhang, D.; Hu, X.Y.; Wang, L. GSH-Responsive supramolecular nanoparticles constructed by β-d-galactose-modified pillar[5]arene and camptothecin prodrug for targeted anticancer drug delivery. Chem. Commun. 2017, 53, 8596–8599. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Liang, M.; Ye, M.; Xu, J.; Liu, H.; Zhang, X.; Sun, W.; Xue, P.; Kang, Y.; Xu, Z. Reduction-triggered polycyclodextrin supramolecular nanocage induces immunogenic cell death for improved chemotherapy. Carbohydr. Polym. 2023, 301, 120365. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, K.; Wei, P.; Huang, S.; Pei, Y.; Zhao, W.; Pei, Z. Cationic vesicles based on amphiphilic pillar[5]arene capped with ferrocenium: A redox-responsive system for drug/siRNA co-delivery. Angew. Chem. Int. Ed. 2014, 53, 13126–13130. [Google Scholar] [CrossRef]
- Yasen, W.; Dong, R.; Zhou, L.; Wu, J.; Cao, C.; Aini, A.; Zhu, X. Synthesis of a cationic supramolecular block copolymer with covalent and noncovalent polymer blocks for gene delivery. ACS Appl. Mater. Interfaces 2017, 9, 9006–9014. [Google Scholar] [CrossRef]
- Lee, H.; Woo, S.M.; Jang, H.; Kang, M.; Kim, S.Y. Cancer depends on fatty acids for ATP production: A possible link between cancer and obesity. Semin. Cancer Biol. 2022, 86, 347–357. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, D.H.; Hong, J.I.; Yoon, J. Chemosensors for pyrophosphate. Acc. Chem. Res. 2009, 42, 23–31. [Google Scholar] [CrossRef]
- Fuentes, E.; Palomo, I. Extracellular ATP metabolism on vascular endothelial cells: A pathway with pro-thrombotic and anti-thrombotic molecules. Vasc. Pharmacol. 2015, 75, 1–6. [Google Scholar] [CrossRef]
- Pellegatti, P.; Raffaghello, L.; Bianchi, G.; Piccardi, F.; Pistoia, V.; Di Virgilio, F. Increased level of extracellular ATP at tumor sites: In vivo imaging with plasma membrane luciferase. PLoS ONE 2008, 3, e2599. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Chai, J.; Wang, Y.; Zhao, X.; Guo, D.-S.; Shi, L.; Zhang, Z.; Liu, Y. Calixarene-integrated nano-drug delivery system for tumor-targeted delivery and tracking of anti-cancer drugs in vivo. Nano Res. 2022, 15, 7295–7303. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Zhao, L.; Zhang, Y.; Chen, L.; Ma, M.; Du, X.; Meng, Z.; Li, C.; Meng, Q. Supramolecular drug delivery system from macrocycle-based self-assembled amphiphiles for effective tumor therapy. ACS Appl. Mater. Interfaces 2021, 13, 53564–53573. [Google Scholar] [CrossRef]
- Yu, G.; Zhou, J.; Shen, J.; Tang, G.; Huang, F. Cationic pillar[6]arene/ATP host-guest recognition: Selectivity, inhibition of ATP hydrolysis, and application in multidrug resistance treatment. Chem. Sci. 2016, 7, 4073–4078. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.X.; Hou, X.; Kong, Y.; Yang, F.; Yue, Y.X.; Shah, M.R.; Li, H.B.; Huang, F.; Liu, J.; Guo, D.S. A hypoxia-responsive supramolecular formulation for imaging-guided photothermal therapy. Theranostics 2022, 12, 396–409. [Google Scholar] [CrossRef]
- Piao, W.; Tsuda, S.; Tanaka, Y.; Maeda, S.; Liu, F.; Takahashi, S.; Kushida, Y.; Komatsu, T.; Ueno, T.; Terai, T.; et al. Development of azo-based fluorescent probes to detect different levels of hypoxia. Angew. Chem. Int. Ed. 2013, 52, 13028–13032. [Google Scholar] [CrossRef]
- Yao, S.Y.; Yue, Y.X.; Ying, A.K.; Hu, X.Y.; Li, H.B.; Cai, K.; Guo, D.S. An antitumor dual-responsive host-guest supramolecular polymer based on hypoxia-cleavable azocalix[4]arene. Angew. Chem. Int. Ed. 2023, 62, e202213578. [Google Scholar] [CrossRef]
- Li, J.J.; Hu, Y.; Hu, B.; Wang, W.; Xu, H.; Hu, X.Y.; Ding, F.; Li, H.B.; Wang, K.R.; Zhang, X.; et al. Lactose azocalixarene drug delivery system for the treatment of multidrug-resistant pseudomonas aeruginosa infected diabetic ulcer. Nat. Commun. 2022, 13, 6279. [Google Scholar] [CrossRef]
- Geng, W.C.; Jia, S.; Zheng, Z.; Li, Z.; Ding, D.; Guo, D.S. A noncovalent fluorescence turn-on strategy for hypoxia imaging. Angew. Chem. Int. Ed. 2019, 58, 2377–2381. [Google Scholar] [CrossRef]
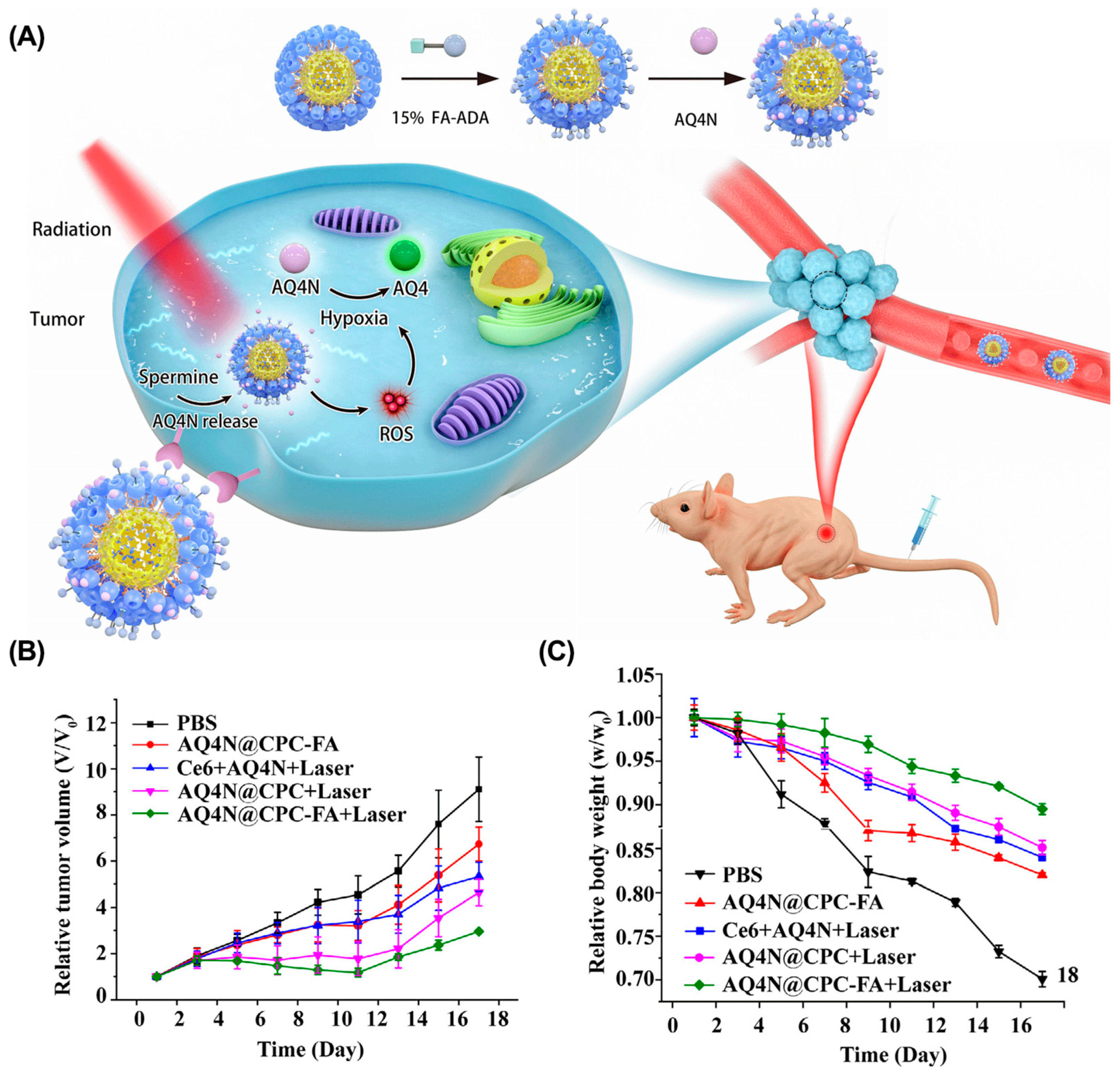
- Zhang, T.X.; Zhang, Z.Z.; Yue, Y.X.; Hu, X.Y.; Huang, F.; Shi, L.; Liu, Y.; Guo, D.S. A general hypoxia-responsive molecular container for tumor-targeted therapy. Adv. Mater. 2020, 32, e1908435. [Google Scholar] [CrossRef]
- Yue, Y.-X.; Zhang, Z.; Wang, Z.-H.; Ma, R.; Chen, M.-M.; Ding, F.; Li, H.-B.; Li, J.-J.; Shi, L.; Liu, Y.; et al. Promoting tumor accumulation of anticancer drugs by hierarchical carrying of exogenous and endogenous vehicles. Small Struct. 2022, 3, 2200067. [Google Scholar] [CrossRef]
- Zhang, Z.; Yue, Y.X.; Xu, L.; Wang, Y.; Geng, W.C.; Li, J.J.; Kong, X.L.; Zhao, X.; Zheng, Y.; Zhao, Y.; et al. Macrocyclic-amphiphile-based self-assembled nanoparticles for ratiometric delivery of therapeutic combinations to tumors. Adv. Mater. 2021, 33, e2007719. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, Z.; Sun, B.; Chen, Q.; Sun, J.; He, Z.; Luo, C. Nanotherapeutics for antimetastatic treatment. Trends Cancer 2020, 6, 645–659. [Google Scholar] [CrossRef]
- Demaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Dai, W.; Mei, D.; Liu, T.; Li, S.; He, B.; He, B.; Yuan, L.; Zhang, H.; Wang, X.; et al. Comprehensively priming the tumor microenvironment by cancer-associated fibroblast-targeted liposomes for combined therapy with cancer cell-targeted chemotherapeutic drug delivery system. J. Control. Release 2016, 241, 68–80. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, X. Theoretical study of macrocyclic host molecules: From supramolecular recognition to self-assembly. Phys. Chem. Chem. Phys. 2022, 24, 19011–19028. [Google Scholar] [CrossRef] [PubMed]
- Mei, K.C.; Bai, J.; Lorrio, S.; Wang, J.T.; Al-Jamal, K.T. Investigating the effect of tumor vascularization on magnetic targeting in vivo using retrospective design of experiment. Biomaterials 2016, 106, 276–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.; Liu, C.; Guo, M.; Cheng, H.; Lu, Y.; Jin, K.; Liu, L.; Long, J.; Xu, J.; Lu, R.; et al. Potential biomarkers in lewis negative patients with pancreatic cancer. Ann. Surg. 2017, 265, 800–805. [Google Scholar] [CrossRef]
- Kline, J.B.; Fernando, S.; Ross, E.N.; Grasso, L.; Nicolaides, N.C. Tumor-shed antigen CA125 blocks complement-mediated killing via suppression of C1q-antibody binding. Eur. J. Immunol. 2018, 48, 1872–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babamiri, B.; Hallaj, R.; Salimi, A. Ultrasensitive electrochemiluminescence immunoassay for simultaneous determination of CA125 and CA15-3 tumor markers based on PAMAM-sulfanilic acid-Ru(bpy)(3)(2+) and PAMAM-CdTe@CdS nanocomposite. Biosens. Bioelectron. 2018, 99, 353–360. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, M.; Chen, Z.; Hu, X.; Pu, L.; Pei, Z.; Pei, Y. Tumor microenvironment responsive supramolecular glyco-nanovesicles based on diselenium-bridged pillar[5]arene dimer for targeted chemotherapy. Chem. Commun. 2020, 56, 10642–10645. [Google Scholar] [CrossRef]
- Lu, Y.; Hou, C.; Ren, J.; Yang, K.; Chang, Y.; Pei, Y.; Dong, H.; Pei, Z. A multifunctional supramolecular vesicle based on complex of cystamine dihydrochloride capped pillar[5]arene and galactose derivative for targeted drug delivery. Int. J. Nanomed. 2019, 14, 3525–3532. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.Y.; Gao, L.; Mosel, S.; Ehlers, M.; Zellermann, E.; Jiang, H.; Knauer, S.K.; Wang, L.; Schmuck, C. From supramolecular vesicles to micelles: Controllable construction of tumor-targeting nanocarriers based on host-guest interaction between a pillar[5]arene-based prodrug and a RGD-sulfonate guest. Small 2018, 14, e1803952. [Google Scholar] [CrossRef]
- Huang, T.; Luan, X.; Xia, Q.; Pan, S.; An, Q.; Wu, Y.; Zhang, Y. Molecularly selective regulation of delivery fluxes by employing supramolecular interactions in layer-by-layer films. Chem. Asian J. 2018, 13, 1067–1073. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, Z.; Qiu, N.; Shen, Y. Rational design of cancer nanomedicine: Nanoproperty integration and synchronization. Adv. Mater. 2017, 29, 1606628. [Google Scholar] [CrossRef]
- Li, Z.; Shan, X.; Chen, Z.; Gao, N.; Zeng, W.; Zeng, X.; Mei, L. Applications of surface modification technologies in nanomedicine for deep tumor penetration. Adv. Sci. 2020, 8, 2002589. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm. Sin. B 2021, 11, 2265–2285. [Google Scholar] [CrossRef]
- Yan, X.; Ma, F.; Chen, Q.; Gou, X.; Li, X.; Zhang, L.; Gao, H. Construction of size-transformable supramolecular nano-platform against drug-resistant colorectal cancer caused by Fusobacterium nucleatum. Chem. Eng. J. 2022, 450, 137605. [Google Scholar] [CrossRef]
- Hu, D.; Deng, Y.; Jia, F.; Jin, Q.; Ji, J. Surface charge switchable supramolecular nanocarriers for nitric oxide synergistic photodynamic eradication of biofilms. ACS Nano 2020, 14, 347–359. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Q.; Yue, L.; Gao, C.; Wei, J.; Ding, Y.; Wang, Y.; Zheng, Y.; Wang, R. Macrophage-hitchhiking supramolecular aggregates of CuS nanoparticles for enhanced tumor deposition and photothermal therapy. Nanoscale Horiz. 2021, 6, 907–912. [Google Scholar] [CrossRef]
- Liang, M.; Gao, Y.; Qiu, W.; Ye, M.; Hu, J.; Xu, J.; Xue, P.; Kang, Y.; Xu, Z. Acid-sensitive supramolecular nanoassemblies with multivalent interaction: Effective tumor retention and deep intratumor infiltration. ACS Appl. Mater. Interfaces 2021, 13, 37680–37692. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Sun, X.; Zhou, J.; Chen, X.; Xi, J.; Fan, L.; Han, J.; Guo, R. Supramolecular core-shell nanoassemblies with tumor microenvironment-triggered size and structure switch for improved photothermal therapy. Small 2022, 18, e2200588. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J., III; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition metal complexes and photodynamic therapy from a tumor-centered approach: Challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Tang, X.L.; Wang, Z.; Zhu, Y.Y.; Xiao, H.; Xiao, Y.; Cui, S.; Lin, B.L.; Yang, K.; Liu, H.Y. Hypoxia-activated ROS burst liposomes boosted by local mild hyperthermia for photo/chemodynamic therapy. J. Control. Release 2020, 328, 100–111. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic therapy: Tumour microenvironment-mediated fenton and fenton-like reactions. Angew. Chem. Int. Ed. 2019, 58, 946–956. [Google Scholar] [CrossRef]
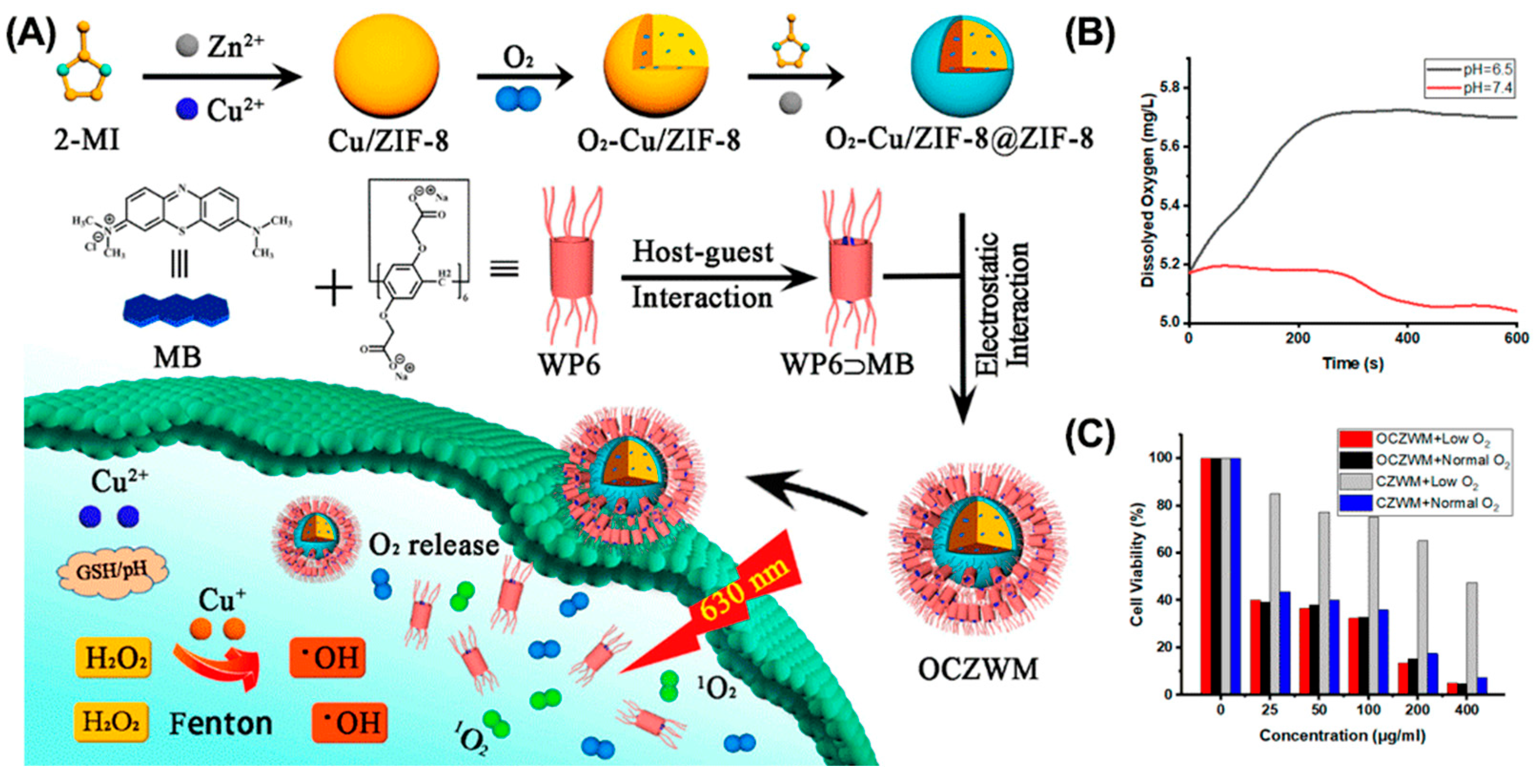
- Yang, K.; Wen, J.; Chao, S.; Liu, J.; Yang, K.; Pei, Y.; Pei, Z. A supramolecular photosensitizer system based on the host-guest complexation between water-soluble pillar[6]arene and methylene blue for durable photodynamic therapy. Chem. Commun. 2018, 54, 5911–5914. [Google Scholar] [CrossRef]
- Khurana, R.; Kakatkar, A.S.; Chatterjee, S.; Barooah, N.; Kunwar, A.; Bhasikuttan, A.C.; Mohanty, J. Supramolecular nanorods of (N-Methylpyridyl) porphyrin with captisol: Effective photosensitizer for anti-bacterial and anti-tumor activities. Front. Chem. 2019, 7, 452. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Wang, Y.; Sun, C.; Bao, L.; Zhao, Y.; Yang, X.; Zhao, Y. A photosensitizer discretely loaded nanoaggregate with robust photodynamic effect for local treatment triggers systemic antitumor responses. ACS Nano 2022, 16, 3070–3080. [Google Scholar] [CrossRef]
- Li, X.; Kim, C.Y.; Shin, J.M.; Lee, D.; Kim, G.; Chung, H.M.; Hong, K.S.; Yoon, J. Mesenchymal stem cell-driven activatable photosensitizers for precision photodynamic oncotherapy. Biomaterials 2018, 187, 18–26. [Google Scholar] [CrossRef]
- Li, Y.; Lin, T.Y.; Luo, Y.; Liu, Q.; Xiao, W.; Guo, W.; Lac, D.; Zhang, H.; Feng, C.; Wachsmann-Hogiu, S.; et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat. Commun. 2014, 5, 4712. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Xia, L.; Wu, J.; Huang, B.; Cao, H.; Zhang, W. Linear alternating supramolecular photosensitizer for enhanced photodynamic therapy. ACS Appl. Mater. Interfaces 2020, 12, 32352–32359. [Google Scholar] [CrossRef]
- Tian, J.; Huang, B.; Cui, Z.; Wang, P.; Chen, S.; Yang, G.; Zhang, W. Mitochondria-targeting and ROS-sensitive smart nanoscale supramolecular organic framework for combinational amplified photodynamic therapy and chemotherapy. Acta Biomater. 2021, 130, 447–459. [Google Scholar] [CrossRef]
- Guo, L.; Niu, G.; Zheng, X.; Ge, J.; Liu, W.; Jia, Q.; Zhang, P.; Zhang, H.; Wang, P. Single near-infrared emissive polymer nanoparticles as versatile phototheranostics. Adv. Sci. 2017, 4, 1700085. [Google Scholar] [CrossRef]
- Lovell, J.F.; Liu, T.W.; Chen, J.; Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef]
- Gao, J.; Li, J.; Geng, W.C.; Chen, F.Y.; Duan, X.; Zheng, Z.; Ding, D.; Guo, D.S. Biomarker displacement activation: A general host-guest strategy for targeted phototheranostics in vivo. J. Am. Chem. Soc. 2018, 140, 4945–4953. [Google Scholar] [CrossRef]
- Feng, H.T.; Li, Y.; Duan, X.; Wang, X.; Qi, C.; Lam, J.W.Y.; Ding, D.; Tang, B.Z. Substitution activated precise phototheranostics through supramolecular assembly of AIEgen and calixarene. J. Am. Chem. Soc. 2020, 142, 15966–15974. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Weston, M.H.; Fuller, P.E.; Tovar, T.M.; Peterson, G.W.; LeVan, M.D.; Farha, O.K. Metal-organic frameworks for oxygen storage. Angew. Chem. Int. Ed. 2014, 53, 14092–14095. [Google Scholar] [CrossRef]
- Rummer, J.L.; McKenzie, D.J.; Innocenti, A.; Supuran, C.T.; Brauner, C.J. Root effect hemoglobin may have evolved to enhance general tissue oxygen delivery. Science 2013, 340, 1327–1329. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Yu, Y.; Chao, S.; Zhu, H.; Pei, Y.; Chen, L.; Pei, Z. A supramolecular photosensitizer system based on nano-Cu/ZIF-8 capped with water-soluble pillar[6]arene and methylene blue host-guest complexations. Molecules 2021, 26, 3878. [Google Scholar] [CrossRef]
- Fang, X.; Cai, S.; Wang, M.; Chen, Z.; Lu, C.; Yang, H. Photogenerated holes mediated nitric oxide production for hypoxic tumor treatment. Angew. Chem. Int. Ed. 2021, 60, 7046–7050. [Google Scholar] [CrossRef] [PubMed]
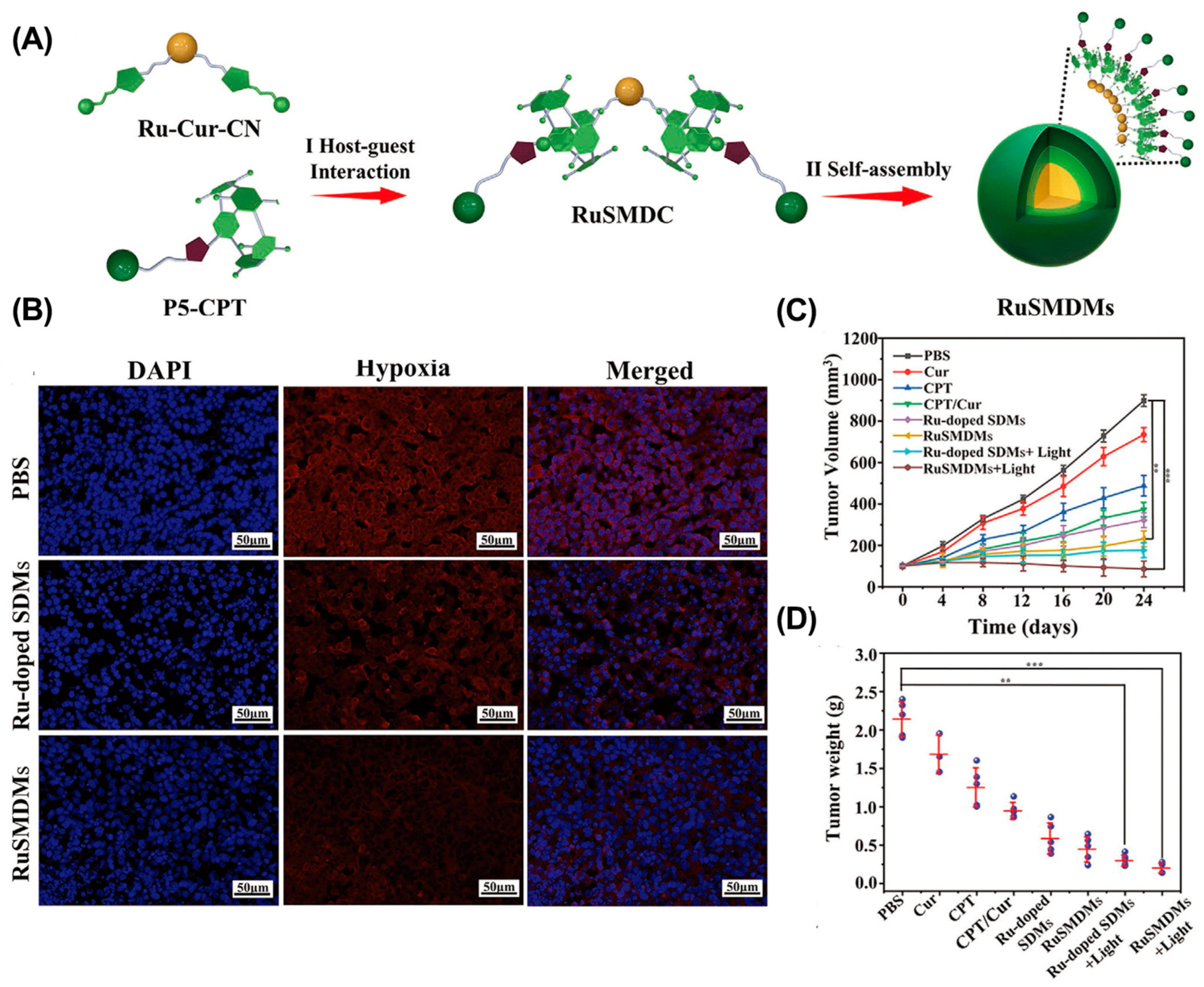
- Liu, C.; Li, M.; Li, P.; Bai, Y.; Pang, J.; Fan, L.; Tian, W. Ruthenium (II)-coordinated supramolecular metallodrug complex realizing oxygen self-supply in situ for overcoming hypoxic tumors. Adv. Fun. Mater. 2021, 31, 2105837. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, W.; Ke, W.; Chen, W.; He, C.; Ge, Z. Multifunctional polymeric micelles with amplified fenton reaction for tumor ablation. Biomacromolecules 2018, 19, 1990–1998. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Han, Y.; Liu, H.; Ren, F.; Zeng, J.; Sun, Q.; Li, Z.; Gao, M. Light-enhanced O(2)-evolving nanoparticles boost photodynamic therapy to elicit antitumor immunity. ACS Appl. Mater. Interfaces 2019, 11, 16367–16379. [Google Scholar] [CrossRef]
- Qin, Y.; Tong, F.; Zhang, W.; Zhou, Y.; He, S.; Xie, R.; Lei, T.; Wang, Y.; Peng, S.; Li, Z.; et al. Self-delivered supramolecular nanomedicine with transformable shape for ferrocene-amplified photodynamic therapy of breast cancer and bone metastases. Adv. Funct. Mater. 2021, 31, 2104645. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.H.; Lee, K.C.; Doudna, J.A. Applications of CRISPR-Cas enzymes in cancer therapeutics and detection. Trends Cancer 2018, 4, 499–512. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Fang, F.; Xu, T.; Lan, M.; Zhang, J. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials 2021, 268, 120557. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-mediated combination therapy: Two-in-one approach for cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.L.; Luo, G.F.; Chen, W.H.; Zhang, X.Z. Recent advances in engineered materials for immunotherapy-involved combination cancer therapy. Adv. Mater. 2021, 33, e2007630. [Google Scholar] [CrossRef]
- Li, F.; Simon, M.C. Cancer cells don’t live alone: Metabolic communication within tumor microenvironments. Dev. Cell 2020, 54, 183–195. [Google Scholar] [CrossRef]
- Kemp, J.A.; Shim, M.S.; Heo, C.Y.; Kwon, Y.J. “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Shen, L.; Li, X.; Song, W.; Liu, Y.; Huang, L. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano 2019, 13, 12511–12524. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Zheng, Z.; Du, N.; Guan, S.; Guo, W.; Tang, X.; Cui, J.; Zhang, L.; Liu, K.; et al. Co-delivery of precisely prescribed multi-prodrug combination by an engineered nanocarrier enables efficient individualized cancer chemotherapy. Adv. Mater. 2022, 34, e2110490. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Zhao, X.; Xu, L.; Zheng, Y.; Li, H.B.; Guo, D.S.; Shi, L.; Liu, Y. Calixarene-modified albumin for stoichiometric delivery of multiple drugs in combination-chemotherapy. Theranostics 2022, 12, 3747–3757. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Hu, J.J.; Qin, S.Y.; Zhang, A.Q.; Zhang, X.Z. Recent advances in functional mesoporous silica-based nanoplatforms for combinational photo-chemotherapy of cancer. Biomaterials 2020, 232, 119738. [Google Scholar] [CrossRef]
- Huang, X.; Chen, T.; Mu, N.; Lam, H.W.; Sun, C.; Yue, L.; Cheng, Q.; Gao, C.; Yuan, Z.; Wang, R. Supramolecular micelles as multifunctional theranostic agents for synergistic photodynamic therapy and hypoxia-activated chemotherapy. Acta Biomater. 2021, 131, 483–492. [Google Scholar] [CrossRef]
- Jia, D.; Ma, X.; Lu, Y.; Li, X.; Hou, S.; Gao, Y.; Xue, P.; Kang, Y.; Xu, Z. ROS-responsive cyclodextrin nanoplatform for combined photodynamic therapy and chemotherapy of cancer. Chin. Chem. Lett. 2021, 32, 162–167. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, Y.; Zhu, L.; Xu, Y.; Wang, C.; Lu, B.; Wang, Y.; Du, C.; Yao, Y. Nitric oxide-containing supramolecular polypeptide nanomedicine based on [2]biphenyl-extended-pillar[6]arenes for drug resistance reversal. J. Mater. Chem. B 2022, 10, 6181–6186. [Google Scholar] [CrossRef]
- Koo, M.; Oh, K.T.; Noh, G.; Lee, E.S. Gold nanoparticles bearing a tumor pH-sensitive cyclodextrin cap. ACS Appl. Mater. Interfaces 2018, 10, 24450–24458. [Google Scholar] [CrossRef]
- Shi, K.; Liu, X.; Liu, Y.; Liu, C.; Wang, Y.; Liu, Y. Supramolecular polypeptide self-assembly mediated in situ elicitation of robust innate and adaptive immune responses boosts immunogenic photothermal therapy toward "cold" tumor. Adv. Healthc. Mater. 2022, 12, e2202017. [Google Scholar] [CrossRef]
- De Angelis, B.; Depalo, N.; Petronella, F.; Quintarelli, C.; Curri, M.L.; Pani, R.; Calogero, A.; Locatelli, F.; De Sio, L. Stimuli-responsive nanoparticle-assisted immunotherapy: A new weapon against solid tumours. J. Mater. Chem. B 2020, 8, 1823–1840. [Google Scholar] [CrossRef]
- Luo, G.F.; Chen, W.H.; Lei, Q.; Qiu, W.X.; Liu, Y.X.; Cheng, Y.J.; Zhang, X.Z. A triple-collaborative strategy for high-performance tumor therapy by multifunctional mesoporous silica-coated gold nanorods. Adv. Fun. Mater. 2016, 26, 4339–4350. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Wang, Y.; Han, M.; Wang, C.; Yan, F. Cyclodextrin-functionalized gold nanorods loaded with meclofenamic acid for improving N(6)-methyladenosine-mediated second near-infrared photothermal immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 40612–40623. [Google Scholar] [CrossRef]
- Tang, H.; Xu, X.; Chen, Y.; Xin, H.; Wan, T.; Li, B.; Pan, H.; Li, D.; Ping, Y. Reprogramming the tumor microenvironment through second-near-infrared-window photothermal genome editing of PD-L1 mediated by supramolecular gold nanorods for enhanced cancer immunotherapy. Adv. Mater. 2021, 33, e2006003. [Google Scholar] [CrossRef]
- Jin, W.; Wang, Q.; Wu, M.; Li, Y.; Tang, G.; Ping, Y.; Chu, P.K. Lanthanide-integrated supramolecular polymeric nanoassembly with multiple regulation characteristics for multidrug-resistant cancer therapy. Biomaterials 2017, 129, 83–97. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Yin, Z.; Tan, L.; Zhu, W.; Tao, K.; Wang, G.; Shi, W.; Gao, J. Interferon regulatory factor-1 reverses chemoresistance by downregulating the expression of P-glycoprotein in gastric cancer. Cancer Lett. 2019, 457, 28–39. [Google Scholar] [CrossRef]
- Xia, L.L.; Tang, Y.B.; Song, F.F.; Xu, L.; Ji, P.; Wang, S.J.; Zhu, J.M.; Zhang, Y.; Zhao, G.P.; Wang, Y.; et al. DCTPP1 attenuates the sensitivity of human gastric cancer cells to 5-fluorouracil by up-regulating MDR1 expression epigenetically. Oncotarget 2016, 7, 68623–68637. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Bai, Y.; Liu, C.; Fan, L.; Tian, W. Supramolecular dual drug nanomicelles for circumventing multidrug resistance. ACS Biomater. Sci. Eng. 2021, 7, 5515–5523. [Google Scholar] [CrossRef]
- Dong, J.; Qin, Z.; Zhang, W.D.; Cheng, G.; Yehuda, A.G.; Ashby, C.R., Jr.; Chen, Z.S.; Cheng, X.D.; Qin, J.J. Medicinal chemistry strategies to discover P-glycoprotein inhibitors: An update. Drug Resist. Updates 2020, 49, 100681. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, C.; Wang, L.; Gao, X.; Wu, J.; Tan, S.; Wu, G. Doxorubicin and adjudin co-loaded pH-sensitive nanoparticles for the treatment of drug-resistant cancer. Acta Biomater. 2019, 94, 469–481. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Zhao, X.; Li, Q.; Zhang, Z.; Liu, Y. Recent Advances in Supramolecular-Macrocycle-Based Nanomaterials in Cancer Treatment. Molecules 2023, 28, 1241. https://doi.org/10.3390/molecules28031241
Pan Z, Zhao X, Li Q, Zhang Z, Liu Y. Recent Advances in Supramolecular-Macrocycle-Based Nanomaterials in Cancer Treatment. Molecules. 2023; 28(3):1241. https://doi.org/10.3390/molecules28031241
Chicago/Turabian StylePan, Zheng, Xinzhi Zhao, Qiushi Li, Zhanzhan Zhang, and Yang Liu. 2023. "Recent Advances in Supramolecular-Macrocycle-Based Nanomaterials in Cancer Treatment" Molecules 28, no. 3: 1241. https://doi.org/10.3390/molecules28031241
APA StylePan, Z., Zhao, X., Li, Q., Zhang, Z., & Liu, Y. (2023). Recent Advances in Supramolecular-Macrocycle-Based Nanomaterials in Cancer Treatment. Molecules, 28(3), 1241. https://doi.org/10.3390/molecules28031241






