Effect of Winemaking on Phenolic Compounds and Antioxidant Activities of Msalais Wine
Abstract
1. Introduction
2. Results and Discussion
2.1. Compounds Identified in Msalais Wine
2.2. Furans
2.3. Phenolic Acids
2.4. Flavonoids
2.5. Others
2.6. Phenolic Compounds
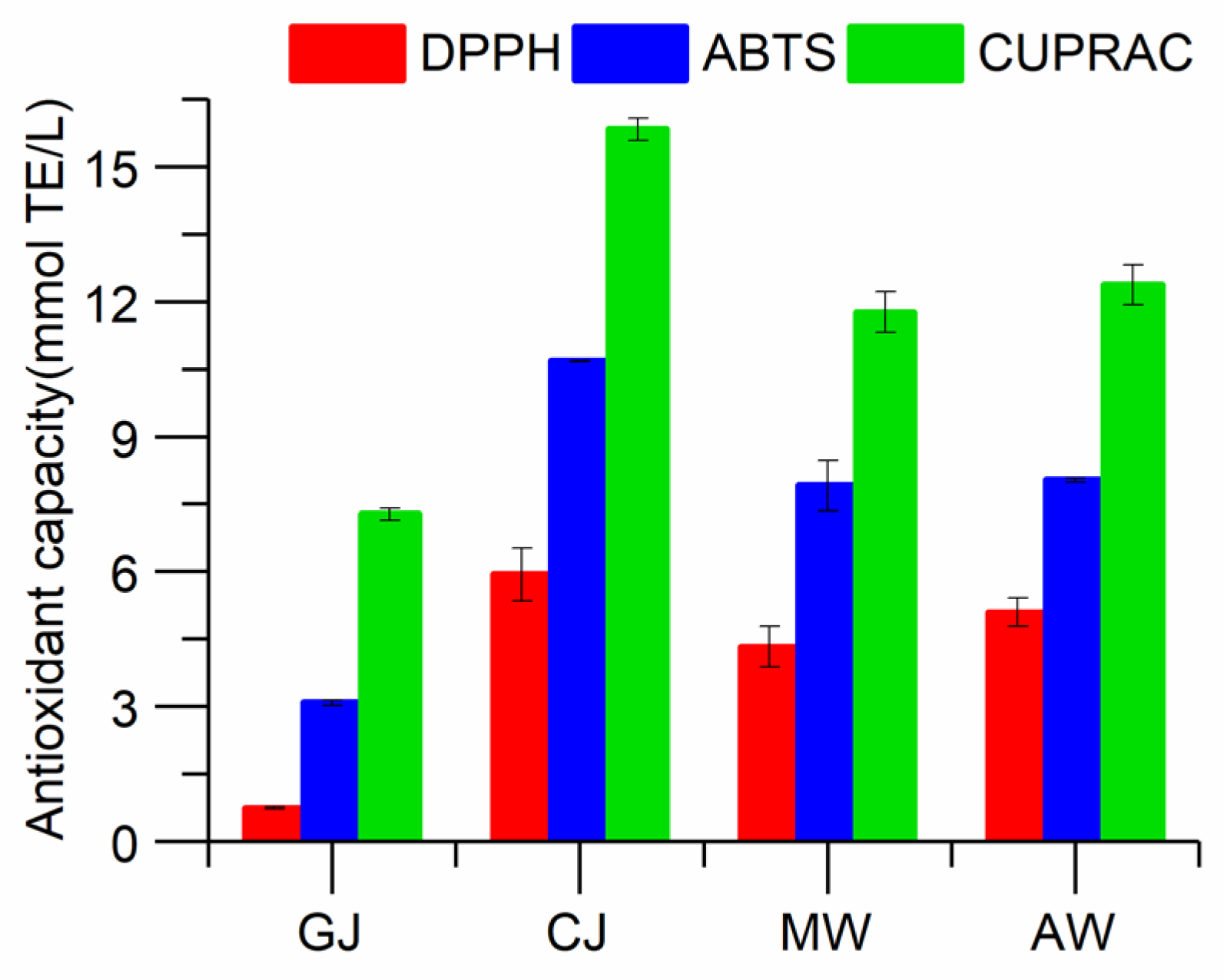
2.7. Antioxidant Activities
3. Materials and Methods
3.1. Chemicals
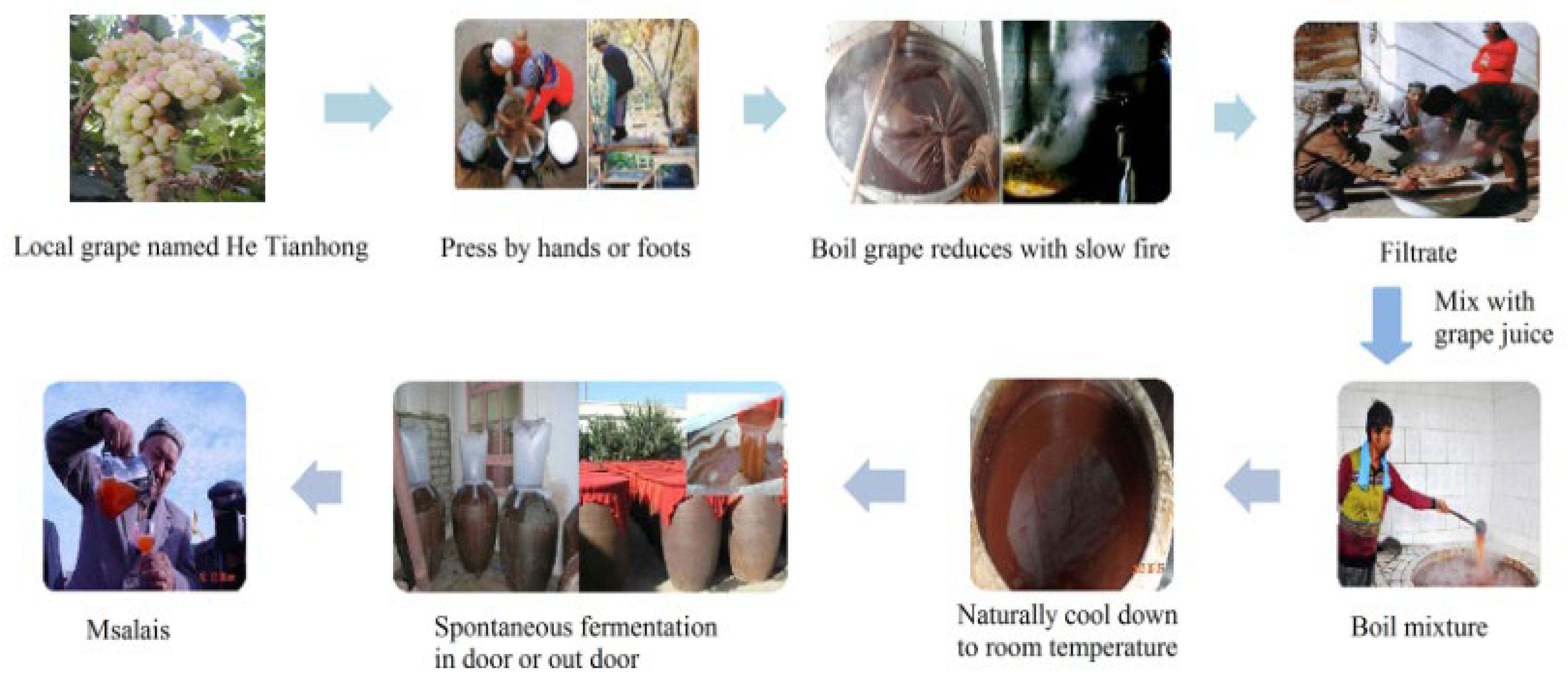
3.2. Process for Preparation of Msalais Wine and Their Procurement
3.3. LC-MS/QTOF Analysis
3.4. HPLC Analysis
3.5. DPPH Radical Scavenging Activity
3.6. ABTS Radical Scavenging Activity
3.7. Cupric-Reducing Antioxidant Capacity (CUPRAC)
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Islam, G.M.R.; Akter, S.S.; Hoque, M.M. Traditional foods for health: In vitro screening for antioxidant capacity of popular traditional tribal foods in the Sylhet territory in Bangladesh-focusing on total phenolic and tannin contents. J. Food Meas. Charact. 2018, 12, 2087–2093. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Yang, W.; Guo, D. Physicochemical Data Mining of Msalais, a Traditional Local Wine in Southern Xinjiang of China. Int. J. Food Prop. 2016, 19, 2385–2395. [Google Scholar] [CrossRef]
- Zhu, L.-X.; Zhang, M.-M.; Shi, Y.; Duan, C.-Q. Evolution of the aromatic profile of traditional Msalais wine during industrial production. Int. J. Food Prop. 2019, 22, 911–924. [Google Scholar] [CrossRef]
- Zhu, L.-X.; Wang, G.-Q.; Xue, J.-L.; Gou, D.-Q.; Duan, C.-Q. Direct stamp of technology or origin on the genotypic and phenotypic variation of indigenous Saccharomyces cerevisiae population in a natural model of boiled grape juice fermentation into traditional Msalais wine in China. Fems Yeast Res. 2017, 17, fow108. [Google Scholar] [CrossRef]
- Jiabin, Z.; Guanqun, W.; Tongguo, C.; Quan, M.; Lixia, Z. Laboratory-scale development of combined starter for Msalais production. China Brew. 2017, 36, 115–120. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, J. Modern technology homogenizes enological traits of indigenous Saccharomyces cerevisiae strains associated with Msalais, a traditional wine in China. World J. Microbiol. Biotechnol. 2017, 33, 63. [Google Scholar] [CrossRef]
- Zhu, L.-X.; Zhang, M.-M.; Liu, Z.; Shi, Y.; Duan, C.-Q. Levels of Furaneol in Msalais Wines: A Comprehensive Overview of Multiple Stages and Pathways of Its Formation during Msalais Winemaking. Molecules 2019, 24, 3104. [Google Scholar] [CrossRef]
- Zhu, L.-X.; Zhang, M.-M.; Xiang, X.-F.; Lan, Y.-B.; Shi, Y.; Duan, C.-q.; Zhang, R.-L. Aromatic characterization of traditional Chinese wine Msalais by partial least-square regression analysis based on sensory quantitative descriptive and odor active values, aroma extract dilution analysis, and aroma recombination and omission tests. Food Chem. 2021, 361, 129781. [Google Scholar] [CrossRef]
- Zhang, R.-l.; Zhang, M.-M.; Pu, Y.-F.; Zhu, L.-X. Evolution of nonenzymatic browning during the simulated Msalais-production process in models of grape juice. Food Sci. Nutr. 2022, 10, 2132–2140. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.-R.; Li, H.-Y.; Wei, S.-Y.; Han, Q.-H.; Zhao, L.; Zhang, Q.; Li, S.-Q.; Qin, W.; Wu, D.-T. Changes of phenolic compounds, antioxidant capacities, and inhibitory effects on digestive enzymes of kiwifruits (Actinidia chinensis) during maturation. J. Food Meas. Charact. 2020, 14, 1765–1774. [Google Scholar] [CrossRef]
- Janhavi, P.; Sindhoora, S.; Muthukumar, S.P. Bioaccessibility and bioavailability of polyphenols from sour mangosteen (Garcinia xanthochymus) fruit. J. Food Meas. Charact. 2020, 14, 2414–2423. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Serreli, G.; Congiu, F.; Montoro, P.; Fenu, M.A. Characterization, phenolic profile, nitrogen compounds and antioxidant activity of Carignano wines. J. Food Compos. Anal. 2017, 58, 60–68. [Google Scholar] [CrossRef]
- Alvarez-Casas, M.; Pajaro, M.; Lores, M.; Garcia-Jares, C. Polyphenolic Composition and Antioxidant Activity of Galician Monovarietal Wines from Native and Experimental Non-Native White Grape Varieties. Int. J. Food Prop. 2016, 19, 2307–2321. [Google Scholar] [CrossRef]
- Garaguso, I.; Nardini, M. Polyphenols content, phenolics profile and antioxidant activity of organic red wines produced without sulfur dioxide/sulfites addition in comparison to conventional red wines. Food Chem. 2015, 179, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Santosh, D.; Balasubramaniam, V.M.; Cocuron, J.C.; Alonso, A.P.; Agcam, E.; Shreya, K. Pressure-thermal kinetics of furan formation in selected fruit and vegetable juices. Food Bioprocess Technol. 2017, 10, 1959–1969. [Google Scholar] [CrossRef]
- Yunfeng, P.; Tian, D.; Na, Z.; Peng, J.; Donghong, L. Identification of bitter compounds from dried fruit of Ziziphus jujuba cv. Junzao. Int. J. Food Prop. 2017, 20, S26–S35. [Google Scholar] [CrossRef]
- Samoticha, J.; Wojdylo, A.; Golis, T. Phenolic composition, physicochemical properties and antioxidant activity of interspecific hybrids of grapes growing in Poland. Food Chem. 2017, 215, 263–273. [Google Scholar] [CrossRef]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Giacomelli, M.; Villegas, T.R.; Nardin, T.; Larcher, R. Targeted and untargeted high resolution mass approach for a putative profiling of glycosylated simple phenols in hybrid grapes. Food Res. Int. 2017, 98, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.C.; Ju, W.-T.; Kim, H.-B.; Sung, G.-B.; Kim, Y.-S. UPLC-DAD-QTOF/MS Analysis of Flavonoids from 12 Varieties of Korean Mulberry Fruit. J. Food Qual. 2019, 2019, 1528917. [Google Scholar] [CrossRef]
- Sasot, G.; Martinez-Huelamo, M.; Vallverdu-Queralt, A.; Mercader-Marti, M.; Estruch, R.; Lamuela-Raventos, R.M. Identification of phenolic metabolites in human urine after the intake of a functional food made from grape extract by a high resolution LTQ-Orbitrap-MS approach. Food Res. Int. 2017, 100, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.B.; Pu, Y.; Fan, L.; Dandago, M.A.; Guo, M.; Liu, D. Characterizing the phenolic constituents of baobab (Adansonia digitata) fruit shell by LC-MS/QTOF and their in vitro biological activities. Sci. Total Environ. 2019, 694, 140396. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Identification of (Poly)phenolic Compounds in Concord Grape Juice and Their Metabolites in Human Plasma and Urine after Juice Consumption. J. Agric. Food Chem. 2011, 59, 9512–9522. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H. LC-DAD-ESI-MS/MS characterization of phenolic constituents in Turkish black tea: Effect of infusion time and temperature. Food Chem. 2016, 204, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Wojdylo, A.; Nowicka, P. Anticholinergic effects of Actinidia arguta fruits and their polyphenol content determined by liquid chromatography-photodiode array detector-quadrupole/time of flight-mass spectrometry (LC-MS-PDA-Q/TOF). Food Chem. 2019, 271, 216–223. [Google Scholar] [CrossRef]
- Cavallini, G.; Straniero, S.; Donati, A.; Bergamini, E. Resveratrol requires red wine polyphenols for optimum antioxidant activity. J. Nutr. Health Aging 2016, 20, 540–545. [Google Scholar] [CrossRef]
- Hashim, S.N.N.S.; Schwarz, L.J.; Boysen, R.I.; Yang, Y.; Danylec, B.; Hearn, M.T.W. Rapid solid-phase extraction and analysis of resveratrol and other polyphenols in red wine. J. Chromatogr. A 2013, 1313, 284–290. [Google Scholar] [CrossRef]
- Granato, D.; Koot, A.; Schnitzler, E.; van Ruth, S.M. Authentication of Geographical Origin and Crop System of Grape Juices by Phenolic Compounds and Antioxidant Activity Using Chemometrics. J. Food Sci. 2015, 80, C584–C593. [Google Scholar] [CrossRef]
- Yongli, L.; Jin, W.; Xuejiao, W.; Xuchun, S.; Hackman, R.M.; Zhixi, L.; Xianchao, F. Evaluation of antioxidant capacity and flavor profile change of pomegranate wine during fermentation and aging process. Food Chem. 2017, 232, 777–787. [Google Scholar] [CrossRef]
- Moser, P.; Nicoletti Telis, V.R.; Neves, N.d.A.; Garcia-Romero, E.; Gomez-Alonso, S.; Hermosin-Gutierrez, I. Storage stability of phenolic compounds in powdered BRS Violeta grape juice microencapsulated with protein and maltodextrin blends. Food Chem. 2017, 214, 308–318. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R.D.; Capanoglu, E. A Review on the Effect of Drying on Antioxidant Potential of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2016, 56, S110–S129. [Google Scholar] [CrossRef]
- Capanoglu, E. Investigating the antioxidant potential of turkish dried fruits. Int. J. Food Prop. 2014, 17, 690–702. [Google Scholar] [CrossRef]
- Bimpilas, A.; Tsimogiannis, D.; Balta-Brouma, K.; Lymperopoulou, T.; Oreopoulou, V. Evolution of phenolic compounds and metal content of wine during alcoholic fermentation and storage. Food Chem. 2015, 178, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Qing-An, Z.; Xue-Hui, F.; Wu-Qi, Z.; Xiao-Yu, W.; Hong-Zhu, L. Evolution of some physicochemical properties in Cornus officinalis wine during fermentation and storage. Eur. Food Res. Technol. 2013, 237, 711–719. [Google Scholar]
- Picariello, L.; Slaghenaufi, D.; Ugliano, M. Fermentative and post-fermentative oxygenation of Corvina red wine: Influence on phenolic and volatile composition, colour and wine oxidative response. J. Sci. Food Agric. 2020, 100, 2522–2533. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Silva Ferreira, A.C.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Versari, A.; Boulton, R.B.; Parpinello, G.P. A comparison of analytical methods for measuring the color components of red wines. Food Chem. 2008, 106, 397–402. [Google Scholar] [CrossRef]
- Motta, S.; Guaita, M.; Cassino, C.; Bosso, A. Relationship between polyphenolic content, antioxidant properties and oxygen consumption rate of different tannins in a model wine solution. Food Chem. 2020, 313, 126045. [Google Scholar] [CrossRef]
- Mengqi, Y.; Tianli, Y.; Yahong, Y. Evolution of polyphenols and organic acids during the fermentation of apple cider. J. Sci. Food Agric. 2014, 94, 2951–2957. [Google Scholar]
- Kallithraka, S.; Salacha, M.I.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Luis Aleixandre-Tudo, J.; Alvarez, I.; Lizama, V.; Jose Garcia, M.; Luis Aleixandre, J.; Du Toit, W.J. Impact of Caffeic Acid Addition on Phenolic Composition of Tempranillo Wines from Different Winemaking Techniques. J. Agric. Food Chem. 2013, 61, 11900–11912. [Google Scholar] [CrossRef]
- Roldan, A.; Palacios, V.; Caro, I.; Perez, L. Evolution of Resveratrol and Piceid Contents during the Industrial Winemaking Process of Sherry Wine. J. Agric. Food Chem. 2010, 58, 4268–4273. [Google Scholar] [CrossRef]
- Figueiredo-Gonzalez, M.; Martinez-Carballo, E.; Cancho-Grande, B.; Santiago, J.L.; Martinez, M.C.; Simal-Gandara, J. Pattern recognition of three Vitis vinifera L. red grapes varieties based on anthocyanin and flavonol profiles, with correlations between their biosynthesis pathways. Food Chem. 2012, 130, 9–19. [Google Scholar] [CrossRef]
- Mengting, Z.; Yousheng, H.; Yangling, W.; Ting, S.; Lulu, Z.; Yi, C.; Mingyong, X. Comparison of (poly)phenolic compounds and antioxidant properties of pomace extracts from kiwi and grape juice. Food Chem. 2019, 271, 425–432. [Google Scholar] [CrossRef]
- Ismail, B.B.; Pu, Y.; Guo, M.; Ma, X.; Liu, D. LC-MS/QTOF identification of phytochemicals and the effects of solvents on phenolic constituents and antioxidant activity of baobab (Adansonia digitata) fruit pulp. Food Chem. 2019, 277, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Neves, A.C.; Fernandes, T.A.; Fernandes, A.L.; Mateus, N.; De Freitas, V.; Leandro, C.; Spranger, M.I. Evolution of Phenolic Composition of Red Wine during Vinification and Storage and Its Contribution to Wine Sensory Properties and Antioxidant Activity. J. Agric. Food Chem. 2011, 59, 6550–6557. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Padilha, C.V.; Telles Biasoto, A.C.; Correa, L.C.; Lima, M.d.S.; Pereira, G.E. Phenolic compounds profile and antioxidant activity of commercial tropical red wines (Vitis vinifera L.) from Sao Francisco Valley, Brazil. J. Food Biochem. 2017, 41, e12346. [Google Scholar] [CrossRef]
- Sun, B.; Spranger, I.; Yang, J.; Leandro, C.; Guo, L.; Canario, S.; Zhao, Y.; Wo, C. Red Wine Phenolic Complexes and Their In Vitro Antioxidant Activity. J. Agric. Food Chem. 2009, 57, 8623–8627. [Google Scholar] [CrossRef]
- Viljanen, K.; Kylli, P.; Hubbermann, E.M.; Schwarz, K.; Heinonen, M. Anthocyanin antioxidant activity and partition behavior in whey protein emulsion. J. Agric. Food Chem. 2005, 53, 2022–2027. [Google Scholar] [CrossRef]
- Gris, E.F.; Mattivi, F.; Ferreira, E.A.; Vrhovsek, U.; Wilhelm Filho, D.; Pedrosa, R.C.; Bordignon-Luiz, M.T. Stilbenes and Tyrosol as Target Compounds in the Assessment of Antioxidant and Hypolipidemic Activity of Vitis vinifera Red Wines from Southern Brazil. J. Agric. Food Chem. 2011, 59, 7954–7961. [Google Scholar] [CrossRef]
- Heinonen, I.M.; Lehtonen, P.J.; Hopia, A.I. Antioxidant activity of berry and fruit wines and liquors. J. Agric. Food Chem. 1998, 46, 25–31. [Google Scholar] [CrossRef]
- Zafrilla, P.; Morillas, J.; Mulero, J.; Cayuela, J.M.; Martinez-Cacha, A.; Pardo, F.; Nicolas, J.M.L. Changes during storage in conventional and ecological wine: Phenolic content and antioxidant activity. J. Agric. Food Chem. 2003, 51, 4694–4700. [Google Scholar] [CrossRef]
- Olivares-Tenorio, M.-L.; Verkerk, R.; van Boekel, M.A.J.S.; Dekker, M. Thermal stability of phytochemicals, HMF and antioxidant activity in cape gooseberry (Physalis peruviana L.). J. Funct. Foods 2017, 32, 46–57. [Google Scholar] [CrossRef]
- Koley, T.K.; Kaur, C.; Nagal, S.; Walia, S.; Jaggi, S.; Sarika. Antioxidant activity and phenolic content in genotypes of Indian jujube (Zizyphus mauritiana Lamk.). Arab. J. Chem. 2016, 9, S1044–S1052. [Google Scholar] [CrossRef]
| Peak | RT (min) | Tentative Identification | Formula | Error (ppm) | MM | [M-H]- | MS/MS Fragments |
|---|---|---|---|---|---|---|---|
| 1 | 1.831 | (3,4-dihydroxy-5-oxotetrahydro-2-furanyl)(hydroxy)-acetic acid | C6H8O7 | 1.4 | 192.0265 | 191.2000 | 191/111/87/85/57 |
| 2 | 2.167 | L-erytho-pent-1-enofuranose | C5H8O5 | 6.8 | 148.0366 | 147.0309 | 147/129/87/85/57 |
| 3 | 2.930 | garlic acid * | C7H6O5 | 2.1 | 170.0210 | 169.0146 | 169/125/124/79 |
| 4 | 3.732 | 3-hydroxy-2-butanyl 6-O-[3,4-dihydroxy-4-(hydroxymethyl)tetrahydro-2-furanyl]-glucopyranoside | C15H28O11 | 5.2 | 384.1626 | 383.1539 | 383/251/191/161/101/73 |
| 5 | 4.182 | 5-(hydroxymethyl)-2-furaldehyde | C6H6O3 | 2.1 | 126.0311 | 127.0387 | 109/81/53 |
| 6 | 4.611 | 3-hydroxyl-5-[1,2,3,4-tetrahydroxybutyl]-2(H)-furanone | C8H12O7 | 2.9 | 220.0583 | 219.0504 | 219/154/111/73 |
| 7 | 4.820 | [4-(methoxycarbonyl)-5-methyl-2furyl]methyl-2,3-dyhydro-1,4-benzodioxine-2-carboxylate | C17H16O7 | 4.1 | 332.0896 | 331.0832 | 331/303/187/143/99 |
| 8 | 5.024 | 2-(3,4-dihydroxyphenyl)ethyl-3-glucopyranosyl- glucopyranoside | C20H30O13 | 4.5 | 478.1686 | 477.1592 | 477/323/153/123 |
| 9 | 5.202 | 3,5-dimethoxyphenyl-glucopyranoside | C14H20O8 | 1.4 | 316.1153 | 315.1081 | 315/153/123/122 |
| 10 | 5.372 | protocatechuic acid * | C7H6O4 | 3.1 | 154.0261 | 153.0198 | 153/123/109/81 |
| 11 | 5.661 | gentistic acid | C8H10O3 | 4.5 | 154.0630 | 153.0564 | 153/109 |
| 12 | 6.174 | caftaric acid | C13H12O9 | 4.0 | 312.0476 | 311.0396 | 179/149/135/87 |
| 13 | 6.825 | 2-isopropyl-5-methylphenyl 6-O-[3,4-dihydroxy-4-(hydroxymethyl)tetrahydro-2-furanyl]-glucopyranoside | C21H32O10 | 3.8 | 444.1995 | 443.1906 | 443/189/119//89/71 |
| 14 | 7.085 | p-hydroxybenzoic acid * | C7H6O3 | 0.9 | 138.1207 | 137.0258 | 137/91/79 |
| 15 | 7.175 | 1-(5-hydroxy-1-benzofuran-3-yl)ethanone | C7H12O5 | 4.6 | 176.0473 | 175.0620 | 175/157/115/85 |
| 16 | 7.346 | Catechin * | C15H14O6 | 1.4 | 290.0743 | 289.0712 | 289/245/205/203/187/151 |
| 17 | 7.711 | coutaric acid | C13H12O8 | 1.2 | 296.0527 | 295.0456 | 163/119/87 |
| 18 | 8.030 | 3-hydroxy-5-methylphenyl-6-O-glucopyranosyl- glucopyranoside | C19H28O12 | 3.8 | 448.1575 | 447.1491 | 447/401/269/161 |
| 19 | 8.315 | ferulic acid * | C10H10O4 | 1.4 | 194.0574 | 193.0511 | 193/178/134/133 |
| 20 | 8.562 | caffeic acid * | C9H8O4 | 5.7 | 180.0417 | 179.0359 | 179/149/135/134/87 |
| 21 | 9.120 | Epicatechin * | C15H14O6 | 1.4 | 290.0743 | 289.0714 | 289/245/205/203/187/151 |
| 22 | 9.317 | eriodictyol-7-O-glucopyranoside | C21H22O11 | 2.5 | 450.1157 | 449.1078 | 449/287/259/178/151 |
| 23 | 9.622 | 2,6-dimethoxy-4-[3-oxo-1-buten-1-yl]phenyl-glucopyranoside | C18H24O9 | 3.7 | 384.1421 | 383.1382 | 206/188/160/118 |
| 24 | 10.080 | eriocitrin | C27H32O15 | 3.3 | 596.1741 | 595.1754 | 595/301/300/271/255 |
| 25 | 10.289 | p-hydroxycinnamic acid * | C9H8O3 | 6.3 | 164.0468 | 163.0413 | 119/117/93 |
| 26 | 10.785 | cinnamic acid | C9H8O2 | 4.4 | 148.0519 | 147.0458 | 119/103/76 |
| 27 | 11.376 | Rutin * | C27H30O16 | 2.5 | 610.1528 | 609.1453 | 609/301/300/271/255/151 |
| 28 | 11.609 | ellagic acid * | C14H6O8 | 2.0 | 302.0057 | 300.9984 | 300/283/244/229/201/145 |
| 29 | 11.762 | quercetin-3-O-glucoside * | C21H20O12 | 3.7 | 464.0949 | 463.0865 | 463/301/300/271/255/179 |
| 30 | 12.182 | quercetin 3-O-glucuronide | C21H18O13 | 3.1 | 478.0742 | 477.0660 | 477/301/255/179/151 |
| 31 | 12.804 | kaempferol-3-O-rutinoside | C27H30O15 | 1.8 | 594.1579 | 593.1511 | 593/285/284/255/227 |
| 32 | 12.910 | ramnazin-3-O-rutinoside | C28H32O16 | 3.7 | 624.1085 | 623.1068 | 623/315/314/300/299/271 |
| 33 | 13.067 | quercetin-3-O-rhamnoside | C21H20O11 | 2.0 | 448.3769 | 447.0908 | 447/301/300/271/255/179 |
| 34 | 13.490 | Resveratrol * | C14H12O3 | 0.7 | 228.0781 | 227.0712 | 227/185/157/143 |
| 35 | 14.085 | Phloridzin * | C21H24O10 | 3.6 | 436.1224 | 435.1276 | 435/273/179/167/93 |
| 36 | 15.965 | kaempferol-7-O-rhamnose | C21H20O10 | 3.9 | 432.0984 | 431.1013 | 431/285/284/255/227/185 |
| 37 | 20.016 | Quercetin * | C15H10O7 | 2.6 | 302.0354 | 301.0349 | 301/273/179/151/121/93 |
| 38 | 21.465 | Kaempferol * | C15H10O6 | 2.3 | 286.1102 | 286.1043 | 285/241/229/187/145 |
| Sample Name | GJ | CJ | MW | AW |
|---|---|---|---|---|
| gallic acid | 1.21 ± 0.07 d | 26.48 ± 0.56 c | 38.01 ± 0.07 b | 41.60 ± 1.13 a |
| p-coumaric acid | 6.43 ± 0.21 d | 13.03 ± 0.21 a | 10.43 ± 0.39 b | 7.38 ± 0.13 c |
| (+)-catechin | 89.65 ± 1.76 d | 272.92 ± 6.51 a | 201.03 ± 0.21 b | 175.94 ± 6.65 c |
| (−)-epicatechin | 6.86 ± 0.13 d | 42.63 ± 0.92 a | 37.13 ± 0.92 b | 28.62 ± 0.85 c |
| caffeic acid | 1.95 ± 0.35 c | 18.31 ± 0.07 b | 21.97 ± 0.21 a | 21.70 ± 0.7 a |
| ferulic acid | 2.16 ± 0.12 a | 1.91 ± 0.07 b | 0.90 ± 0.15 c | 1.01 ± 0.07 c |
| rutin | 6.96 ± 0.04 d | 10.68 ± 0.06 c | 11.36 ± 0.17 b | 11.81 ± 0.12 a |
| resveratrol | 0.99 ± 0.05 d | 7.81 ± 0.07 a | 4.78 ± 0.06 c | 6.33 ± 0.02 b |
| quercetin | ND | 1.98 ± 0.06 b | 3.89 ± 0.06 a | 3.60 ± 0.08 a |
| CUPRAC | ABTS | DPPH | |
|---|---|---|---|
| quercetin | 0.519 | 0.619 | 0.718 |
| resveratrol | 0.978 * | 0.979 * | 0.990 * |
| rutin | 0.749 | 0.818 | 0.899 |
| ferulic acid | −0.238 | −0.352 | −0.481 |
| caffeic acid | 0.757 | 0.830 | 0.897 |
| epicatechin | 0.932 | 0.966 * | 0.935 |
| catechin | 0.978 * | 0.978 * | 0.918 |
| p-coumaric acid | 0.848 | 0.840 | 0.720 |
| gallic acid | 0.636 | 0.718 | 0.820 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, X.; Chen, S.; Pu, Y.; Wang, T.; Xu, H.; Li, H.; Ma, P.; Hou, X. Effect of Winemaking on Phenolic Compounds and Antioxidant Activities of Msalais Wine. Molecules 2023, 28, 1250. https://doi.org/10.3390/molecules28031250
Hou X, Chen S, Pu Y, Wang T, Xu H, Li H, Ma P, Hou X. Effect of Winemaking on Phenolic Compounds and Antioxidant Activities of Msalais Wine. Molecules. 2023; 28(3):1250. https://doi.org/10.3390/molecules28031250
Chicago/Turabian StyleHou, Xiaojie, Shenghuizi Chen, Yunfeng Pu, Tingting Wang, Heng Xu, Hu Li, Peng Ma, and Xujie Hou. 2023. "Effect of Winemaking on Phenolic Compounds and Antioxidant Activities of Msalais Wine" Molecules 28, no. 3: 1250. https://doi.org/10.3390/molecules28031250
APA StyleHou, X., Chen, S., Pu, Y., Wang, T., Xu, H., Li, H., Ma, P., & Hou, X. (2023). Effect of Winemaking on Phenolic Compounds and Antioxidant Activities of Msalais Wine. Molecules, 28(3), 1250. https://doi.org/10.3390/molecules28031250





