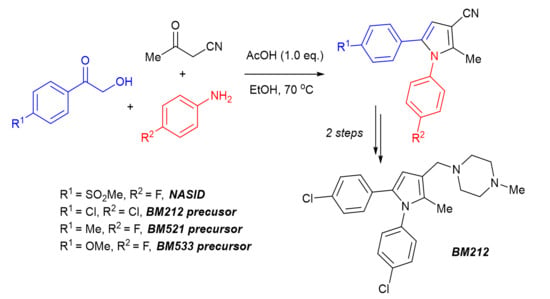
A Concise Synthesis of Pyrrole-Based Drug Candidates from α-Hydroxyketones, 3-Oxobutanenitrile, and Anilines
Abstract
:1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. Materials and Methods
3.2. General Procedure 1: Preparation of Substituted Phenacyl Alcohols 3 and 11–13
3.3. General Procedure 2: Synthesis of N-Substituted 1,2,3,5-Substituted Pyrroles 1a, 9–10 and 15–17






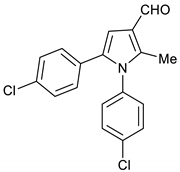

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Colhoun, H.; Thomason, M.; Mackness, M.; Maton, S.; Betteridge, D.; Durrington, P.; Hitman, G.; Neil, H.; Fuller, J. Collaborative AtoRvastatin Diabetes Study (CARDS)., Design of the Collaborative AtoRvastatin Diabetes Study (CARDS) in patients with type 2 diabetes. Diabet. Med. 2002, 19, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Demetri, G.; Sargent, W.; Raymond, E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 2007, 6, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Etcheverry, S.B.; Barrio, D.A.; Cortizo, A.M.; Williams, P.A.M. Three new vanadyl (IV) complexes with non-steroidal anti-inflammatory drugs (Ibuprofen, Naproxen and Tolmetin). Bioactivity on osteoblast-like cells in culture. J. Inorg. Biochem. 2002, 88, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battilocchio, C.; Poce, G.; Alfonso, S.; Porretta, G.C.; Consalvi, S.; Sautebin, L.; Pace, S.; Rossi, A.; Ghelardini, C.; Mannelli, L.D.C. A class of pyrrole derivatives endowed with analgesic/anti-inflammatory activity. Bioorganic Med. Chem. 2013, 21, 3695–3701. [Google Scholar] [CrossRef] [PubMed]
- Fatahala, S.S.; Hasabelnaby, S.; Goudah, A.; Mahmoud, G.I.; Helmy Abd-El Hameed, R. Pyrrole and fused pyrrole compounds with bioactivity against inflammatory mediators. Molecules 2017, 22, 461. [Google Scholar] [CrossRef] [Green Version]
- Biava, M.; Porretta, G.; Manetti, F. New derivatives of BM212: A class of antimycobacterial compounds based on the pyrrole ring as a scaffold. Mini Rev. Med. Chem. 2007, 7, 65–78. [Google Scholar] [CrossRef]
- Pegklidou, K.; Papastavrou, N.; Gkizis, P.; Komiotis, D.; Balzarini, J.; Nicolaou, I. N-substituted pyrrole-based scaffolds as potential anticancer and antiviral lead structures. Med. Chem. 2015, 11, 602–608. [Google Scholar] [CrossRef]
- Khanna, I.K.; Weier, R.M.; Yu, Y.; Collins, P.W.; Miyashiro, J.M.; Koboldt, C.M.; Veenhuizen, A.W.; Currie, J.L.; Seibert, K.; Isakson, P.C. 1, 2-Diarylpyrroles as potent and selective inhibitors of cyclooxygenase-2. J. Med. Chem. 1997, 40, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Poce, G.; Bates, R.H.; Alfonso, S.; Cocozza, M.; Porretta, G.C.; Ballell, L.; Rullas, J.; Ortega, F.; De Logu, A.; Agus, E. Improved BM212 MmpL3 inhibitor analogue shows efficacy in acute murine model of tuberculosis infection. PLoS ONE 2013, 8, e56980. [Google Scholar] [CrossRef] [PubMed]
- Poce, G.; Consalvi, S.; Venditti, G.; Scarpecci, C.; Biava, M. Development of MmpL3 inhibitors for tuberculosis treatment. In Annual Reports in Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 52, pp. 71–96. [Google Scholar]
- More, N.A.; Patil, M.D.; Garud, D.R.; Gajbhiye, J.M. An efficient synthesis of potent anti-tubercular drug candidate BM212. Rasayan J. Chem. 2016, 9, 806–811. [Google Scholar]
- Liu, P.; Yang, Y.; Ju, Y.; Tang, Y.; Sang, Z.; Chen, L.; Yang, T.; An, Q.; Zhang, T.; Luo, Y. Design, synthesis and biological evaluation of novel pyrrole derivatives as potential ClpP1P2 inhibitor against Mycobacterium tuberculosis. Bioorganic Chem. 2018, 80, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Moussa, Z.; Judeh, Z. Selective One-pot multicomponent synthesis of N-substituted 2,3,5-functionalized 3-cyanopyrroles via the reaction between α-hydroxyketones, oxoacetonitriles, and primary amines. Molecules 2022, 27, 5285. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-J.; He, H.; Bertekap, R.; Westphal, R.; Lelas, S.; Newton, A.; Wallace, T.; Taber, M.; Davis, C.; Macor, J.E. Discovery of disubstituted piperidines and homopiperidines as potent dual NK1 receptor antagonists–serotonin reuptake transporter inhibitors for the treatment of depression. Bioorganic Med. Chem. 2013, 21, 2217–2228. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, M.; Santoso, M.; Moussa, Z.; Judeh, Z.M.A. A Concise Synthesis of Pyrrole-Based Drug Candidates from α-Hydroxyketones, 3-Oxobutanenitrile, and Anilines. Molecules 2023, 28, 1265. https://doi.org/10.3390/molecules28031265
Xia M, Santoso M, Moussa Z, Judeh ZMA. A Concise Synthesis of Pyrrole-Based Drug Candidates from α-Hydroxyketones, 3-Oxobutanenitrile, and Anilines. Molecules. 2023; 28(3):1265. https://doi.org/10.3390/molecules28031265
Chicago/Turabian StyleXia, Mengxin, Mardi Santoso, Ziad Moussa, and Zaher M. A. Judeh. 2023. "A Concise Synthesis of Pyrrole-Based Drug Candidates from α-Hydroxyketones, 3-Oxobutanenitrile, and Anilines" Molecules 28, no. 3: 1265. https://doi.org/10.3390/molecules28031265
APA StyleXia, M., Santoso, M., Moussa, Z., & Judeh, Z. M. A. (2023). A Concise Synthesis of Pyrrole-Based Drug Candidates from α-Hydroxyketones, 3-Oxobutanenitrile, and Anilines. Molecules, 28(3), 1265. https://doi.org/10.3390/molecules28031265