Dynamic Interfacial Tensions of Surfactant and Polymer Solutions Related to High-Temperature and High-Salinity Reservoir
Abstract
:1. Introduction
2. Results and Discussion
2.1. Interfacial Tension between Surfactant Solution and n-Alkanes
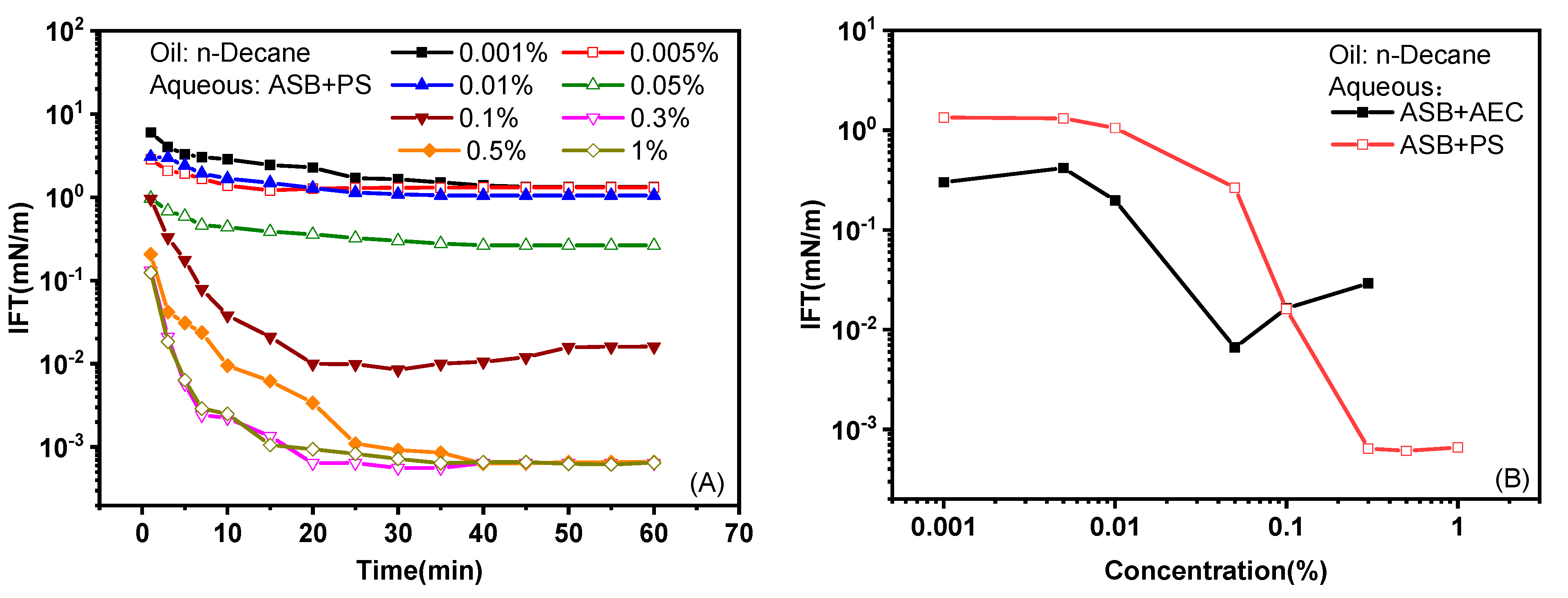
2.1.1. Effect of Concentration
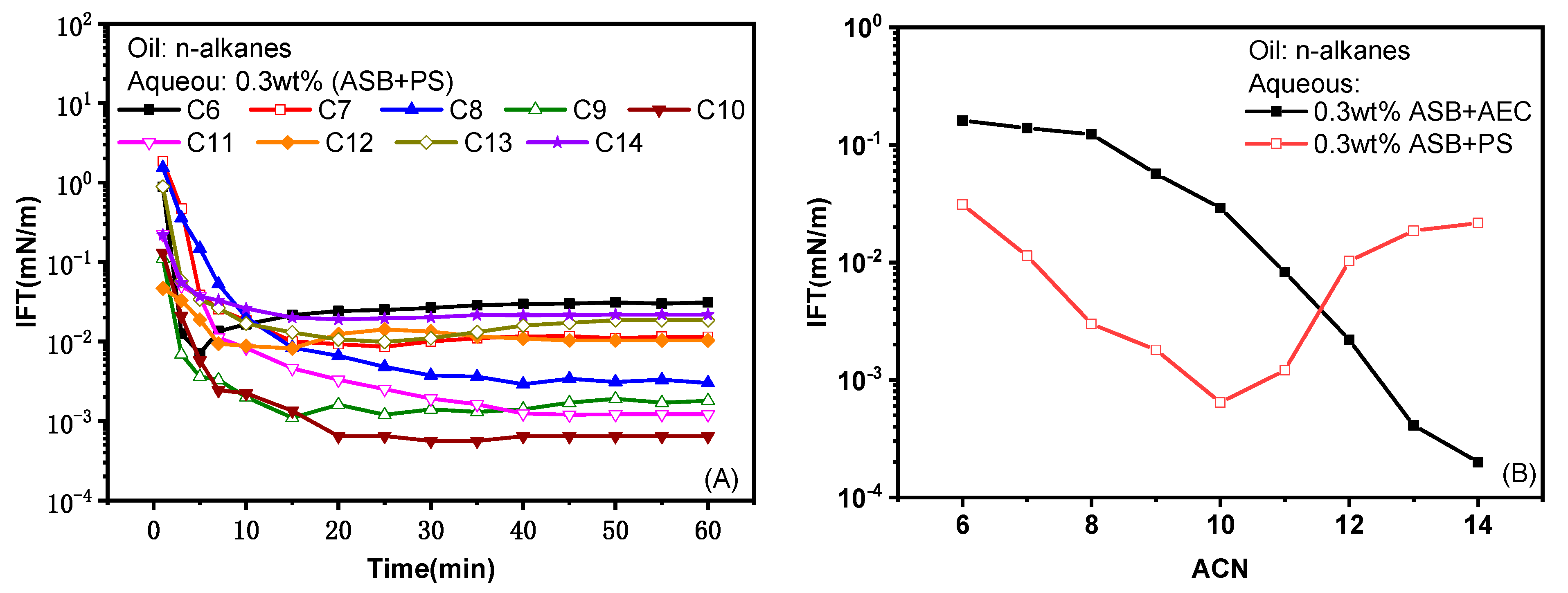
2.1.2. Effect of Carbon Chain Length of Oil Molecule
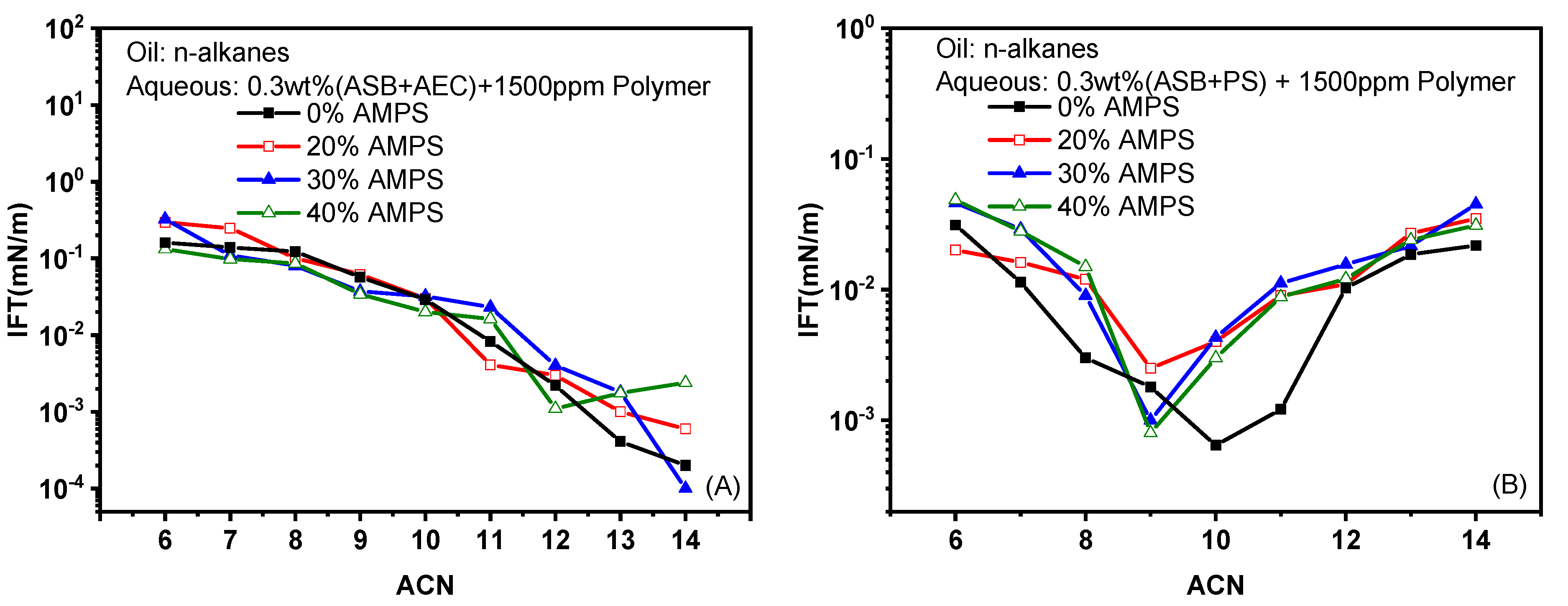
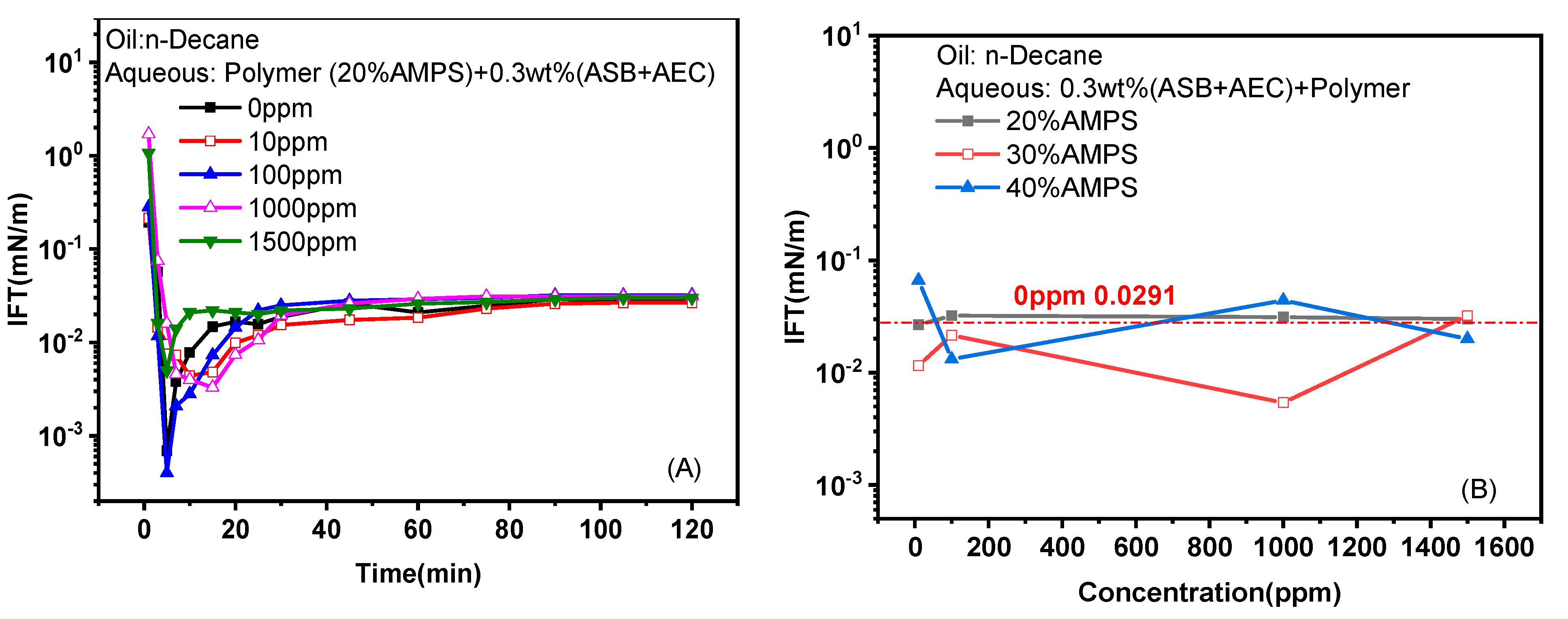
2.1.3. Effects of Polymers
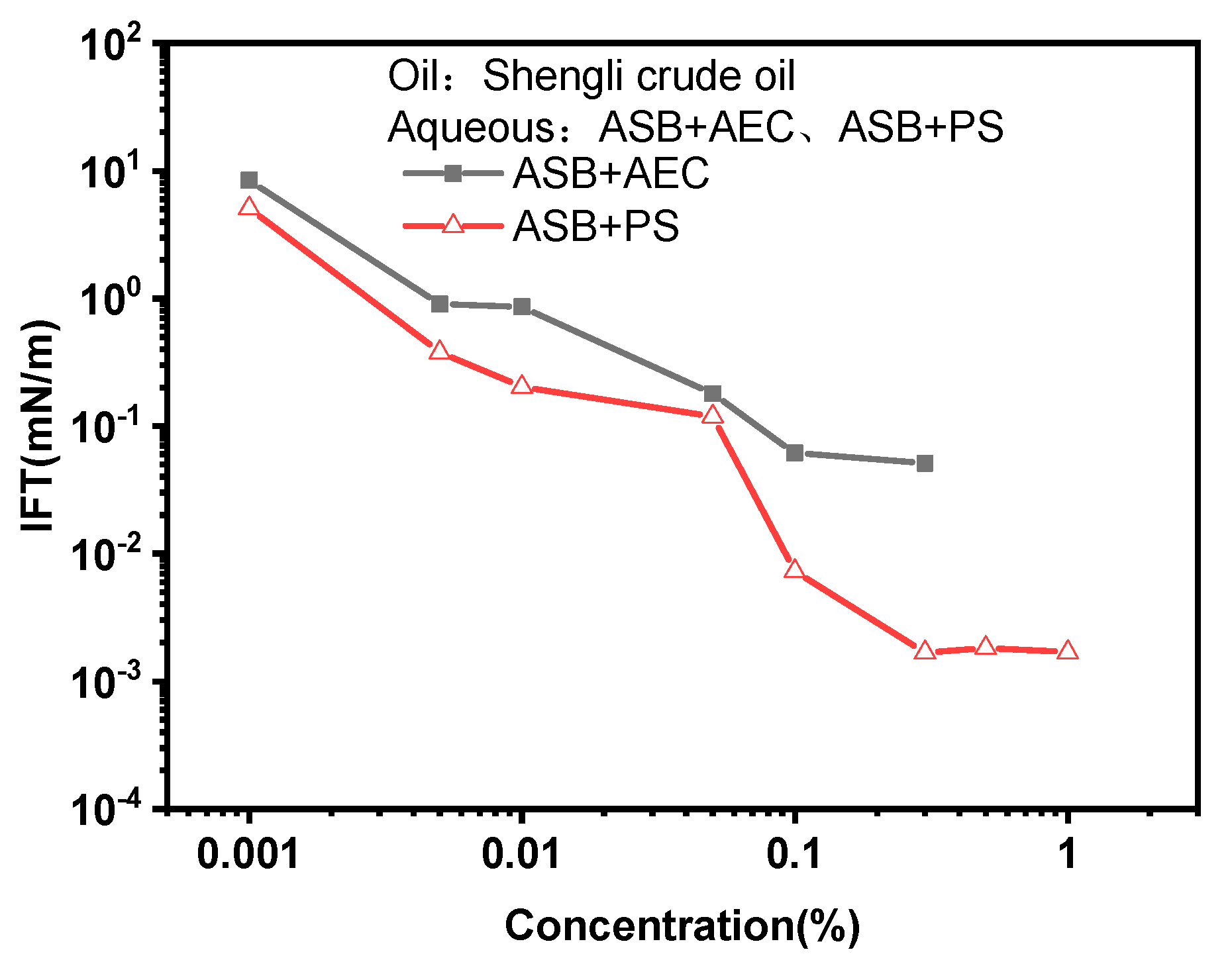
2.2. IFT between Surfactant Solution and Shengli Crude Oil
3. Experimental Section
3.1. Materials
3.2. Apparatus and Methods
4. Conclusions
- (1)
- The IFT between betaine ASB and n-alkanes can be reduced to an ultra-low value through compounding with an anionic surfactant PS and an extended anionic surfactant AEC, respectively. ASB@AEC is very oil-soluble, with an nmin value ≥14 and can produce ultra-low IFTs against oils C12–C14. On the other hand, ASB@PS is relatively water-soluble, with an nmin value of 10 and can achieve an ultra-low IFT against oils C8–C11.
- (2)
- The water-solubility of both ASB@PS and ASB@AEC is enhanced by the addition of water-soluble polymers. The HLB of ASB@AEC solution becomes better when the oil is decane after the addition of polymers, and the IFT decreases as a result. On the contrary, the antagonistic effect can be observed for ASB@PS in reducing IFT against decane when polymers are added, which can be attributed to the deviation of the HLB.
- (3)
- Similar to the IFT against decane, the addition of polymers decreases the nmin value of the surfactant solution and results in better and worse HLBs for ASB@AEC and ASB@PS against crude oil, respectively. Therefore, the IFTs of ASB@AEC solutions against crude oil decrease to ultra-low values therough the addition of polymers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Bera, A.; Ojha, K.; Mandal, A.; Kumar, T. Interfacial tension and phase behavior of surfactant-brine-oil system. Colloids Surf. A Physicochem. Eng. Asp. 2011, 383, 114–119. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, L.; Zhao, S.; Yu, J.Y. Ultra low interfacial tension and interfacial dilational properties related to enhanced oil recovery. In Petroleum Science Research Progress; Montclaire, K.L., Ed.; Nova Science Publishers: New York, NY, USA, 2008; pp. 81–139. [Google Scholar]
- Bashir, A.; Haddad, A.; Rafati, R. A review of fluid displacement mechanisms in surfactant-based chemical enhanced oil recovery processes: Analyses of key influencing factors. Pet. Sci. 2021, 19, 1211–1235. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Q.; Li, M.; Wu, Z.; Christy, A.A. The effect of alkali on crude oil/water interfacial properties and the stability of crude oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 273, 213–218. [Google Scholar] [CrossRef]
- Zhang, L.H.; Xiao, H.; Zhang, H.T.; Xu, L.; Zhang, D. Optimal design of a novel oil–water separator for raw oil produced from ASP flooding. J. Pet. Sci. Eng. 2007, 59, 213–218. [Google Scholar] [CrossRef]
- Zhao, Z.; Bi, C.; Qiao, W.; Li, Z.; Cheng, L. Dynamic interfacial tension behavior of the novel surfactant solutions and Daqing crude oil. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 191–202. [Google Scholar] [CrossRef]
- Zhu, P.; Zhu, Y.; Xu, Z.C.; Zhang, L.; Zhang, L.; Zhao, S. Effect of Polymer on Dynamic Interfacial Tensions of Anionic–nonionic Surfactant Solutions. J. Dispers. Sci. Technol. 2015, 37, 820–829. [Google Scholar] [CrossRef]
- Hua, Z.; Lin, M.; Dong, Z.; Li, M.; Zhang, G.; Yang, J. Study of deep profile control and oil displacement technologies with nanoscale polymer microspheres. J. Colloid Interface Sci. 2014, 424, 67–74. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, R.; Huang, D.; Shen, Y.; Gao, X.; Shi, W. Membrane fouling in microfiltration of alkali/surfactant/polymer flooding oilfield wastewater: Effect of interactions of key foulants. J. Colloid Interface Sci. 2020, 570, 20–30. [Google Scholar] [CrossRef]
- Chang, H.L.; Zhang, Z.Q.; Wang, Q.M.; Xu, Z.S.; Guo, Z.D.; Sun, H.Q.; Cao, X.L.; Qiao, Q. Advances in Polymer Flooding and Alkaline/Surfactant/Polymer Processes as Developed and Applied in the People’s Republic of China. J. Pet. Technol. 2006, 58, 84–89. [Google Scholar] [CrossRef]
- Pal, N.; Saxena, N.; Mandal, A. Characterization of alkali-surfactant-polymer slugs using synthesized gemini surfactant for potential application in enhanced oil recovery. J. Pet. Sci. Eng. 2018, 168, 283–300. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, F.; Han, L.; Luo, P.; Yang, J.; Chen, H. The effect of temperature on the interfacial tension between crude oil and gemini surfactant solution. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 138–141. [Google Scholar] [CrossRef]
- Gong, H.; Xu, G.; Zhu, Y.; Wang, Y.; Wu, D.; Niu, M.; Wang, L.; Guo, H.; Wang, H. Influencing Factors on the Properties of Complex Systems Consisting of Hydrolyzed Polyacrylamide/Triton X-100/Cetyl Trimethylammonium Bromide: Viscosity and Dynamic Interfacial Tension Studies. Energy Fuels 2009, 23, 300–305. [Google Scholar] [CrossRef]
- Danov, K.D.; Kralchevska, S.D.; Kralchevsky, P.A.; Ananthapadmanabhan, K.P.; Lips, A. Mixed solutions of anionic and zwitterionic surfactant (Betaine): Surface-tension isotherms, adsorption, and relaxation kinetics. Langmuir 2004, 20, 5445–5453. [Google Scholar] [CrossRef] [Green Version]
- Mafi, A.; Hu, D.; Chou, K.C. Interactions of Sulfobetaine Zwitterionic Surfactants with Water on Water Surface. Langmuir 2016, 32, 10905–10911. [Google Scholar] [CrossRef]
- Jiang, P.; Li, N.; Ge, J.; Zhang, G.; Wang, Y.; Chen, L.; Zhang, L. Efficiency of a sulfobetaine-type surfactant on lowering IFT at crude oil–formation water interface. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 141–148. [Google Scholar] [CrossRef]
- Li, N.; Zhang, G.; Ge, J.; Zhang, L.; Liu, X.; Wang, J. Ultra-Low Interfacial Tension Between Heavy Oil and Betaine-Type Amphoteric Surfactants. J. Dispers. Sci. Technol. 2012, 33, 258–264. [Google Scholar] [CrossRef]
- Qiao, W.; Cui, Y.; Zhu, Y.; Cai, H. Dynamic interfacial tension behaviors between Guerbet betaine surfactants solution and Daqing crude oil. Fuel 2012, 102, 746–750. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zhang, Q.; Liu, Y.; Wang, H.Z.; Cai, H.Y.; Zhang, F.; Tian, M.Z.; Liu, Z.Y.; Zhang, L.; Zhang, L. Effect of Fatty Acids on Interfacial Tensions of Novel Sulfobetaines Solutions. Energy Fuels 2014, 28, 1020–1027. [Google Scholar] [CrossRef]
- Cao, J.H.; Zhou, Z.H.; Xu, Z.C.; Zhang, Q.; Li, S.H.; Cui, H.B.; Zhang, L.; Zhang, L. Synergism/Antagonism between Crude Oil Fractions and Novel Betaine Solutions in Reducing Interfacial Tension. Energy Fuels 2016, 30, 924–932. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, Z.H.; Zhang, Q.; Zhang, F.; Ma, G.Y.; Zhang, L.; Zhang, L. Effect of Electrolyte on Synergism for Reducing Interfacial Tension between Betaine and Petroleum Sulfonate. Energy Fuels 2020, 34, 3188–3198. [Google Scholar] [CrossRef]
- Li, F.F.; Xu, H.J.; Kang, P. Properties of Binary Surfactant Mixtures of Anionic Gemini Surfactant and Amphoteric Surfactant. Tenside Surfactants Det. 2016, 53, 64–69. [Google Scholar] [CrossRef]
- Zhong, Q.L.; Cao, X.L.; Zhu, Y.W.; Ma, B.D.; Xu, Z.C.; Zhang, L.; Ma, G.Y.; Zhang, L. Studies on interfacial tensions of betaine and anionic-nonionic surfactant mixed solutions. J. Mol. Liq. 2020, 311, 113262. [Google Scholar] [CrossRef]
- Zhong, Q.L.; Zhou, Z.H.; Zhang, Q.; Ma, D.S.; Luan, H.-X.; Zhang, L.; Ma, G.Y.; Zhang, L. Studies on Interfacial Tensions of Ionic Surfactant and Alkyl Sulfobetaine Mixed Solutions. Energy Fuels 2018, 32, 8202–8209. [Google Scholar] [CrossRef]
- Zhou, B.; Kang, W.; Wang, Q.; Li, X.; He, Y.; Jia, R.; Wang, K.; Zhao, Z.; Yang, H.; Li, Z. Enhanced oil recovery performance and mechanism of a wormlike micelles flooding system with zwitterionic-anionic surfactants. J. Mol. Liq. 2022, 362, 119726. [Google Scholar] [CrossRef]
- Han, X.; Chen, Z.; Zhang, G.; Yu, J. Surfactant-polymer flooding formulated with commercial surfactants and enhanced by negative salinity gradient. Fuel. 2020, 274, 117874. [Google Scholar] [CrossRef]
- Ma, B.-d.; Gao, B.-y.; Zhang, L.; Gong, Q.-t.; Jin, Z.-q.; Zhang, L.; Zhao, S. Influence of polymer on dynamic interfacial tensions of EOR surfactant solutions. J. Appl. Polym. Sci. 2014, 131, 40562. [Google Scholar] [CrossRef]
- Li, H.R.; Li, Z.Q.; Song, X.W.; Li, C.B.; Guo, L.L.; Zhang, L.; Zhang, L.; Zhao, S. Effect of Organic Alkalis on Interfacial Tensions of Surfactant/Polymer Solutions against Hydrocarbons. Energy Fuels 2015, 29, 459–466. [Google Scholar] [CrossRef]
- SiTu, W.X.; Lu, H.M.; Ruan, C.Y.; Zhang, L.; Zhu, Y.; Zhang, L. Effect of polymer on dynamic interfacial tensions of sulfobetaine solutions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 231–240. [Google Scholar] [CrossRef]
- Li, H.T.; Cui, C.Z.; Guo, L.L.; Yuan, F.Q.; Xu, Z.C.; Gong, Q.T.; Jin, Z.Q.; Zhang, L.; Zhang, L. Dynamic interfacial tensions of sulfobetaine and polymers solutions: Effect of structures. J. Mol. Liq. 2022, 356, 119018. [Google Scholar] [CrossRef]
- Seright, R.S.S.; Campbell, A.R.R.; Mozley, P.S.S.; Han, P. Stability of Partially Hydrolyzed Polyacrylamides at Elevated Temperatures in the Absence of Divalent Cations. SPE J. 2009, 15, 341–348. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, L.; Ge, J.; Jiang, P.; Zhu, X. Experimental research of syneresis mechanism of HPAM/Cr3+ gel. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 96–103. [Google Scholar] [CrossRef]
- Zhu, D.; Hou, J.; Wei, Q.; Wu, X.; Bai, B. Terpolymer Gel System Formed by Resorcinol–Hexamethylenetetramine for Water Management in Extremely High-Temperature Reservoirs. Energy Fuels 2017, 31, 1519–1528. [Google Scholar] [CrossRef]
- Nurmi, L.; Sandengen, K.; Hanski, S.; Molesworth, P. Sulfonated Polyacrylamides—Evaluation of Long Term Stability by Accelerated Aging at Elevated Temperature. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14–18 April 2018. SPE-190184-MS. [Google Scholar]
- Unomah, M.; Thach, S.; Shong, R.; App, J.; Zhang, T.; Kim, D.H.; Malik, T.; Dwarakanath, V. Performance of Conformance Gels Under Harsh Conditions. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14–18 April 2018. SPE-190266-MS. [Google Scholar]
- Song, X.W.; Zhao, R.H.; Cao, X.L.; Zhang, J.C.; Zhang, L.; Zhang, L.; Zhao, S. Dynamic Interfacial Tensions Between Offshore Crude Oil and Enhanced Oil Recovery Surfactants. J. Dispers. Sci. Technol. 2013, 34, 234–239. [Google Scholar] [CrossRef]
- Chan, K.S.; Shah, D.O. The molecular mechanism for achiecing ultra low interfacial tension minimum in a petroleum sulfonate/oil/brine/oil/brine system. J. Dispers. Sci. Technol. 1980, 1, 55–95. [Google Scholar] [CrossRef]
- Chan, K.S.; Shah, D.O. The Physico-Chemical Conditions Necessary to Produce Ultralow Interfacial Tension at the Oil/Brine Interface. In Surface Phenomena in Enhanced Oil Recovery; Shah, D.O., Ed.; Springer: Boston, MA, USA, 1981; pp. 53–72. [Google Scholar]
- Zhao, R.H.; Zhang, L.; Zhang, L.; Zhao, S.; Yu, J.Y. Effect of the Hydrophilic-Lipophilic Ability on Dynamic Interfacial Tensions of Alkylbenzene Sulfonates. Energy Fuels 2010, 24, 5048–5052. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, L.; Zhao, S.; Yu, J. Studies of synergism/antagonism for lowering dynamic interfacial tensions in surfactant/alkali/acidic oil systems. 1. Synergism/Antagonism in surfactant/model oil systems. J. Colloid Interface Sci. 2002, 249, 187–193. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, R.H.; Jin, Z.Q.; Zhang, L.; Zhang, L.; Luo, L.; Zhao, S. Influence of Crude Oil Fractions on Interfacial Tensions of Alkylbenzene Sulfonate Solutions. Energy Fuels 2013, 27, 4648–4653. [Google Scholar] [CrossRef]
- Zhao, R.H.; Huang, H.Y.; Wang, H.Y.; Zhang, J.C.; Zhang, L.; Zhang, L.; Zhao, S. Effect of Organic Additives and Crude Oil Fractions on Interfacial Tensions of Alkylbenzene Sulfonates. J. Dispers. Sci. Technol. 2013, 34, 623–631. [Google Scholar] [CrossRef]
- He, L.; Li, X.; Wu, G.; Lin, F.; Sui, H. Distribution of Saturates, Aromatics, Resins, and Asphaltenes Fractions in the Bituminous Layer of Athabasca Oil Sands. Energy Fuels. 2013, 27, 4677–4683. [Google Scholar] [CrossRef]
- Rane, J.; Harbottle, D.; Pauchard, V.; Couzis, A.; Banerjee, S. Adsorption Kinetics of Asphaltenes at the Oil-Water Interface and Nanoaggregation in the Bulk. Langmuir ACS J. Surf. Colloids 2012, 28, 9986–9995. [Google Scholar] [CrossRef]
- Tangparitkul, S.; Charpentier, T.V.J.; Pradilla, D.; Harbottle, D. Interfacial and Colloidal Forces Governing Oil Droplet Displacement: Implications for Enhanced Oil Recovery. Colloids Interfaces 2018, 2, 30. [Google Scholar] [CrossRef] [Green Version]
- Tangparitkul, S.; Hodges, C.S.; Ballard, D.A.; Niu, Z.; Pradilla, D.; Charpentier, T.V.J.; Xu, Z.; Harbottle, D. Dewetting dynamics of heavy crude oil droplet in low-salinity fluids at elevated pressures and temperatures. J. Colloid Interface Sci. 2021, 596, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, L.; Lu, X.; Shi, C.; Tang, T.; Wang, X.G.; Huang, Q.X.; Zeng, H.B. Adsorption kinetics of asphaltenes at oil/water interface: Effects of concentration and temperature. Fuel 2018, 212, 387–394. [Google Scholar] [CrossRef]




| Components | Cl− | SO42− | CO32− | HCO3- | Na++K+ | Ca2+ | Mg2+ | TDS |
|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | 17422 | 0 | 0 | 596 | 9665 | 1079 | 355 | 29117 |
| Solutions | pH |
|---|---|
| Formation brine | 7.78 |
| 0.3 wt%ASB@AEC | 7.92 |
| 0.3 wt% ASB@PS | 8.05 |
| 0.3 wt% ASB@AEC + 1500 ppm 20 wt%AMPS polymer | 7.89 |
| 0.3 wt% ASB@PS + 1500 ppm 20 wt%AMPS polymer | 8.03 |
| Polymer Concentration (ppm) | 10 | 100 | 1000 | 1500 |
|---|---|---|---|---|
| 0.3 wt%ASB@AEC + 20 wt%AMPS polymer | 0.9 | 1.1 | 4.1 | 6.9 |
| 0.3 wt%ASB@PS + 20 wt%AMPS polymer | 0.8 | 1 | 3.8 | 6.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.-L.; Pan, Y.; Hu, F.-T.; Han, L.; Zhu, X.-Y.; Zhang, L.; Zhou, Z.-H.; Li, G.; Ma, G.-Y.; Zhang, L. Dynamic Interfacial Tensions of Surfactant and Polymer Solutions Related to High-Temperature and High-Salinity Reservoir. Molecules 2023, 28, 1279. https://doi.org/10.3390/molecules28031279
Cui X-L, Pan Y, Hu F-T, Han L, Zhu X-Y, Zhang L, Zhou Z-H, Li G, Ma G-Y, Zhang L. Dynamic Interfacial Tensions of Surfactant and Polymer Solutions Related to High-Temperature and High-Salinity Reservoir. Molecules. 2023; 28(3):1279. https://doi.org/10.3390/molecules28031279
Chicago/Turabian StyleCui, Xiang-Long, Yi Pan, Fu-Tang Hu, Lu Han, Xiu-Yu Zhu, Lei Zhang, Zhao-Hui Zhou, Gen Li, Gui-Yang Ma, and Lu Zhang. 2023. "Dynamic Interfacial Tensions of Surfactant and Polymer Solutions Related to High-Temperature and High-Salinity Reservoir" Molecules 28, no. 3: 1279. https://doi.org/10.3390/molecules28031279
APA StyleCui, X.-L., Pan, Y., Hu, F.-T., Han, L., Zhu, X.-Y., Zhang, L., Zhou, Z.-H., Li, G., Ma, G.-Y., & Zhang, L. (2023). Dynamic Interfacial Tensions of Surfactant and Polymer Solutions Related to High-Temperature and High-Salinity Reservoir. Molecules, 28(3), 1279. https://doi.org/10.3390/molecules28031279





