A Review of Fibraurea tinctoria and Its Component, Berberine, as an Antidiabetic and Antioxidant
Abstract
:1. Introduction
2. Fibraurea tinctoria
3. Berberine
3.1. Pharmacokinetics of Berberine
3.2. Berberine Toxicity
3.3. In Silico Studies of Berberine
4. Antioxidant Activity of Berberine
4.1. Berberine Scavenges ROS and Blocks ROS Generation
4.2. Berberine Chelates Metal Ions
4.3. Berberine Regulates Enzyme Activity
4.3.1. Berberine Enhances Antioxidant Enzyme Activity and Inhibits Lipid/Protein Peroxidation
4.3.2. Berberine Inhibits the Production of Oxidase
4.4. Berberine Effects on Signal Transduction Pathway and Cell Antioxidant Response
5. Antidiabetic Activity of Berberine
5.1. Berberine Increases Insulin Secretion
5.2. Berberine Improves Insulin Resistance
5.3. Berberine Inhibits Gluconeogenesis
5.4. Berberine Increases Glucose Uptake
5.5. Berberine Induces Glycolysis
5.6. Berberine Inhibits Enzyme Activity
5.7. Berberine Regulates Gut Microbiota
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ADA. Standards of Medical Care in Diabetes—2020. Diabetes Care J. Clin. Appl. Res. Educ. 2020, 43, S1–S212. [Google Scholar]
- IDF. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; ISBN 9782930229980. [Google Scholar]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar] [PubMed]
- Tabatabaei-Malazy, O.; Fakhrzadeh, H.; Sharifi, F.; Mirarefin, M.; Arzaghi, S.M.; Badamchizadeh, Z.; Khoee, M.A.; Larijani, B. Effect of metabolic control on oxidative stress, subclinical atherosclerosis and peripheral artery disease in diabetic patients. J. Diabetes Metab. Disord. 2015, 14, 84. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.N.; Khan, R.A.; Ahmad, M.; Mushtaq, N. Role of antioxidant in oxidative stress and diabetes mellitus. J. Pharmacogn. Phytochem. 2015, 3, 217–220. [Google Scholar] [CrossRef]
- Zonszein, J.; Groop, P.H. Strategies for diabetes management: Using newer oral combination therapies early in the disease. Diabetes Ther. 2016, 7, 621–639. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Jadhav, P.; Deshmukh, Y. Prescribing pattern and efficacy of anti-diabetic drugs in maintaining optimal glycemic levels in diabetic patients. J. Basic Clin. Pharm. 2014, 5, 79. [Google Scholar] [CrossRef] [Green Version]
- Lipska, K.J.; Yao, X.; Herrin, J.; McCoy, R.G.; Ross, J.S.; Steinman, M.A.; Inzucchi, S.E.; Gill, T.M.; Krumholz, H.M.; Shah, N.D. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care 2017, 40, 468–475. [Google Scholar] [CrossRef] [Green Version]
- Casagrande, S.S.; Fradkin, J.E.; Saydah, S.H.; Rust, K.F.; Cowie, C.C. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013, 36, 2271–2279. [Google Scholar] [CrossRef] [Green Version]
- Thorpe, L.E.; Kanchi, R.; Chamany, S.; Rodriguez-Lopez, J.S.; Chernov, C.; Freeman, A.; Perlman, S.E. Change in Diabetes Prevalence and Control among New York City Adults: NYC Health and Nutrition Examination Surveys 2004–2014. J. Urban Health 2018, 95, 826–831. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Yin, M.; Zhang, Y.; Huang, L.; Chen, R.; Ni, J. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: A systematic literature review and a meta-analysis. Endocr. J. 2019, 66, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Triplitt, C.; Repas, T.; Alvarez, C. Chapter 74: Diabetes Mellitus. In Pharmacotherapy a Pathophysiologic Approach; Mc Graw Hill Education: New York, NY, USA, 2016; pp. 3211–3292. [Google Scholar]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Sease, J.; Shealy, K. Chapter 43: Diabetes Mellitus. In Pharmacotherapy Principles & Practice; Chicholm-Burns, M., Schwinghammer, T., Wells, B., Malone, P., Kolesar, J., Dipiro, J., Eds.; Mc Graw Hill Education: New York, NY, USA, 2016; pp. 651–678. ISBN 9781626239777. [Google Scholar]
- Defronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezuruike, U.F.; Prieto, J.M. The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations. J. Ethnopharmacol. 2014, 155, 857–924. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.L.; Chen, B.C.; Mou, C.H.; Sun, M.F.; Yen, H.R. Association of traditional Chinese medicine therapy and the risk of vascular complications in patients with type II diabetes mellitus: A nationwide, retrospective, Taiwanese-registry, cohort study. Medicine 2016, 95, e2536. [Google Scholar] [CrossRef]
- Alam, F.; Islam, M.A.; Kamal, M.A.; Gan, S.H. Updates on managing type 2 diabetes mellitus with natural products: Towards antidiabetic drug development. Curr. Med. Chem. 2016, 23, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhao, L.; Zhou, Q.; Zhao, T.; Wang, H.; Gu, C.; Tong, X. Application of Berberine on Treating Type 2 Diabetes Mellitus. Int. J. Endocrinol. 2015, 2015, 905749. [Google Scholar] [CrossRef] [Green Version]
- Teoh, S.L.; Das, S. Phytochemicals and their effective role in the treatment of diabetes mellitus: A short review. Phytochem. Rev. 2018, 17, 1111–1128. [Google Scholar] [CrossRef]
- Rios, J.L.; Francini, F.; Schinella, G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [Green Version]
- Ota, A.; Ulrih, N.P. An overview of herbal products and secondary metabolites used for management of type two diabetes. Front. Pharmacol. 2017, 8, 436. [Google Scholar] [CrossRef]
- Galappathie, S.; Palombo, E.A.; Chia, T.; Lim, D.; Ley, S.; Lee, C.; Mahon, P.J. Comparative antimicrobial activity of South East Asian plants used in Bornean folkloric medicine. J. Herb. Med. 2014, 4, 96–105. [Google Scholar] [CrossRef]
- Noorcahyati, N.; Sulandjari, S.; Dewi, W.S. Asosiasi Akar Kuning (Fibraurea tinctoria Lour.) dengan tumbuhan berpotensi obat di Samboja, Kalimantan Timur. J. Hutan Trop. 2016, 4, 232–239. [Google Scholar]
- Nguyen-pouplin, J.; Tran, H.; Tran, H.; Anh, T.; Dolecek, C. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J. Ethnopharmacol. 2007, 109, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kiyotani, T.; Maeda, M.; Katayama, T.; Tomita-yokotani, K.; Syafii, W.; Muladi, S. Furanoditerpenes from Arcangelisia flava (L.) Merr. and their antifungal activity. Phytochem. Lett. 2011, 4, 333–336. [Google Scholar] [CrossRef]
- TPL. The Plant List. Available online: http://www.theplantlist.org/ (accessed on 16 August 2022).
- Barbosa-Filho, J.M.; Da-Cunha, E.V.L.; Gray, A.I. Alkaloids of the Menispermaceae. Alkaloids Chem. Biol. 2000, 54, 1–190. [Google Scholar] [CrossRef]
- Chang, W.; Chen, L.; Hatch, G.M. Berberine as a therapy for type 2 diabetes and its complications: From mechanism of action to clinical studies. Biochem. Cell Biol. 2015, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, N.; Zhao, L.; Lu, F. Berberine in the treatment of type 2 diabetes mellitus: A systemic review and meta-analysis. Evid.-Based Complement. Altern. Med. 2012, 2012, 591654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Geng, Y.N.; Jiang, J.D.; Kong, W.J. Antioxidant and anti-inflammatory activities of Berberine in the treatment of diabetes mellitus. Evid.-Based Complement. Altern. Med. 2014, 2014, 289264. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Chen, Y.; Liou, M.; Tsai, H.; Chang, W.; Wu, T. Anti-inflammatory activities of furanoditerpenoids and other constituents from Fibraurea tinctoria. Bioorg. Med. Chem. 2008, 16, 9603–9609. [Google Scholar] [CrossRef] [PubMed]
- Watthanachaiyingcharoen, R.; Komatsu, K.; Zhu, S.; Vajragupta, O.; Leelamanit, W. Authentication of Coscinium fenestratum among the other Menispermaceae plants prescribed in Thai folk medicines. Biol. Pharm. Bull. 2010, 33, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Ueng, Y.; Nguyen, X.; Reddy, M.; Wu, T. Cytochrome P3A4 inhibitors and other constituents of Fibraurea tinctoria. J. Nat. Prod. 2007, 70, 1930–1933. [Google Scholar] [CrossRef]
- Keawpradub, N.; Dej-Adisai, S.; Yuenyongsawad, S. Antioxidant and cytotoxic activities of Thai medicinal plants named Khaminkhruea: Arcangelisia flava, Coscinium blumeanum and Fibraurea tinctoria. Songklanakarin J. Sci. Technol. 2005, 27, 455–467. [Google Scholar]
- Manosroi, A.; Akazawa, H.; Pattamapun, K.; Akihisa, T.; Manosroi, W.; Manosroi, J. Potent anti-proliferative effects against oral and cervical cancers of Thai medicinal plants selected from the Thai/Lanna medicinal plant recipe database “MANOSROI III” Potent anti-proliferative effects against oral and cervical cancers of Thai medici. Pharm. Biol. 2015, 53, 1075–1081. [Google Scholar] [CrossRef]
- Manosroi, A.; Akazawa, H.; Kitdamrongtham, W.; Akihisa, T.; Manosroi, W.; Manosroi, J. Potent antiproliferative effect on liver cancer of medicinal plants selected from the Thai/Lanna medicinal plant recipe database “MANOSROI III”. Evid.-Based Complement. Altern. Med. 2015, 2015, 397181. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef]
- Ehteshamfar, S.M.; Akhbari, M.; Afshari, J.T.; Seyedi, M.; Nikfar, B.; Shapouri-Moghaddam, A.; Ghanbarzadeh, E.; Momtazi-Borojeni, A.A. Anti-inflammatory and immune-modulatory impacts of berberine on activation of autoreactive T cells in autoimmune inflammation. J. Cell. Mol. Med. 2020, 24, 13573–13588. [Google Scholar] [CrossRef]
- Chang, Y. Effectiveness of berberine in bacillary dysentery. Zhonghua Nei Ke Za Zhi 1959, 7, 741–743. [Google Scholar]
- Xia, X.; Wang, H.; Niu, X.; Wang, H.; Liu, Z.; Liu, Y.; Qi, Z.; Wang, S.; Liu, S.; Liu, S. Assessment of the anti-diarrhea function of compound Chinese herbal medicine Cangpo Oral Liquid. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Jin, X.; Liang, C.; Bu, F.; Pan, D.; He, Q.; Ming, Y.; Little, P.; Du, H.; Liang, S.; et al. Berberine for diarrhea in children and adults: A systematic review and meta-analysis. Therap. Adv. Gastroenterol. 2020, 13, 1756284820961299. [Google Scholar] [CrossRef]
- Yue, S.J.; Liu, J.; Wang, W.X.; Wang, A.T.; Yang, X.Y.; Guan, H.S.; Wang, C.Y.; Yan, D. Berberine treatment-emergent mild diarrhea associated with gut microbiota dysbiosis. Biomed. Pharmacother. 2019, 116, 109002. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, Z.; Li, Y.; Fichna, J.; Storr, M. Effects of berberine in the gastrointestinal tract-A review of actions and therapeutic implications. Am. J. Chin. Med. 2014, 42, 1053–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pierro, F.; Bertuccioli, A.; Giuberti, R.; Saponara, M.; Ivaldi, L. Role of a berberine-based nutritional supplement in reducing diarrhea in subjects with functional gastrointestinal disorders. Minerva Gastroenterol. Dietol. 2020, 66, 29–34. [Google Scholar] [CrossRef]
- Li, L.; Cui, H.; Li, T.; Qi, J.; Chen, H.; Gao, F.; Tian, X.; Mu, Y.; He, R.; Lv, S.; et al. Synergistic effect of berberine-based Chinese medicine assembled nanostructures on diarrhea-predominant irritable bowel syndrome in vivo. Front. Pharmacol. 2020, 11, 1210. [Google Scholar] [CrossRef]
- Jiang, X.W.; Zhang, Y.; Zhu, Y.L.; Zhang, H.; Lu, K.; Li, F.F.; Peng, H.Y. Effects of berberine gelatin on recurrent aphthous stomatitis: A randomized, placebo-controlled, double-blind trial in a Chinese cohort. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 212–217. [Google Scholar] [CrossRef]
- Li, H.L.; Han, T.; Liu, R.H.; Zhang, C.; Chen, H.S.; Zhang, W.D. Alkaloids from Corydalis saxicola and their anti-hepatitis B virus activity. Chem. Biodivers. 2008, 5, 777–783. [Google Scholar] [CrossRef]
- Hung, T.C.; Jassey, A.; Liu, C.H.; Lin, C.J.; Lin, C.C.; Wong, S.H.; Wang, J.Y.; Yen, M.H.; Lin, L.T. Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine 2019, 53, 62–69. [Google Scholar] [CrossRef]
- Och, A.; Podgórski, R.; Nowak, R. Biological activity of Berberine-A summary update. Toxins 2020, 12, 713. [Google Scholar] [CrossRef]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.T.S.; Smejkal, K.; Malaník, M.; et al. Berberine in cardiovascular and metabolic diseases: From mechanisms to therapeutics. Theranostics 2019, 9, 1923–1951. [Google Scholar] [CrossRef]
- Jin, Y.; Khadka, D.B.; Cho, W.J. Pharmacological effects of berberine and its derivatives: A patent update. Expert Opin. Ther. Pat. 2016, 26, 229–243. [Google Scholar] [CrossRef]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crisan, G.; Buzoianu, A.D. Berberine: Botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, I.; Zhu, W. Dose-dependent effect of berberine on SARS-CoV-2 spike protein induced inflammatory host cell response. Int. J. Med. Sci. Health Res. 2021, 5, 169–181. [Google Scholar] [CrossRef]
- Varghese, F.S.; van Woudenbergh, E.; Overheul, G.J.; Eleveld, M.J.; Kurver, L.; van Heerbeek, N.; van Laarhoven, A.; Miesen, P.; Den Hartog, G.; De Jonge, M.I.; et al. Berberine and Obatoclax inhibit SARS-CoV-2 replication in primary human nasal epithelial cells in vitro. Viruses 2021, 13, 282. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, B.A.; Noval, M.G.; Kaczmarek, M.E.; Jang, K.K.; Thannickal, S.A.; Kottkamp, A.C.; Brown, R.S.; Kielian, M.; Cadwell, K.; Stapleford, K.A. Atovaquone and Berberine Chloride reduce SARS-CoV-2 replication in vitro. Viruses 2021, 13, 2437. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Chen, M.; Chen, X.C.; Cao, K.; You, Y.; Qian, Y.J.; Yu, W.K. Berberine reduces circulating inflammatory mediators in patients with severe COVID-19. Br. J. Surg. 2021, 108, e9–e11. [Google Scholar] [CrossRef]
- Xue, M.; Yang, M.; Zhang, W.; Li, X.; Gao, D.; Ou, Z.; Li, Z.; Liu, S.; Li, X.; Yang, S. Characterization, pharmacokinetics, and hypoglycemic effect of berberine loaded solid lipid nanoparticles. Int. J. Nanomed. 2013, 8, 4677–4687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.X.; Hu, Y.N.; Li, J.W.; Yuan, K.; Guo, Y. Preparation and evaluation of antidiabetic agents of berberine organic acid salts for enhancing the bioavailability. Molecules 2019, 24, 103. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.S.; Zheng, Y.R.; Zhang, Y.F.; Long, X.Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, S.; Colliva, C.; Camborata, C.; Roberti, M.; Ianni, C.; Neri, F.; Calvarese, C.; Lisotti, A.; Mazzella, G.; Roda, A. Berberine and its metabolites: Relationship between physicochemical properties and plasma levels after administration to human subjects. J. Nat. Prod. 2014, 77, 766–772. [Google Scholar] [CrossRef]
- Liu, Y.T.; Hao, H.P.; Xie, H.G.; Lai, L.; Wang, Q.; Liu, C.X.; Wang, G.J. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab. Dispos. 2010, 38, 1779–1784. [Google Scholar] [CrossRef] [Green Version]
- Kwon, M.; Lim, D.Y.; Lee, C.H.; Jeon, J.H.; Choi, M.K.; Song, I.S. Enhanced intestinal absorption and pharmacokinetic modulation of berberine and its metabolites through the inhibition of p-glycoprotein and intestinal metabolism in rats using a berberine mixed micelle formulation. Pharmaceutics 2020, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.-Q.; Zhu, Y.-P.; Pang, J.; Wang, Y.-X.; Song, D.-Q.; Kong, W.-J.; Jiang, J.-D. Tetrandrine potentiates the hypoglycemic efficacy of berberine by inhibiting P-glycoprotein function. Biol. Pharm. Bull. 2013, 36, 1562–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Miao, Y.Q.; Fan, D.J.; Yang, S.S.; Lin, X.; Meng, L.K.; Tang, X. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, X.; Wang, Q.; Wang, S.; Kuang, Y.; Li, K.; Song, W.; Ye, M. A 42-markers pharmacokinetic study reveals interactions of berberine and glycyrrhizic acid in the anti-diabetic chinese medicine formula Gegen-Qinlian decoction. Front. Pharmacol. 2018, 9, 622. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, F.; Jiang, J.; Gao, C.; Tan, Y. Intestinal absorption mechanisms of berberine, palmatine, jateorhizine, and coptisine: Involvement of P-glycoprotein. Xenobiotica 2011, 41, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Wang, T.; Chen, K.; Wang, H.; Jia, Q.; Li, Y. Enhancement of berberine hypoglycemic activity by oligomeric proanthocyanidins. Molecules 2018, 23, 3318. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tong, Q.; Shou, J.W.; Zhao, Z.X.; Li, X.Y.; Zhang, X.F.; Ma, S.R.; He, C.Y.; Lin, Y.; Wen, B.Y.; et al. Gut microbiota-mediated personalized treatment of hyperlipidemia using berberine. Theranostics 2017, 7, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Shou, J.W.; Zhao, Z.X.; He, C.Y.; Ma, C.; Huang, M.; Fu, J.; Tan, X.S.; Li, X.Y.; Wen, B.Y.; et al. Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci. Rep. 2015, 5, 12155. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.S.; Ma, J.Y.; Feng, R.; Ma, C.; Chen, W.J.; Sun, Y.P.; Fu, J.; Huang, M.; He, C.Y.; Shou, J.W.; et al. Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS ONE 2013, 8, e77969. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Hao, H.; Xie, H.; LV, H.; Liu, C.; Wang, G. Oxidative demethylenation and subsequent glucuronidation are the major metabolic pathways of berberine in rats. J. Pharm. Sci. 2009, 99, 4391–4401. [Google Scholar] [CrossRef]
- Alolga, R.N.; Fan, Y.; Chen, Z.; Liu, L.W.; Zhao, Y.J.; Li, J.; Chen, Y.; Lai, M.D.; Li, P.; Qi, L.W. Significant pharmacokinetic differences of berberine are attributable to variations in gut microbiota between Africans and Chinese. Sci. Rep. 2016, 6, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Chai, L.; Feng, X.; Liu, Z.; Liu, H.; Ding, L.; Qiu, F. Metabolites identification of berberine in rats using ultra-high performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2017, 139, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xiang, Y.; Shi, Y.; Tang, X.; Pan, L.; Gao, J.; Bi, R.; Lai, X. Pharmacokinetics and pharmacological activities of berberine in diabetes mellitus treatment. Evid.-Based Complement. Altern. Med. 2021, 2021, 9987097. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-L.; Tsai, T.-H. Hepatobiliary excretion of berberine. Drug Metab. Dispos. 2004, 32, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, F.; Ma, X.; Cheng, X.; Zhou, H.; Klaassen, C.D. CYP2D plays a major role in berberine metabolism in liver of mice and humans. Xenobiotica 2011, 41, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xu, C.; Li, X.; Li, D.; Li, Y.; Jiang, J.; Yang, P.; Duan, G. Rapid identification of berberine metabolites in rat plasma by UHPLC-Q-TOF-MS. Molecules 2019, 24, 1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Wang, K.; Cao, S.; Ding, L.; Qiu, F. Pharmacokinetics and excretion of berberine and its nine metabolites in rats. Front. Pharmacol. 2021, 11, 594852. [Google Scholar] [CrossRef]
- Ma, J.Y.; Feng, R.; Tan, X.S.; Ma, C.; Shou, J.W.; Fu, J.; Huang, M.; He, C.Y.; Chen, S.N.; Zhao, Z.X.; et al. Excretion of berberine and its metabolites in oral administration in rats. J. Pharm. Sci. 2013, 102, 4181–4192. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, H.; Ren, J.-Y.; Pan, J.-F.; Hong, Y.-C.; Zhu, D.-Y.; Xu, X.-R. Determination and preliminary studies of metabolism of berberine in human urine after oral administration. Chin. J. Clin. Pharmacol. 2000, 16, 36–39. [Google Scholar]
- Kheir, M.M.; Wang, Y.; Hua, L.; Hu, J.; Li, L.; Lei, F.; Du, L. Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem. Toxicol. 2010, 48, 1105–1110. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, B. Toxicological effects of berberine and sanguinarine. Front. Mol. Biosci. 2018, 5, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anis, K.V.; Kuttan, G.; Kuttan, R. Role of berberine as an adjuvant response modifier during tumour therapy in mice. Pharm. Pharmacol. Commun. 1999, 5, 697–700. [Google Scholar] [CrossRef] [Green Version]
- Lan, J.; Zhao, Y.; Dong, F.; Yan, Z.; Zheng, W.; Fan, J.; Sun, G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 2015, 161, 69–81. [Google Scholar] [CrossRef]
- Barnett, L.M.A.; Cummings, B.S. Nephrotoxicity and renal pathophysiology: A contemporary perspective. Toxicol. Sci. 2018, 164, 379–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, E.; Lee, T.W.; Park, D.J. Drug-induced nephrotoxicity. J. Korean Med. Assoc. 2020, 63, 30–35. [Google Scholar] [CrossRef]
- Domitrović, R.; Cvijanović, O.; Pernjak-Pugel, E.; Škoda, M.; Mikelić, L.; Crnčević-Orlić, Ž. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem. Toxicol. 2013, 62, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Riahi, H. Preventive use of berberine in inhibition of lead-induced renal injury in rats. Environ. Sci. Pollut. Res. 2018, 25, 4896–4903. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.M.; Shalkami, A.G.S.; Khalaf, M.M.; Mohamed, W.R.; Hemeida, R.A.M. The impact of Keap1/Nrf2, P 38 MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed. Pharmacother. 2019, 109, 47–56. [Google Scholar] [CrossRef]
- Allameh, H.; Fatemi, I.; Malayeri, A.R.; Nesari, A.; Mehrzadi, S.; Goudarzi, M. Pretreatment with berberine protects against cisplatin-induced renal injury in male Wistar rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1825–1833. [Google Scholar] [CrossRef]
- Zhao, X.; Tong, N. Protective effects of berberine on doxorubicin-induced nephrotoxicity in mice. J. Transl. Med. 2012, 10, 400716. [Google Scholar] [CrossRef] [Green Version]
- Hussien, N.R.; Al-Kuraishy, H.M.; Al-Gareeb, A.I. Reno-protective effect of berberine. J. Pak. Med. Assoc. 2019, 69, S83–S87. [Google Scholar]
- Adil, M.; Kandhare, A.D.; Dalvi, G.; Ghosh, P.; Venkata, S.; Raygude, K.S.; Bodhankar, S.L. Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Ren. Fail. 2016, 38, 996–1006. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim Fouad, G.; Ahmed, K.A. The protective impact of berberine against doxorubicin-induced nephrotoxicity in rats. Tissue Cell 2021, 73, 101612. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.K.; Malik, S.; Mutneja, E.; Sahu, A.K.; Rupashi, K.; Dinda, A.K.; Arya, D.S.; Bhatia, J. Mechanism involved in fortification by berberine in CDDP-induced nephrotoxicity. Curr. Mol. Pharmacol. 2020, 13, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Gholampour, F.; Keikha, S. Berberine protects the liver and kidney against functional disorders and histological damages induced by ferrous sulfate. Iran. J. Basic Med. Sci. 2018, 21, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.S.; Safwat, G.; Aboulkhair, M.; Abdel Moneim, A.E. The potential effect of berberine in mercury-induced hepatorenal toxicity in albino rats. Food Chem. Toxicol. 2014, 69, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, W.; Shao, X.; Wu, J.; Li, S.; Che, X.; Ni, Z. Integrated analysis of m6A methylome in cisplatin-induced acute kidney injury and berberine alleviation in mouse. Front. Genet. 2020, 11, 584460. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Xue, Q.; Kuang, L.; Xie, L.; Luo, R.; Nie, X. Berberine alleviates cisplatin-induced acute kidney injury by regulating mitophagy via PINK 1/Parkin pathway. Transl. Androl. Urol. 2020, 9, 1712–1724. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, Z.; Zhang, X.; Wu, A.; Huang, Y.; Miao, Y.; Yang, M. Effect of berberine on the renal tubular epithelial-to-mesenchymal transition by inhibition of the notch/snail pathway in diabetic nephropathy model KKAy mice. Drug Des. Dev. Ther. 2017, 11, 1065–1079. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; He, H.; Liang, D.; Jiang, Y.; Liang, W.; Chi, Z.H.; Ma, J. Protective effects of berberine on renal injury in streptozotocin (STZ)-Induced diabetic mice. Int. J. Mol. Sci. 2016, 17, 1327. [Google Scholar] [CrossRef] [Green Version]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Hussien, N.R. Synergistic effect of berberine and pentoxifylline in attenuation of acute kidney injury. Int. J. Crit. Illn. Inj. Sci. 2019, 9, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, Z.; Liu, D.; Zheng, J.; Xie, J.; Chen, J.; Zeng, H.; Su, Z.; Li, Y. Effect of berberine on hyperuricemia and kidney injury: A network pharmacology analysis and experimental validation in a mouse model. Drug Des. Dev. Ther. 2021, 15, 3241–3254. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, W. Protective effect of berberine on renal fibrosis caused by diabetic nephropathy. Mol. Med. Rep. 2017, 16, 1055–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, G.; Wang, H.; Gao, Y.; Chen, G.; Pei, Y.; Bai, J. 1H-NMR-Based metabonomics of the protective effect of coptis chinensis and berberine on cinnabar-induced hepatotoxicity and nephrotoxicity in rats. Molecules 2017, 22, 1855. [Google Scholar] [CrossRef] [Green Version]
- Pervez, S.; Saeed, M.; Khan, H.; Shahid, M.; Ullah, I. Nephroprotective effect of berberis baluchistanica against gentamicin-induced nephrotoxicity in rabbit. Bangladesh J. Pharmacol. 2018, 13, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Laamech, J.; El-hilaly, J.; Fetoui, H.; Chtourou, Y.; Tahraoui, A.; Lyoussi, B. Nephroprotective effects of Berberis Vulgaris L. total extract on lead acetate-induced toxicity in mice. Indian J. Pharm. Sci. 2016, 78, 326–333. [Google Scholar] [CrossRef]
- Rao, V.S.; Srinivas, K. Modern drug discovery process: An in silico approach. J. Bioinform. Seq. Anal. 2011, 2, 89–94. [Google Scholar]
- Ekins, S.; Mestres, J.; Testa, B. In silico pharmacology for drug discovery: Applications to targets and beyond. Br. J. Pharmacol. 2007, 152, 21–37. [Google Scholar] [CrossRef] [Green Version]
- Terstappen, G.C.; Reggiani, A. In silico research in drug discovery. Trends Pharmacol. Sci. 2001, 22, 23–26. [Google Scholar] [CrossRef]
- Kazmi, S.R.; Jun, R.; Yu, M.S.; Jung, C.; Na, D. In silico approaches and tools for the prediction of drug metabolism and fate: A review. Comput. Biol. Med. 2019, 106, 54–64. [Google Scholar] [CrossRef]
- Brogi, S.; Ramalho, T.C.; Kuca, K.; Medina-Franco, J.L.; Valko, M. Editorial: In silico methods for drug design and discovery. Front. Chem. 2020, 8, 612. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Discussing the mechanism of Dahuang Huanglian Xiexin decoction in the treatment of type 2 diabetes mellitus via network pharmacology and molecular docking. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Zabidi, N.A.; Ishak, N.A.; Hamid, M.; Ashari, S.E.; Mohammad Latif, M.A. Inhibitory evaluation of Curculigo latifolia on α-glucosidase, DPP (IV) and in vitro studies in antidiabetic with molecular docking relevance to type 2 diabetes mellitus. J. Enzyme Inhib. Med. Chem. 2021, 36, 109–121. [Google Scholar] [CrossRef]
- Jhong, C.H.; Riyaphan, J.; Lin, S.H.; Chia, Y.C.; Weng, C.F. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. BioFactors 2015, 41, 242–251. [Google Scholar] [CrossRef]
- Mandar, B.K.; Khanal, P.; Patil, B.M.; Dey, Y.N.; Pasha, I. In silico analysis of phytoconstituents from Tinospora cordifolia with targets related to diabetes and obesity. In Silico Pharmacol. 2021, 9, 3. [Google Scholar] [CrossRef]
- Rahman, N.; Muhammad, I.; Nayab, G.E.; Khan, H.; Aschner, M.; Filosa, R.; Daglia, M. Molecular docking of isolated alkaloids for possible α-Glucosidase inhibition. Biomolecules 2019, 9, 544. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, I.; Kumar, S.; Rajesh, S. Dipeptidyl Peptidase IV inhibitory activity of Berberine and Mangiferin: An in silico approach. Int. J. Clin. Endocrinol. Metab. 2017, 3, 18–22. [Google Scholar] [CrossRef]
- Herowati, R.; Widodo, G.P. Molecular docking studies of chemical constituents of Tinospora cordifolia on glycogen phosphorylase. Procedia Chem. 2014, 13, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Dou, Y.; Huang, R.; Li, Q.; Liu, Y.; Li, Y.; Chen, H.; Ai, G.; Xie, J.; Zeng, H.; Chen, J.; et al. Oxyberberine, an absorbed metabolite of berberine, possess superior hypoglycemic effect via regulating the PI3K/Akt and Nrf2 signaling pathways. Biomed. Pharmacother. 2021, 137, 111312. [Google Scholar] [CrossRef]
- Kaboli, P.J.; Ismail, P.; Ling, K.H. Molecular modeling, dynamics simulations, and binding efficiency of berberine derivatives: A new group of RAF inhibitors for cancer treatment. PLoS ONE 2018, 13, e0193941. [Google Scholar] [CrossRef]
- Song, L.; Luo, Y.; Wang, X.; Almutairi, M.M.; Pan, H.; Li, W.; Liu, Y.; Wang, Q.; Hong, M. Exploring the active mechanism of berberine against HCC by systematic pharmacology and experimental validation. Mol. Med. Rep. 2019, 20, 4654–4664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbarzadeh Kaboli, P.; Leong, M.P.Y.; Ismail, P.; Ling, K.H. Antitumor effects of berberine against EGFR, ERK1/2, P38 and AKT in MDA-MB231 and MCF-7 breast cancer cells using molecular modelling and in vitro study. Pharmacol. Rep. 2019, 71, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Khan, I.A.; Rehman, A.; Bibi, Z. Determination of effectiveness of berberine, a characteristic phytochemical of Berberis species, against human proteome using in-silico analysis. J. Biodivers. Environ. Sci. 2014, 4, 53–63. [Google Scholar]
- Foroughi, K.; Jahanbani, S.; Nazarnezhad, S.; Khastar, H.; Jafarisani, M. Survivin as a target for anti-cancer phytochemicals according to the molecular docking analysis. Int. J. Pept. Res. Ther. 2019, 26, 1115–1126. [Google Scholar] [CrossRef]
- Subair, T.I.; Soremekun, O.S.; Olotu, F.A.; Soliman, M.E.S. Prospecting the therapeutic edge of a novel compound (B12) over berberine in the selective targeting of Retinoid X Receptor in colon cancer. J. Mol. Model. 2021, 27, 231. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, K.; Samanta, S.; Bagoband, V.; Murugan, N.A.; Govindaraju, T. Antioxidant berberine-derivative inhibits multifaceted amyloid toxicity. iScience 2020, 23, 101005. [Google Scholar] [CrossRef]
- Chu, M.; Chen, X.; Wang, J.; Guo, L.; Wang, Q.; Gao, Z.; Kang, J.; Zhang, M.; Feng, J.; Guo, Q.; et al. Polypharmacology of berberine based on multi-target binding motifs. Front. Pharmacol. 2018, 9, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marasco, D.; Vicidomini, C.; Krupa, P.; Cioffi, F.; Dinh, P.; Huy, Q.; Suan, M.; Florio, D.; Broersen, K.; Francesca, M.; et al. Plant isoquinoline alkaloids as potential neurodrugs: A comparative study of the effects of benzo[c]phenanthridine and berberine-based compounds on β-amyloid aggregation. Chem. Biol. Interact. 2021, 334, 109300. [Google Scholar] [CrossRef]
- Hussien, H.M.; Abd-Elmegied, A.; Ghareeb, D.A.; Hafez, H.S.; Ahmed, H.E.A.; El-Moneam, N.A. Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem. Toxicol. 2018, 111, 432–444. [Google Scholar] [CrossRef]
- Sarkar, B.; Ullah, M.A.; Prottoy, M.N.I. A computational approach for exploring herbal inhibitors of acetylcholinesterase in Alzheimer’s disease. medRxiv 2020. [Google Scholar] [CrossRef]
- Amat-ur-rasool, H.; Ahmed, M.; Hasnain, S.; Ahmed, A.; Carter, W.G. In silico design of dual-binding site anti-cholinesterase phytochemical heterodimers as treatment options for Alzheimer’s disease. Curr. Issues Mol. Biol. 2022, 44, 152–175. [Google Scholar] [CrossRef]
- Ribaudo, G.; Zanforlin, E.; Canton, M.; Bova, S.; Zagotto, G. Preliminary studies of berberine and its semi-synthetic derivatives as a promising class of multi-target anti-parkinson agents. Nat. Prod. Res. 2018, 32, 1395–1401. [Google Scholar] [CrossRef]
- Jaitrong, M.; Boonsri, P.; Samosorn, S. Molecular docking studies of berberine derivative as novel multitarget Pcsk9 and Hmgcr inhibitors. Srinakharinwirot Sci. J. 2021, 37, 124–142. [Google Scholar]
- Olǧaç, A.; Orhan, I.E.; Banoglu, E. The potential role of in silico approaches to identify novel bioactive molecules from natural resources. Future Med. Chem. 2017, 9, 1663–1684. [Google Scholar] [CrossRef] [PubMed]
- Jenny, J.; Kumar, P.B. Dihydroxy berberine from tinospora cordifolia: In silico evidences for the mechanism of anti-inflammatory action through dual inhibition of lipoxygenase and cyclooxygenase. Indian J. Biochem. Biophys. 2021, 58, 244–252. [Google Scholar]
- Liu, X.; Fan, Y.; Du, L.; Mei, Z.; Fu, Y. In silico and in vivo studies on the mechanisms of chinese medicine formula (Gegen Qinlian decoction) in the treatment of ulcerative colitis. Front. Pharmacol. 2021, 12, 665102. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, T.; Zhao, J.; Li, C.; Zou, H.; Li, F.; Zhang, J.; Ren, L. Glucocorticoid receptor-mediated alleviation of inflammation by berberine: In vitro, in silico and in vivo investigations. Food Funct. 2021, 12, 11974–11986. [Google Scholar] [CrossRef] [PubMed]
- Shalkami, A.-G.S.; Hassanein, E.H.M.; Sayed, A.M.; Mohamed, W.R.; Khalaf, M.M.; Hemeida, R.A.M. Hepatoprotective effects of phytochemicals berberine and umbelliferone against methotrexate-induced hepatic intoxication: Experimental studies and in silico evidence. Environ. Sci. Pollut. Res. 2021, 28, 67593–67607. [Google Scholar] [CrossRef]
- Shinde, S.D.; Cheke, R.S.; Tathe, P.R.; Jain, P.G.; Narkhede, R.R. The Berberis aristata ameliorates oxazolone induced contact dermatitis: In-vivo and in silico evidences. Adv. Tradit. Med. 2021, 21, 685–692. [Google Scholar] [CrossRef]
- Alamzeb, M.; Ali, S.; Mamoon-Ur-Rashid; Khan, B.; Ihsanullah; Adnan; Omer, M.; Ullah, A.; Ali, J.; Setzer, W.N.; et al. Antileishmanial potential of berberine alkaloids from Berberis glaucocarpa roots: Molecular docking suggests relevant leishmania protein targets. Nat. Prod. Commun. 2021, 16, 1934578X211031148. [Google Scholar] [CrossRef]
- Nguyen, C.Q.; Nguyen, T.H.M.; Nguyen, T.T.T.; Bui, T.B.H.; Nguyen, T.T.; Huynh, N.T.; Le, T.D.; Nguyen, T.M.P.; Nguyen, D.T.; Nguyen, M.T.; et al. Designs, synthesis, docking studies, and biological evaluation of novel berberine derivatives targeting zika virus. J. Chem. 2021, 2021, 5567111. [Google Scholar] [CrossRef]
- Jhanji, R.; Bhati, V.; Singh, A.; Kumar, A. Phytomolecules against bacterial biofilm and efflux pump: An in silico and in vitro study. J. Biomol. Struct. Dyn. 2020, 38, 5500–5512. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, E.; Cedraro, N.; Mangiaterra, G.; Citterio, B.; Mobbili, G.; Minnelli, C.; Bizzaro, D.; Biavasco, F.; Galeazzi, R. Natural alkaloid berberine activity against Pseudomonas aeruginosa MexXY-mediated aminoglycoside resistance: In silico and in vitro studies. J. Nat. Prod. 2019, 82, 1935–1944. [Google Scholar] [CrossRef]
- Giorgini, G.; Mangiaterra, G.; Cedraro, N.; Laudadio, E.; Sabbatini, G.; Cantarini, M.; Minnelli, C.; Mobbili, G.; Frangipani, E.; Biavasco, F.; et al. Berberine derivatives as pseudomonas aeruginosa mexxy-oprm inhibitors: Activity and in silico insights. Molecules 2021, 26, 6644. [Google Scholar] [CrossRef] [PubMed]
- Milani, G.; Cavalluzzi, M.M.; Solidoro, R.; Salvagno, L.; Quintieri, L.; Di Somma, A.; Rosato, A.; Corbo, F.; Franchini, C.; Duilio, A.; et al. Molecular simplification of natural products: Synthesis, antibacterial activity, and molecular docking studies of berberine open models. Biomedicines 2021, 9, 452. [Google Scholar] [CrossRef]
- Sun, N.; Chan, F.Y.; Lu, Y.J.; Neves, M.A.C.; Lui, H.K.; Wang, Y.; Chow, K.Y.; Chan, K.F.; Yan, S.C.; Leung, Y.C.; et al. Rational design of berberine-based FtsZ inhibitors with broad-spectrum antibacterial activity. PLoS ONE 2014, 9, e97514. [Google Scholar] [CrossRef] [Green Version]
- Domadia, P.N.; Bhunia, A.; Sivaraman, J.; Swarup, S.; Dasgupta, D. Berberine targets assembly of Escherichia coli cell division protein FtsZ. Biochemistry 2008, 47, 3225–3234. [Google Scholar] [CrossRef]
- Kwatra, B.; Bhattacharya, B.; Khokhawat, T.; Raphael Jes, A.; Bhati, M.; Ahuja, S. Drug repurposing: In silico modeling of Mucormycosis. J. Sci. Res. Rep. 2021, 27, 53–61. [Google Scholar] [CrossRef]
- Da Silva, G.D.; de Lima, H.G.; de Freitas, H.F.; da Rocha Pita, S.S.; dos Santos Luz, Y.; de Figueiredo, M.P.; Uzêda, R.S.; Branco, A.; Costa, S.L.; Batatinha, M.J.M.; et al. In vitro and in silico studies of the larvicidal and anticholinesterase activities of berberine and piperine alkaloids on Rhipicephalus microplus. Ticks Tick-Borne Dis. 2021, 12, 101643. [Google Scholar] [CrossRef]
- Kumar, M.; Chung, S.M.; Enkhtaivan, G.; Patel, R.V.; Shin, H.S.; Mistry, B.M. Molecular docking studies and biological evaluation of berberine–benzothiazole derivatives as an anti-influenza agent via blocking of neuraminidase. Int. J. Mol. Sci. 2021, 22, 2368. [Google Scholar] [CrossRef]
- Enkhtaivan, G.; Muthuraman, P.; Kim, D.H.; Mistry, B. Discovery of berberine based derivatives as anti-influenza agent through blocking of neuraminidase. Bioorg. Med. Chem. 2017, 25, 5185–5193. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Tran, D.; Nguyen, A.; Le, L. A screening of neuraminidase inhibition activities of isoquinolone alkaloids in Coptis chinensis using molecular docking and pharmacophore analysis. ACS Omega 2020, 5, 30315–30322. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Chaturvedi, A.; Shrivastava, A.; Dubey, N.; Jain, S.; Saxena, N.; Gupta, P.; Mujariya, R. In silico interaction of Berberine with some immunomodulatory targets: A docking analysis. Indian J. Biochem. Biophys. 2022, 59, 848–853. [Google Scholar] [CrossRef]
- Yu, R.; Li, P. Screening of potential spike glycoprotein/ACE2 dual antagonists against COVID-19 in silico molecular docking. J. Virol. Methods 2020, 301, 114424. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Kumar, S.; Prasad, A.K.; Bhatt, M.L.B.; Saxena, S.K. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. VirusDisease 2020, 31, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Li, K.; Maskey, A.R.; Huang, W.; Toutov, A.A.; Yang, N.; Srivastava, K.; Geliebter, J.; Tiwari, R.; Miao, M.; et al. A small molecule compound berberine as an orally active therapeutic candidate against COVID-19 and SARS: A computational and mechanistic study. FASEB J. 2021, 35, e21360. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P. In silico investigation of phytoconstituents from Indian medicinal herb ‘Tinospora cordifolia (giloy)’ against SARS-CoV-2 (COVID-19) by molecular dynamics approach. J. Biomol. Struct. Dyn. 2021, 39, 6792–6809. [Google Scholar] [CrossRef]
- Agrawal, A.; Jain, N.K.; Kumar, N.; Kulkarni, G.T. Molecular Docking Study to Identify Potential Inhibitor of COVID-19 Main Protease Enzyme: An In-Silico Approach; Cambridge University Press: Washington, DC, USA, 2020. [Google Scholar]
- Narkhede, R.R.; Pise, A.V.; Cheke, R.S.; Shinde, S.D. Recognition of natural products as potential inhibitors of COVID-19 Main Protease (Mpro): In silico evidences. Nat. Prod. Bioprospect. 2020, 10, 297–306. [Google Scholar] [CrossRef]
- Krupanidhi, S.; Abraham Peele, K.; Venkateswarulu, T.C.; Ayyagari, V.S.; Nazneen Bobby, M.; John Babu, D.; Venkata Narayana, A.; Aishwarya, G. Screening of phytochemical compounds of Tinospora cordifolia for their inhibitory activity on SARS-CoV-2: An in silico study. J. Biomol. Struct. Dyn. 2020, 39, 5799–5803. [Google Scholar] [CrossRef]
- Garg, S.; Roy, A. In silico analysis of selected alkaloids against main protease (Mpro) of SARS-CoV-2. Chem. Biol. Interact. 2020, 332, 109309. [Google Scholar] [CrossRef]
- Gopalasatheeskumar, K.; Lakshmanan, K.; Moulishankar, A.; Suresh, J.; Kalaichelvan, V.K.; Kumudhaveni, B. Screening of Kabasura Kudineer Chooranam against COVID-19 through targeting of main protease and RNA-dependent RNA polymerase of SARS-CoV-2 by molecular docking studies. SSRN Electron. J. 2020, 2020, 3625653. [Google Scholar] [CrossRef]
- Cheke, R.S.; Narkhede, R.R.; Shinde, S.D.; Ambhore, J.P.; Jain, P.G. Natural product emerging as potential sars spike glycoproteins-ace2 inhibitors to combat COVID-19 attributed by in-silico investigations. Biointerface Res. Appl. Chem. 2021, 11, 10628–10639. [Google Scholar] [CrossRef]
- Kumar, S.; Kashyap, P.; Chowdhury, S.; Kumar, S.; Panwar, A.; Kumar, A. Identification of phytochemicals as potential therapeutic agents that binds to Nsp15 protein target of coronavirus (SARS-CoV-2) that are capable of inhibiting virus replication. Phytomedicine 2020, 85, 153317. [Google Scholar] [CrossRef]
- Matkovics, B.; Varga, S.I.; Szabo, L.; Witas, H. The effect of diabetes on the activities of the peroxide metabolism enzymes. Horm. Metab. Res. 1982, 14, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.H.; Pang, Q.Q.; Jung, P.; Cho, E.J. Antioxidant activity and acteoside analysis of Abeliophyllum distichum. Antioxidants 2020, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, S.; Tang, J.; Zhang, K.; Guang, L.; Huang, Y.; Xu, Y.; Ying, Y. Protective effect of berberine on beta cells in streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Eur. J. Pharmacol. 2009, 606, 262–268. [Google Scholar] [CrossRef]
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J. Diabetes Res. 2017, 2017, 4826724. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine and barberry (Berberis vulgaris): A clinical review. Phytother. Res. 2019, 33, 504–523. [Google Scholar] [CrossRef]
- Jung, H.A.; Min, B.S.; Yokozawa, T.; Lee, J.H.; Kim, Y.S.; Choi, J.S. Anti-Alzheimer and antioxidant activities of coptidis rhizoma alkaloids. Biol. Pharm. Bull. 2009, 32, 1433–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.-S.; Kim, S.-J.; Jung, M.Y. Inhibitory activity of berberine on DNA strand cleavage induced by hydrogen peroxide and cytochrome c. Biosci. Biotechnol. Biochem. 2001, 65, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Stoyanovsky, D.; Tyurina, Y.; Shrivastava, I.; Bahar, I.; Tyurin, V.; Protchenko, O.; Jadhav, S.; Bolevich, S.; Kozlov, A.; Vladimirov, Y.; et al. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free Radic. Biol. Med. 2019, 133, 153–161. [Google Scholar] [CrossRef]
- Jang, M.H.; Kim, H.Y.; Kang, K.S.; Yokozawa, T.; Park, J.H. Hydroxyl radical scavenging activities of isoquinoline alkaloids isolated from Coptis chinensis. Arch. Pharm. Res. 2009, 32, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Parvin, M.S.; Chlebek, J.; Hošťálková, A.; Catapano, M.C.; Lomozová, Z.; Macáková, K.; Mladěnka, P. Interactions of isoquinoline alkaloids with transition metals iron and copper. Molecules 2022, 27, 6429. [Google Scholar] [CrossRef]
- Aalikhani, M.; Alikhani, M.; Jahanshahi, M.; Elyasi, L.; Khalili, M. Berberine is a promising alkaloid to attenuate iron toxicity efficiently in iron-overloaded mice. Nat. Prod. Commun. 2022, 17, 1934578X211029522. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxidative Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, A.; Kumar, A. Targeting oxidative stress through antioxidants in diabetes mellitus. J. Drug Target. 2018, 26, 766–776. [Google Scholar] [CrossRef]
- Ji, M.; Gong, X.; Li, X.; Wang, C.; Li, M. Mechanism of polyphenols from Hippophae species—A review. Molecules 2020, 25, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecker, M.; Wagner, A.H. Role of protein carbonylation in diabetes. J. Inherit. Metab. Dis. 2018, 41, 29–38. [Google Scholar] [CrossRef]
- Lao-Ong, T.; Chatuphonprasert, W.; Nemoto, N.; Jarukamjorn, K. Alteration of hepatic glutathione peroxidase and superoxide dismutase expression in streptozotocin-induced diabetic mice by berberine. Pharm. Biol. 2012, 50, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Chatuphonprasert, W.; Lao-Ong, T.; Jarukamjorn, K. Improvement of superoxide dismutase and catalase in streptozotocin-nicotinamide-induced type 2-diabetes in mice by berberine and glibenclamide. Pharm. Biol. 2014, 52, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, P.; Tao, S.; Deng, Y.; Li, X.; Lan, T.; Zhang, X.; Guo, F.; Huang, W.; Chen, F.; et al. Berberine inhibits aldose reductase and oxidative stress in rat mesangial cells cultured under high glucose. Arch. Biochem. Biophys. 2008, 475, 128–134. [Google Scholar] [CrossRef]
- Xie, X.; Chang, X.; Chen, L.; Huang, K.; Huang, J.; Wang, S.; Shen, X.; Liu, P.; Huang, H. Berberine ameliorates experimental diabetes-induced renal inflammation and fibronectin by inhibiting the activation of RhoA/ROCK signaling. Mol. Cell. Endocrinol. 2013, 381, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Zhou, S.W. Protective effect of berberine on antioxidant enzymes and positive transcription elongation factor b expression in diabetic rat liver. Fitoterapia 2011, 82, 184–189. [Google Scholar] [CrossRef]
- Wu, D.; Wen, W.; Qi, C.L.; Zhao, R.X.; Lü, J.H.; Zhong, C.Y.; Chen, Y.Y. Ameliorative effect of berberine on renal damage in rats with diabetes induced by high-fat diet and streptozotocin. Phytomedicine 2012, 19, 712–718. [Google Scholar] [CrossRef]
- Tang, L.Q.; Wei, W.; Chen, L.M.; Liu, S. Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J. Ethnopharmacol. 2006, 108, 109–115. [Google Scholar] [CrossRef]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Tawari, S.; Patil, S.; Dixit, P.; Umathe, S.; Mundhada, D. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav. Brain Res. 2011, 220, 30–41. [Google Scholar] [CrossRef]
- Moghaddam, H.K.; Baluchnejadmojarad, T.; Roghani, M.; Khaksari, M.; Norouzi, P.; Ahooie, M.; Mahboobi, F. Berberine ameliorate oxidative stress and astrogliosis in the hippocampus of STZ-induced diabetic rats. Mol. Neurobiol. 2014, 49, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Z.; Song, Y.; Wu, D.; Zheng, X.; Li, P.; Jin, J.; Xu, N.; Li, L. Effects of berberine on amelioration of hyperglycemia and oxidative stress in high glucose and high fat diet-induced diabetic hamsters in vivo. Biomed. Res. Int. 2015, 2015, 313808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Campbell, T.; Perry, B.; Beaurepaire, C.; Qin, L. Hypoglycemic and insulin-sensitizing effects of berberine in high-fat diet- and streptozotocin-induced diabetic rats. Metabolism. 2011, 60, 298–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Kakkar, P. Antihyperglycemic and antioxidant effect of Berberis aristata root extract and its role in regulating carbohydrate metabolism in diabetic rats. J. Ethnopharmacol. 2009, 123, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Hei, Z.Q.; Nie, H.; Tang, F.T.; Huang, H.Q.; Li, X.J.; Deng, Y.H.; Chen, S.R.; Guo, F.F.; Huang, W.G.; et al. Berberine ameliorates renal injury in streptopzotocin-induced diabetic rats by suppression of both oxidative stress and aldose reductase. Chin. Med. J. 2008, 121, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Kim, H.J. Berberine ameliorates cold and mechanical allodynia in a rat model of diabetic neuropathy. J. Med. Food 2013, 16, 511–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Yang, K. Berberine alleviates oxidative stress in islets of diabetic mice by inhibiting miR-106b expression and up-regulating SIRT1. J. Cell. Biochem. 2017, 118, 4349–4357. [Google Scholar] [CrossRef]
- Chandirasegaran, G.; Elanchezhiyan, C.; Ghosh, K. Berberine chloride ameliorates oxidative stress, inflammation and apoptosis in the pancreas of streptozotocin induced diabetic rats. Biomed. Pharmacother. 2017, 95, 175–185. [Google Scholar] [CrossRef]
- Chandirasegaran, G.; Elanchezhiyana, C.; Ghosh, K. Effects of berberine chloride on the liver of streptozotocin-induced diabetes in albino wistar rats. Biomed. Pharmacother. 2018, 99, 227–236. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Abdel-rahman, M.M.; Bastawy, N.A.; Eissa, H.M. Modulatory effect of berberine on adipose tissue PPAR γ, adipocytokines and oxidative stress in high fat diet/streptozotocin-induced diabetic rats. J. Appl. Pharm. Sci. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Q.; Zhang, X.; Wang, T.; Hu, W.; Manicum, T.; Chen, H.; Sun, L. Possible therapeutic potential of berberine in the treatment of STZ plus HFD-induced diabetic osteoporosis. Biomed. Pharmacother. 2018, 108, 280–287. [Google Scholar] [CrossRef]
- Zych, M.; Wojnar, W.; Kielanowska, M.; Folwarczna, J.; Kaczmarczyk-sedlak, I. Effect of berberine on glycation, aldose reductase activity, and oxidative stress in the lenses of streptozotocin-induced diabetic rats in vivo—A preliminary study. Int. J. Mol. Sci. 2020, 21, 4278. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Dada, F.A.; Oyeleye, S.I.; Oboh, G. Effects of berberine on cholinesterases and monoamine oxidase activities, and antioxidant status in the brain of streptozotocin (STZ)-induced diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2022, 33, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Elumalai, S.; Karunakaran, U.; Moon, J.S.; Won, K.C. NADPH Oxidase (NOX) targeting in diabetes: A special emphasis on pancreatic β-cell dysfunction. Cells 2021, 10, 1573. [Google Scholar] [CrossRef]
- Sedeek, M.; Montezano, A.C.; Hebert, R.L.; Gray, S.P.; Di Marco, E.; Jha, J.C.; Cooper, M.E.; Jandeleit-Dahm, K.; Schiffrin, E.L.; Wilkinson-Berka, J.L.; et al. Oxidative stress, Nox isoforms and complications of diabetes-potential targets for novel therapies. J. Cardiovasc. Transl. Res. 2012, 5, 509–518. [Google Scholar] [CrossRef]
- Sarna, L.K.; Wu, N.; Hwang, S.Y.; Siow, Y.L.; Karmin, O. Berberine inhibits NADPH oxidase mediated superoxide anion production in macrophages. Can. J. Physiol. Pharmacol. 2010, 88, 369–378. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, Y.; Li, J.; Su, C.; Wu, F.; Xia, W.H.; Yang, Z.; Yu, B.B.; Qiu, Y.X.; Tao, J. Berberine improves endothelial function by reducing endothelial microparticles-mediated oxidative stress in humans. Int. J. Cardiol. 2013, 167, 936–942. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Lv, X.; Zhang, M.; Song, Y.; Chen, L.; Liu, Y. Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur. J. Pharmacol. 2009, 620, 131–137. [Google Scholar] [CrossRef]
- Ding, H.; Hashem, M.; Triggle, C. Increased oxidative stress in the streptozotocin-induced diabetic apoE-deficient mouse: Changes in expression of NADPH oxidase subunits and eNOS. Eur. J. Pharmacol. 2007, 561, 121–128. [Google Scholar] [CrossRef]
- Liang, C.F.; Liu, J.T.; Wang, Y.; Xu, A.; Vanhoutte, P.M. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Goettsch, C.; Goettsch, W.; Muller, G.; Seebach, J.; Schnittler, H.J.; Morawietz, H. Nox4 overexpression activates reactive oxygen species and p38 MAPK in human endothelial cells. Biochem. Biophys. Res. Commun. 2009, 380, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Rueckschloss, U.; Duerrschmidt, N.; Morawietz, H. NADPH oxidase in endothelial cells: Impact on atherosclerosis. Antioxidants Redox Signal. 2003, 5, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Inoue, N.; Kawashima, S. Role of the vascular NADH/NADPH oxidase system in atherosclerosis. Ann. N. Y. Acad. Sci. 2000, 902, 241–248. [Google Scholar] [CrossRef]
- Eid, A.A.; Ford, B.M.; Block, K.; Kasinath, B.S.; Gorin, Y.; Ghosh-Choudhury, G.; Barnes, J.L.; Abboud, H.E. AMP-activated Protein Kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J. Biol. Chem. 2010, 285, 37503–37512. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, M.; Liang, B.; Xu, J.; Xie, Z.; Liu, C.; Viollet, B.; Yan, D.; Zou, M.H. AMPKα2 deletion causes aberrant expression and activation of NAD(P)H Oxidase and consequent endothelial dysfunction in vivo: Role of 26S proteasomes. Circ. Res. 2010, 106, 1117–1128. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Ye, J.; Jia, W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm. Sin. B 2012, 2, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Xu, J.; Song, P.; Viollet, B.; Zou, M.H. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes 2009, 58, 1893–1901. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Zou, M.-H. Regulation of NAD(P)H Oxidase by AMPK in Cardiovascular Systems. Free Radic. Biol. Med. 2012, 52, 1607–1619. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, T.; Hayashi, T.; Miyamoto, L.; Yonemitsu, S.; Nakano, M.; Tanaka, S.; Ebihara, K.; Masuzaki, H.; Hosoda, K.; Inoue, G.; et al. Possible involvement of the α1 isoform of 5′AMP-activated protein kinase in oxidative stress-stimulated glucose transport in skeletal muscle. Am. J. Physiol.—Endocrinol. Metab. 2004, 287, 166–173. [Google Scholar] [CrossRef]
- Salt, I.; Celler, J.W.; Hawley, S.A.; Prescott, A.; Woods, A.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Greater AMP dependence, and preferential nuclear localization, of complexes containing the α2 isoform. Biochem. J. 1998, 334, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Kukidome, D.; Nishikawa, T.; Sonoda, K.; Imoto, K.; Fujisawa, K.; Yano, M.; Motoshima, H.; Taguchi, T.; Matsumura, T.; Araki, E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 2006, 55, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, X.; Gao, X.; Pang, X.; Wang, Y.; Wu, X.; Deng, X.; Zhang, Q.; Sun, C.; Li, Y. Elevated serum xanthine oxidase activity is associated with the development of type 2 diabetes: A prospective cohort study. Diabetes Care 2018, 41, 884–890. [Google Scholar] [CrossRef] [Green Version]
- Sunagawa, S.; Shirakura, T.; Hokama, N.; Kozuka, C.; Yonamine, M.; Namba, T.; Morishima, S.; Nakachi, S.; Nishi, Y.; Ikema, T.; et al. Activity of xanthine oxidase in plasma correlates with indices of insulin resistance and liver dysfunction in patients with type 2 diabetes mellitus and metabolic syndrome: A pilot exploratory study. J. Diabetes Investig. 2019, 10, 94–103. [Google Scholar] [CrossRef]
- Liu, S.; Xing, J.; Zheng, Z.; Song, F.; Liu, Z.; Liu, S. Ultrahigh performance liquid chromatography-triple quadrupole mass spectrometry inhibitors fishing assay: A novel method for simultaneously screening of xanthine oxidase inhibitor and superoxide anion scavenger in a single analysis. Anal. Chim. Acta 2012, 715, 64–70. [Google Scholar] [CrossRef]
- Assmann, T.S.; Brondani, L.A.; Bouças, A.P.; Rheinheimer, J.; de Souza, B.M.; Canani, L.H.; Bauer, A.C.; Crispim, D. Nitric oxide levels in patients with diabetes mellitus: A systematic review and meta-analysis. Nitric Oxide—Biol. Chem. 2016, 61, 1–9. [Google Scholar] [CrossRef]
- Duicu, O.M.; Lighezan, R.; Sturza, A.; Balica, R.; Vaduva, A.; Feier, H.; Gaspar, M.; Ionac, A.; Noveanu, L.; Borza, C.; et al. Assessment of mitochondrial dysfunction and monoamine oxidase contribution to oxidative stress in human diabetic hearts. Oxidative Med. Cell. Longev. 2016, 2016, 8470394. [Google Scholar] [CrossRef] [Green Version]
- Vomhof-DeKrey, E.E.; Picklo, M.J. The Nrf2-antioxidant response element pathway: A target for regulating energy metabolism. J. Nutr. Biochem. 2012, 23, 1201–1206. [Google Scholar] [CrossRef]
- Jia, L.; Xue, K.; Liu, J.; Habotta, O.A.; Hu, L.; Abdel Moneim, A.E. Anticolitic effect of berberine in rat experimental model: Impact of PGE2/p38 MAPK pathways. Mediat. Inflamm. 2020, 2020, 9419085. [Google Scholar] [CrossRef]
- Hsu, Y.Y.; Chen, C.S.; Wu, S.N.; Jong, Y.J.; Lo, Y.C. Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mechanism in NSC34 motor neuron-like cells. Eur. J. Pharm. Sci. 2012, 46, 415–425. [Google Scholar] [CrossRef]
- Hsu, Y.Y.; Tseng, Y.T.; Lo, Y.C. Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2-related neurite outgrowth. Toxicol. Appl. Pharmacol. 2013, 272, 787–796. [Google Scholar] [CrossRef]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of Berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Tang, K.; Chen, R.; Nie, H.; Liang, S.; Zhang, J.; Zhang, Y.; Yang, Q. Berberine attenuates hepatic oxidative stress in rats with non-alcoholic fatty liver disease via the Nrf2/ARE signalling pathway. Exp. Ther. Med. 2019, 17, 2091–2098. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Guo, X.; Mao, G.; Gao, Z.; Wang, H.; He, Q.; Li, D. Hepatoprotection of berberine against hydrogen peroxide-induced apoptosis by upregulation of sirtuin 1. Phyther. Res. 2013, 27, 417–421. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between oxidative stress and SIRT1: Impact on the aging process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [Green Version]
- Alam, F.; Syed, H.; Amjad, S.; Baig, M.; Khan, T.A.; Rehman, R. Interplay between oxidative stress, SIRT1, reproductive and metabolic functions. Curr. Res. Physiol. 2021, 4, 119–124. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Q.; Zeng, Z.; Wu, J.; Zhang, Y.; Chen, Z. Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxidative Med. Cell. Longev. 2017, 2017, 7543973. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Shentu, T.P.; Wen, L.; Johnson, D.A.; Shyy, J.Y.J. Regulation of SIRT1 by oxidative stress-responsive miRNAs and a systematic approach to identify its role in the endothelium. Antioxid. Redox Signal. 2013, 19, 1522–1538. [Google Scholar] [CrossRef] [Green Version]
- Chong, Z.Z.; Shang, Y.C.; Wang, S.; Maiese, K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert Opin. Ther. Targets 2012, 16, 167–178. [Google Scholar] [CrossRef]
- Meng, T.; Qin, W.; Liu, B. SIRT1 antagonizes oxidative stress in diabetic vascular complication. Front. Endocrinol. 2020, 11, 568861. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, X.; Feng, K.; Wang, T.; Li, W.; Yuan, T.; Sun, X.; Sun, Q.; Xiang, H.; Wang, H. Berberine moderates glucose and lipid metabolism through multipathway mechanism. Evid.-Based Complement. Altern. Med. 2011, 2011, 924851. [Google Scholar] [CrossRef] [Green Version]
- Donadelli, M.; Dando, I.; Fiorini, C.; Palmieri, M. UCP2, a mitochondrial protein regulated at multiple levels. Cell. Mol. Life Sci. 2014, 71, 1171–1190. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zheng, Y.; Huang, J.; Peng, W.; Chen, X.; Kang, X.; Zeng, Q. UCP2 ameliorates mitochondrial dysfunction, inflammation, and oxidative stress in lipopolysaccharide-induced acute kidney injury. Int. Immunopharmacol. 2019, 71, 336–349. [Google Scholar] [CrossRef]
- Lee, K.U.; Lee, I.K.; Han, J.; Song, D.K.; Kim, Y.M.; Song, H.S.; Kim, H.S.; Lee, W.J.; Koh, E.H.; Song, K.H.; et al. Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ. Res. 2005, 96, 1200–1207. [Google Scholar] [CrossRef] [Green Version]
- Teshima, Y.; Akao, M.; Jones, S.P.; Marbán, E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ. Res. 2003, 93, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Hou, G.; Jin, Y.; Liu, M.; Wang, C.; Song, G. UCP2–866G/A polymorphism is associated with prediabetes and type 2 diabetes. Arch. Med. Res. 2020, 51, 556–563. [Google Scholar] [CrossRef]
- Čater, M.; Bombek, L.K. Protective role of mitochondrial uncoupling proteins against age-related oxidative stress in type 2 diabetes mellitus. Antioxidants 2022, 11, 1473. [Google Scholar] [CrossRef]
- Hass, D.T.; Barnstable, C.J. Uncoupling proteins in the mitochondrial defense against oxidative stress. Prog. Retin. Eye Res. 2021, 83, 100941. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Patel, S.; Santani, D. Role of NF-κB in the pathogenesis of diabetes and its associated complications. Pharmacol. Rep. 2009, 61, 595–603. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Liu, P.; Shen, X.; Lan, T.; Li, W.; Jiang, Q.; Xie, X.; Huang, H. Effects of berberine on matrix accumulation and NF-kappa B signal pathway in alloxan-induced diabetic mice with renal injury. Eur. J. Pharmacol. 2010, 638, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Li, Z.; Zhang, H.; Ma, L.; Ma, Z.; Zhang, Y.; Zou, J.; Li, M.; Ma, L.; Wang, X.; et al. Berberine protects against diabetic retinopathy by inhibiting cell apoptosis via deactivation of the NF-κB signaling pathway. Mol. Med. Rep. 2020, 22, 4227–4235. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xie, M. Studies on the hypoglycemic effect of Coptis chinensis and berberine. Acta Pharm. Sin. 1986, 21, 401–406. [Google Scholar] [CrossRef]
- Ni, Y.X. Therapeutic effect of berberine on 60 patients with type II diabetes mellitus and experimental research. Chin. J. Mod. Dev. Tradit. 1988, 8, 707, 711–713. [Google Scholar]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wei, J.; Xue, R.; Wu, J.; Zhao, W.; Wang, Z.; Kong, W.; Jiang, J. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metab. Clin. Exp. 2010, 59, 285–292. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, L.; Wang, C. Efficacy and safety of berberine in patients with type 2 diabetes mellitus: A meta-analysis. Chin. Herb. Med. 2015, 7, 344–353. [Google Scholar] [CrossRef]
- Li, A.; Lin, C.; Xie, F.; Jin, M.; Lin, F. Berberine ameliorates insulin resistance by inhibiting IKK/NF-κB, JNK, and IRS-1/AKT signaling pathway in liver of gestational diabetes mellitus rats. Metab. Syndr. Relat. Disord. 2022, 20, 480–488. [Google Scholar] [CrossRef]
- Cole, L.K.; Zhang, M.; Chen, L.; Sparagna, G.C.; Vandel, M.; Xiang, B.; Dolinsky, V.W.; Hatch, G.M. Supplemental berberine in a high-fat diet reduces adiposity and cardiac dysfunction in offspring of mouse dams with gestational diabetes mellitus. J. Nutr. 2021, 151, 892–901. [Google Scholar] [CrossRef]
- Cole, L.K.; Sparagna, G.C.; Vandel, M.; Xiang, B.; Dolinsky, V.W.; Hatch, G.M. Berberine elevates cardiolipin in heart of offspring from mouse dams with high fat diet-induced gestational diabetes mellitus. Sci. Rep. 2021, 11, 15770. [Google Scholar] [CrossRef]
- Mejia, E.M.; Hatch, G.M. Mitochondrial phospholipids: Role in mitochondrial function. J. Bioenerg. Biomembr. 2016, 48, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yi, H.; Wu, J.; Kuang, T.; Zhang, J.; Li, Q.; Du, H.; Xu, T.; Jiang, G.; Fan, G. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed. Pharmacother. 2021, 133, 110984. [Google Scholar] [CrossRef]
- Leng, S.H.; Lu, F.E.; Xu, L.J. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol. Sin. 2004, 25, 496–502. [Google Scholar] [PubMed]
- Yamagata, K. Roles of HNF1α and HNF4α in pancreatic β-cells: Lessons from a monogenic form of diabetes (MODY). Vitam. Horm. 2014, 95, 407–423. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Lu, F.E.; Leng, S.H.; Fang, X.S.; Chen, G.; Wang, Z.S.; Dong, L.P.; Yan, Z.Q. Facilitating effects of berberine on rat pancreatic islets through modulating hepatic nuclear factor 4 alpha expression and glucokinase activity. World J. Gastroenterol. 2008, 14, 6004–6011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Lu, F.E.; Chen, G.; Xu, L.J.; Wang, K.F.; Zou, X. Effect of berberine on insulin secretion and glucokinase activity of NIT-1 cells. Acta Pharm. Sin. 2007, 42, 1045–01049. [Google Scholar]
- Toulis, K.A.; Nirantharakumar, K.; Pourzitaki, C.; Barnett, A.H.; Tahrani, A.A. Glucokinase activators for type 2 diabetes: Challenges and future developments. Drugs 2020, 80, 467–475. [Google Scholar] [CrossRef]
- Lu, S.S.; Yu, Y.L.; Zhu, H.J.; Liu, X.D.; Liu, L.; Liu, Y.W.; Wang, P.; Xie, L.; Wang, G.J. Berberine promotes glucagon-like peptide-1 (7-36) amide secretion in streptozotocin-induced diabetic rats. J. Endocrinol. 2009, 200, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Xiao, X.; Li, M.; Li, W.; Yu, M.; Zhang, H.; Ping, F.; Wang, Z.; Zheng, J. Berberine moderates glucose metabolism through the GnRH-GLP-1 and MAPK pathways in the intestine. BMC Complement. Altern. Med. 2014, 14, 188. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Boer, G.A.; Holst, J.J. Increatin hormones and type 2 diabetes mellitus—Mechanistic insights and therapeutic approches. Biology 2020, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rubio, K.G.; González-Ortiz, M.; Martínez-Abundis, E.; Robles-Cervantes, J.A.; Espinel-Bermúdez, M.C. Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2013, 11, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Liu, Y.; Zhou, F.; Zhang, Y.; Zhu, Q.; Zhang, L.; Zhang, Q.; Wang, S.; Zhu, K.; Wang, X.; et al. Berberine inhibits glucose oxidation and insulin secretion in rat islets. Endocr. J. 2018, 65, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szkudelski, T.; Szkudelska, K. The relevance of AMP-activated protein kinase in insulin-secreting β cells: A potential target for improving β cell function? J. Physiol. Biochem. 2019, 75, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Xavier, G.; Leclerc, I.; Varadi, A.; Tsuboi, T.; Moule, S.K.; Rutter, G.A. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem. J. 2003, 371, 761–774. [Google Scholar] [CrossRef]
- Fu, A.; Eberhard, C.E.; Screaton, R.A. Role of AMPK in pancreatic beta cell function. Mol. Cell. Endocrinol. 2013, 366, 127–134. [Google Scholar] [CrossRef]
- Salt, I.P.; Johnson, G.; Ashcroft, S.J.H.; Hardie, D.G. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic β cells, and may regulate insulin release. Biochem. J. 1998, 335, 533–539. [Google Scholar] [CrossRef] [Green Version]
- Targonsky, E.D.; Dai, F.; Koshkin, V.; Karaman, G.T.; Gyulkhandanyan, A.V.; Zhang, Y.; Chan, C.B.; Wheeler, M.B. α-Lipoic acid regulates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Diabetologia 2006, 49, 1587–1598. [Google Scholar] [CrossRef] [Green Version]
- Langelueddecke, C.; Jakab, M.; Ketterl, N.; Lehner, L.; Hufnagl, C.; Schmidt, S.; Geibel, J.P.; Fuerst, J.; Ritter, M. Effect of the AMP-kinase modulators AICAR, metformin and compound C on insulin secretion of INS-1E rat insulinoma cells under standard cell culture conditions. Cell. Physiol. Biochem. 2012, 29, 75–86. [Google Scholar] [CrossRef]
- Zhang, F.; Dey, D.; Bränstrom, R.; Forsberg, L.; Lu, M.; Zhang, Q.; Sjöholm, Å. BLX-1002, a novel thiazolidinedione with no PPAR affinity, stimulates AMP- Activated protein kinase activity, raises cytosolic Ca2+, and enhances glucose- Stimulated insulin secretion in a PI3K-dependent manner. Am. J. Physiol.—Cell Physiol. 2009, 296, 346–354. [Google Scholar] [CrossRef]
- Elazzouny, M.A.; Evans, C.R.; Burant, C.F.; Kennedy, R.T. Metabolomics analysis reveals that AICAR affects glycerolipid, ceramide and nucleotide synthesis pathways in INS-1 cells. PLoS ONE 2015, 10, e0129029. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Lu, J.; Li, S.; Wang, H.; Cao, X.; Li, Q.; Shi, T.T.; Matsunaga, K.; Chen, C.; Huang, H.; et al. Berberine is an insulin secretagogue targeting the KCNH6 potassium channel. Nat. Commun. 2021, 12, 5616. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Lu, J.; Yuan, S.S.; Asan; Cao, X.; Qiu, H.Y.; Shi, T.T.; Yang, F.Y.; Li, Q.; Liu, C.P.; et al. From hyper- to hypoinsulinemia and diabetes: Effect of KCNH6 on insulin secretion. Cell Rep. 2018, 25, 3800–3810.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Félix-Martínez, G.J.; Godínez-Fernández, J.R. Mathematical models of electrical activity of the pancreatic β-cell: A physiological review. Islets 2014, 6, e949195. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.N.; Shi, Y.; Yang, G.; Li, Y.; Yu, J.; Berggren, P.O. Ionic mechanisms in pancreatic β cell signaling. Cell. Mol. Life Sci. 2014, 71, 4149–4177. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E.; Sewing, S.; Wang, J.; Joseph, J.W.; Smukler, S.R.; Sakellaropoulos, G.; Wang, J.; Saleh, M.C.; Chan, C.B.; Tsushima, R.G.; et al. Inhibition of Kv2.1 voltage-dependent K+ channels in pancreatic β-cells enhances glucose-dependent insulin secretion. J. Biol. Chem. 2002, 277, 44938–44945. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, P.E.; Ha, X.F.; Wang, J.; Smukler, S.R.; Sun, A.M.; Gaisano, H.Y.; Salapatek, A.M.F.; Backx, P.H.; Wheeler, M.B. Members of the Kv1 and Kv2 voltage-dependent K+ channel families regulate insulin secretion. Mol. Endocrinol. 2001, 15, 1423–1435. [Google Scholar] [CrossRef]
- Herrington, J.; Zhou, Y.P.; Bugianesi, R.M.; Dulski, P.M.; Feng, Y.; Warren, V.A.; Smith, M.M.; Kohler, M.G.; Garsky, V.M.; Sanchez, M.; et al. Blockers of the delayed-rectifier potassium current in pancreatic β-cells enhance glucose-dependent insulin secretion. Diabetes 2006, 55, 1034–1042. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.C.; Hardie, D.G. AMPK: Sensing glucose as well as cellular energy status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef] [Green Version]
- Nawrocki, A.R.; Rajala, M.W.; Tomas, E.; Pajvani, U.B.; Saha, A.K.; Trumbauer, M.E.; Pang, Z.; Chen, A.S.; Ruderman, N.B.; Chen, H.; et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. J. Biol. Chem. 2006, 281, 2654–2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Zhang, M.; Li, J.; Meng, Z.; Wei, S.; Du, H.; Chen, L.; Hatch, G.M. Berberine improves insulin resistance in cardiomyocytes via activation of 5′-adenosine monophosphate-activated protein kinase. Metabolism 2013, 62, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.; Li, J.; Gosby, A.; To, S.W.C.; Cheng, Z.; Miyoshi, H.; Taketo, M.M.; Cooney, G.J.; Kraegen, E.W.; James, D.E.; et al. Berberine and Its More Biologically Available Derivative, Dihydroberberine, Inhibit Mitochondrial Respiratory Complex I. Diabetes 2008, 57, 1414–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, W.L.; Yin, J.; Alimujiang, M.; Yu, X.Y.; Ai, L.G.; Bao, Y.Q.; Liu, F.; Jia, W.P. Inhibition of mitochondrial complex I improves glucose metabolism independently of AMPK activation. J. Cell. Mol. Med. 2018, 22, 1316–1328. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, B.; Shen, J.; Bai, M.; Xu, E. Berberine attenuates fructose-induced insulin resistance by stimulating the hepatic LKB1/AMPK/PGC1α pathway in mice. Pharm. Biol. 2020, 58, 385–392. [Google Scholar] [CrossRef]
- Neeland, I.J.; Singh, S.; McGuire, D.K.; Vega, G.L.; Roddy, T.; Reilly, D.F.; Castro-Perez, J.; Kozlitina, J.; Scherer, P.E. Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes mellitus: The Dallas Heart Study. Diabetologia 2018, 61, 2570–2579. [Google Scholar] [CrossRef] [Green Version]
- Hilvo, M.; Salonurmi, T.; Havulinna, A.S.; Kauhanen, D.; Pedersen, E.R.; Tell, G.S.; Meyer, K.; Teeriniemi, A.M.; Laatikainen, T.; Jousilahti, P.; et al. Ceramide stearic to palmitic acid ratio predicts incident diabetes. Diabetologia 2018, 61, 1424–1434. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.M.; Pratipanawatr, T.; Berria, R.; Wang, E.; DeFronzo, R.A.; Sullards, M.C.; Mandarino, L.J. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 2004, 53, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Wu, F.; Wu, W.; Dong, H.; Huang, Z.; Xu, L.; Lu, F.; Gong, J. Berberine reduces hepatic ceramide levels to improve insulin resistance in HFD-fed mice by inhibiting HIF-2α. Biomed. Pharmacother. 2022, 150, 112955. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Nakayama, K.; Nakayama, M.; Mori, T.; Matsushima, M.; Okamura, M.; Senda, M.; Nako, K.; Miyata, T.; Ito, S. Methylglyoxal is a predictor in type 2 diabetic patients of intima-media thickening and elevation of blood pressure. Hypertension 2010, 56, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-B.; Tian, M.; Jin, J.; Zou, G.-L.; Sui, Y.-B.; Peng, P.; Liu, L. Berberine improves metabolic syndrome insulin resistance by inducing macrophage M2 polarization. Int. J. Clin. Exp. Med. 2018, 11, 11191–11197. [Google Scholar]
- Yin, J.; Gao, Z.; Liu, D.; Liu, Z.; Ye, J. Berberine improves glucose metabolism through induction of glycolysis. Am. J. Physiol.—Endocrinol. Metab. 2008, 294, 148–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, W.J.; Zhang, H.; Song, D.Q.; Xue, R.; Zhao, W.; Wei, J.; Wang, Y.M.; Shan, N.; Zhou, Z.X.; Yang, P.; et al. Berberine reduces insulin resistance through protein kinase C-dependent up-regulation of insulin receptor expression. Metabolism 2009, 58, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Fy, G.; Ty, Z. A preliminary investigation of the mechanisms underlying the effect of berberine in preventing high-fat diet-induced insulin resistance in rats. J. Physiol. Pharmacol. 2012, 63, 505–513. [Google Scholar] [PubMed]
- Yue, S.J.; Liu, J.; Wang, A.T.; Meng, X.T.; Yang, Z.R.; Peng, C.; Guan, H.S.; Wang, C.Y.; Yan, D. Berberine alleviates insulin resistance by reducing peripheral branched-chain amino acids. Am. J. Physiol.—Endocrinol. Metab. 2019, 316, E73–E85. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Z.; Cheung, S.C.K.; Lan, L.L.; Ho, S.K.S.; Xu, H.X.; Chan, J.C.N.; Tong, P.C.Y. Berberine modulates insulin signaling transduction in insulin-resistant cells. Mol. Cell. Endocrinol. 2010, 317, 148–153. [Google Scholar] [CrossRef]
- Tasdelen, I.; Van Beekum, O.; Gorbenko, O.; Fleskens, V.; Van Den Broek, N.J.F.; Koppen, A.; Hamers, N.; Berger, R.; Coffer, P.J.; Brenkman, A.B.; et al. The serine/threonine phosphatase PPM1B (PP2Cβ) selectively modulates PPARγ activity. Biochem. J. 2013, 451, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.S.; Li, Z.M.; Chen, Y.T.; Dai, S.J.; Zhou, X.J.; Yang, Y.X.; Lou, J.S.; Ji, L.T.; Bao, Y.T.; Xuan, L.; et al. Berberine improves inflammatory responses of diabetes mellitus in zucker diabetic fatty rats and insulin-resistant HepG2 cells through the PPM1B pathway. J. Immunol. Res. 2020, 2020, 2141508. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, W.; Lv, S.; Qu, H.; He, Y. Berberine improves insulin resistance in adipocyte models by regulating the methylation of hypoxia-inducible factor-3a. Biosci. Rep. 2019, 39, BSR20192059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Qu, H.; Wang, Y. Methylation of HIF3A promoter CpG islands contributes to insulin resistance in gestational diabetes mellitus. Mol. Genet. Genom. Med. 2019, 7, e00583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatting, M.; Tavares, C.D.J.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin regulation of gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Gluconeogenesis and glycogenolysis in health and diabetes. J. Investig. Med. 2004, 52, 375–378. [Google Scholar] [CrossRef]
- Chung, S.T.; Hsia, D.S.; Chacko, S.K.; Rodriguez, L.M.; Haymond, M.W. Increased gluconeogenesis in youth with newly diagnosed type 2 diabetes. Diabetologia 2015, 58, 596–603. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Yan, J.; Shen, Y.; Tang, K.; Yin, J.; Zhang, Y.; Yang, D.; Liang, H.; Ye, J.; Weng, J. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS ONE 2011, 6, e16556. [Google Scholar] [CrossRef] [Green Version]
- Benchoula, K.; Arya, A.; Parhar, I.S.; Hwa, W.E. FoxO1 signaling as a therapeutic target for type 2 diabetes and obesity. Eur. J. Pharmacol. 2021, 891, 173758. [Google Scholar] [CrossRef]
- Jiang, S.J.; Dong, H.; Li, J.B.; Xu, L.J.; Zou, X.; Wang, K.F.; Lu, F.E.; Yi, P. Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats. World J. Gastroenterol. 2015, 21, 7777–7785. [Google Scholar] [CrossRef]
- Xu, X.H.; Hu, Q.; Zhou, L.S.; Xu, L.J.; Zou, X.; Lu, F.E.; Yi, P. Berberine inhibits gluconeogenesis in skeletal muscles and adipose tissues in streptozotocin-induced diabetic rats via LKB1-AMPK-TORC2 signaling pathway. Curr. Med. Sci. 2020, 40, 530–538. [Google Scholar] [CrossRef]
- Chen, M.; Huang, N.; Liu, J.; Huang, J.; Shi, J.; Jin, F. AMPK: A bridge between diabetes mellitus and Alzheimer’s disease. Behav. Brain Res. 2021, 400, 113043. [Google Scholar] [CrossRef]
- Miller, R.; Chu, Q.; Le Lay, J.; Scherer, P.; Ahima, R.; Kaestner, K.; Foretz, M.; Viollet, B.; Birnbaum, M. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. J. Clin. Investig. 2011, 121, 2518–2528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canettieri, G.; Koo, S.H.; Berdeaux, R.; Heredia, J.; Hedrick, S.; Zhang, X.; Montminy, M. Dual role of the coactivator TORC2 in modulating hepatic glucose output and insulin signaling. Cell Metab. 2005, 2, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Vera, L.; Fischer, W.H.; Montminy, M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature 2009, 460, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Jin, J.; Liu, P.; Song, Y.; Zhang, H.; Sheng, L.; Zhou, H.; Jiang, B. Berberine attenuates hyperglycemia by inhibiting the hepatic glucagon pathway in diabetic mice. Oxidative Med. Cell. Longev. 2020, 2020, 6210526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Pan, Y.; Xu, L.; Tang, D.; Dorfman, R.G.; Zhou, Q.; Yin, Y.; Li, Y.; Zhou, L.; Zhao, S.; et al. Berberine promotes glucose uptake and inhibits gluconeogenesis by inhibiting deacetylase SIRT3. Endocrine 2018, 62, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Shima, A.; Kuramoto, D.; Kikumoto, D.; Matsui, T.; Michihara, A. Phosphoenolpyruvate carboxykinase, a key enzyme that controls blood glucose, is a target of retinoic acid receptor-related orphan receptor α. PLoS ONE 2015, 10, e0137955. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, D.; Kuang, H.; Feng, X.; Ai, W.; Wang, Y.; Shi, S.; Chen, J.; Fan, R. Berberine increases glucose uptake and intracellular ROS levels by promoting Sirtuin 3 ubiquitination. Biomed. Pharmacother. 2020, 121, 109563. [Google Scholar] [CrossRef]
- Xu, S.; Liu, C.X.; Xu, W.; Huang, L.; Zhao, J.Y.; Zhao, S.M. Butyrate induces apoptosis by activating PDC and inhibiting complex i through SIRT3 inactivation. Signal Transduct. Target. Ther. 2017, 2, 16035. [Google Scholar] [CrossRef] [Green Version]
- Ahn, B.H.; Kim, H.S.; Song, S.; In, H.L.; Liu, J.; Vassilopoulos, A.; Deng, C.X.; Finkel, T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 14447–14452. [Google Scholar] [CrossRef] [Green Version]
- Cok, A.; Plaisier, C.; Salie, M.J.; Oram, D.S.; Chenge, J.; Louters, L.L. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie 2011, 93, 1187–1192. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Pang, T.; Gu, M.; Gao, A.-H.; Xie, C.-M.; Li, J.-Y.; Nan, F.-J.; Li, J. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim. Biophys. Acta 2006, 1760, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, Y.; Wang, X.; Liu, S.; Shang, W.; Yuan, G.; Li, F.; Tang, J.; Chen, M.; Chen, J. Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism 2007, 56, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shin, E.J.; Kim, E.D.; Bayaraa, T.; Frost, S.C.; Hyun, C.K. Berberine activates GLUT1-mediated glucose uptake in 3T3-L1 adipocytes. Biol. Pharm. Bull. 2007, 30, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, W.; Wang, X.; Chen, W. Effect of Astragalus polysaccharides and berberine on carbohydrate metabolism and cell differentiation in 3T3-L1 adipocytes. Zhongguo Zhong Xi Yi Jie He Za Zhi 2004, 24, 926–928. [Google Scholar] [PubMed]
- Guasch-ferré, M.; Santos, J.L.; Martínez-gonzález, M.A.; Clish, C.B.; Razquin, C.; Wang, D. Glycolysis/gluconeogenesis- and tricarboxylic acid cycle—Related metabolites, Mediterranean diet, and type 2 diabetes. Am. J. Clin. Nutr. 2020, 111, 835–844. [Google Scholar] [CrossRef]
- Guo, X.; Li, H.; Xu, H.; Woo, S.; Dong, H.; Lu, F.; Lange, A.J.; Wu, C. Glycolysis in the control of blood glucose homeostasis. Acta Pharm. Sin. B 2012, 2, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Li, X.B.; Gu, J.D.; Zhou, Q.H. Review of aerobic glycolysis and its key enzymes—New targets for lung cancer therapy. Thorac. Cancer 2015, 6, 17–24. [Google Scholar] [CrossRef]
- Yan, S.; Wei, X.; Xu, S.; Sun, H.; Wang, W.; Liu, L.; Jiang, X.; Zhang, Y.; Che, Y. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoform 3 spatially mediates autophagy through the AMPK signaling pathway. Oncotarget 2017, 8, 80909–80922. [Google Scholar] [CrossRef] [Green Version]
- Marsin, A.S.; Bertrand, L.; Rider, M.H.; Deprez, J.; Beauloye, C.; Vincent, M.F.; Van den Berghe, G.; Carling, D.; Hue, L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 2000, 10, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Xiao, Y.; Yin, J.; Hou, W.; Yu, X.; Shen, L.; Liu, F.; Wei, L.; Jia, W. Berberine promotes glucose consumption independently of AMP-activated protein kinase activation. PLoS ONE 2014, 9, e103702. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Hu, R.; Chen, M.; Li, F.; Yang, Y.; Chen, J. Effects of berberine on glucose metabolism in vitro. Metab. Clin. Exp. 2002, 51, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Guo, J.H.; Qian, Y.Z.; Kong, W.J.; Jiang, J.D. Berberine improves glucose and lipid metabolism in HepG2 cells through AMPKα1 activation. Front. Pharmacol. 2020, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Magaji, U.F.; Sacan, O.; Yanardag, R. Alpha amylase, alpha glucosidase and glycation inhibitory activity of Moringa oleifera extracts. S. Afr. J. Bot. 2020, 128, 225–230. [Google Scholar] [CrossRef]
- Riyaphan, J.; Jhong, C.-H.; Tsai, M.-J.; Lee, D.-N.; Leong, M.K.; Weng, C.-F. Potent natural inhibitors of alpha-glucosidase and alpha-amylase against hyperglycemia in vitro and in vivo. Preprints 2017, 2017, 2017030116. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-Safi, I.; Louba, A.; Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alharbi, M.S.; Alqahtani, A.; Bari, A.; et al. In vitro alpha-amylase and alpha-glucosidase inhibitory activity and in vivo antidiabetic activity of withania frutescens l. Foliar extract. Molecules 2021, 26, 293. [Google Scholar] [CrossRef]
- Que, L.; Huang, K.; Ding, Y.; Chu, N.; Yang, J.; Qian, Z.; He, Q. Acarbose bioequivalence: Exploration of eligible protocol design. J. Clin. Pharm. Ther. 2021, 46, 492–503. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Karrar, E.; Xu, D.; Sun, X. Inhibition mechanism of berberine on α-Amylase and α-glucosidase in vitro. Starch 2022, 74, 2100231. [Google Scholar] [CrossRef]
- Pan, G.-Y.; Huang, Z.-J.; Wang, G.-J.; Fawcett, J.; Liu, X.-D.; Zhao, X.-C.; Sun, J.-G.; Xie, Y.-Y. The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta Med. 2003, 69, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Zuo, D.Y.; Qie, X.D.; Qi, H.; Zhao, M.Q.; Wu, Y.L. Berberine acutely inhibits the digestion of maltose in the intestine. J. Ethnopharmacol. 2012, 142, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Deng, Y.; Yu, S.; Lu, S.; Xie, L.; Liu, X. Berberine attenuates intestinal disaccharidases in streptozotocin-induced diabetic rats. Pharmazie 2008, 63, 384–388. [Google Scholar] [CrossRef]
- Lu, T.; Liang, Y.; Song, J.; Xie, L.; Wang, G.J.; Liu, X.D. Simultaneous determination of berberine and palmatine in rat plasma by HPLC-ESI-MS after oral administration of traditional Chinese medicinal preparation Huang-Lian-Jie-Du decoction and the pharmacokinetic application of the method. J. Pharm. Biomed. Anal. 2006, 40, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.P.; Pratley, R.E. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: Review of head-to-head clinical trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef]
- Makrilakis, K. The role of dpp-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: When to select, what to expect. Int. J. Environ. Res. Public Health 2019, 16, 2720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argun-Kurum, G.; Kaya-Dagistanli, F.; Ozturk, M. DPP4 inhibitor induces beta cell regeneration and DDR-1 protein expression as an endocrine progenitor cell marker in neonatal STZ-diabetic rats. Pharmacol. Rep. 2019, 71, 721–731. [Google Scholar] [CrossRef]
- Wu, Y.J.; Guo, X.; Li, C.J.; Li, D.Q.; Zhang, J.; Yang, Y.; Kong, Y.; Guo, H.; Liu, D.M.; Chen, L.M. Dipeptidyl peptidase-4 inhibitor, vildagliptin, inhibits pancreatic beta cell apoptosis in association with its effects suppressing endoplasmic reticulum stress in db/db mice. Metabolism 2015, 64, 226–235. [Google Scholar] [CrossRef]
- Bugliani, M.; Syed, F.; Paula, F.M.M.; Omar, B.A.; Suleiman, M.; Mossuto, S.; Grano, F.; Cardarelli, F.; Boggi, U.; Vistoli, F.; et al. DPP-4 is expressed in human pancreatic beta cells and its direct inhibition improves beta cell function and survival in type 2 diabetes. Mol. Cell. Endocrinol. 2018, 473, 186–193. [Google Scholar] [CrossRef]
- Al-Masri, I.M.; Mohammad, M.K.; Tahaa, M.O. Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine. J. Enzym. Inhib. Med. Chem. 2009, 24, 1061–1066. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P.-F. Diabetes and gut microbiota. World J. Diabetes 2021, 12, 1693–1703. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Ren, H.; Wang, S.; Zhong, H.; Zhao, X.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat. Commun. 2020, 11, 5015. [Google Scholar] [CrossRef]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagar, N.M.; Cree, I.A.; Covington, J.A.; Arasaradnam, R.P. The interplay of the gut microbiome, bile acids, and volatile organic compounds. Gastroenterol. Res. Pract. 2015, 2015, 398585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Wang, X.; Li, J.; Zhang, Y.; Zhong, H.; Liu, R.; Zhang, D.; Feng, Q.; Xie, X.; Hong, J.; et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat. Commun. 2017, 8, 1785. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Yan, L.; Wang, W.; Wang, D. Berberine alleviates type 2 diabetic symptoms by altering gut microbiota and reducing aromatic amino acids. Biomed. Pharmacother. 2020, 131, 110669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.D.; Yu, C.J.; Li, J.Y.; Wang, S.H.; Yang, D.; Guo, C.L.; Du, X.; Zhang, W.J.; Cheng, R.D.; Diao, X.C.; et al. Effect of berberine on hyperglycaemia and gut microbiota composition in type 2 diabetic Goto-Kakizaki rats. World J. Gastroenterol. 2021, 27, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Cai, J.; Gui, W.; Nichols, R.G.; Koo, I.; Zhang, J.; Anitha, M.; Patterson, A.D. Berberine directly affects the gut microbiota to promote intestinal farnesoid X receptor activation. Drug Metab. Dispos. 2019, 47, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.X.; Hu, Y.N.; Li, J.W.; Yuan, K. Hypoglycemic mechanism of the berberine organic acid salt under the synergistic effect of intestinal flora and oxidative stress. Oxidative Med. Cell. Longev. 2018, 2018, 8930374. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Xu, J.H.; Yu, T.; Chen, Q.K. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed. Pharmacother. 2019, 118, 109131. [Google Scholar] [CrossRef]
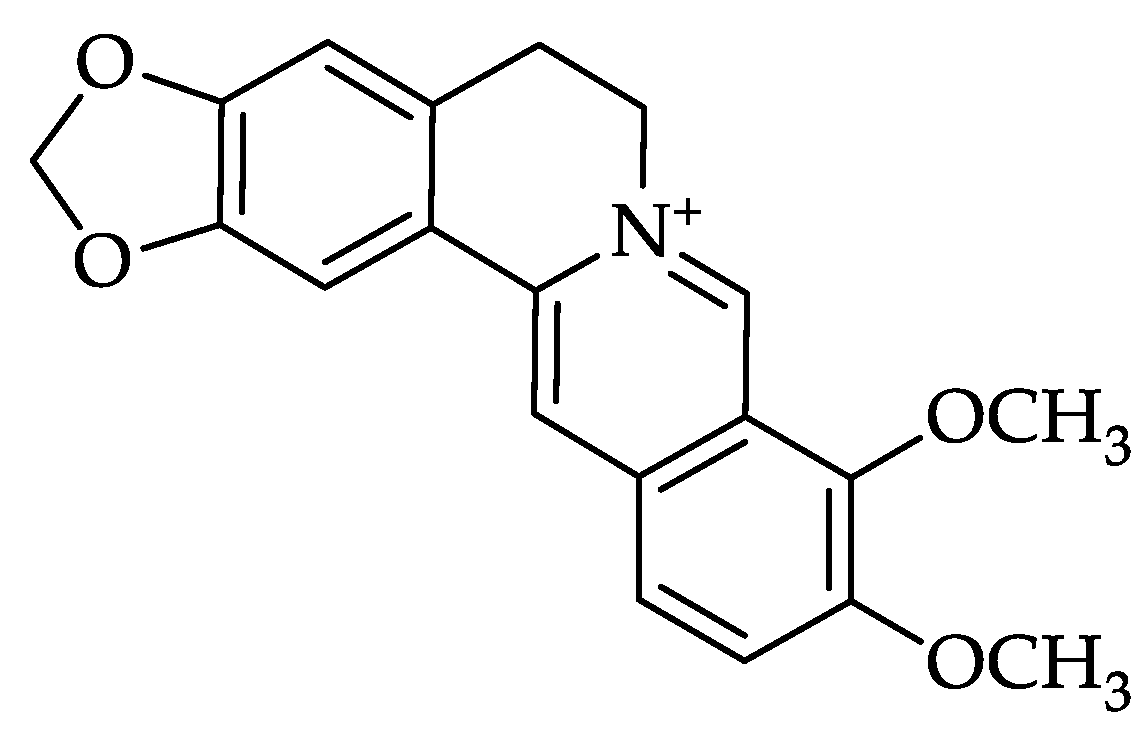
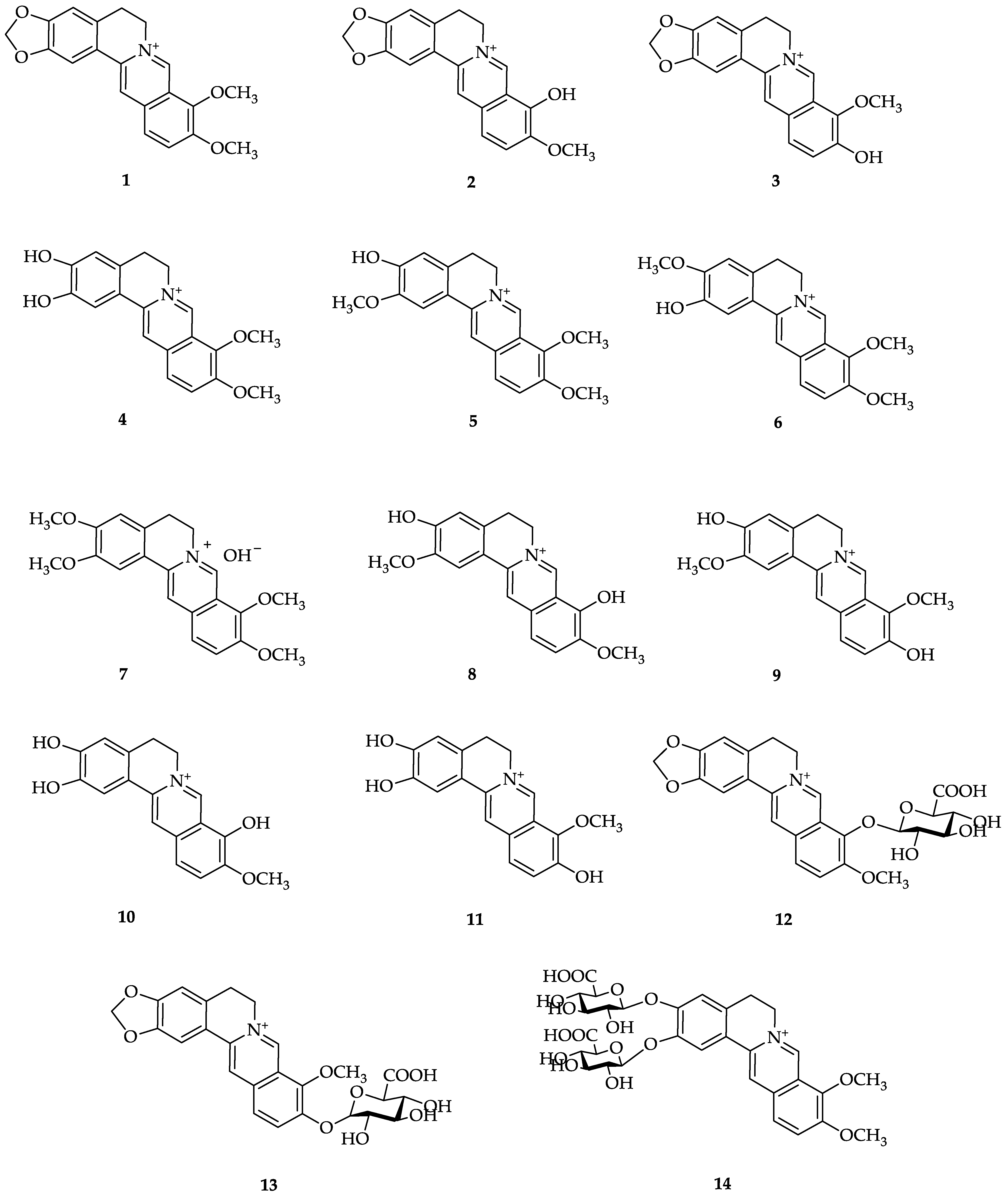
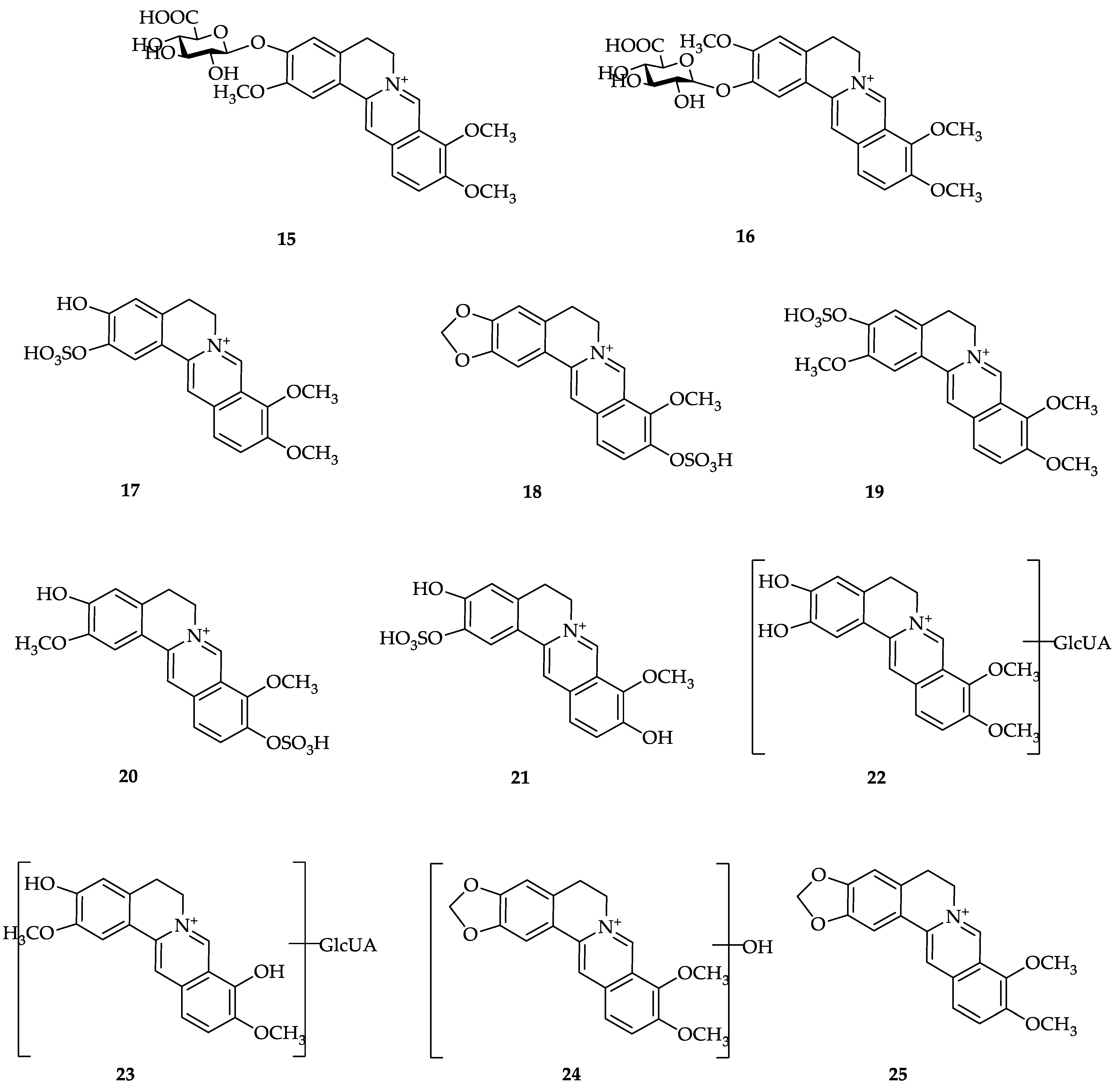
| No. | Berberine Metabolites | No. | Berberine Metabolites |
|---|---|---|---|
| 1 | Berberine | 7 | Palmatine |
| 2 | Berberrubine | 8 | 3,9-Demethylpalmatine |
| 3 | Thalifendine | 9 | 3,10-Demethylpalmatine |
| 4 | Demethyleneberberine | 10 | 2,3,9-Trihydroxiberberine |
| 5 | Jathorrhizine | 11 | 2,3,10-Trihydroxiberberine |
| 6 | Columbamine | 12 | Berberrubine-9-O-β-D-glucuronide |
| 13 | Thalifendine-10-O-β-D-glucuronide | 20 | 3,10-Demethylpalmatine-10-O-sulfate |
| 14 | Demethyleneberberine-2,3-di-O-β-D-glucuronide | 21 | 2,3,10-Trihydroxyberberine-2-O-sulfate |
| 15 | Jatrorrhizine-3-O-β-D-glucuronide | 22 | Glucuronide of demethyleneberberine |
| 16 | Columbamine-3-O-β-D-glucuronide | 23 | Glucuronide of 3,9-demethylpalmatine |
| 17 | Demethyleneberberine-2-O-sulfate | 24 | Hydroxylated berberine |
| 18 | Thalifendine-10-O-sulfate | 25 | Dihydroberberine |
| 19 | Jatrorrhizine-3-O-sulfate |
| Diseases | Protein Targets | References |
|---|---|---|
| Diabetes mellitus | Insulin receptor (IR) | [116,117] |
| α-Amylase | [118,119] | |
| α-Glucosidase | [117,119,120] | |
| Dipeptidyl peptidase IV (DPP-IV) | [117,121] | |
| Glycogen phosphorylase | [122] | |
| Glucose transporter type 4 (GLUT-4) | [116] | |
| Phosphoinositide 3-kinase (PI3K), protein kinase B (Akt), glycogen synthase kinase 3 beta (GSK-3β), and Kelch-like ECH-associated protein-1 (Keap-1) | [123] | |
| Cytokine signaling 3 (Socs3), Cholesteryl Ester Transfer Protein (CETP), C-Jun N-terminal kinases-1 (JNK1), lamin A/C, Peroxisome Proliferator-Activated Receptor γ (PPAR-γ), adiponectin, aldose reductase | [119] | |
| Cancer | BRAF and CRAF kinases | [124] |
| AKT | [125,126] | |
| Epidermal growth factor receptor (EGFR), p38 mitogen-activated protein kinases (p38 MAPK), Extracellular-Regulated Kinase (ERK1/2) | [126] | |
| Human proteome | [127] | |
| Survivin | [128] | |
| Retinoid X Receptor (RXRα) | [129] | |
| Alzheimer’s | Amyloid beta (Aβ) peptide | [130,131,132] |
| Beta-secretase 1 (BACE1) | [131] | |
| Cyclooxygenase-2 (COX-2), TNF Alpha Converting Enzyme (TACE) | [133] | |
| Acetylcholinesterase (AChE) | [133,134,135] | |
| Butyrylcholinesterase (BuChE) | [135] | |
| Parkinson’s | Phosphodiesterase (PDE4 and PDE10), α-synuclein, monoamine oxidase (MAO-B), Adenosine A2A receptors (A2Ar) | [136] |
| Hyperlipidemia | Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9), HMG-CoA reductase (HMGCR) | [137] |
| Niemann Pick C1 Like-1 (NPC1L1), Lanosterol 14α-Demethylase (LDM), Squalene Synthase (SqS) | [138] | |
| Hyperuricemia | Urate transporter 1 (URAT1) | [106] |
| Anti-inflammatory | 5-Lipoxygenase (5-LOX), cyclooxygenase-2 (COX-2) | [139] |
| Epidermal growth factor receptor erbB1 (EGFR) | [140] | |
| Glucocorticoid Receptor (GR) | [141] | |
| Hepatoprotective | p38 mitogen-activated protein kinases (p38 MAPK), Nuclear factor kappa-B (NF-κB), and Kelch-like ECH-associated protein 1 (Keap-1) | [142] |
| Dermatitis | Toll-like receptors (TLR1-TLR2 heterodimer) | [143] |
| Leishmanial | N-Myristoyltransferase (NMT), Methionyl-tRNA synthetase (MetRS), Pteridine reductase 1 (PTR1), Oligopeptidase B (OPB) | [144] |
| Zika Virus | ZIKV NS2B-NS3 protease | [145] |
| Antibacterial | Bacterial efflux pump proteins and biofilm proteins of bacteria of Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus | [146] |
| The inner membrane transporter (MexY) | [147,148] | |
| The filamentous temperature-sensitive Z protein (FtsZ) | [149,150,151] | |
| Antifungal | Mucormycosis Proteins | [152] |
| Anticholinesterase | R. microplus acetylcholinesterase (RmAChE 1) | [153] |
| Anti-Influenza | Neuraminidase (NA) | [154,155] |
| Neuraminidase (NA) of different subtypes of influenza A: A/H1N1/1918, A/H1N1/2009pdm, H3N2/2010 wild type, H3N2/2010 D151G mutant, H5N1 wild type, and H5N1 H274Y mutant | [156] | |
| Immunomodulatory | Tumor necrosis factor-α (TNF-α), Interleukin (IL-1β and IL-6), cyclooxygenase-2 (COX-2) | [157] |
| COVID-19 | ACE2 receptor | [158,159,160] |
| COVID-19 main protease (Mpro or 3CLpro) | [160,161,162,163,164,165,166] | |
| Spike glycoprotein of SARS-CoV-2 | [159,160] | |
| SARS spike glycoprotein–Human ACE2 complex | [167] | |
| RNA-Dependent RNA Polymerase of SARS-CoV-2 | [166] | |
| Non-structural protein 15 (Nsp15) | [168] | |
| NFκB1, CHUK, MAPK3, MAPK1, NFκB1A, CASP3, IL6, MAPK8, BAX, TNF, TMPRSS2, PLpro, RdRp | [160] |
| Animal Test | Diabetes Inducer | Berberine Dose (mg/kg/day) | Treatment Duration (Weeks) | Specimen | Findings | Ref. |
|---|---|---|---|---|---|---|
| Wistar rats | STZ 60 mg/kg, single i.p. injection High glucose | 200 (Berberine) | 12 | Serum | - | [190] |
| Cultured rat mesangial cells (in vitro) | MDA *, SOD ** | |||||
| SD rats | STZ, 60 mg/kg, single tail-vein injection | 200 (Berberine) | 12 | Serum | MDA *, SOD ** | [191] |
| Wistar rats | STZ 35 mg/kg, single i.p. injection and HFD | 75, 150, 300 (Berberine) | 16 | Serum | MDA *, GSH **, SOD **, GSH-Px **, CAT ** | [192] |
| Liver | MDA *, GSH **, SOD **, GSH-Px **, CAT ** | |||||
| Mice | STZ 100 mg/kg, single i.p. injection | 200 (Berberine chloride) | 2 | Liver | GSH *, SOD **, GSH-Px * | [188] |
| ICR Mice | STZ 100 mg/kg, single i.p. injection and Nicotinamide 1000 mg/kg, single i.p. injection | 100 (Berberine chloride) | 2 | Liver | MDA *, SOD ***, CAT ** | [189] |
| Brain | MDA *, SOD **, CAT ** | |||||
| Kidney | MDA ***, SOD **, CAT * | |||||
| SD rats | HFD | 100, 200 (Berberine chloride) | 8 | Kidney | MDA *, SOD ** | [193] |
| Wistar rats | STZ 35 mg/kg, single i.p. injection and HFD | 75, 150, 300 (Berberine chloride) | 16 | Pancreas | MDA *, SOD ** | [171] |
| Wistar rats | Alloxan 55 mg/kg, single tail-vein injection and HFD | 100, 200 (Rhizoma coptidis) | 3 | Heart | MDA *, SOD **, GSH-Px ** | [194] |
| Wistar rats | STZ 60 mg/kg, single i.p. injection | 25, 50, 100 (Berberine hydrochloride) | 4 | Cortex | MDA *, GSH ** | [195] |
| Hippocampus | MDA *, GSH ** | |||||
| Albino Wistar rats | STZ 55 mg/kg, single i.p. injection | 50, 100 (Berberine hydrochloride) | 8 | Hippocampus | MDA *, SOD ** | [196] |
| Hamster | High glucose and HFD | 50, 100 (Berberine chloride) | 6 | Serum | MDA *, TBARS * 8-isoprostane *, SOD ** | [197] |
| SD rats | STZ 35 mg/kg, single i.p. injection and HFD | 50, 100, 150 (Berberine chloride) | 6 | Liver | MDA **, SOD **, GSH **, GSSG ***, GSH:GSGG *** | [198] |
| Albino Wistar rats | Alloxan 100 mg/kg | 250 (50% aqueous ethanolic root extract of Berberis aristate) | 3 | Liver | CAT **, SOD **, GR **, GSH **, GPx **, MDA *, Protein Carbonyl * | [199] |
| Wistar rats | STZ 60 mg/kg, single i.p. injection | 200 (Berberine) | 12 | Serum | MDA *, SOD ** | [200] |
| SD rats | STZ 80 mg/kg, single i.p. injection (twice) | 5, 10, 20 (Berberine chloride) | 2 | Liver | MDA *, SOD *, CAT *, GPx * | [201] |
| ICR Mice | STZ 30 mg/kg, single i.p. injection | 5 (Berberine) | 3 | Pancreas | MDA *, SOD ** | [202] |
| Albino Wistar rats | STZ 40 mg/kg, intragastric intubation | 50 (Berberine chloride) | 7 | Pancreas | SOD **, TBARS *, LOOH *, CAT **, GPx **, GSH ** | [203] |
| Albino Wistar rats | STZ 40 mg/kg, intragastric intubation | 50 (Berberine chloride) | 7 | Liver | SOD **, TBARS *, LOOH *, CAT **, GPx **, GSH ** | [204] |
| Albino rats | STZ 35 mg/kg, single i.p. injection and HFD | 50, 100 (Berberine chloride) | 4 | Liver | MDA *, SOD **, CAT **, GPx **, GSH ** | [205] |
| Wistar rats | STZ 35 mg/kg, single i.p. injection and HFD | 50, 100 (Berberine) | 12 | Serum | SOD **, CAT **, GPx **, GST ** | [206] |
| Wistar rats | STZ 60 mg/kg, single i.p. injection | 50 (Berberine chloride) | 4 | Lense | SOD ***, CAT ***, GPx ***, TBARS *** | [207] |
| Rats | STZ 50 mg/kg, single i.p. injection | 50, 100 (Berberine) | 2 | Brain | MDA *, SOD **, GPx **, GSH ** | [208] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purwaningsih, I.; Maksum, I.P.; Sumiarsa, D.; Sriwidodo, S. A Review of Fibraurea tinctoria and Its Component, Berberine, as an Antidiabetic and Antioxidant. Molecules 2023, 28, 1294. https://doi.org/10.3390/molecules28031294
Purwaningsih I, Maksum IP, Sumiarsa D, Sriwidodo S. A Review of Fibraurea tinctoria and Its Component, Berberine, as an Antidiabetic and Antioxidant. Molecules. 2023; 28(3):1294. https://doi.org/10.3390/molecules28031294
Chicago/Turabian StylePurwaningsih, Indah, Iman Permana Maksum, Dadan Sumiarsa, and Sriwidodo Sriwidodo. 2023. "A Review of Fibraurea tinctoria and Its Component, Berberine, as an Antidiabetic and Antioxidant" Molecules 28, no. 3: 1294. https://doi.org/10.3390/molecules28031294
APA StylePurwaningsih, I., Maksum, I. P., Sumiarsa, D., & Sriwidodo, S. (2023). A Review of Fibraurea tinctoria and Its Component, Berberine, as an Antidiabetic and Antioxidant. Molecules, 28(3), 1294. https://doi.org/10.3390/molecules28031294






