Modified Supporting Materials to Fabricate Form Stable Phase Change Material with High Thermal Energy Storage
Abstract
:1. Introduction
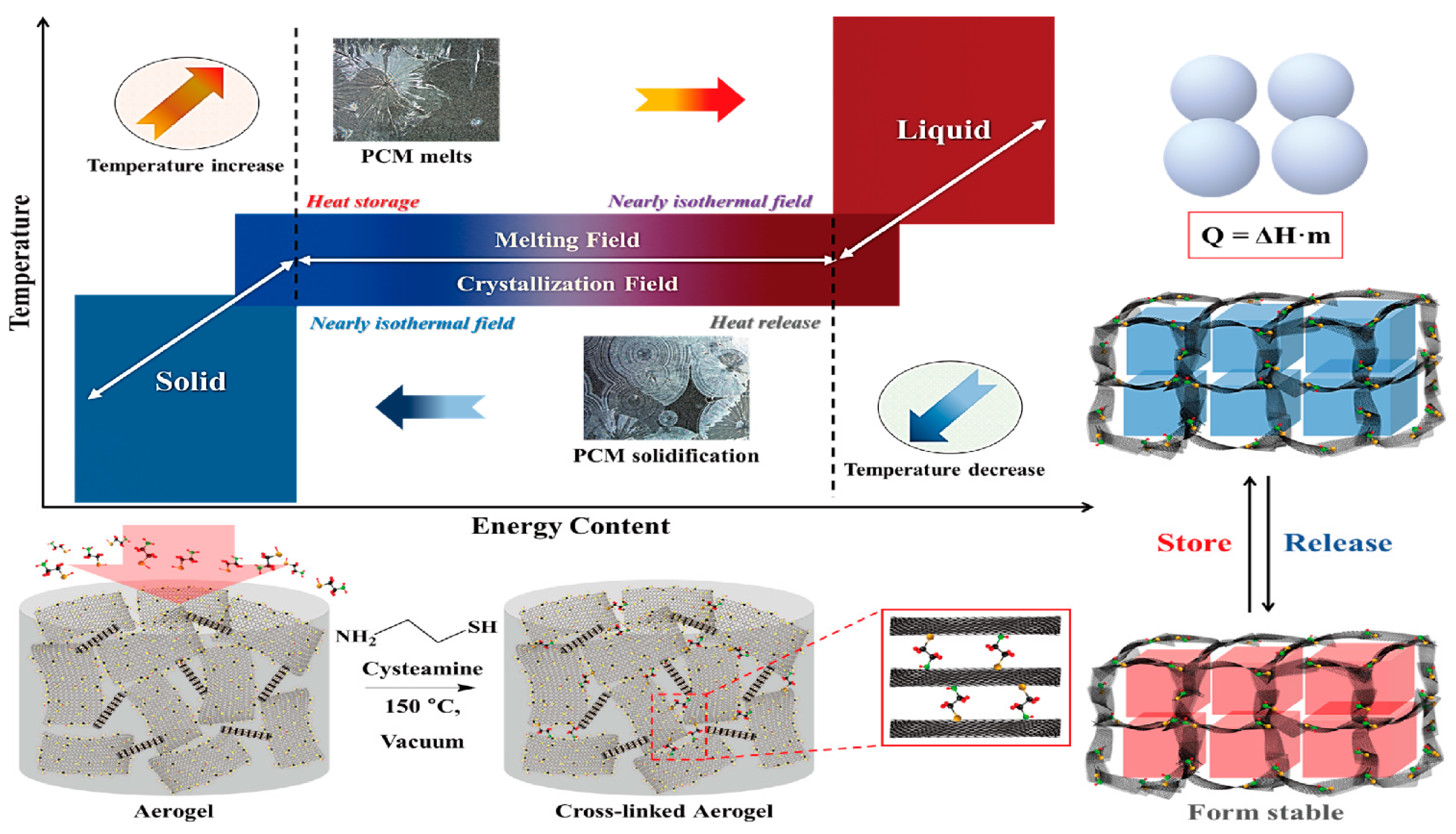
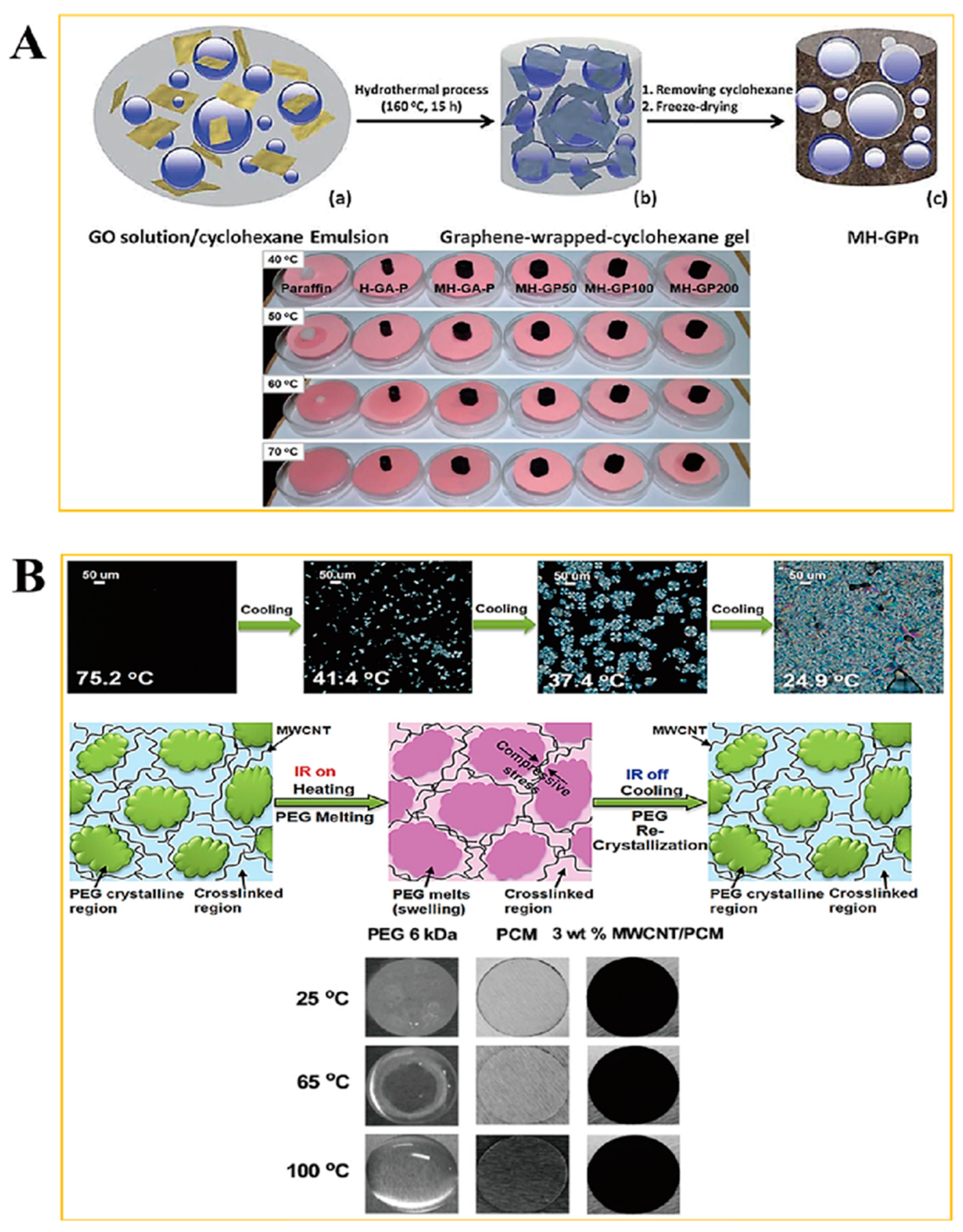
2. Results and Discussion
2.1. Form Stable Microencapsulated PCM
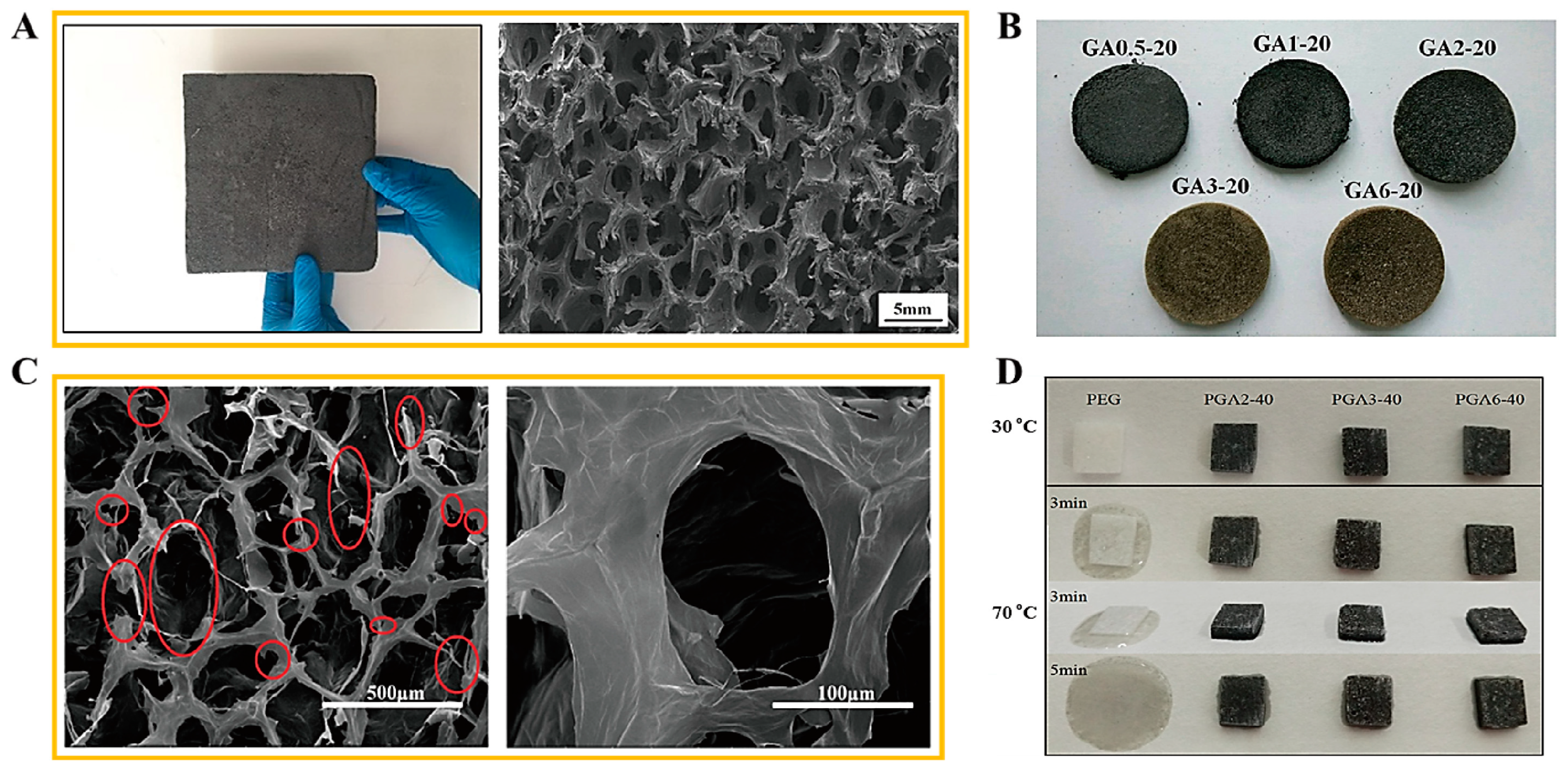
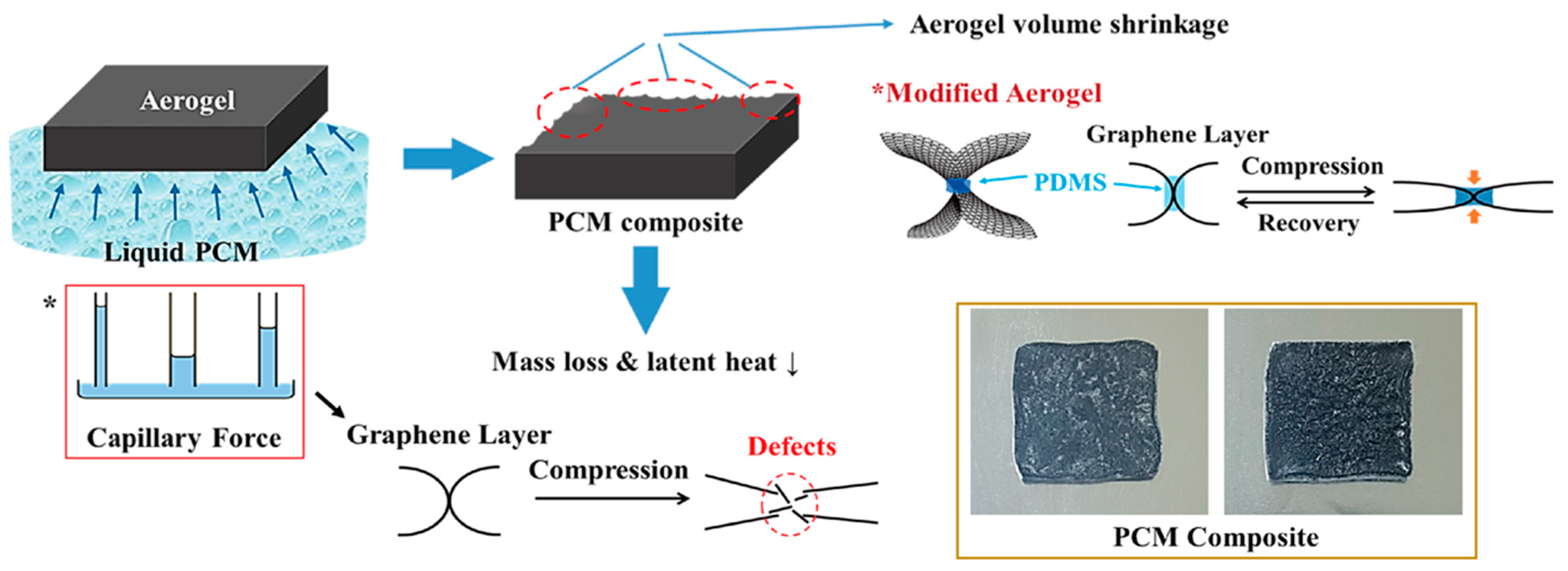
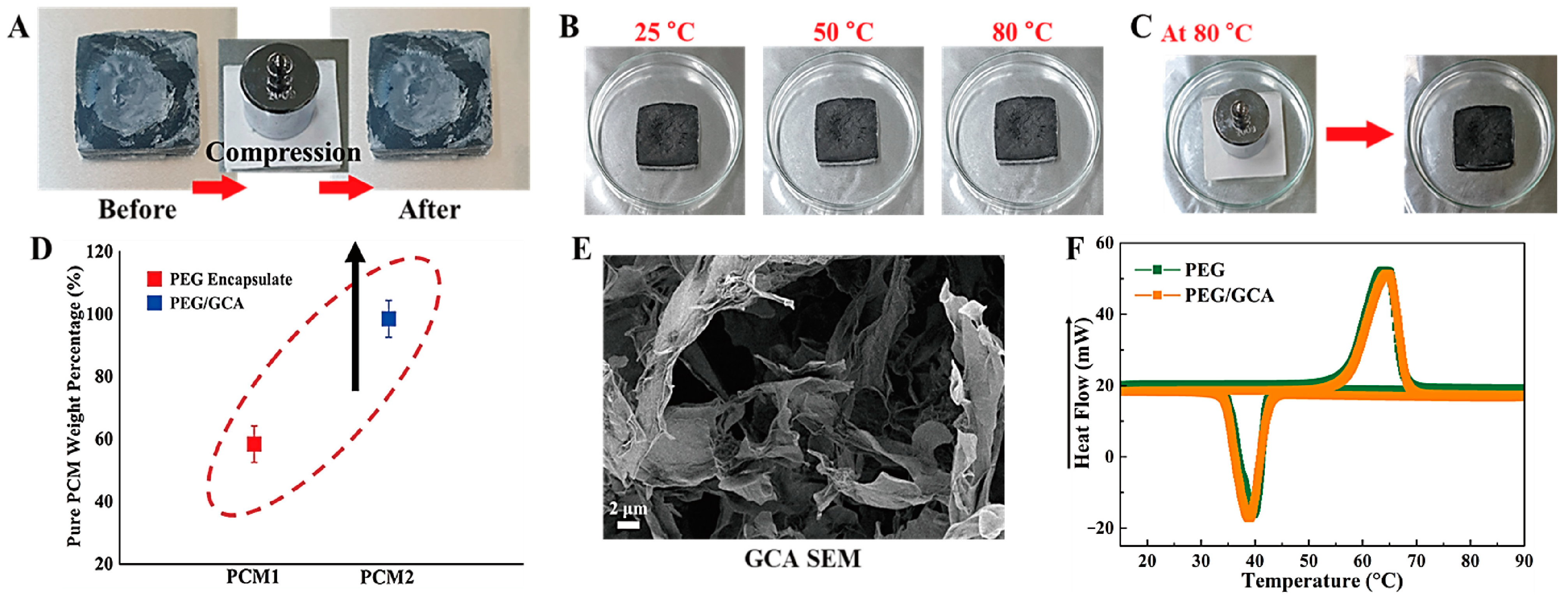
2.2. Aerogel Supported PCM Composite
3. Experimental Sections
3.1. Fabrication of Microencapsulated PCM Composite
3.2. Fabrication of PCM/Aerogel Composite
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, P. Is the digital economy driving clean energy development? -New evidence from 276 cities in China. J. Clean. Prod. 2022, 372, 133783. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, S.; Wang, Y.; He, Z. Does Clean Energy Use Have Threshold Effects on Economic Development? A Case of Theoretical and Empirical Analyses from China. Int. J. Environ. Res. Public Health 2022, 19, 9757. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xiang, M.; Zhu, Y.; Hui, J.; Jiang, Y.; Dong, S.; Yu, C.; Ou, J.; Qin, H. Single-atom platinum or ruthenium on C4N as 2D high-performance electrocatalysts for oxygen reduction reaction. Chem. Eng. J. 2021, 426, 131347. [Google Scholar] [CrossRef]
- Pandey, A.; Pandey, P.; Tumuluru, J.S. Solar Energy Production in India and Commonly Used Technologies—An Overview. Energies 2022, 15, 500. [Google Scholar] [CrossRef]
- Yang, Z.; Xiang, M.; Niu, H.; Xie, X.; Yu, C.; Hui, J.; Dong, S. A novel 2D sulfide gallium heterojunction as a high-performance electrocatalyst for overall water splitting. J. Solid State Chem. 2022, 314, 123365. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, H.; Xiang, M.; Yu, C.; Hui, J.; Dong, S. Coral reef structured cobalt-doped vanadate oxometalate nanoparticle for a high-performance electrocatalyst in water splitting. Int. J. Hydrogen Energy 2022, 47, 31566–31574. [Google Scholar] [CrossRef]
- Pingkuo, L.; Xue, H. Comparative analysis on similarities and differences of hydrogen energy development in the World’s top 4 largest economies: A novel framework. Int. J. Hydrogen Energy 2022, 47, 9485–9503. [Google Scholar] [CrossRef]
- Yang, Z.; Xiang, M.; Wu, Z.; Fan, W.; Hui, J.; Yu, C.; Dong, S.; Qin, H. Single-atom lanthanum on 2D N-doped graphene oxide as a novel bifunctional electrocatalyst for rechargeable zinc–air battery. Mater. Today Chem. 2022, 26, 101147. [Google Scholar] [CrossRef]
- Khan, T.; Yu, M.; Waseem, M. Review on recent optimization strategies for hybrid renewable energy system with hydrogen technologies: State of the art, trends and future directions. Int. J. Hydrogen Energy 2022, 47, 25155–25201. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, X.; Zhang, Z.; Yang, J.; Yu, C.; Dong, S.; Xiang, M.; Qin, H. NiS2@ V2O5/VS2 ternary heterojunction for a high-performance electrocatalyst in overall water splitting. Int. J. Hydrogen Energy 2022, 47, 27338–27346. [Google Scholar] [CrossRef]
- Abdin, Z.; Alim, M.; Saidur, R.; Islam, M.; Rashmi, W.; Mekhilef, S.; Wadi, A. Solar energy harvesting with the application of nanotechnology. Renew. Sustain. Energy Rev. 2013, 26, 837–852. [Google Scholar] [CrossRef]
- Liang, H.; Wang, F.; Xu, C.; Li, G.; Shuai, Y. Full-spectrum solar energy utilization and enhanced solar energy harvesting via photon anti-reflection and scattering performance using biomimetic nanophotonic structure. ES Energy Environ. 2020, 8, 29–41. [Google Scholar]
- Yu, C.; Song, Y. Modification of Graphene Aerogel Embedded Form-Stable Phase Change Materials for High Energy Har-vesting Efficiency. Macromol. Res. 2022, 30, 198–204. [Google Scholar] [CrossRef]
- Ai, H.; Lv, L.; Chen, T.; Zhang, Y.; Dong, L.; Song, S. An eco-friendly and facile montmorillonite nanosheets aerogel based phase change materials for efficient solar-to-thermal energy conversion. Energy Convers. Manag. 2022, 253, 115172. [Google Scholar] [CrossRef]
- Burnete, N.V.; Mariasiu, F.; Depcik, C.; Barabas, I.; Moldovanu, D. Review of thermoelectric generation for internal combustion engine waste heat recovery. Prog. Energy Combust. Sci. 2022, 91, 101009. [Google Scholar] [CrossRef]
- Yu, C.; Youn, J.R.; Song, Y.S. Enhancement of Thermo-Electric Energy Conversion Using Graphene Nano-platelets Embedded Phase Change Material. Macromol. Res. 2021, 29, 534–542. [Google Scholar] [CrossRef]
- Zeng, S.; Tanveer, A.; Fu, X.; Gu, Y.; Irfan, M. Modeling the influence of critical factors on the adoption of green energy tech-nologies. Renew. Sustain. Energy Rev. 2022, 168, 112817. [Google Scholar] [CrossRef]
- Fayaz, H.; Afzal, A.; Samee, A.D.M.; Soudagar, M.E.M.; Akram, N.; Mujtaba, M.A.; Jilte, R.D.; Islam, T.; Ağbulut, Ü.; Saleel, C.A. Optimization of Thermal and Structural Design in Lithium-Ion Batteries to Obtain Energy Efficient Battery Thermal Management System (BTMS): A Critical Review. Arch. Comput. Methods Eng. 2021, 29, 129–194. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Q.; Huang, F.; Lin, S.; Chen, F.; Gao, W. Thermal stability, latent heat and flame retardant properties of the thermal energy storage phase change materials based on paraffin/high density polyethylene composites. Renew. Energy 2009, 34, 2117–2123. [Google Scholar] [CrossRef]
- Yu, C.; Park, J.; Youn, J.; Song, Y. Sustainable solar energy harvesting using phase change material (PCM) embedded pyro-electric system. Energy Convers. Manag. 2022, 253, 115145. [Google Scholar] [CrossRef]
- Alkan, C.; Sari, A. Fatty acid/poly(methyl methacrylate) (PMMA) blends as form-stable phase change materials for latent heat thermal energy storage. Sol. Energy 2008, 82, 118–124. [Google Scholar] [CrossRef]
- Yu, C.; Song, Y.S. Analysis of Thermoelectric Energy Harvesting with Graphene Aerogel-Supported Form-Stable Phase Change Materials. Nanomaterials 2021, 11, 2192. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Javadi, F.; Metselaar, H.; Ganesan, P. Performance improvement of solar thermal systems integrated with phase change mate-rials (PCM): A review. Sol. Energy 2020, 206, 330–352. [Google Scholar] [CrossRef]
- Yu, C.; Song, Y.S. Advanced internal porous skeleton supported phase change materials for thermo-electric energy conversion applications. J. Polym. Res. 2022, 29, 79. [Google Scholar] [CrossRef]
- Chang, C.; Nie, X.; Li, X.; Tao, P.; Fu, B.; Wang, Z.; Xu, J.; Ye, Q.; Zhang, J.; Song, C.; et al. Bioinspired roll-to-roll solar-thermal energy harvesting within form-stable flexible composite phase change materials. J. Mater. Chem. A 2020, 8, 20970–20978. [Google Scholar] [CrossRef]
- Kenisarin, M.M. Thermophysical properties of some organic phase change materials for latent heat storage. A review. Sol. Energy 2014, 107, 553–575. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic phase change materials and their textile applications: An overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.; Tang, L.-S.; Feng, C.-P.; Bai, L.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Hierarchically Porous PVA Aerogel for Leakage-Proof Phase Change Materials with Superior Energy Storage Capacity. Energy Fuels 2020, 34, 2471–2479. [Google Scholar] [CrossRef]
- Yu, C.; Yang, S.H.; Pak, S.Y.; Youn, J.R.; Song, Y.S. Graphene embedded form stable phase change materials for drawing the thermo-electric energy harvesting. Energy Convers. Manag. 2018, 169, 88–96. [Google Scholar] [CrossRef]
- Yu, C.; Youn, J.R.; Song, Y.S. Multiple Energy Harvesting Based on Reversed Temperature Difference Between Graphene Aerogel Filled Phase Change Materials. Macromol. Res. 2019, 27, 606–613. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, J.; Yu, C.; Bai, J.; Xie, X.; Jiang, N.; Chen, B.; Dong, S.; Xiang, M.; Qin, H. Rare-Earth Lanthanum Tailoring Mott–Schottky Heterojunction by Sulfur Vacancy Modification as a Bifunctional Electrocatalyst for Zinc–Air Battery. Small Struct. 2022, 2200267. [Google Scholar] [CrossRef]
- Abdeali, G.; Bahramian, A.R.; Abdollahi, M. Review on Nanostructure Supporting Material Strategies in Shape-stabilized Phase Change Materials. J. Energy Storage 2020, 29, 101299. [Google Scholar] [CrossRef]
- Yu, C.; Youn, J.R.; Song, Y.S. Reversible thermo-electric energy harvesting with phase change material (PCM) composites. J. Polym. Res. 2021, 28, 279. [Google Scholar] [CrossRef]
- Su, W.; Hu, M.; Wang, L.; Kokogiannakis, G.; Chen, J.; Gao, L.; Li, A.; Xu, C. Microencapsulated phase change materials with graphene-based materials: Fabrication, characterisation and prospects. Renew. Sustain. Energy Rev. 2022, 168, 112806. [Google Scholar] [CrossRef]
- Pathak, L.; Trivedi, G.; Parameshwaran, R.; Deshmukh, S.S. Microencapsulated phase change materials as slurries for thermal energy storage: A review. Mater. Today Proc. 2021, 44, 1960–1963. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, K.; Zhang, H.; Liu, H.; Wu, D.; Wang, X. Development of poly (ethylene glycol)/silica phase-change microcapsules with well-defined core-shell structure for reliable and durable heat energy storage. Sol. Energy Mater. Sol. Cells 2021, 225, 111069. [Google Scholar] [CrossRef]
- Huo, X.; Li, W.; Wang, Y.; Han, N.; Wang, J.; Wang, N.; Zhang, X. Chitosan composite microencapsulated comb-like polymeric phase change material via coacervation microencapsulation. Carbohydr. Polym. 2018, 200, 602–610. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, T.D.; Feng, H.X.; Zhang, H. Stearic acid/polymethylmethacrylate composite as form-stable phase change materials for latent heat thermal energy storage. Renew. Energy 2011, 36, 1814–1820. [Google Scholar] [CrossRef]
- Shi, X.; Yazdani, M.R.; Ajdary, R.; Rojas, O.J. Leakage-proof microencapsulation of phase change materials by emulsification with acetylated cellulose nanofibrils. Carbohydr. Polym. 2020, 254, 117279. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, C.; Wu, H.; Guo, S. Construction of hybrid graphene oxide/graphene nanoplates shell in paraffin microencapsulated phase change materials to improve thermal conductivity for thermal energy storage. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124780. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, W.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K. Microencapsulated Paraffin Phase-Change Material with Calcium Carbonate Shell for Thermal Energy Storage and Solar-Thermal Conversion. Langmuir 2018, 34, 14254–14264. [Google Scholar] [CrossRef]
- Huang, X.; Xia, W.; Zou, R. Nanoconfinement of phase change materials within carbon aerogels: Phase transition behaviours and photo-to-thermal energy storage. J. Mater. Chem. A 2014, 2, 19963–19968. [Google Scholar] [CrossRef]
- Yu, C.; Song, Y.S. Phase Change Material (PCM) Composite Supported by 3D Cross-Linked Porous Graphene Aerogel. Materials 2022, 15, 4541. [Google Scholar] [CrossRef]
- Ghalambaz, M.; Aljaghtham, M.; Chamkha, A.; Abdullah, A.; Mansir, I.; Ghalambaz, M. Mathematical modeling of heteroge-neous metal foams for phase-change heat transfer enhancement of latent heat thermal energy storage units. Appl. Mathe-Matical Model. 2023, 115, 398–413. [Google Scholar] [CrossRef]
- Yu, C.; Song, Y.S. Enhancing energy harvesting efficiency of form stable phase change materials by decreasing surface roughness. J. Energy Storage 2023, 58, 106360. [Google Scholar] [CrossRef]
- Ghalambaz, M.; Aljaghtham, M.; Chamkha, A.J.; Abdullah, A.; Alshehri, A.; Ghalambaz, M. Anisotropic metal foam design for improved latent heat thermal energy storage in a tilted enclosure. Int. J. Mech. Sci. 2023, 238, 107830. [Google Scholar] [CrossRef]
- NKhedher, B.; Mahdi, J.; Majdi, H.; Al-Azzawi, W.; Dhahbi, S.; Talebizadehsardari, P. A hybrid solidification enhance-ment in a latent-heat storage system with nanoparticles, porous foam, and fin-aided foam strips. J. Energy Storage 2022, 56, 106070. [Google Scholar] [CrossRef]
- Ye, S.; Zhang, Q.; Hu, D.; Feng, J. Core–shell-like structured graphene aerogel encapsulating paraffin: Shape-stable phase change material for thermal energy storage. J. Mater. Chem. A 2014, 3, 4018–4025. [Google Scholar] [CrossRef]
- Wang, Y.; Mi, H.; Zheng, Q.; Ma, Z.; Gong, S. Flexible Infrared Responsive Multi-Walled Carbon Nanotube/Form-Stable Phase Change Material Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 21602–21609. [Google Scholar] [CrossRef]
- Yu, C.; Youn, J.R.; Song, Y.S. Tunable Electrical Resistivity of Carbon Nanotube Filled Phase Change Material Via Solid-solid Phase Transitions. Fibers Polym. 2020, 21, 24–32. [Google Scholar] [CrossRef]
- Yu, C.; Youn, J.R.; Song, Y.S. Encapsulated Phase Change Material Embedded by Graphene Powders for Smart and Flexible Thermal Response. Fibers Polym. 2019, 20, 545–554. [Google Scholar] [CrossRef]
- Wu, W.; Huang, X.; Li, K.; Yao, R.; Chen, R.; Zou, R. A functional form-stable phase change composite with high efficiency electro-to-thermal energy conversion. Appl. Energy 2017, 190, 474–480. [Google Scholar] [CrossRef]
- Tang, L.-S.; Yang, J.; Bao, R.-Y.; Liu, Z.-Y.; Xie, B.-H.; Yang, M.-B.; Yang, W. Polyethylene glycol/graphene oxide aerogel shape-stabilized phase change materials for photo-to-thermal energy conversion and storage via tuning the oxidation degree of graphene oxide. Energy Convers. Manag. 2017, 146, 253–264. [Google Scholar] [CrossRef]
- Yu, C.; Youn, J.R.; Song, Y.S. Enhancement in thermo-electric energy harvesting efficiency by embedding PDMS in form-stable PCM composites. Polym. Adv. Technol. 2021, 33, 700–709. [Google Scholar] [CrossRef]
- Yu, C.; Song, Y.S. Characterization of Phase Change Materials Fabricated with Cross-Linked Graphene Aerogels. Gels 2022, 8, 572. [Google Scholar] [CrossRef]
- Yu, C.; Park, J.; Youn, J.R.; Song, Y.S. Integration of form-stable phase change material into pyroelectric energy harvesting system. Appl. Energy 2021, 307, 118212. [Google Scholar] [CrossRef]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Yu, C.; Song, Y.S. Graphene Aerogel-Supported Phase-Change Material for Pyroelectric Energy Harvesting: Structural Modification and Form Stability Analysis. Energy Technol. 2023; Early View. [Google Scholar] [CrossRef]
- Yu, C.; Kim, H.; Youn, J.R.; Song, Y.S. Enhancement of Structural Stability of Graphene Aerogel for Thermal Energy Harvesting. ACS Appl. Energy Mater. 2021, 4, 11666–11674. [Google Scholar] [CrossRef]

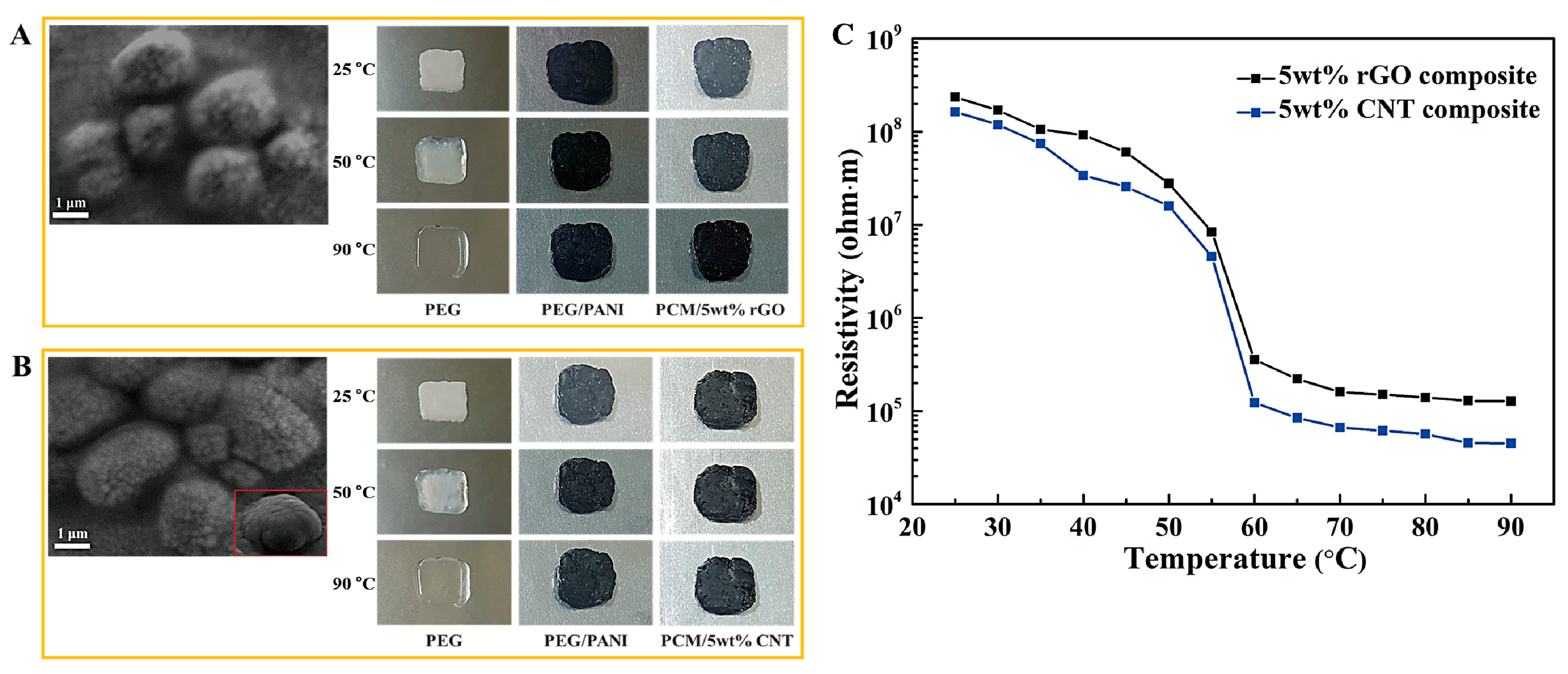
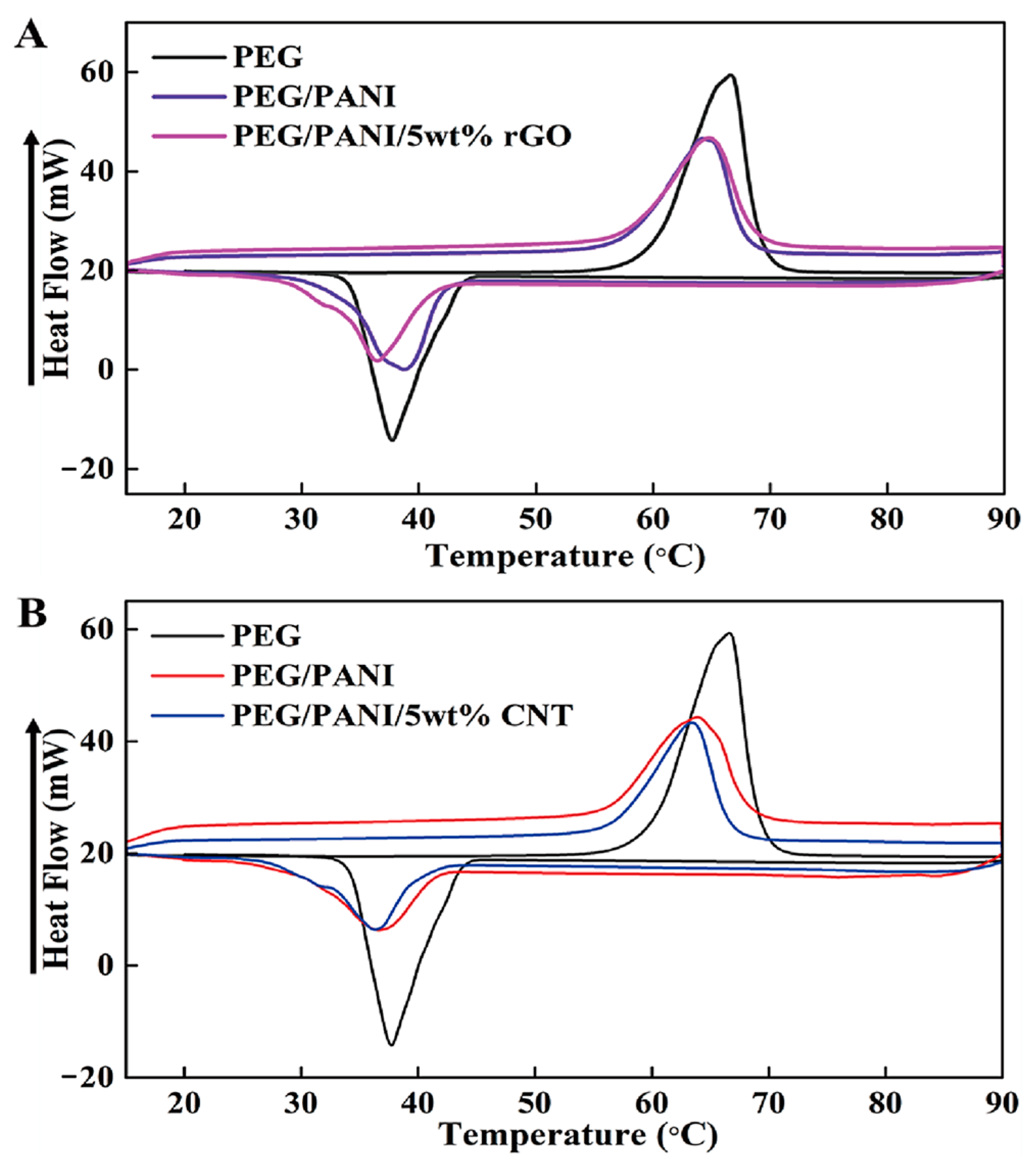
| Samples | Tmp (°C) | Tcp (°C) | ΔHm (J/g) | ΔHc (J/g) |
|---|---|---|---|---|
| PEG | 66.55 | 37.66 | 179.44 | 153.75 |
| PEG/PANI | 64.77 | 38.78 | 118.01 | 109.65 |
| PEG/PANI/rGO | 64.27 | 36.37 | 115.97 | 105.53 |
| PEG/PANI/CNT | 63.38 | 36.38 | 110.96 | 105.89 |
| Samples | Tmp (°C) | Tcp (°C) | ΔHm (J/g) | ΔHc (J/g) |
|---|---|---|---|---|
| PEG | 65.72 | 39.04 | 181.77 | 160.02 |
| PEG/GCA | 64.84 | 39.01 | 178.90 | 159.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Song, Y. Modified Supporting Materials to Fabricate Form Stable Phase Change Material with High Thermal Energy Storage. Molecules 2023, 28, 1309. https://doi.org/10.3390/molecules28031309
Yu C, Song Y. Modified Supporting Materials to Fabricate Form Stable Phase Change Material with High Thermal Energy Storage. Molecules. 2023; 28(3):1309. https://doi.org/10.3390/molecules28031309
Chicago/Turabian StyleYu, Chengbin, and Youngseok Song. 2023. "Modified Supporting Materials to Fabricate Form Stable Phase Change Material with High Thermal Energy Storage" Molecules 28, no. 3: 1309. https://doi.org/10.3390/molecules28031309





