New Insight into the Concanavalin A-Induced Apoptosis in Hepatocyte of an Animal Model: Possible Involvement of Caspase-Independent Pathway
Abstract
:1. Introduction
2. Results
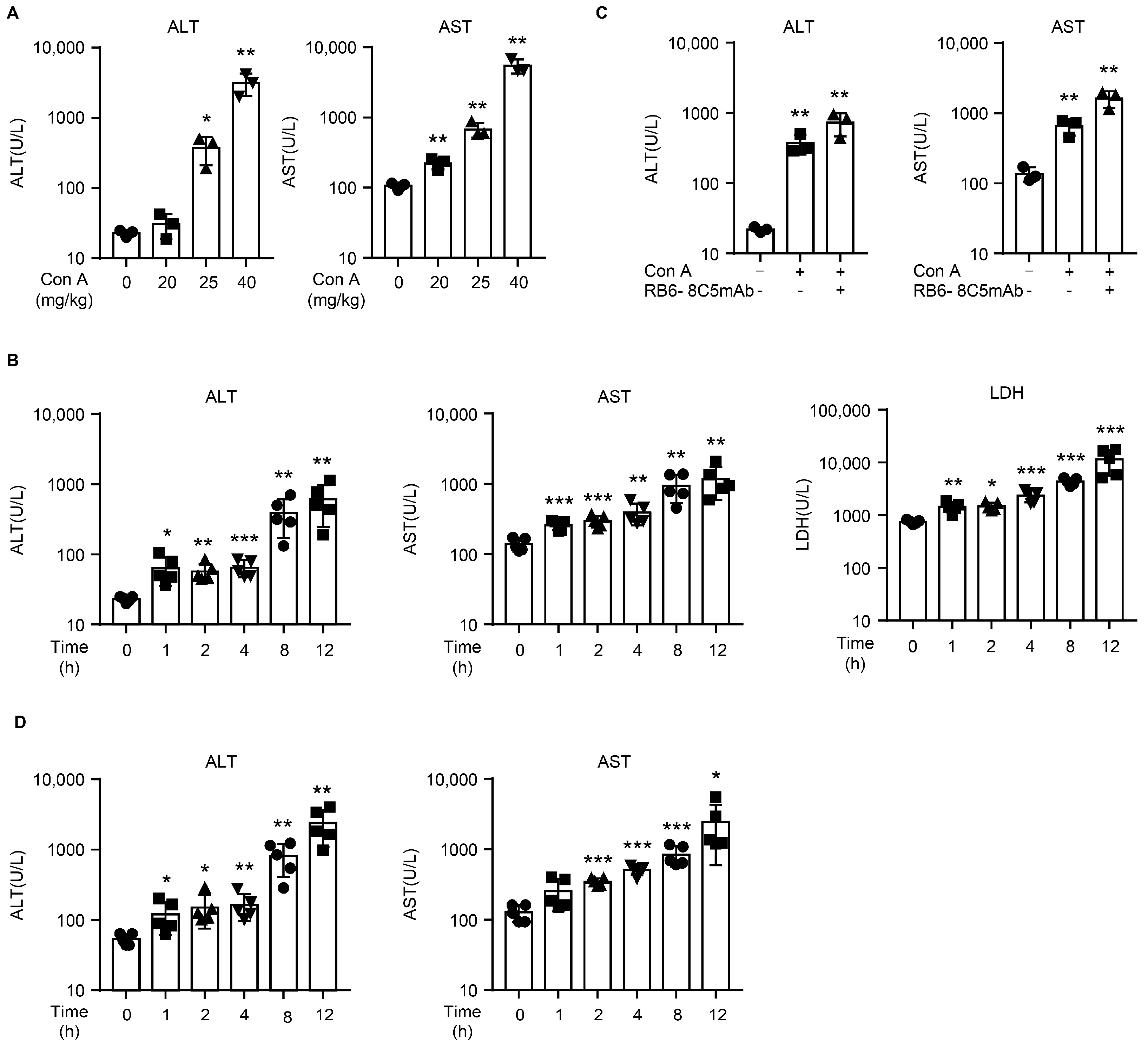
2.1. Con A Induces Liver Injury in NOD SCID Mice
2.2. Con A-Injected NOD SCID Mice Exhibit Histological Changes Typical of Liver Injury
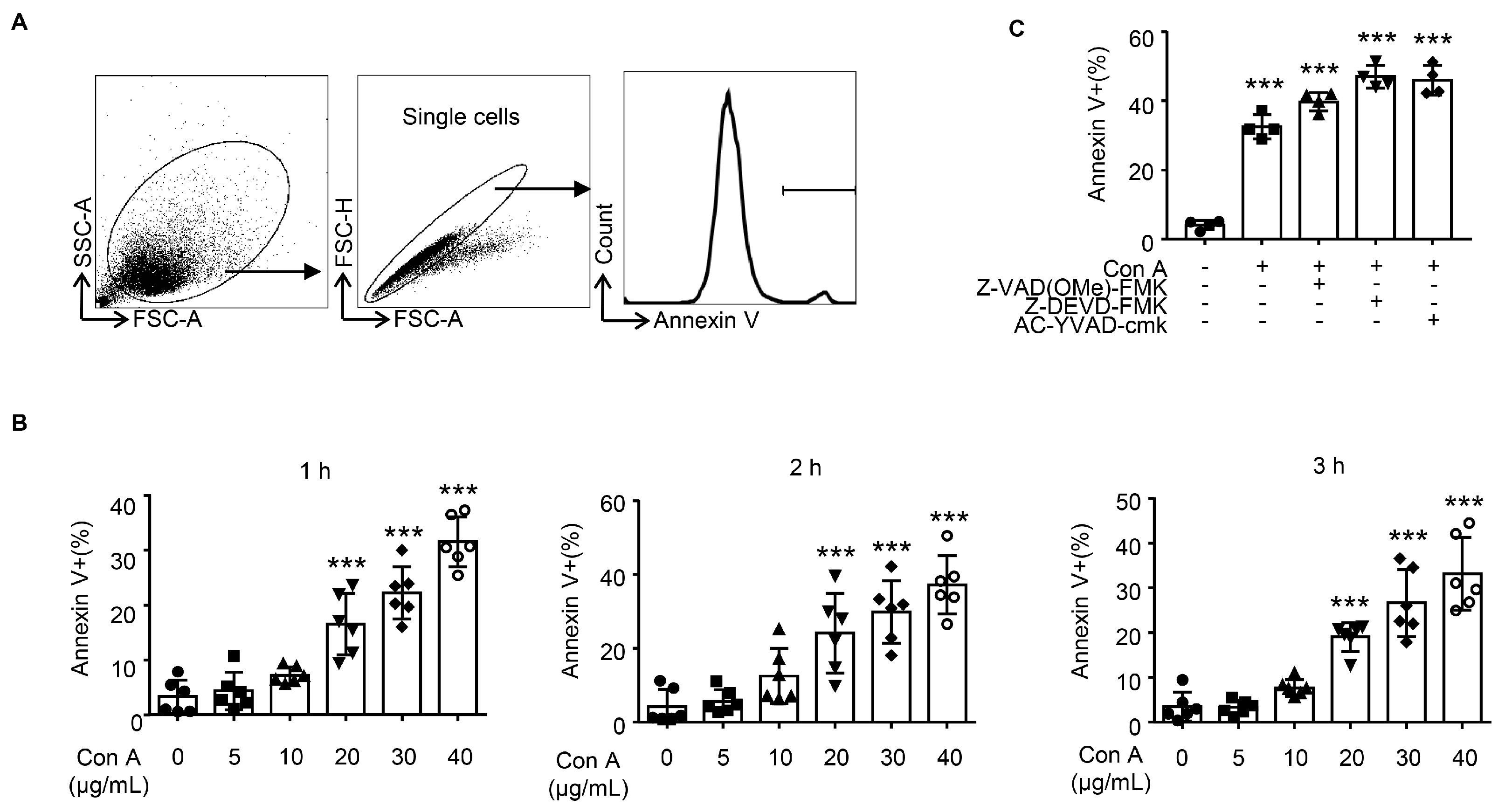
2.3. Con A Treatment Leads to Hepatocyte Apoptosis In Vitro
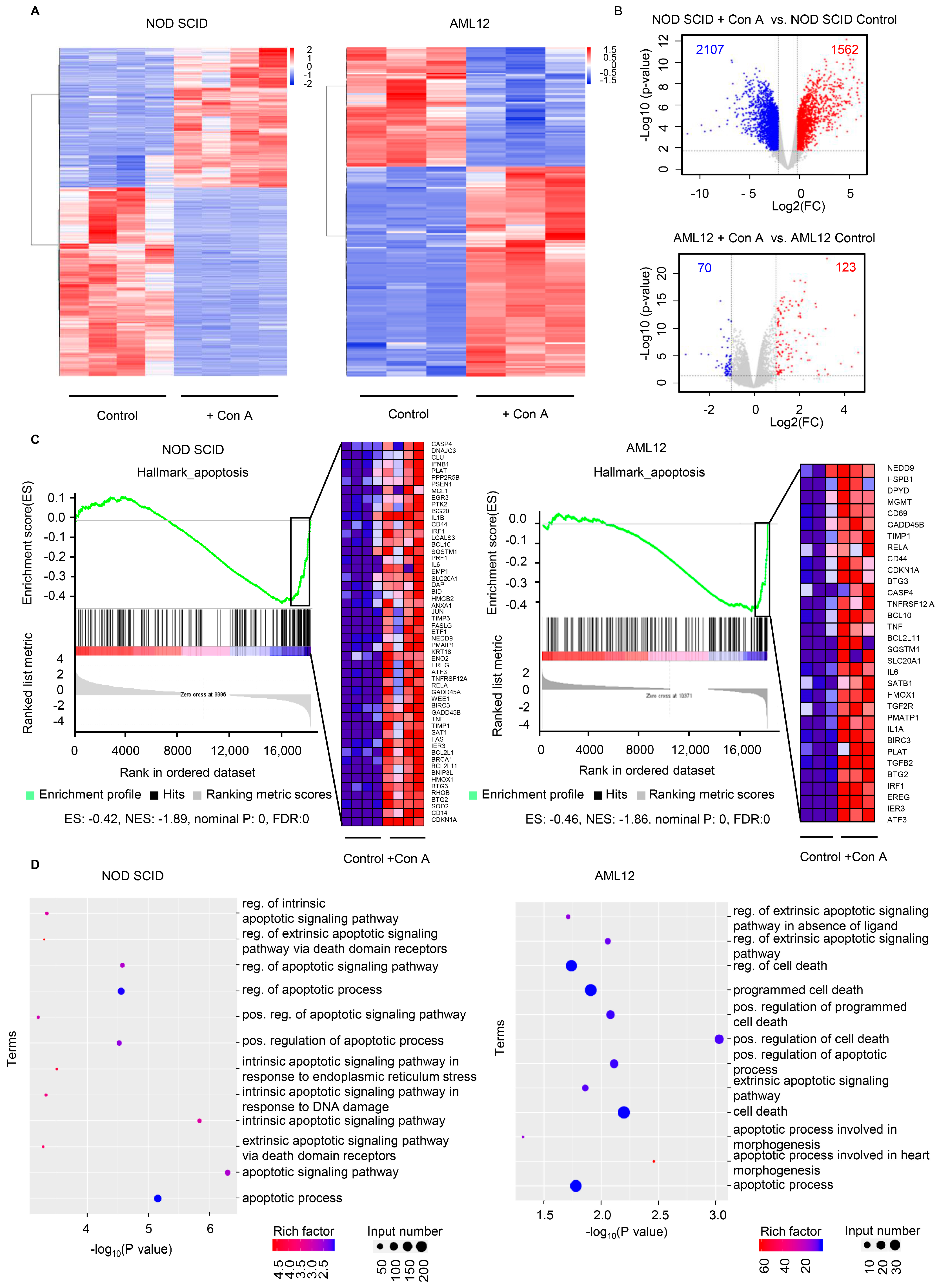
2.4. The Expression of Apoptosis-Related Genes Is Elevated in Response to Con A Treatment Both In Vivo and In Vitro
3. Discussion
4. Materials and Methods
4.1. Reagents and Cell Lines
4.2. Mice and Treatment
4.3. Serum Biochemical
4.4. Histological Examination
4.5. Immunohistochemistry and Immunofluorescent Staining
4.6. Flow Cytometry
4.7. RNA-Seq Analysis
4.8. Quantitative RT-PCR
4.9. Protein-Protein Interaction (PPI) Network
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heymann, F.; Hamesch, K.; Weiskirchen, R.; Tacke, F. The concanavalin A model of acute hepatitis in mice. Lab. Anim. 2015, 49 (Suppl. S1), 12–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Liu, M.; Weng, S.Y.; Li, J.J.; Xie, C.; He, H.L.; Guan, W.; Yuan, Y.S.; Gao, J. Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World J. Gastroenterol. 2012, 18, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, W.; Feng, Z.; Gao, Y.; Zhang, L.; Feng, X.; Tian, D. Plasma proteomic analysis of autoimmune hepatitis in an improved AIH mouse model. J. Transl. Med. 2020, 18, 3. [Google Scholar] [CrossRef]
- Hao, J.; Sun, W.; Xu, H. Pathogenesis of Concanavalin A induced autoimmune hepatitis in mice. Int. Immunopharmacol. 2022, 102, 108411. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sun, L.; Li, Q.; Qi, C.; Fu, C.; Zhu, H.; Yang, X.; Feng, H.; Li, Y.; Zhang, Y.; et al. Secoemestrin C inhibits activation of NKT/conventional T cells and protects against concanavalin A-induced autoimmune hepatitis in mice. Am. J. Transl. Res. 2020, 12, 3389–3401. [Google Scholar]
- Takeda, K.; Hayakawa, Y.; Van Kaer, L.; Matsuda, H.; Yagita, H.; Okumura, K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc. Natl. Acad. Sci. USA 2000, 97, 5498–5503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sass, G.; Heinlein, S.; Agli, A.; Bang, R.; Schumann, J.; Tiegs, G. Cytokine expression in three mouse models of experimental hepatitis. Cytokine 2002, 19, 115–120. [Google Scholar] [CrossRef]
- Mathews, S.; Feng, D.; Maricic, I.; Ju, C.; Kumar, V.; Gao, B. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration. Cell Mol. Immunol. 2016, 13, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Bonder, C.S.; Ajuebor, M.N.; Zbytnuik, L.D.; Kubes, P.; Swain, M.G. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J. Immunol. 2004, 172, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, H.; Kinoshita, M.; Nakashima, M.; Habu, Y.; Shono, S.; Uchida, T.; Shinomiya, N.; Seki, S. Superoxide produced by Kupffer cells is an essential effector in concanavalin A-induced hepatitis in mice. Hepatology 2008, 48, 1979–1988. [Google Scholar] [CrossRef]
- Chang, C.P.; Yang, M.C.; Liu, H.S.; Lin, Y.S.; Lei, H.Y. Concanavalin A induces autophagy in hepatoma cells and has a therapeutic effect in a murine in situ hepatoma model. Hepatology 2007, 45, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.Y.; Chang, C.P. Induction of autophagy by concanavalin A and its application in anti-tumor therapy. Autophagy 2007, 3, 402–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pink, J.R.; Hoessli, D.; Tartakoff, A.; Hooghe, R. Characterisation of Concanavalin A-binding glycoproteins from mouse splenic leukocytes by two-dimensional electrophoresis: Preferential binding of incompletely glycosylated forms of H-2 antigen to the lectin. Mol. Immunol. 1983, 20, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Peschke, T.; Wollweber, L.; Gabert, A.; Augsten, K.; Stracke, R. Effect of different fixatives on Con A surface receptors of mouse peritoneal macrophages. Histochemistry 1990, 93, 443–446. [Google Scholar] [CrossRef] [PubMed]
- McMillan, P.N.; Ferayorni, L.S.; Gerhardt, C.O.; Jauregui, H.O. Light and electron microscope analysis of lectin binding to adult rat liver in situ. Lab. Investig. 1984, 50, 408–420. [Google Scholar] [PubMed]
- Cline, M.J.; Livingston, D.C. Binding of 3 H-concanavalin A by normal and transformed cells. Nat. New Biol. 1971, 232, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, B.; Sambrook, J. Binding of radioactively labelled concanavalin A and wheat germ agglutinin to normal and virus-transformed cells. Nat. New Biol. 1971, 232, 156–160. [Google Scholar] [CrossRef]
- Gantner, F.; Leist, M.; Lohse, A.W.; Germann, P.G.; Tiegs, G. Concanavalin A-induced T-cell-mediated hepatic injury in mice: The role of tumor necrosis factor. Hepatology 1995, 21, 190–198. [Google Scholar]
- Chang, C.P.; Lei, H.Y. Autophagy induction in T cell-independent acute hepatitis induced by concanavalin A in SCID/NOD mice. Int. J. Immunopathol. Pharmacol. 2008, 21, 817–826. [Google Scholar] [CrossRef]
- Leist, M.; Wendel, A. A novel mechanism of murine hepatocyte death inducible by concanavalin A. J. Hepatol. 1996, 25, 948–959. [Google Scholar] [CrossRef] [Green Version]
- Hershkoviz, R.; Bruck, R.; Aeed, H.; Shirin, H.; Halpern, Z. Treatment of concanavalin A-induced hepatitis in mice with low molecular weight heparin. J. Hepatol. 1999, 31, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Otsuka, H.; Yamaguchi, K.; Kuroishi, T.; Sasano, T.; Sugawara, S.; Nakamura, M.; Endo, Y. Roles of platelets and macrophages in the protective effects of lipopolysaccharide against concanavalin A-induced murine hepatitis. Biochim. Biophys. Acta 2011, 1812, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Leist, M.; Gantner, F.; Bohlinger, I.; Tiegs, G.; Germann, P.G.; Wendel, A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am. J. Pathol. 1995, 146, 1220–1234. [Google Scholar] [PubMed]
- Lapidot, T.; Fajerman, Y.; Kollet, O. Immune-deficient SCID and NOD/SCID mice models as functional assays for studying normal and malignant human hematopoiesis. J. Mol. Med. 1997, 75, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Harada, M.; Kawano, T.; Yamashita, M.; Shibata, Y.; Gejyo, F.; Nakayama, T.; Taniguchi, M. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J. Exp. Med. 2000, 191, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Tiegs, G.; Hentschel, J.; Wendel, A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Investig. 1992, 90, 196–203. [Google Scholar] [CrossRef]
- Noel, G.; Arshad, M.I.; Filliol, A.; Genet, V.; Rauch, M.; Lucas-Clerc, C.; Lehuen, A.; Girard, J.P.; Piquet-Pellorce, C.; Samson, M. Ablation of interaction between IL-33 and ST2+ regulatory T cells increases immune cell-mediated hepatitis and activated NK cell liver infiltration. Am. J. Physiol. Gastrointest. Liver. Physiol. 2016, 311, G313-23. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Harms, C.; Lattig-Tunnemann, G.; Sellge, G.; Mandic, A.D.; Malato, Y.; Heuser, A.; Endres, M.; Trautwein, C.; Donath, S. TAT-apoptosis repressor with caspase recruitment domain protein transduction rescues mice from fulminant liver failure. Hepatology 2012, 56, 715–726. [Google Scholar] [CrossRef]
- Wang, X.M.; Liu, X.M.; Wang, Y.; Chen, Z.Y. Activating transcription factor 3 (ATF3) regulates cell growth, apoptosis, invasion and collagen synthesis in keloid fibroblast through transforming growth factor beta (TGF-beta)/SMAD signaling pathway. Bioengineered 2021, 12, 117–126. [Google Scholar] [CrossRef]
- Shi, Q.; Hu, B.; Yang, C.; Zhao, L.; Wu, J.; Qi, N. ATF3 Promotes Arsenic-Induced Apoptosis and Oppositely Regulates DR5 and Bcl-xL Expression in Human Bronchial Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 4223. [Google Scholar] [CrossRef]
- Gingras, S.; Pelletier, S.; Boyd, K.; Ihle, J.N. Characterization of a family of novel cysteine- serine-rich nuclear proteins (CSRNP). PLoS ONE 2007, 2, e808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younce, C.W.; Wang, K.; Kolattukudy, P.E. Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1 production and induction of a novel zinc-finger protein MCPIP. Cardiovasc. Res. 2010, 87, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Zhao, J.; Bu, S.; Teng, H.; Yang, J.; Zhang, X.; Li, X.; Dong, L. KLF6 Induces Apoptosis in Human Lens Epithelial Cells Through the ATF4-ATF3-CHOP Axis. Drug Des. Devel. Ther. 2020, 14, 1041–1055. [Google Scholar] [CrossRef] [Green Version]
- Younce, C.W.; Kolattukudy, P.E. MCP-1 causes cardiomyoblast death via autophagy resulting from ER stress caused by oxidative stress generated by inducing a novel zinc-finger protein, MCPIP. Biochem. J. 2010, 426, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zhang, W.; Liu, H.; Zhou, Z.; Dai, X.; Cheng, Y.; Fang, S.; Zhang, Y.; Yao, H.; et al. MCPIP1 Regulates Alveolar Macrophage Apoptosis and Pulmonary Fibroblast Activation After in vitro Exposure to Silica. Toxicol. Sci. 2016, 151, 126–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; Ning, H.; Gu, L.; Peng, H.; Wang, Q.; Hou, R.; Fu, M.; Hoft, D.F.; Liu, J. MCPIP1 Selectively Destabilizes Transcripts Associated with an Antiapoptotic Gene Expression Program in Breast Cancer Cells That Can Elicit Complete Tumor Regression. Cancer Res. 2016, 76, 1429–1440. [Google Scholar] [CrossRef] [Green Version]
- Dobosz, E.; Wadowska, M.; Kaminska, M.; Wilamowski, M.; Honarpisheh, M.; Bryzek, D.; Potempa, J.; Jura, J.; Lech, M.; Koziel, J. MCPIP-1 Restricts Inflammation via Promoting Apoptosis of Neutrophils. Front. Immunol. 2021, 12, 627922. [Google Scholar] [CrossRef]
- Zhou, L.; Azfer, A.; Niu, J.; Graham, S.; Choudhury, M.; Adamski, F.M.; Younce, C.; Binkley, P.F.; Kolattukudy, P.E. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ. Res. 2006, 98, 1177–1185. [Google Scholar] [CrossRef]
- Li, Y.; Pan, D.; Wang, X.; Huo, Z.; Wu, X.; Li, J.; Cao, J.; Xu, H.; Du, L.; Xu, B. Silencing ATF3 Might Delay TBHP-Induced Intervertebral Disc Degeneration by Repressing NPC Ferroptosis, Apoptosis, and ECM Degradation. Oxid. Med. Cell. Longev. 2022, 2022, 4235126. [Google Scholar] [CrossRef]
- Wei, J.; Long, L.; Zheng, W.; Dhungana, Y.; Lim, S.A.; Guy, C.; Wang, Y.; Wang, Y.D.; Qian, C.; Xu, B.; et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature 2019, 576, 471–476. [Google Scholar] [CrossRef]
- Tang, Q.; Ren, L.; Liu, J.; Li, W.; Zheng, X.; Wang, J.; Du, G. Withaferin A triggers G2/M arrest and intrinsic apoptosis in glioblastoma cells via ATF4-ATF3-CHOP axis. Cell Prolif. 2020, 53, e12706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Sun, Y.; Gao, A.; Zhang, N.; Jia, Y.; Yang, S.; Na, M.; Liu, H.; Cheng, X.; Fang, X.; et al. Ac-YVAD-cmk improves neurological function by inhibiting caspase-1-mediated inflammatory response in the intracerebral hemorrhage of rats. Int. Immunopharmacol. 2019, 75, 105771. [Google Scholar] [CrossRef] [PubMed]
- Ilangovan, R.; Marshall, W.L.; Hua, Y.; Zhou, J. Inhibition of apoptosis by Z-VAD-fmk in SMN-depleted S2 cells. J. Biol. Chem. 2003, 278, 30993–30999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanthasamy, A.G.; Anantharam, V.; Zhang, D.; Latchoumycandane, C.; Jin, H.; Kaul, S.; Kanthasamy, A. A novel peptide inhibitor targeted to caspase-3 cleavage site of a proapoptotic kinase protein kinase C delta (PKCdelta) protects against dopaminergic neuronal degeneration in Parkinson’s disease models. Free Radic. Biol. Med. 2006, 41, 1578–1589. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Fu, C.; Sun, L.; Feng, H.; Xie, P.; Wu, M.; Tan, X.; Chen, G. New Insight into the Concanavalin A-Induced Apoptosis in Hepatocyte of an Animal Model: Possible Involvement of Caspase-Independent Pathway. Molecules 2023, 28, 1312. https://doi.org/10.3390/molecules28031312
Zhao X, Fu C, Sun L, Feng H, Xie P, Wu M, Tan X, Chen G. New Insight into the Concanavalin A-Induced Apoptosis in Hepatocyte of an Animal Model: Possible Involvement of Caspase-Independent Pathway. Molecules. 2023; 28(3):1312. https://doi.org/10.3390/molecules28031312
Chicago/Turabian StyleZhao, Xiangli, Cheng Fu, Lingjuan Sun, Hao Feng, Peiling Xie, Meng Wu, Xiaosheng Tan, and Gang Chen. 2023. "New Insight into the Concanavalin A-Induced Apoptosis in Hepatocyte of an Animal Model: Possible Involvement of Caspase-Independent Pathway" Molecules 28, no. 3: 1312. https://doi.org/10.3390/molecules28031312




