Impacts of Binary Oxide Nanoparticles on the Soybean Plant and Its Rhizosphere, Associated Phytohormones, and Enzymes
Abstract
1. Introduction
2. Methods of Synthesizing Binary Oxide Nanoparticles

| Synthetic Method | Brief Description | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Sol–gel | Preparation via transformation of liquid precursors to sol and finally to a gel structure | Control of morphology is possible by changing the precursors | A toxic organic solvent may be required; processing is associated with contraction | [21,32] |
| Hydrothermal | Reaction of solid material with aqueous solution at high temperature and pressure | The reaction is usually carried out in a closed system which minimizes pollution; easy to control the nucleation; low temperature required in a suitable solvent; it saves energy | Longer reaction time than techniques such as vapor deposition technique | [24,33] |
| Sonochemical | The production of nanoparticles using ultrasound under high intensity of sound, high pressure, and high temperature | Possibility of initiating reaction without external agents | Lack of ultrasonic reactors that can produce in commercial quantities | [23,34] |
| Spray-drying | It involves atomization by using hot drying gas to give dry powder of nanoparticles | Reproducible, fast, and cheap | Reduced yield due to the sticking of the products to the walls of the drying chamber | [35] |
| Solvothermal | Precursors are stoichiometrically mixed with organic solvent at an elevated temperature to generate nanoparticles | Materials produced have high degree of crystallization | Long time reaction; contamination which requires several washing steps | [24,36,37] |
| Deposition of gas phase/vapor | Conversion of vapor phase to condensed phase to produce nanoparticles | Thin films of nanoparticles are formed easily | It has high cost and gives low yield | [21,25] |
| Mechanical/ball milling | Employing impacts from mechanical energy to generate inorganic materials | Cheap, easy to optimize, and gives pure product | Contamination is possible; it requires a long time; high energy is required | [28] |
| Microwave | Utilizing microwave irradiation to raise the temperature of reactants in solution leading to the formation of nanoparticles | Easy to reproduce; short reaction time needed; high yield is obtained | High synthetic cost; commercialization is tedious | [24] |
| Laser/spray pyrolysis | Laser beam is used to heat up or decompose the precursor leading to the formation of nanoparticles | Relatively cheap; morphological modulation is possible | The reactors needed for pyrolysis are expensive | [21] |
3. The Methods of Characterizing Binary Oxide Nanoparticles Inside Soybean Plant

| Characterization Tools | Application | Principle of Operation | Ref. |
|---|---|---|---|
| X-ray diffraction (XRD) | To determine the dimensions of the lattice, particle size and crystallinity; it is also used for crystal characterization | The interaction of a light having a single wavelength with the oxide nanoparticles. | [43,44] |
| UV/visible absorption spectroscopy | To determine the stability of the oxide nanoparticles and for identification purposes | The plot of coefficient of extinction against wavelength obtained when light of known intensity passes through the sample to the detector | [45] |
| Fourier-transform infrared spectroscopy (FTIR) | To determine the functional group of molecules attached to the oxide nanoparticles | Interaction of infrared radiation causing vibration and interaction of molecules | [46] |
| Dynamic light scattering (DLS) | To measure the distribution of the particle size in colloid or suspension | Detection of the scattered light at a known angle after the sample is focused with a laser beam | [47] |
| Scanning electron microscopy (SEM) | To determine the surface images of oxide nanoparticles | Interaction of the electrons in the sample with the beam of electron from the machine to generate captured signals | [48,49] |
| Transmission electron microscopy (TEM) | To determine the morphology, size, and internal morphology of oxide nanoparticles | The beams of electrons pass through the oxide nanoparticles; the beam is scattered, while the lens captures the scattered electrons to form an image | [50] |
| Energy-dispersive X-ray analysis (EDAX) | To determine the elements that are present in oxide nanoparticles | Electrons are knocked off from the inner shell of electrons when it is bombarded with a beam of electrons, leading to the generation of a positively charged hole which takes up another electron from the valence shell due to electrostatic forces of attraction | [48,49] |
| X-ray phosphorescence (XPS) | To determine the purity of oxide nanoparticles | Bombardment of nanoparticles with high energy radiations to give a characteristic fluorescent emission. | [51] |
| Atomic force microscopy (AFM) | To determine the volume distribution, surface area, roughness, morphology, and size of oxide nanoparticles | A micro-cantilever is used with the side having weaker force contacting the sample; the fluctuation of the probe is measured | [52,53,54] |
| Thermal gravimetric analysis (TGA) | To determine the stability of oxide nanoparticles under heat | The change in weight is plotted as a function of temperature | [55] |
| Dynamic light scattering (DLS) | To determine the state of aggregation of oxide nanoparticles | It operates on the basis of “Brownian motion” | [56] |
4. Effects of Binary Oxide Nanoparticles on Soybean Plant
4.1. Effects of Binary Oxide Nanoparticles on the Rhizosphere Microbial Community of Soybean Plant
4.2. Effects of Binary Oxide Nanoparticles on the Leaf of Soybean Plant
4.3. The Effects of Binary Oxide Nanoparticles on the Stem of Soybean Plant
4.4. The Effects of Binary Oxide Nanoparticles on the Root of Soybean Plant
4.5. The Effects of Binary Oxide Nanoparticles on the Seeds of Soybean Plant
4.6. The Effects of Binary Oxide Nanoparticles on Phytohormones and Enzymes of Soybean Plant
5. Metagenomics as a Tool for Identifying Microbiomes
Metagenomics as a Tool for Investigating the Microbiome of Soybean Rhizosphere
6. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Roriz, M.; Carvalho, S.M.P.; Vasconcelos, M.W. High relative air humidity influences mineral accumulation and growth in iron deficient soybean plants. Front. Plant Sci. 2014, 5, 726. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.W.; Grusak, M.A. Morpho-physiological parameters affecting iron deficiency chlorosis in soybean (Glycine max L.). Plant Soil 2014, 374, 161–172. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Particle size and concentration dependent toxicity of copper oxide nanoparticles (CuONPs) on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci. Total Environ. 2020, 715, 136994. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, M.L.; de la Rosa, G.; Hernández-Viezcas, J.Á.; Castillo-Michel, H.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Evidence of the Differential Biotransformation and Genotoxicity of ZnO and CeO2 Nanoparticles on Soybean (Glycine max) Plants. Environ. Sci. Technol. 2010, 44, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Ajilogba, C.F.; Babalola, O.O.; Nikoro, D.O. Nanotechnology as Vehicle for Biocontrol of Plant Diseases in Crop Production. In Food Security and Safety; Springer: Berlin/Heidelberg, Germany, 2021; pp. 709–724. [Google Scholar]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Villamor, E.; Begines, B.; Alcudia, A. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2021, 14, 166. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Marzouki, R.; Onwudiwe, D.C. Photocatalytic Reduction of Hexavalent Chromium Using Cu3.21Bi4.79S9/g-C3N4 Nanocomposite. Catalysts 2022, 12, 1075. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Kwak, J.I.; Lee, W.-M.; Holden, P.A.; An, Y.-J. Zinc oxide nanoparticles delay soybean development: A standard soil microcosm study. Ecotoxicol. Environ. Saf. 2014, 100, 131–137. [Google Scholar] [CrossRef]
- Capaldi Arruda, S.C.; Diniz Silva, A.L.; Moretto Galazzi, R.; Antunes Azevedo, R.; Zezzi Arruda, M.A. Nanoparticles applied to plant science: A review. Talanta 2015, 131, 693–705. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. The performance of bismuth-based compounds in photocatalytic applications. Surf. Interfaces 2021, 23, 100927. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Photocatalytic removal of parabens and halogenated products in wastewater: A review. Environ. Chem. Lett. 2021, 19, 3789–3819. [Google Scholar] [CrossRef]
- Ahlenstiel, C.L.; Suzuki, K.; Marks, K.; Symonds, G.P.; Kelleher, A.D. Controlling HIV-1: Non-Coding RNA Gene Therapy Approaches to a Functional Cure. Front. Immunol. 2015, 6, 474. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Babalola, S.O.; Onwudiwe, D.C. Photocatalytic Inactivation as a Method of Elimination of E. coli from Drinking Water. Appl. Sci. 2021, 11, 1313. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; Thomas, R.M.; John, H. Antibacterial self-cleaning binary and ternary hybrid photocatalysts of titanium dioxide with silver and graphene. J. Environ. Chem. Eng. 2022, 10, 107275. [Google Scholar] [CrossRef]
- García-Gómez, C.; Fernández, M.D. Chapter Four—Impacts of metal oxide nanoparticles on seed germination, plant growth and development. In Comprehensive Analytical Chemistry; Verma, S.K., Das, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 84, pp. 75–124. [Google Scholar]
- Maroufpoor, N.; Mousavi, M.; Hatami, M.; Rasoulnia, A.; Lajayer, B.A. Chapter 5—Mechanisms Involved in Stimulatory and Toxicity Effects of Nanomaterials on Seed Germination and Early Seedling Growth. In Advances in Phytonanotechnology; Ghorbanpour, M., Wani, S.H., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 153–181. [Google Scholar] [CrossRef]
- Priester, J.H.; Ge, Y.; Mielke, R.E.; Horst, A.M.; Moritz, S.C.; Espinosa, K.; Gelb, J.; Walker, S.L.; Nisbet, R.M.; An, Y.-J. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc. Natl. Acad. Sci. USA 2012, 109, E2451–E2456. [Google Scholar] [CrossRef]
- Tsuno, Y.; Fujimatsu, T.; Endo, K.; Sugiyama, A.; Yazaki, K. Soyasaponins: A New Class of Root Exudates in Soybean (Glycine max). Plant Cell Physiol. 2017, 59, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Mukerji, K.G. Root Exudates as Determinant of Rhizospheric Microbial Biodiversity. In Microbial Activity in the Rhizoshere; Mukerji, K.G., Manoharachary, C., Singh, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 39–53. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Onwudiwe, D.C. Bismuth sulfide based compounds: Properties, synthesis and applications. Results Chem. 2021, 3, 100151. [Google Scholar] [CrossRef]
- Shiva Samhitha, S.; Raghavendra, G.; Quezada, C.; Hima Bindu, P. Green synthesized TiO2 nanoparticles for anticancer applications: Mini review. Mater. Today: Proc. 2022, 54, 765–770. [Google Scholar] [CrossRef]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.; Thomas, S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–139. [Google Scholar]
- Teimouri, M.; Khosravi-Nejad, F.; Attar, F.; Saboury, A.A.; Kostova, I.; Benelli, G.; Falahati, M. Gold nanoparticles fabrication by plant extracts: Synthesis, characterization, degradation of 4-nitrophenol from industrial wastewater, and insecticidal activity—A review. J. Clean. Prod. 2018, 184, 740–753. [Google Scholar] [CrossRef]
- Sastry, A.B.S.; Karthik Aamanchi, R.B.; Sree Rama Linga Prasad, C.; Murty, B.S. Large-scale green synthesis of Cu nanoparticles. Environ. Chem. Lett. 2013, 11, 183–187. [Google Scholar] [CrossRef]
- Kumar, J.A.; Krithiga, T.; Manigandan, S.; Sathish, S.; Renita, A.A.; Prakash, P.; Prasad, B.S.N.; Kumar, T.R.P.; Rajasimman, M.; Hosseini-Bandegharaei, A.; et al. A focus to green synthesis of metal/metal based oxide nanoparticles: Various mechanisms and applications towards ecological approach. J. Clean. Prod. 2021, 324, 129198. [Google Scholar] [CrossRef]
- Goutam, S.; Saxena, G.; Roy, D.; Yadav, A.; Bharagava, R. Green Synthesis of Nanoparticles and Their Applications in Water and Wastewater Treatment. In Bioremediation of Industrial Waste for Environmental Safety; SaxenaRam, G., Bharagava, N., Eds.; Springer: Singapore, 2019; pp. 349–379. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Selvaraj Janaki, N.J.; Ivan Jebakumar, D.S.; Sumithraj Premkumar, P. Studies on the physical properties of green synthesized cerium oxide nanoparticles using Melia dubia leaf extract. Mater. Today Proc. 2021, 58, 850–854. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal processing of materials: Past, present and future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Ashokkumar, M. Advantages, disadvantages and challenges of ultrasonic technology. In Ultrasonic Synthesis of Functional Materials; Springer: Berlin/Heidelberg, Germany, 2016; pp. 41–42. [Google Scholar]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Conventional and Current Methods of Toxic Metals Removal from Water Using g-C3N4-Based Materials. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1419–1442. [Google Scholar] [CrossRef]
- Reverón, H.; Aymonier, C.; Loppinet-Serani, A.; Elissalde, C.; Maglione, M.; Cansell, F. Single-step synthesis of well-crystallized and pure barium titanate nanoparticles in supercritical fluids. Nanotechnology 2005, 16, 1137. [Google Scholar] [CrossRef]
- Pinto, T.L.F.; Cicero, S.; França-Neto, J.; Forti, V. An assessment of mechanical and stink bug damage in soybean seed using X-ray analysis test. Seed Sci. Technol. 2009, 37, 110–120. [Google Scholar] [CrossRef]
- Grassl, J.; Taylor, N.L.; Millar, A. Matrix-assisted laser desorption/ionisation mass spectrometry imaging and its development for plant protein imaging. Plant Methods 2011, 7, 21. [Google Scholar] [CrossRef]
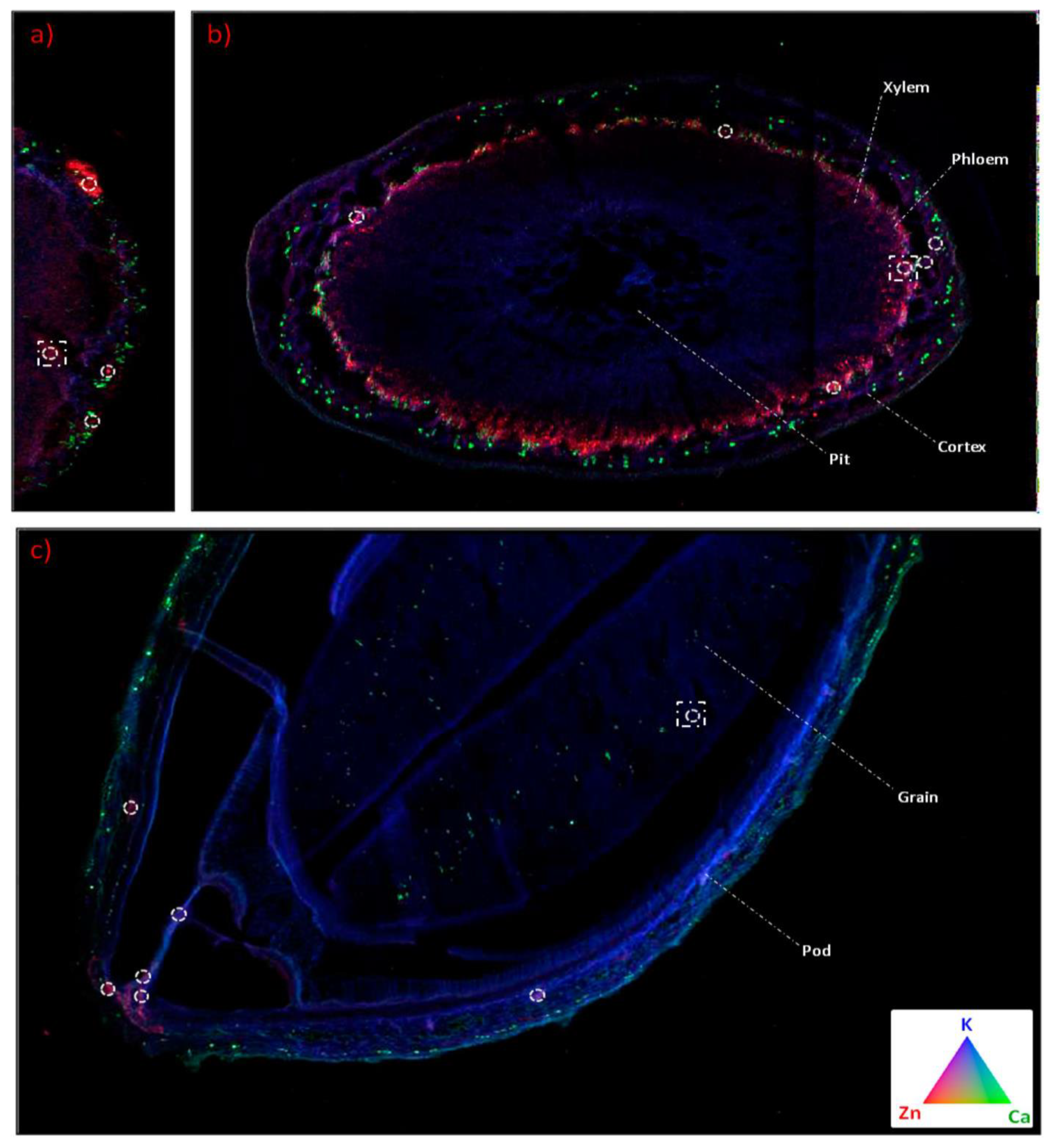
- Hernandez-Viezcas, J.A.; Castillo-Michel, H.; Andrews, J.C.; Cotte, M.; Rico, C.; Peralta-Videa, J.R.; Ge, Y.; Priester, J.H.; Holden, P.A.; Gardea-Torresdey, J.L. In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max). ACS Nano 2013, 7, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, M.; Srivastav, A.; Gandhi, S.; Rao, S.; Roychoudhury, A.; Kumar, A.; Singhal, R.K.; Jha, S.K.; Singh, S.D. Monitoring of engineered nanoparticles in soil-plant system: A review. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100218. [Google Scholar] [CrossRef]
- Ahmad, F.; Siddiqui, M.A.; Babalola, O.O.; Wu, H.-F. Biofunctionalization of nanoparticle assisted mass spectrometry as biosensors for rapid detection of plant associated bacteria. Biosens. Bioelectron. 2012, 35, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles- a comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Iniewski, K.; Grosser, A. Semiconductor Sensors for XRD Imaging. In X-Ray Diffraction Imaging; CRC Press: Boca Raton, FL, USA, 2018; pp. 53–82. [Google Scholar]
- Hussain, C.M.; Rawtani, D.; Pandey, G.; Tharmavaram, M. Chapter 3—UV-visible and fluorescence spectroscopy for forensic samples. In Handbook of Analytical Techniques for Forensic Samples; Hussain, C.M., Rawtani, D., Pandey, G., Tharmavaram, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 37–54. [Google Scholar] [CrossRef]
- Singh, M.K.; Singh, A. Chapter 13—Fourier transform infrared (FTIR) analysis. In Characterization of Polymers and Fibres; Singh, M.K., Singh, A., Eds.; Woodhead Publishing: Sawston, England, 2022; pp. 295–320. [Google Scholar] [CrossRef]
- Babick, F. Chapter 3.2.1—Dynamic light scattering (DLS). In Characterization of Nanoparticles; Hodoroaba, V.-D., Unger, W.E.S., Shard, A.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–172. [Google Scholar] [CrossRef]
- Abd Mutalib, M.; Rahman, M.A.; Othman, M.H.D.; Ismail, A.F.; Jaafar, J. Chapter 9—Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray (EDX) Spectroscopy. In Membrane Characterization; Hilal, N., Ismail, A.F., Matsuura, T., Oatley-Radcliffe, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 161–179. [Google Scholar] [CrossRef]
- Nair, G.M.; Sajini, T.; Mathew, B. Advanced Green Approaches for Metal and Metal Oxide Nanoparticles Synthesis and Their Environmental Applications. Talanta Open 2021, 5, 100080. [Google Scholar] [CrossRef]
- Kannan, M. Transmission Electron Microscope -Principle, Components and Applications Illumination system (Electron Gun and Condenser Lenses); Electron gun: Amsterdam, The Netherlands, 2018; pp. 93–101. [Google Scholar]
- Beckhoff, B.; Kanngießer, B.; Langhoff, N.; Wedell, R.; Wolff, H. Handbook of Practical X-ray Fluorescence Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Deng, X.; Xiong, F.; Li, X.; Xiang, B.; Li, Z.; Wu, X.; Guo, C.; Li, X.; Li, Y.; Li, G.; et al. Application of atomic force microscopy in cancer research. J. Nanobiotechnology 2018, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.R. Atomic Force Microscopy. Chem. Educ. 1996, 1, 1–8. [Google Scholar] [CrossRef]
- Wang, K.; Taylor, K.G.; Ma, L. Advancing the application of atomic force microscopy (AFM) to the characterization and quantification of geological material properties. Int. J. Coal Geol. 2021, 247, 103852. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K. Thermo Gravimetric Analysis. In Essentials of Pharmaceutical Analysis; Springer: Singapore, 2020; pp. 215–222. [Google Scholar]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef]
- Afsheen, S.; Naseer, H.; Iqbal, T.; Abrar, M.; Bashir, A.; Ijaz, M. Synthesis and characterization of metal sulphide nanoparticles to investigate the effect of nanoparticles on germination of soybean and wheat seeds. Mater. Chem. Phys. 2020, 252, 123216. [Google Scholar] [CrossRef]
- Changmei, L.; Chaoying, Z.; Junqiang, W.; Guorong, W.; Mingxuan, T. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002, 21, 168–171. [Google Scholar]
- Stowers, C.; King, M.; Rossi, L.; Zhang, W.; Arya, A.; Ma, X. Initial Sterilization of Soil Affected Interactions of Cerium Oxide Nanoparticles and Soybean Seedlings (Glycine max (L.) Merr.) in a Greenhouse Study. ACS Sustain. Chem. Eng. 2018, 6, 10307–10314. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Below-ground-above-ground Plant-microbial Interactions: Focusing on Soybean, Rhizobacteria and Mycorrhizal Fungi. Open Microbiol. J. 2018, 12, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Mendes, L.W.; Kuramae, E.E.; Navarrete, A.A.; Van Veen, J.A.; Tsai, S.M. Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J. 2014, 8, 1577–1587. [Google Scholar] [CrossRef]
- Alonso, D.; Etienne, R.S.; McKane, A.J. The merits of neutral theory. Trends Ecol. Evol. 2006, 21, 451–457. [Google Scholar] [CrossRef]
- Godsoe, W.; Jankowski, J.; Holt, R.D.; Gravel, D. Integrating Biogeography with Contemporary Niche Theory. Trends Ecol. Evol. 2017, 32, 488–499. [Google Scholar] [CrossRef]
- Prosser, J.I.; Bohannan, B.J.M.; Curtis, T.P.; Ellis, R.J.; Firestone, M.K.; Freckleton, R.P.; Green, J.L.; Green, L.E.; Killham, K.; Lennon, J.J.; et al. The role of ecological theory in microbial ecology. Nat. Rev. Microbiol. 2007, 5, 384–392. [Google Scholar] [CrossRef]
- Frenk, S.; Ben-Moshe, T.; Dror, I.; Berkowitz, B.; Minz, D. Effect of metal oxide nanoparticles on microbial community structure and function in two different soil types. PloS ONE 2013, 8, e84441. [Google Scholar] [CrossRef]
- Burke, D.J.; Zhu, S.; Pablico-Lansigan, M.P.; Hewins, C.R.; Samia, A.C.S. Titanium oxide nanoparticle effects on composition of soil microbial communities and plant performance. Biol. Fertil. Soils 2014, 50, 1169–1173. [Google Scholar] [CrossRef]
- Ge, Y.; Priester, J.H.; Van De Werfhorst, L.C.; Walker, S.L.; Nisbet, R.M.; An, Y.-J.; Schimel, J.P.; Gardea-Torresdey, J.L.; Holden, P.A. Soybean Plants Modify Metal Oxide Nanoparticle Effects on Soil Bacterial Communities. Environ. Sci. Technol. 2014, 48, 13489–13496. [Google Scholar] [CrossRef]
- Allard, P.; Darnajoux, R.; Phalyvong, K.; Bellenger, J.-P. Effects of tungsten and titanium oxide nanoparticles on the diazotrophic growth and metals acquisition by Azotobacter vinelandii under molybdenum limiting condition. Environ. Sci. Technol. 2013, 47, 2061–2068. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, X.; He, S.; Dong, G.; Chen, M.; Wang, J.; Lin, X. The Role of Metal Nanoparticles in Influencing Arbuscular Mycorrhizal Fungi Effects on Plant Growth. Environ. Sci. Technol. 2013, 47, 9496–9504. [Google Scholar] [CrossRef]
- Mustafa, G.; Sakata, K.; Komatsu, S. Proteomic analysis of flooded soybean root exposed to aluminum oxide nanoparticles. J. Proteom. 2015, 128, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Ghafariyan, M.H.; Malakouti, M.J.; Dadpour, M.R.; Stroeve, P.; Mahmoudi, M. Effects of Magnetite Nanoparticles on Soybean Chlorophyll. Environ. Sci. Technol. 2013, 47, 10645–10652. [Google Scholar] [CrossRef]
- Yang, X.; Alidoust, D.; Wang, C. Effects of iron oxide nanoparticles on the mineral composition and growth of soybean (Glycine max L.) plants. Acta Physiol. Plant. 2020, 42, 128. [Google Scholar] [CrossRef]
- Singh, J.; Lee, B.-K. Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): A possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manag. 2016, 170, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.J.; Pietrasiak, N.; Situ, S.F.; Abenojar, E.C.; Porche, M.; Kraj, P.; Lakliang, Y.; Samia, A.C.S. Iron Oxide and Titanium Dioxide Nanoparticle Effects on Plant Performance and Root Associated Microbes. Int. J. Mol. Sci. 2015, 16, 23630–23650. [Google Scholar] [CrossRef]
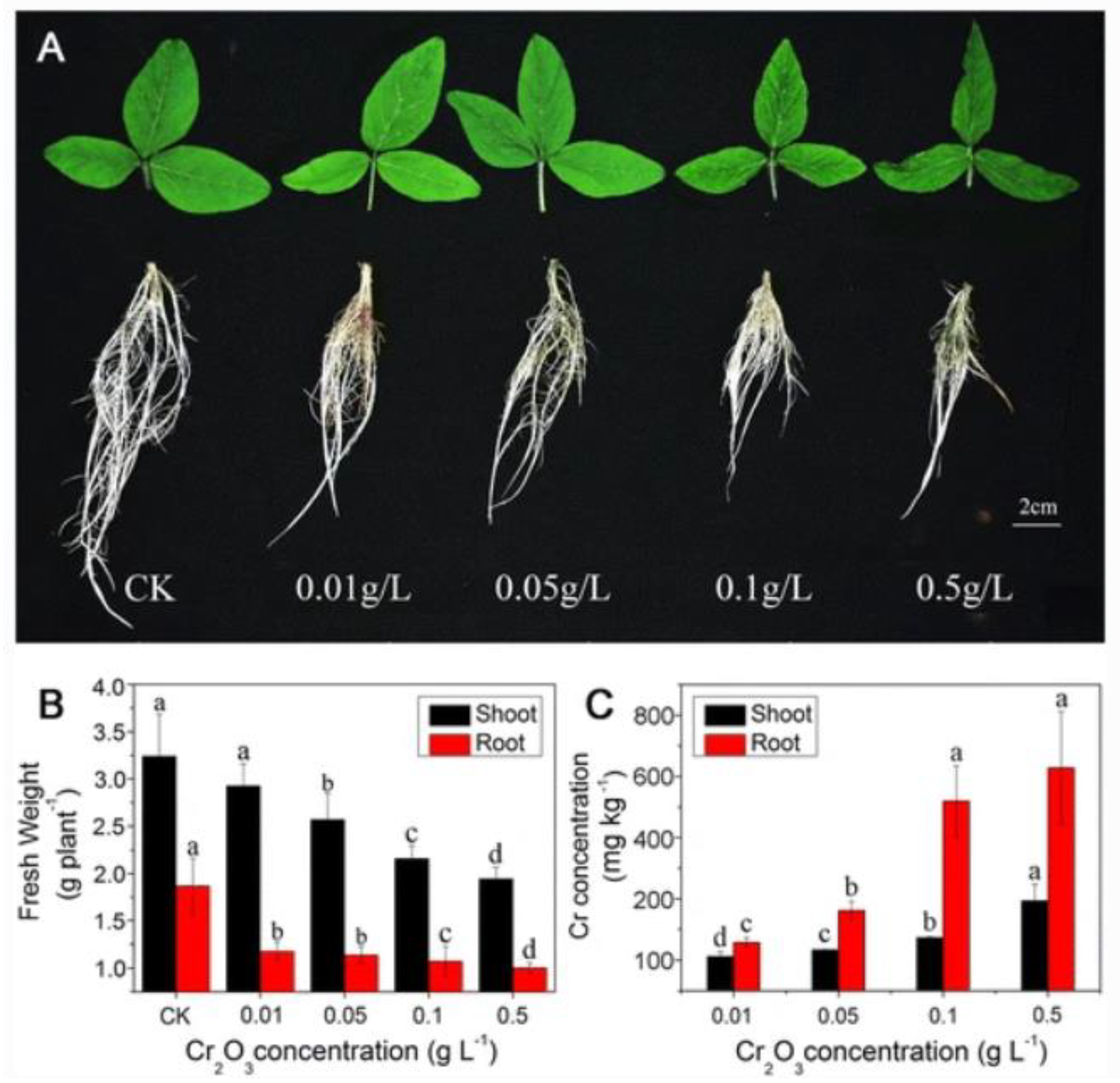
- Li, J.; Song, Y.; Wu, K.; Tao, Q.; Liang, Y.; Li, T. Effects of Cr2O3 nanoparticles on the chlorophyll fluorescence and chloroplast ultrastructure of soybean (Glycine max). Environ. Sci. Pollut. Res. 2018, 25, 19446–19457. [Google Scholar] [CrossRef] [PubMed]
- Jayarambabu, N.; Kumari; Rao, K.; Prabhu, Y. beneficial Role of zinc oxide nanoparticles on green crop production. Int. J. Multidiscip. Adv. Res. Trends 2015, 2, 2349–7408. [Google Scholar]
- Amano, Y.; Tong, X.; Sun, R.; Umemoto, J.; Kobayashi, M.; Shiratsuki, Y.; Okuno, T.; Tanino, A.; Nakao, M.; Hotta, T.; et al. Therapeutic Efficacy of Zinc Oxide Nanoparticles Against Small Cell Lung Cancer in an Orthotopic Xenograft Model. Am. J. Respir. Crit. Care Med. 2019, 199, A3948. [Google Scholar] [CrossRef]
- Vesali-Kermani, E.; Habibi-Yangjeh, A.; Diarmand-Khalilabad, H.; Ghosh, S. Nitrogen photofixation ability of g-C3N4 nanosheets/Bi2MoO6 heterojunction photocatalyst under visible-light illumination. J. Colloid Interface Sci. 2020, 563, 81–91. [Google Scholar] [CrossRef]
- Stowers, C.E. The Impact of the Rhizosphere Microbial Community on the Interactions of Engineered Nanoparticles with Plants. Master’s Thesis, Texas A & M University, Colledge Station, TX, USA, 2017. [Google Scholar]
- Zhang, P.; Ma, Y.; Xie, C.; Guo, Z.; He, X.; Valsami-Jones, E.; Lynch, I.; Luo, W.; Zheng, L.; Zhang, Z. Plant species-dependent transformation and translocation of ceria nanoparticles. Environ. Sci. Nano 2019, 6, 60–67. [Google Scholar] [CrossRef]
- Almeida, G.H.G.d.; Siqueira-Soares, R.d.C.; Mota, T.R.; Oliveira, D.M.d.; Abrahão, J.; Foletto-Felipe, M.d.P.; dos Santos, W.D.; Ferrarese-Filho, O.; Marchiosi, R. Aluminum oxide nanoparticles affect the cell wall structure and lignin composition slightly altering the soybean growth. Plant Physiol. Biochem. 2021, 159, 335–346. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. A Mechanistic Study on the Toxic Effect of Copper Oxide Nanoparticles in Soybean (Glycine max L.) Root Development and Lignification of Root Cells. Biol. Trace Elem. Res. 2014, 162, 342–352. [Google Scholar] [CrossRef]
- Shankramma, K.; Yallappa, S.; Shivanna, M.B.; Manjanna, J. Fe2O3 magnetic nanoparticles to enhance S. lycopersicum (tomato) plant growth and their biomineralization. Appl. Nanosci. 2016, 6, 983–990. [Google Scholar] [CrossRef]
- Palchoudhury, S.; Jungjohann, K.L.; Weerasena, L.; Arabshahi, A.; Gharge, U.; Albattah, A.; Miller, J.; Patel, K.; Holler, R.A. Enhanced legume root growth with pre-soaking in α-Fe2O3 nanoparticle fertilizer. RSC Adv. 2018, 8, 24075–24083. [Google Scholar] [CrossRef]
- Diab, R.; shanshory, a.; Gaafar, R.; Hamouda, M. Role of Zinc Oxide Nanoparticles in Ameliorating Salt Tolerance in Soybean. Egypt. J. Bot. 2020, 60, 733–747. [Google Scholar]
- Adhikari, T.; Kundu, S.; Biswas, A.K.; Tarafdar, J.C.; Rao, A.S. Effect of copper oxide nano particle on seed germination of selected crops. J. Agric. Sci. Technol. A 2012, 2, 815. [Google Scholar]
- Peralta-Videa, J.R.; Hernandez-Viezcas, J.A.; Zhao, L.; Diaz, B.C.; Ge, Y.; Priester, J.H.; Holden, P.A.; Gardea-Torresdey, J.L. Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol. Biochem. 2014, 80, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Galazzi, R.M.; Lopes Júnior, C.A.; de Lima, T.B.; Gozzo, F.C.; Arruda, M.A.Z. Evaluation of some effects on plant metabolism through proteins and enzymes in transgenic and non-transgenic soybeans after cultivation with silver nanoparticles. J. Proteom. 2019, 191, 88–106. [Google Scholar] [CrossRef]
- Ma, C.; White, J.C.; Zhao, J.; Zhao, Q.; Xing, B. Uptake of engineered nanoparticles by food crops: Characterization, mechanisms, and implications. Annu. Rev. Food Sci. Technol. 2018, 9, 129–153. [Google Scholar] [CrossRef] [PubMed]
- Chanu Thounaojam, T.; Thounaojam, T.M.; Upadhyaya, H. Chapter 16—Role of zinc oxide nanoparticles in mediating abiotic stress responses in plant. In Zinc-Based Nanostructures for Environmental and Agricultural Applications; Abd-Elsalam,, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 323–337. [Google Scholar] [CrossRef]
- Ma, C.; Borgatta, J.; Hudson, B.G.; Tamijani, A.A.; De La Torre-Roche, R.; Zuverza-Mena, N.; Shen, Y.; Elmer, W.; Xing, B.; Mason, S.E.; et al. Advanced material modulation of nutritional and phytohormone status alleviates damage from soybean sudden death syndrome. Nat. Nanotechnol. 2020, 15, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Radić, S.; Radić-Stojković, M.; Pevalek-Kozlina, B. Influence of NaCl and mannitol on peroxidase activity and lipid peroxidation in Centaurea ragusina L. roots and shoots. J. Plant Physiol. 2006, 163, 1284–1292. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-J.; Wang, C.-S.; Chen, Y.-H.; Toh, J.-T.; Zheng, Y.-F.; Hong, X.-G.; Lin, H.-Y.; Lai, C.-C. Rapid determination of isoflavones and other bioactive compounds in soybean using SWATH-MS. Anal. Chim. Acta 2020, 1103, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Babalola, O.O.; Glick, B.R. The use of microbial inoculants in African agriculture: Current practice and future prospects. J. Food Agric. Env. 2012, 10, 540–549. [Google Scholar]
- Nazir, A. Review on metagenomics and its applications. Imp. J. Intersd. Res. 2016, 2. [Google Scholar]
- Sharon, I.; Kertesz, M.; Hug, L.A.; Pushkarev, D.; Blauwkamp, T.A.; Castelle, C.J.; Amirebrahimi, M.; Thomas, B.C.; Burstein, D.; Tringe, S.G. Accurate, multi-kb reads resolve complex populations and detect rare microorganisms. Genome Res. 2015, 25, 534–543. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.F.K.; Istvan, P.; Quirino, B.F.; Kruger, R.H. Functional Metagenomics as a Tool for Identification of New Antibiotic Resistance Genes from Natural Environments. Microb. Ecol. 2017, 73, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, A.; Gupta, A.; Sar, P.; Maiti, M.K. Metagenomics of two gnotobiotically grown aromatic rice cultivars reveals genotype-dependent and tissue-specific colonization of endophytic bacterial communities attributing multiple plant growth promoting traits. World J. Microbiol. Biotechnol. 2021, 37, 59. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Kumar, V.; Suyal, D.C.; Jain, L.; Goel, R. Metagenomics of plant rhizosphere microbiome. In Understanding Host-Microbiome Interactions-An Omics Approach; Springer: Berlin/Heidelberg, Germany, 2017; pp. 193–205. [Google Scholar]
- Sugiyama, A.; Ueda, Y.; Zushi, T.; Takase, H.; Yazaki, K. Changes in the bacterial community of soybean rhizospheres during growth in the field. PloS ONE 2014, 9, e100709. [Google Scholar] [CrossRef] [PubMed]
- Lagos, L.; Maruyama, F.; Nannipieri, P.; Mora, M.; Ogram, A.; Jorquera, M. Current overview on the study of bacteria in the rhizosphere by modern molecular techniques: A mini—Review. J. Soil Sci. Plant Nutr. 2015, 15, 504–523. [Google Scholar]
- LeBlanc, N.; Kinkel, L.L.; Kistler, H.C. Soil Fungal Communities Respond to Grassland Plant Community Richness and Soil Edaphics. Microb. Ecol. 2015, 70, 188–195. [Google Scholar] [CrossRef]
- Spence, C.; Alff, E.; Johnson, C.; Ramos, C.; Donofrio, N.; Sundaresan, V.; Bais, H. Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol. 2014, 14, 130. [Google Scholar] [CrossRef]
- Singh, B.K.; Millard, P.; Whiteley, A.S.; Murrell, J.C. Unravelling rhizosphere–microbial interactions: Opportunities and limitations. Trends Microbiol. 2004, 12, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, P.R.; Mauchline, T.H.; Clark, I.M. Culture-independent molecular techniques for soil microbial ecology. Soil Biol. Biochem. 2010, 42, 878–887. [Google Scholar] [CrossRef]
- Marsh, T.L. Terminal restriction fragment length polymorphism (T-RFLP): An emerging method for characterizing diversity among homologous populations of amplification products. Curr. Opin. Microbiol. 1999, 2, 323–327. [Google Scholar] [CrossRef]
- Emoto, T.; Yamashita, T.; Kobayashi, T.; Sasaki, N.; Hirota, Y.; Hayashi, T.; So, A.; Kasahara, K.; Yodoi, K.; Matsumoto, T.; et al. Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: Gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessel. 2017, 32, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Fujii, T. A new approach to retrieve full lengths of functional genes from soil by PCR-DGGE and metagenome walking. Appl. Microbiol. Biotechnol. 2009, 83, 389–396. [Google Scholar] [CrossRef]
- Hong, S.; Bunge, J.; Leslin, C.; Jeon, S.; Epstein, S.S. Polymerase chain reaction primers miss half of rRNA microbial diversity. ISME J. 2009, 3, 1365–1373. [Google Scholar] [CrossRef]
- Isenbarger, T.A.; Carr, C.E.; Johnson, S.S.; Finney, M.; Church, G.M.; Gilbert, W.; Zuber, M.T.; Ruvkun, G. The Most Conserved Genome Segments for Life Detection on Earth and Other Planets. Orig. Life Evol. Biosph. 2008, 38, 517–533. [Google Scholar] [CrossRef]
- Keegan, K.P.; Glass, E.M.; Meyer, F. MG-RAST, a Metagenomics Service for Analysis of Microbial Community Structure and Function. In Microbial Environmental Genomics (MEG); Martin, F., Uroz, S., Eds.; Springer: New York, NY, USA, 2016; pp. 207–233. [Google Scholar]
- Angiuoli, S.V.; Matalka, M.; Gussman, A.; Galens, K.; Vangala, M.; Riley, D.R.; Arze, C.; White, J.R.; White, O.; Fricke, W.F. CloVR: A virtual machine for automated and portable sequence analysis from the desktop using cloud computing. BMC Bioinform. 2011, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.R.; Harper, S.L.; Johnson, M.G.; Andersen, C.P.; Reichman, J.R. CeO2 nanoparticles affect soybeans and their root-associated microbiome at low, environmentally relevant concentrations. A Nano-Sized Dose Toxicol. Elucidating Disconnect Between Nanomater. Dosim. Biol. Effects. 2019, 53. [Google Scholar]
- Dai, Y.; Chen, F.; Yue, L.; Li, T.; Jiang, Z.; Xu, Z.; Wang, Z.; Xing, B. Uptake, Transport, and Transformation of CeO2 Nanoparticles by Strawberry and Their Impact on the Rhizosphere Bacterial Community. ACS Sustain. Chem. Eng. 2020, 8, 4792–4800. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajiboye, T.T.; Ajiboye, T.O.; Babalola, O.O. Impacts of Binary Oxide Nanoparticles on the Soybean Plant and Its Rhizosphere, Associated Phytohormones, and Enzymes. Molecules 2023, 28, 1326. https://doi.org/10.3390/molecules28031326
Ajiboye TT, Ajiboye TO, Babalola OO. Impacts of Binary Oxide Nanoparticles on the Soybean Plant and Its Rhizosphere, Associated Phytohormones, and Enzymes. Molecules. 2023; 28(3):1326. https://doi.org/10.3390/molecules28031326
Chicago/Turabian StyleAjiboye, Titilope Tinu, Timothy Oladiran Ajiboye, and Olubukola Oluranti Babalola. 2023. "Impacts of Binary Oxide Nanoparticles on the Soybean Plant and Its Rhizosphere, Associated Phytohormones, and Enzymes" Molecules 28, no. 3: 1326. https://doi.org/10.3390/molecules28031326
APA StyleAjiboye, T. T., Ajiboye, T. O., & Babalola, O. O. (2023). Impacts of Binary Oxide Nanoparticles on the Soybean Plant and Its Rhizosphere, Associated Phytohormones, and Enzymes. Molecules, 28(3), 1326. https://doi.org/10.3390/molecules28031326








