Inclusions of Pesticides by β-Cyclodextrin in Solution and Solid State: Chlorpropham, Monuron, and Propanil
Abstract
1. Introduction
2. Results
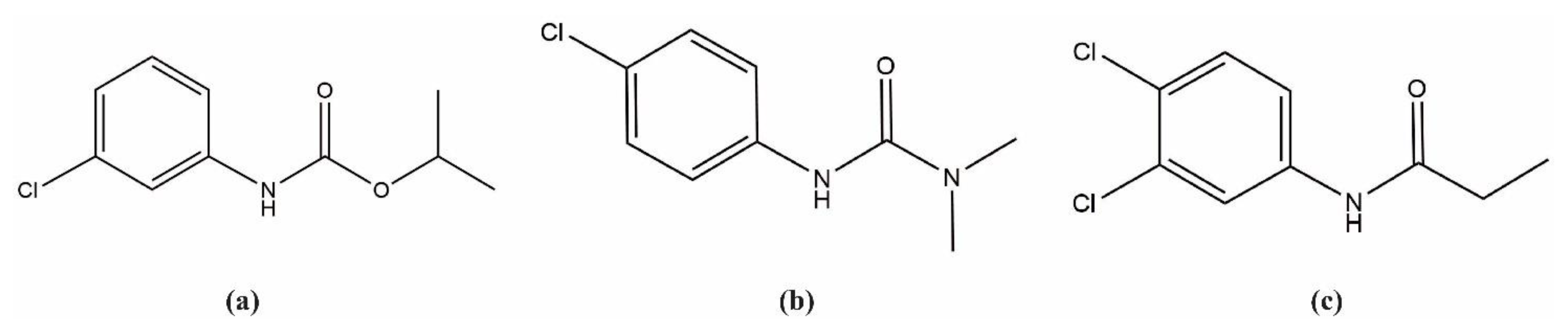
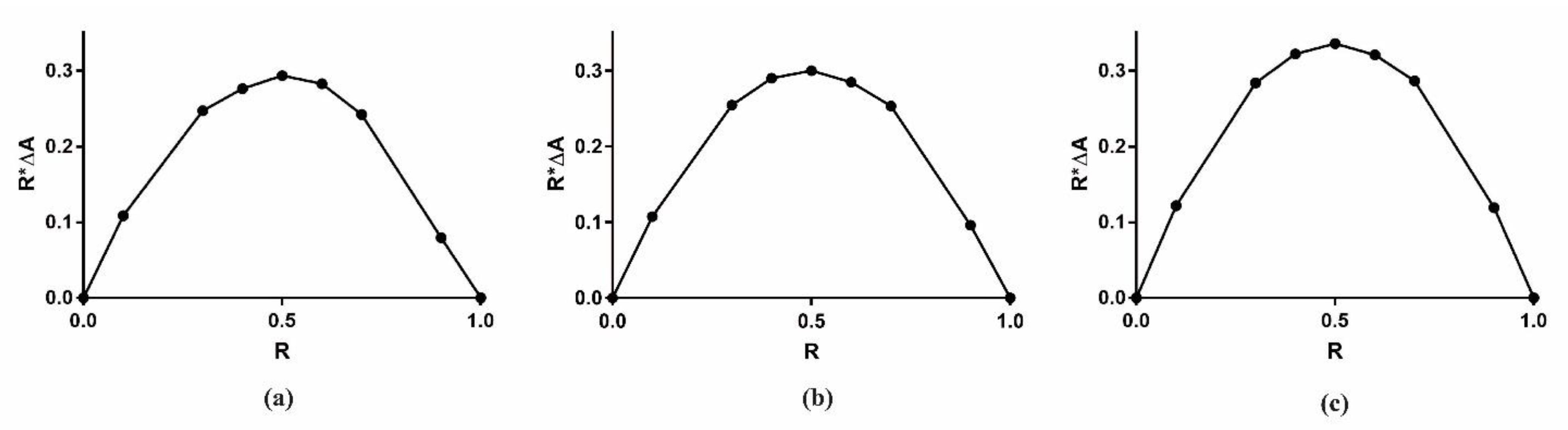
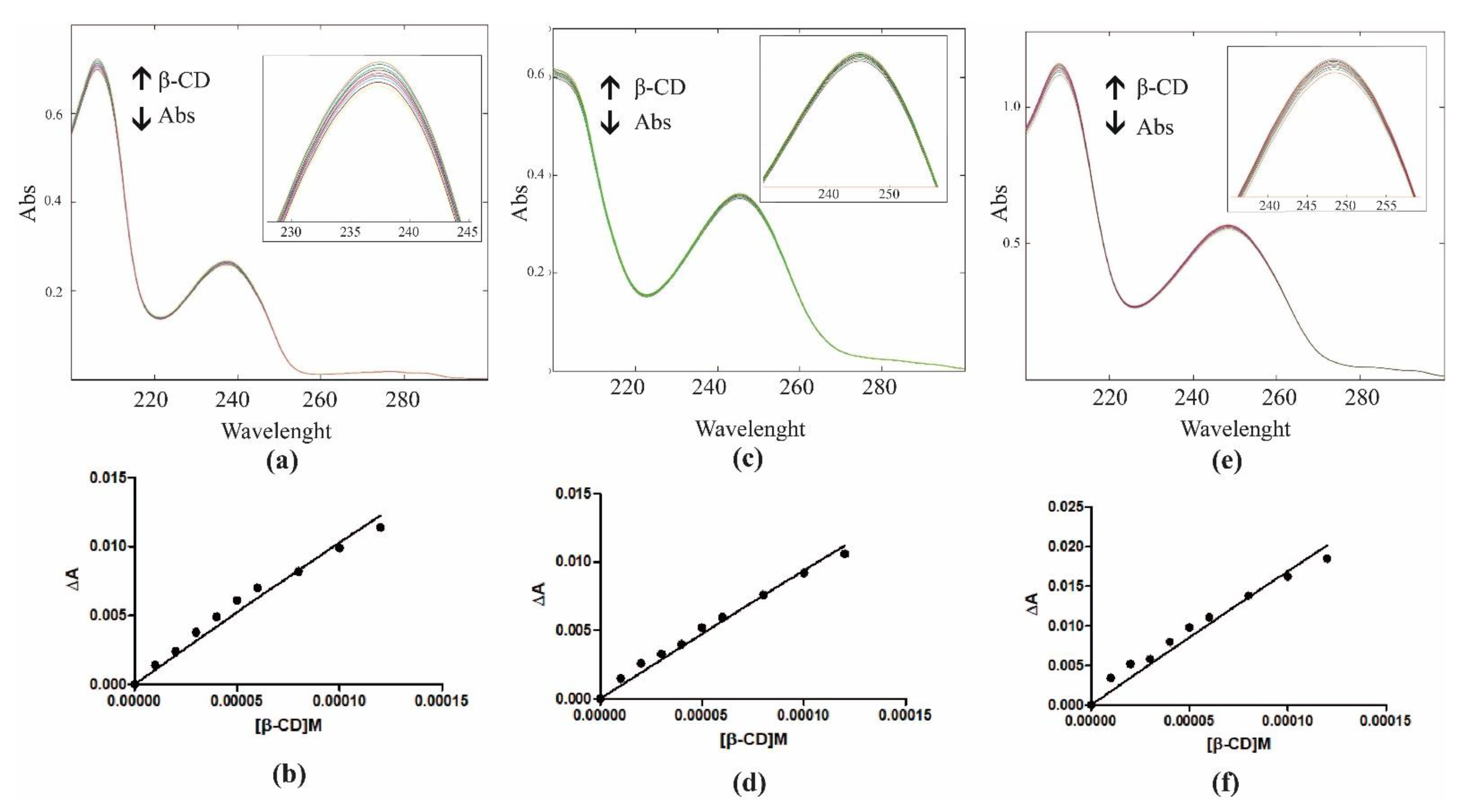
2.1. Characterization of Inclusion Complexes in Solution
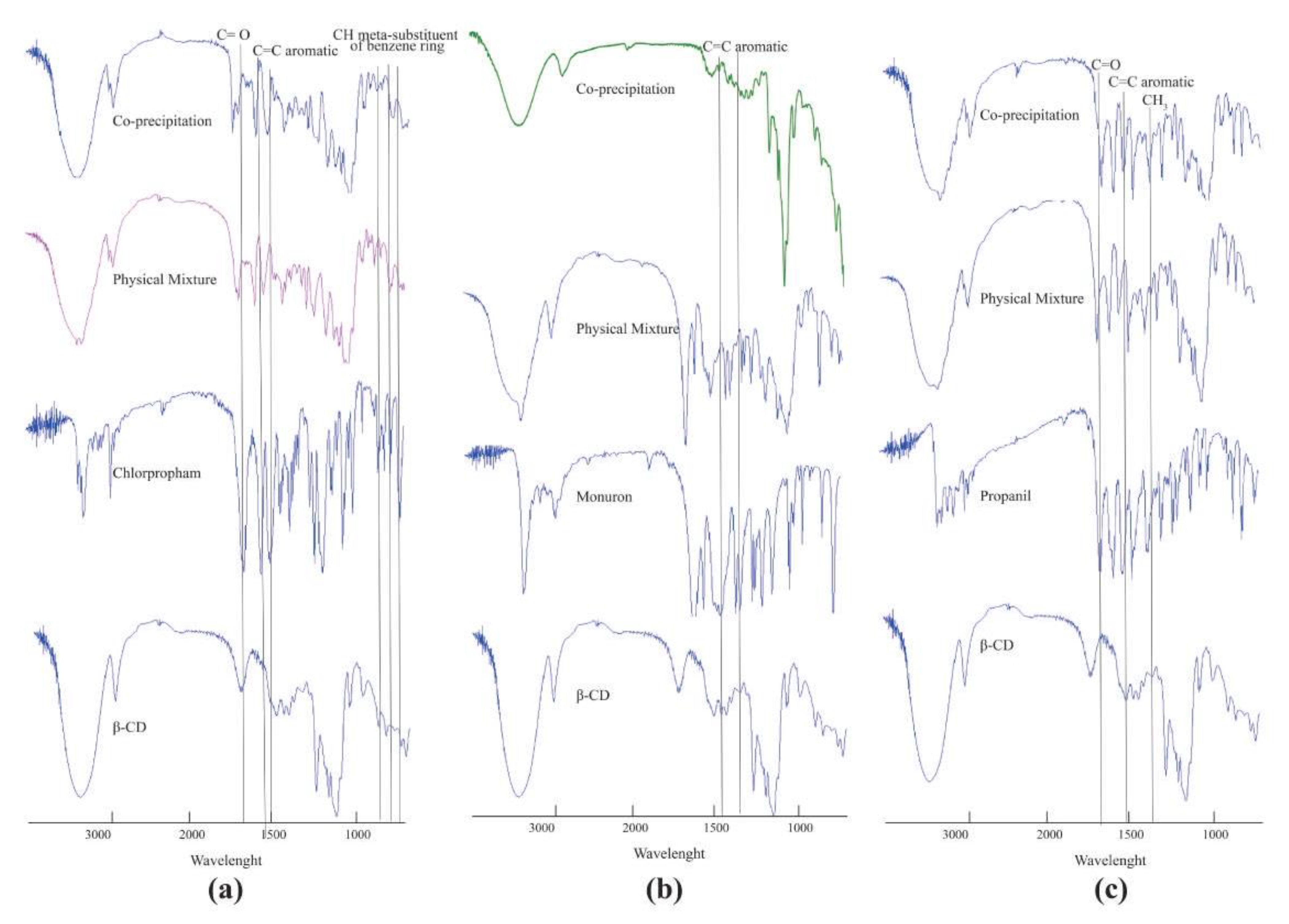
2.2. Characterization of Inclusion Complexes in Solid State FT-IR Spectroscopy
2.2.1. Chlorpropham: β-CD
2.2.2. Monuron: β-CD
2.2.3. Propanil: β-CD
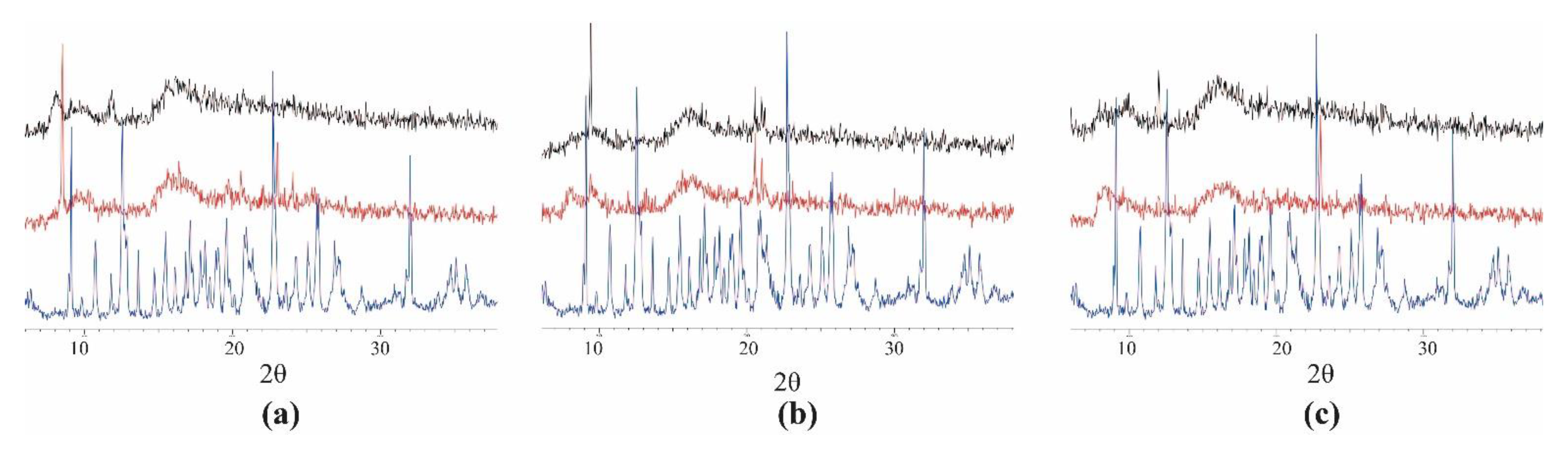
2.3. Powder X-ray Diffraction
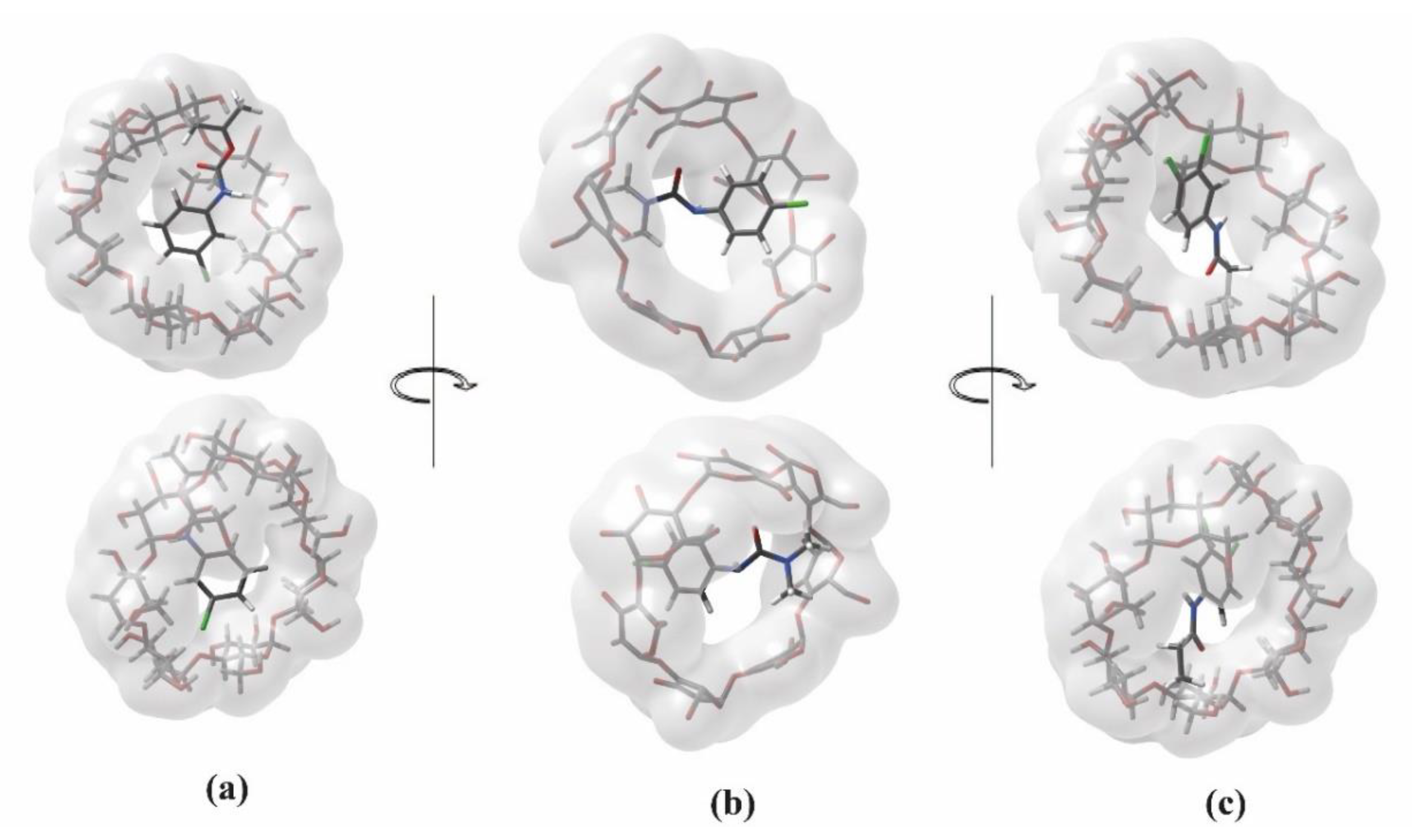
2.4. Molecular Docking
3. Materials and Methods
3.1. Determination of Binding Constants by UV-Vis Spectroscopy
3.2. Stoichiometry Determination Using Job Plot Method
3.3. Preparation of Inclusion Complexes Pesticides: β-CD in Solid State
3.4. X-ray Powder Diffraction (XRD)
3.5. Fourier Transform Infrared (FT-IR) Spectroscopy
3.6. Molecular Docking Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Khan, M.S.; Rahman, M.S. Pesticide Residue in Foods; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, A.M. A review of environmental and health effects of organochlorine pesticide residues in Africa. Chemosphere 2019, 220, 1126–1140. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, M.I.; Sajjad, A.; Shakeel, Q.; Hussain, A. Environmental and health effects of pesticide residues. In Sustainable Agriculture Reviews 48: Pesticide Occurrence, Analysis and Remediation, Vol. 2: Analysis; Springer: Berlin/Heidelberg, Germany, 2021; pp. 311–336. [Google Scholar]
- Debnath, M.; Khan, M.S. Health Concerns of Pesticides. In Pesticide Residue in Foods; Springer: Berlin/Heidelberg, Germany, 2017; pp. 103–118. [Google Scholar]
- Tudi, M.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; Phung, D.T.; et al. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics 2022, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Collotta, M.; Bertazzi, P.A.; Bollati, V. Epigenetics and pesticides. Toxicology 2013, 307, 35–41. [Google Scholar] [CrossRef]
- Shah, H.K.; Sharma, T.; Banerjee, B.D. Organochlorine pesticides induce inflammation, ROS production, and DNA damage in human epithelial ovary cells: An in vitro study. Chemosphere 2020, 246, 125691. [Google Scholar] [CrossRef]
- Sebastian, R.; Raghavan, S.C. Induction of DNA damage and erroneous repair can explain genomic instability caused by endosulfan. Carcinogenesis 2016, 37, 929–940. [Google Scholar] [CrossRef]
- Costa, M.B.; Farias, I.R.; da Silva Monte, C.; Filho, L.I.P.F.; de Paula Borges, D.; de Oliveira, R.T.G.; Ribeiro-Junior, H.L.; Magalhães, S.M.M.; Pinheiro, R.F. Chromosomal abnormalities and dysregulated DNA repair gene expression in farmers exposed to pesticides. Environ. Toxicol. Pharmacol. 2021, 82, 103564. [Google Scholar] [CrossRef]
- Ataei, M.; Abdollahi, M. A systematic review of mechanistic studies on the relationship between pesticide exposure and cancer induction. Toxicol. Appl. Pharmacol. 2022, 456, 116280. [Google Scholar] [CrossRef]
- Islam, M.S.; Azim, F.; Saju, H.; Zargaran, A.; Shirzad, M.; Kamal, M.; Fatema, K.; Rehman, S.; Azad, M.A.M.; Ebrahimi-Barough, S. Pesticides and Parkinson’s disease: Current and future perspective. J. Chem. Neuroanat. 2021, 115, 101966. [Google Scholar] [CrossRef]
- He, X.; Tu, Y.; Song, Y.; Yang, G.; You, M. The relationship between pesticide exposure during critical neurodevelopment and autism spectrum disorder: A narrative review. Environ. Res. 2022, 203, 111902. [Google Scholar] [CrossRef]
- Salazar Mercado, S.A.; Quintero Caleño, J.D.; Rojas Suárez, J.P. Cytogenotoxic effect of propanil using the Lens culinaris Med and Allium cepa L test. Chemosphere 2020, 249, 126193. [Google Scholar] [CrossRef]
- Rittilert, P.; Sriapha, C.; Tongpoo, A.; Pradoo, A.-O.; Wananukul, W.; Satariya, T. Clinical characteristics, treatment and outcomes of acute propanil poisoning in a 7-year retrospective cohort study. Toxicol. Rep. 2022, 9, 1180–1188. [Google Scholar] [CrossRef]
- Ellis, J.K.; Athersuch, T.J.; Cavill, R.; Radford, R.; Slattery, C.; Jennings, P.; McMorrow, T.; Ryan, M.P.; Ebbels, T.M.; Keun, H.C. Metabolic response to low-level toxicant exposure in a novel renal tubule epithelial cell system. Mol. Biosyst. 2011, 7, 247–257. [Google Scholar] [CrossRef]
- Bloch, K.M.; Yaqoob, N.; Sharma, S.; Evans, A.; Aschauer, L.; Radford, R.; Jennings, P.; Ryan, M.P.; van Delft, J.H.M.; Lock, E.A. Transcriptomic alterations induced by Monuron in rat and human renal proximal tubule cells in vitro and comparison to rat renal-cortex in vivo. Toxicol. Res. 2015, 4, 423–431. [Google Scholar] [CrossRef]
- Sepp, K.; Molnár, Z.; László, A.M.; Alapi, T.; Tóth, L.; Serester, A.; Valkusz, Z.; Gálfi, M.; Radács, M. Study of the Potential Endocrine-Disrupting Effects of Phenylurea Compounds on Neurohypophysis Cells. Int. J. Endocrinol. 2019, 2019, 1546131. [Google Scholar] [CrossRef]
- Tanaka, T. Reproductive and neurobehavioral effects of chlorpropham administered to mice in the diet. Toxicol. Ind. Health 1997, 13, 715–726. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakajima, K.; Suzuki, T. Chlorpropham induces mitochondrial dysfunction in rat hepatocytes. Toxicology 2004, 200, 123–133. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Tao, M. Binding properties of herbicide chlorpropham to DNA: Spectroscopic, chemometrics and modeling investigations. J. Photochem. Photobiol. B 2014, 138, 109–117. [Google Scholar] [CrossRef]
- Landy, D.; Mallard, I.; Ponchel, A.; Monflier, E.; Fourmentin, S. Remediation technologies using cyclodextrins: An overview. Environ. Chem. Lett. 2012, 10, 225–237. [Google Scholar] [CrossRef]
- Morillo, E.; Villaverde, J. Advanced technologies for the remediation of pesticide-contaminated soils. Sci. Total Environ. 2017, 586, 576–597. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, R.J.; Nshime, B.; Delamarre, M.; Dejong, S.; Scott, P.; Lantz, A.W. Cyclodextrins as complexation and extraction agents for pesticides from contaminated soil. Chemosphere 2013, 91, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Waris, K.H.; Lee, V.S.; Mohamad, S. Pesticide remediation with cyclodextrins: A review. Environ. Sci. Pollut. Res. Int. 2021, 28, 47785–47799. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, S.; Kamari, A.; Aljafree, N. A review of materials used as carrier agents in pesticide formulations. Int. J. Environ. Sci. Technol. (Tehran) 2016, 13, 2977–2994. [Google Scholar] [CrossRef]
- Martin, J.; Díaz-Montaña, E.J.; Asuero, A.G. Cyclodextrins: Past and Present. In Cyclodextrin; Arora, P., Dhingra, N., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Iacovino, R.; Caso, J.V.; Rapuano, F.; Russo, A.; Isidori, M.; Lavorgna, M.; Malgieri, G.; Isernia, C. Physicochemical characterization and cytotoxic activity evaluation of hydroxymethylferrocene:β-cyclodextrin inclusion complex. Molecules 2012, 17, 6056–6070. [Google Scholar] [CrossRef]
- Iacovino, R.; Rapuano, F.; Caso, J.V.; Russo, A.; Lavorgna, M.; Russo, C.; Isidori, M.; Russo, L.; Malgieri, G.; Isernia, C. β-Cyclodextrin inclusion complex to improve physicochemical properties of pipemidic acid: Characterization and bioactivity evaluation. Int. J. Mol. Sci. 2013, 14, 13022–13041. [Google Scholar] [CrossRef]
- Lavorgna, M.; Iacovino, R.; Russo, C.; di Donato, C.; Piscitelli, C.; Isidori, M. A New Approach for Improving the Antibacterial and Tumor Cytotoxic Activities of Pipemidic Acid by Including It in Trimethyl-β-cyclodextrin. Int. J. Mol. Sci. 2019, 20, 416. [Google Scholar] [CrossRef]
- Bezerra, F.M.; Lis, M.J.; Firmino, H.B.; Dias da Silva, J.G.; Curto Valle, R.d.C.S.; Borges Valle, J.A.; Scacchetti, F.A.P.; Tessaro, A.L. The role of β-cyclodextrin in the textile industry. Molecules 2020, 25, 3624. [Google Scholar] [CrossRef]
- Iacovino, R.; Caso, J.V.; di Donato, C.; Malgieri, G.; Palmieri, M.; Russo, L.; Isernia, C. Cyclodextrins as complexing agents: Preparation and applications. Curr. Org. Chem. 2017, 21, 162–176. [Google Scholar] [CrossRef]
- Gao, S.; Feng, W.; Sun, H.; Zong, L.; Li, X.; Zhao, L.; Ye, F.; Fu, Y. Fabrication and Characterization of Antifungal Hydroxypropyl-β-Cyclodextrin/Pyrimethanil Inclusion Compound Nanofibers Based on Electrospinning. J. Agric. Food Chem. 2022, 70, 7911–7920. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Yang, G.; Fang, W.; Zong, L.; Zhao, L.; Ye, F.; Fu, Y. Antibacterial perillaldehyde/hydroxypropyl-γ-cyclodextrin inclusion complex electrospun polymer-free nanofiber: Improved water solubility, thermostability, and antioxidant activity. Ind. Crops Prod. 2022, 176, 114300. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, J.; Li, X.; Ye, F.; Fu, Y.; Zhao, L. Electrospun Polymer-Free Nanofibers Incorporating Hydroxypropyl-β-cyclodextrin/Difenoconazole via Supramolecular Assembly for Antifungal Activity. J. Agric. Food Chem. 2021, 69, 5871–5881. [Google Scholar] [CrossRef]
- Yadav, M.; Thakore, S.; Jadeja, R. A review on remediation technologies using functionalized Cyclodextrin. Environ. Sci. Pollut. Res. 2021, 1–15. [Google Scholar] [CrossRef]
- Kavitha, K.; Srinivasa Rao, A.; Nalini, C.N. An investigation on enhancement of solubility of 5 fluorouracil by applying complexation technique-characterization, dissolution and molecular-modeling studies. J. Appl. Pharm. Sci. 2013, 3, 162–166. [Google Scholar] [CrossRef]
- Huang, C.Y. Determination of binding stoichiometry by the continuous variation method: The Job plot. Methods Enzymol. 1982, 87, 509–525. [Google Scholar] [CrossRef]
- Negi, J.S.; Singh, S. Spectroscopic investigation on the inclusion complex formation between amisulpride and γ-cyclodextrin. Carbohydr. Polym. 2013, 92, 1835–1843. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Perkins, G.; Hirota, K. Targeting cholesterol with β-cyclodextrin sensitizes cancer cells for apoptosis. FEBS Lett. 2015, 589, 4097–4105. [Google Scholar] [CrossRef]
- Loukas, Y.L.; Vraka, V.; Gregoriadis, G. Use of a nonlinear least-squares model for the kinetic determination of the stability constant of cyclodextrin inclusion complexes. Int. J. Pharm. 1996, 144, 225–231. [Google Scholar] [CrossRef]
- Caso, J.V.; Russo, L.; Palmieri, M.; Malgieri, G.; Galdiero, S.; Falanga, A.; Isernia, C.; Iacovino, R. Investigating the inclusion properties of aromatic amino acids complexing beta-cyclodextrins in model peptides. Amino Acids 2015, 47, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, L.; Mu, T.-W.; Guo, Q.-X. The Performance of the Benesi-Hildebrand Method in Measuring the Binding Constants of the Cyclodextrin Complexation. Anal. Sci. 2000, 16, 537–539. [Google Scholar] [CrossRef]
- Miller, L.A.; Carrier, R.L.; Ahmed, I. Practical considerations in development of solid dosage forms that contain cyclodextrin. J. Pharm. Sci. 2007, 96, 1691–1707. [Google Scholar] [CrossRef]
- Kurozumi, M.; Nambu, N.; Nagai, T. Inclusion compounds of non-steroidal antiinflammatory and other slightly water soluble drugs with alpha- and beta-cyclodextrins in powdered form. Chem. Pharm. Bull. (Tokyo) 1975, 23, 3062–3068. [Google Scholar] [CrossRef]
- Huang, Z.; Tian, S.; Ge, X.; Zhang, J.; Li, S.; Li, M.; Cheng, J.; Zheng, H. Complexation of chlorpropham with hydroxypropyl-β-cyclodextrin and its application in potato sprout inhibition. Carbohydr. Polym. 2014, 107, 241–246. [Google Scholar] [CrossRef]
- Ge, X.; He, J.; Qi, F.; Yang, Y.; Huang, Z.; Lu, R.; Huang, L. Inclusion complexation of chloropropham with β-cyclodextrin: Preparation, characterization and molecular modeling. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 81, 397–403. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Ghoorah, A.W.; Devignes, M.D.; Smaïl-Tabbone, M.; Ritchie, D.W. Protein docking using case-based reasoning. Proteins Struct. Funct. Genet. 2013, 81, 2150–2158. [Google Scholar] [CrossRef]
- Di Donato, C.; Lavorgna, M.; Fattorusso, R.; Isernia, C.; Isidori, M.; Malgieri, G.; Piscitelli, C.; Russo, C.; Russo, L.; Iacovino, R. Alpha- and Beta-Cyclodextrin Inclusion Complexes with 5-Fluorouracil: Characterization and Cytotoxic Activity Evaluation. Molecules 2016, 21, 1644. [Google Scholar] [CrossRef]
- Delogu, G.; Fois, X.; Mannu, R.; Pantaleoni, R.A. Enhancing insecticide activity using a physical mixture with cyclodextrin: A witch’s cauldron or an opportunity? J. Pest Sci. 2019, 92, 943–950. [Google Scholar] [CrossRef]
- Smith, V.J.; Bogdan, D.; Caira, M.R.; Bogdan, M.; Bourne, S.A.; Fărcaş, S.I. Cyclodextrin inclusion of four phenylurea herbicides: Determination of complex stoichiometries and stability constants using solution 1H NMR spectroscopy. Supramol. Chem. 2010, 22, 172–177. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Numanoğlu, U.; Sen, T.; Tarimci, N.; Kartal, M.; Koo, O.M.; Onyüksel, H. Use of cyclodextrins as a cosmetic delivery system for fragrance materials: Linalool and benzyl acetate. AAPS PharmSciTech 2007, 8, 34–42. [Google Scholar] [CrossRef]
- Gonzalez Pereira, A.; Carpena, M.; García Oliveira, P.; Mejuto, J.C.; Prieto, M.A.; Simal Gandara, J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host-Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef]
| Chlorpropham | Monuron | Propanil |
|---|---|---|
| Kb = 369.9 M−1 | Kb = 292.3 M−1 | Kb = 298.3 M−1 |
| R2 = 0.9737 | R2 = 0.9841 | R2 = 0.9580 |
| Chlorpropham | Chlorpropham: β-CD | Monuron | Monuron: β-CD | Propanil | Propanil: β-CD | |
|---|---|---|---|---|---|---|
| C=O | 1702.01 nm | 1735.75 nm | 1670.29 nm | 1667.98 nm | ||
| C=C aromatic | 1594.77 nm 1542.4 nm | 1596.07 nm 1523.42 nm | 1589.06 nm 1514.81 nm | 1587.13 nm 1516.74 nm | 1590.54 nm 1534.95 nm | 1593.23 nm 1535.62 nm |
| C–Cl | 773.68 nm | 761.11 nm | 814.84 nm | 816.05 nm | ||
| Metil group | 1397.17 nm | 1403.92 nm | 1474.98 nm | 1475.27 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragone, M.; Shitaye, G.; D’Abrosca, G.; Russo, L.; Fattorusso, R.; Isernia, C.; Malgieri, G.; Iacovino, R. Inclusions of Pesticides by β-Cyclodextrin in Solution and Solid State: Chlorpropham, Monuron, and Propanil. Molecules 2023, 28, 1331. https://doi.org/10.3390/molecules28031331
Dragone M, Shitaye G, D’Abrosca G, Russo L, Fattorusso R, Isernia C, Malgieri G, Iacovino R. Inclusions of Pesticides by β-Cyclodextrin in Solution and Solid State: Chlorpropham, Monuron, and Propanil. Molecules. 2023; 28(3):1331. https://doi.org/10.3390/molecules28031331
Chicago/Turabian StyleDragone, Martina, Getasew Shitaye, Gianluca D’Abrosca, Luigi Russo, Roberto Fattorusso, Carla Isernia, Gaetano Malgieri, and Rosa Iacovino. 2023. "Inclusions of Pesticides by β-Cyclodextrin in Solution and Solid State: Chlorpropham, Monuron, and Propanil" Molecules 28, no. 3: 1331. https://doi.org/10.3390/molecules28031331
APA StyleDragone, M., Shitaye, G., D’Abrosca, G., Russo, L., Fattorusso, R., Isernia, C., Malgieri, G., & Iacovino, R. (2023). Inclusions of Pesticides by β-Cyclodextrin in Solution and Solid State: Chlorpropham, Monuron, and Propanil. Molecules, 28(3), 1331. https://doi.org/10.3390/molecules28031331









