Brain Targeting by Intranasal Drug Delivery: Effect of Different Formulations of the Biflavone “Cupressuflavone” from Juniperus sabina L. on the Motor Activity of Rats
Abstract
1. Introduction
2. Results and Discussion
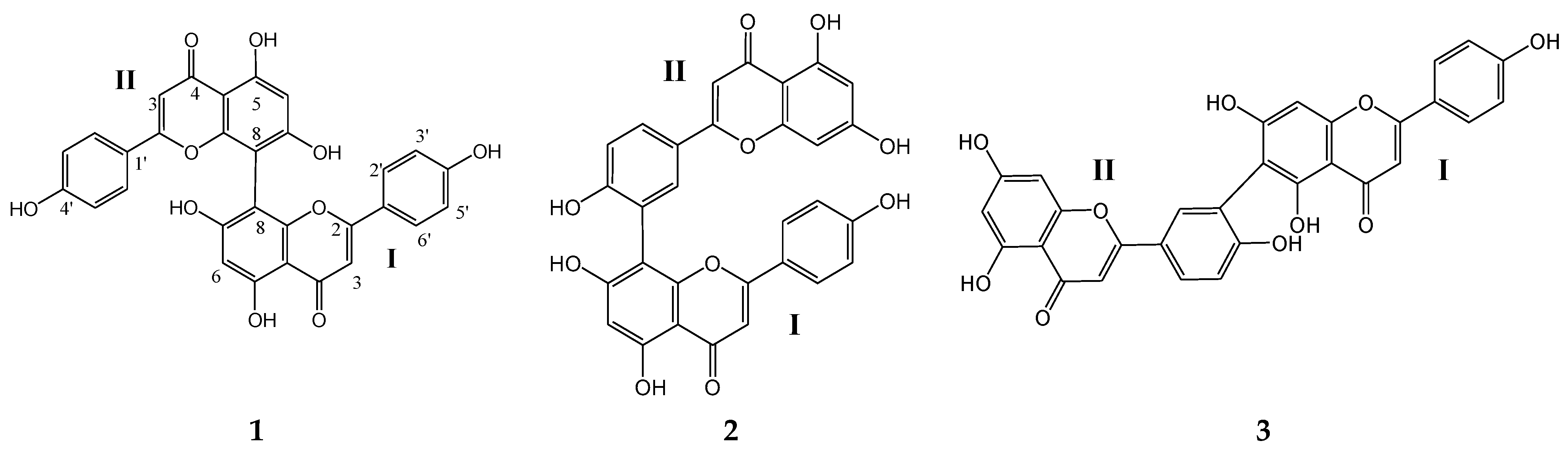
2.1. Characterization of the Isolated Compounds
2.2. Excipients Selection and Formulation Optimization
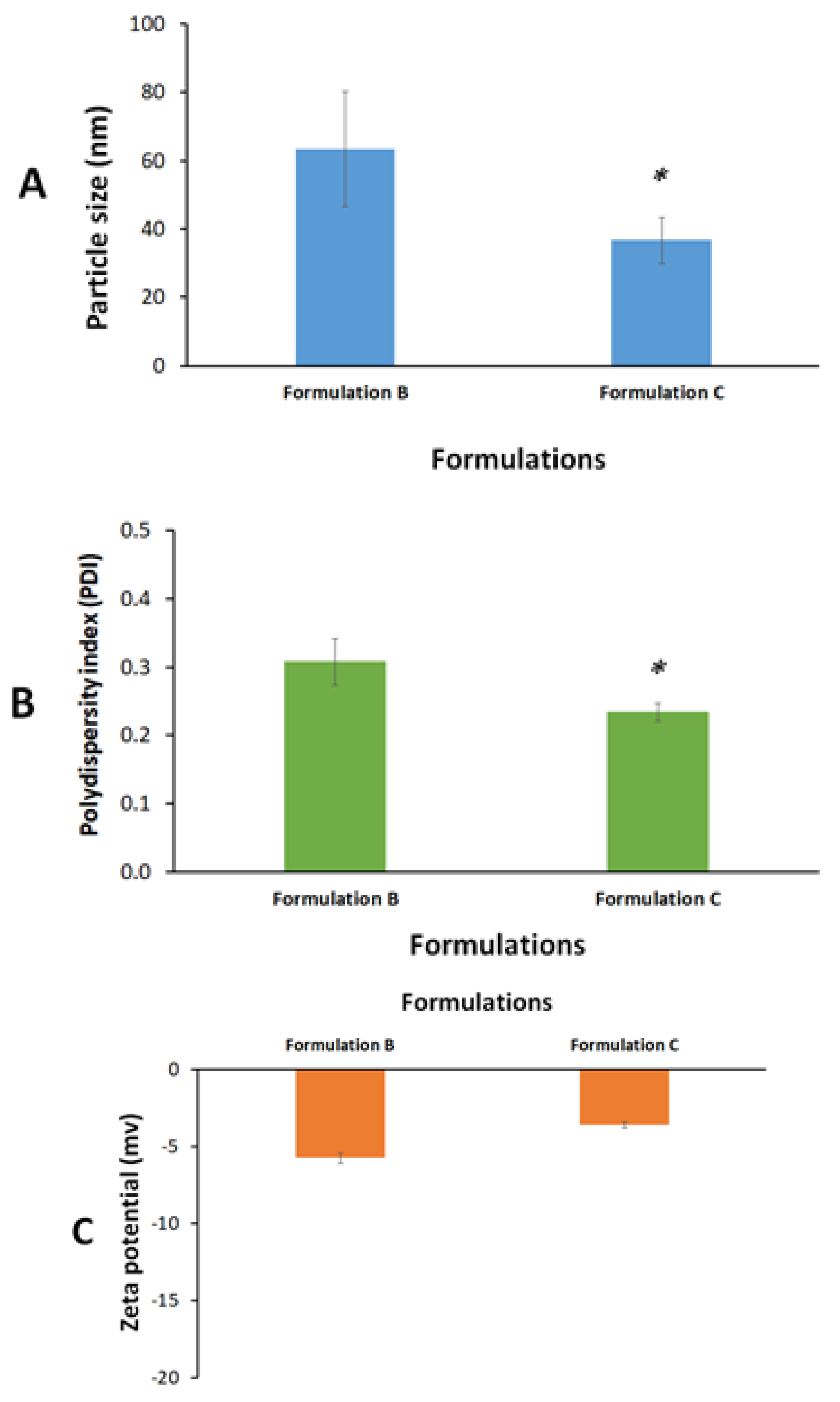
2.3. Formulation Characterization
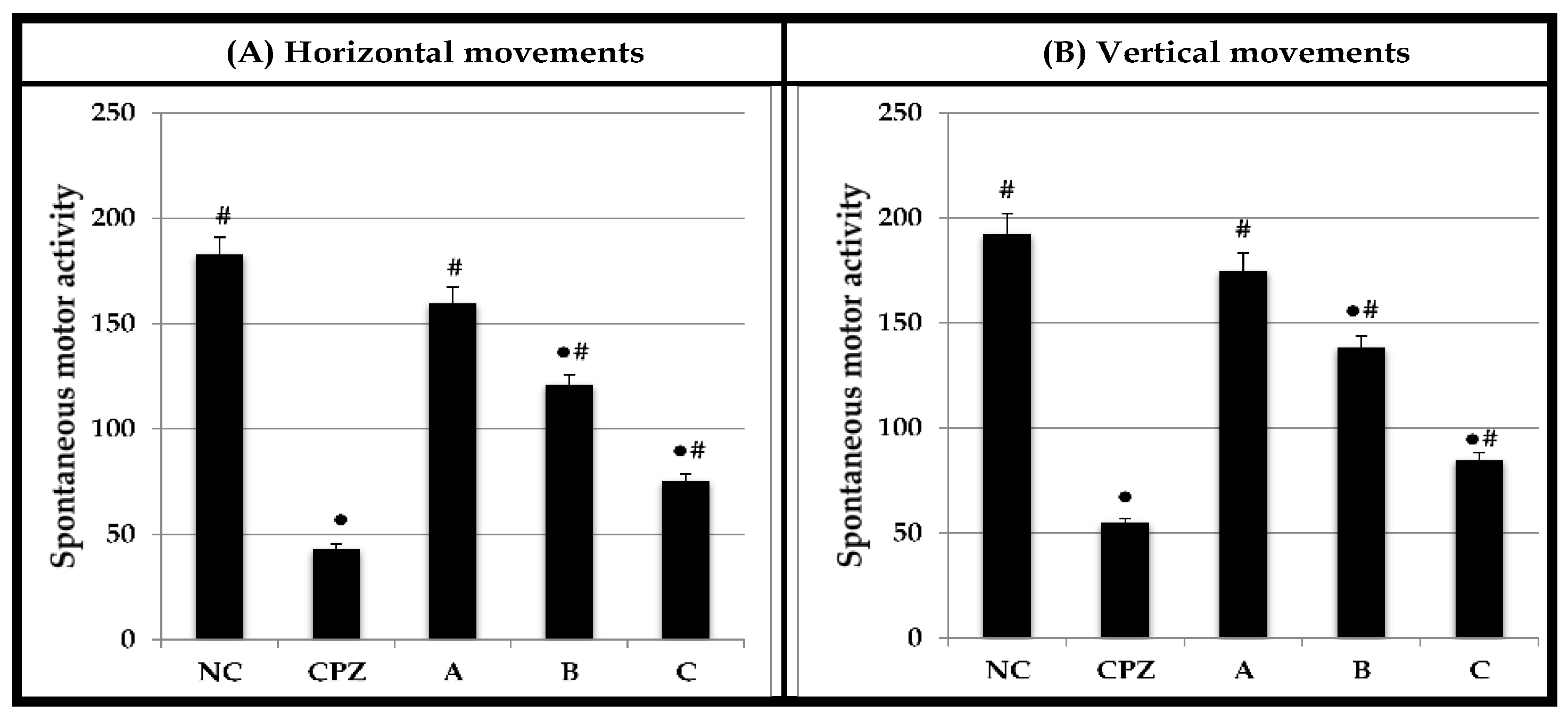
2.4. Effect on Spontaneous Motor Activity
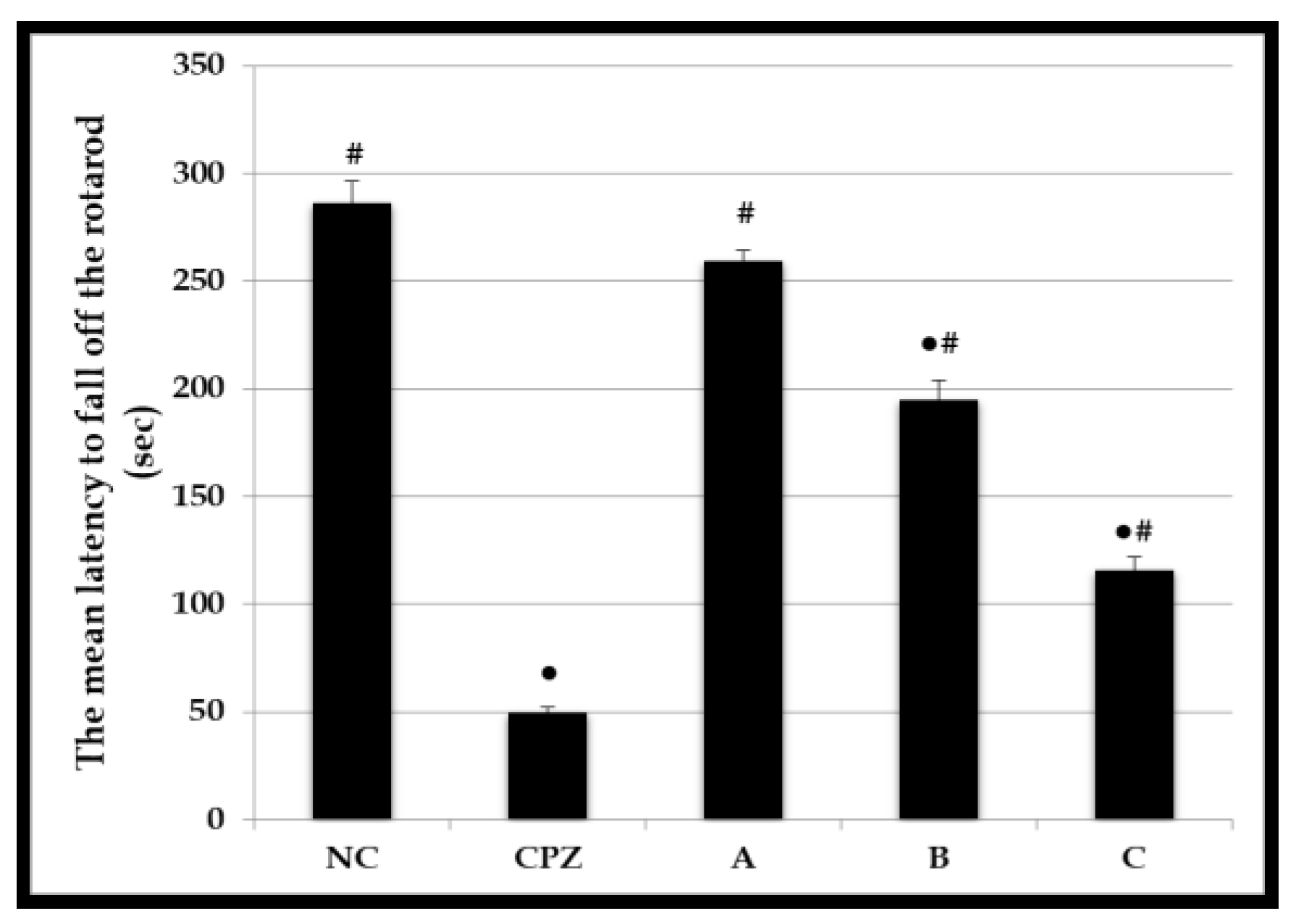
2.5. Effect on Motor Coordination and Balance
2.6. Systemic Absorption of CPF after Intranasal Administration
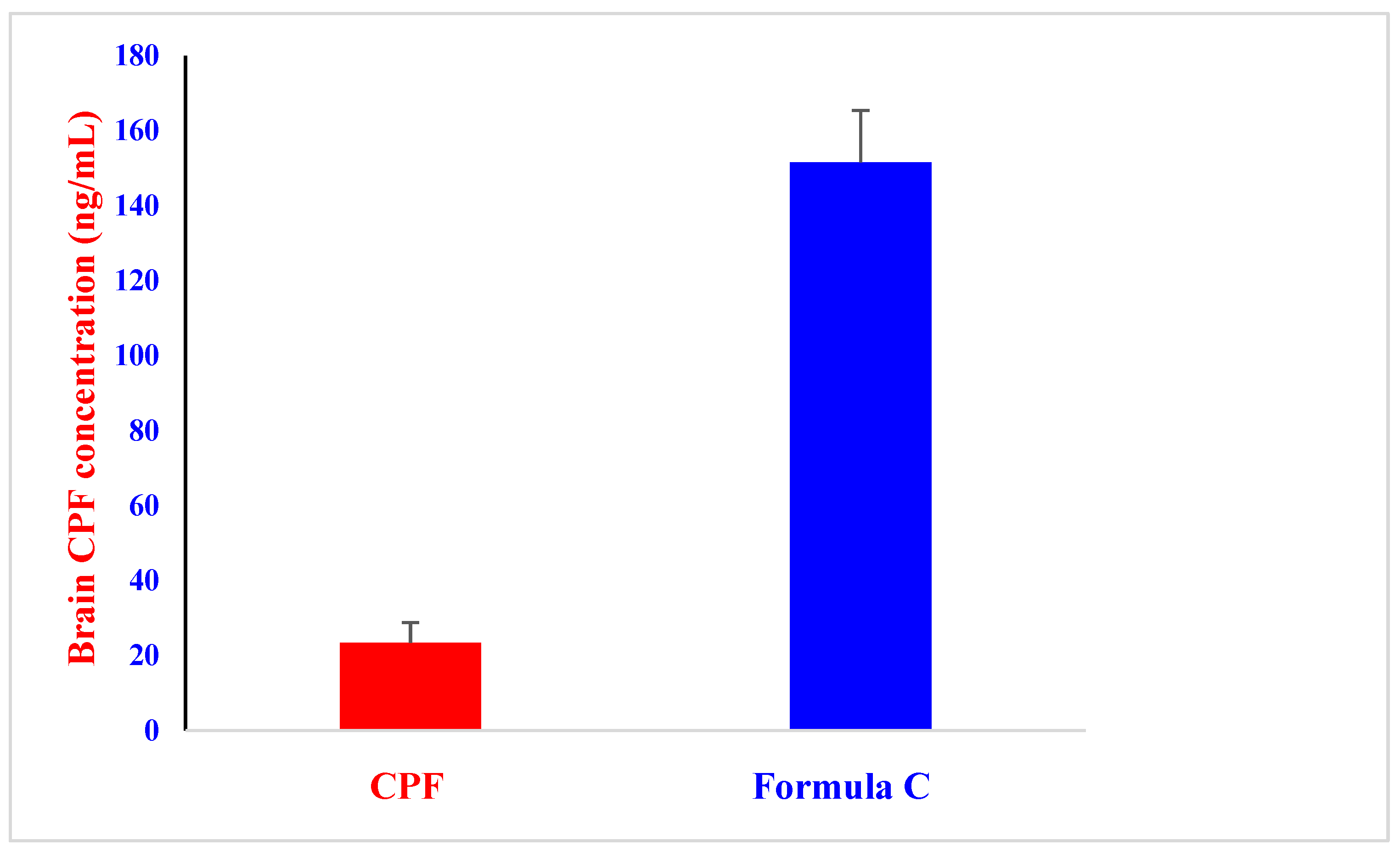
2.7. In Vivo Brain Distribution of CPF after an Intranasal Administration
3. Materials and Methods
3.1. General
3.2. Plant Materials
3.3. Extraction, Fractionation and Purification
3.4. Characterization of the Isolated Compounds
3.5. Estimation of Apparent Drug Solubility
3.6. Preparation of CPF Formulations for Intranasal Delivery
3.6.1. Drug Solution
3.6.2. Microwave Irradiation Solid Dispersion (MW-SD)
3.6.3. Solid-Dispersion Loaded Self-Nano Emulsifying Drug Delivery System (SNEDDS)
3.7. Formulation Characterization
Particle Size, Polydispersity Index (PDI), and Zeta Potential
3.8. Experimental Animals
3.9. Intranasal Delivery of Cupressuflavone Formulations to Rats
3.10. Assessment of Spontaneous Motor Activity Using Activity Cage
3.11. Assessment of Motor Coordination and Balance Using Accelerating Rotarod
3.12. In Vivo CPF Pharmacokinetic and Biodistribution Study
3.13. LC-MS/MS
3.14. Pharmacokinetic Parameters
3.15. Data and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- World Health Organization. Neurological Disorders Affect Millions Globally: WHO Report. Available online: https://www.who.int/news/item/27-02-2007-neurological-disorders-affect-millions-globally-who-report (accessed on 2 May 2022).
- Pandey, M.; Jain, N.; Kanoujia, J.; Hussain, Z.; Gorain, B. Advances and Challenges in Intranasal Delivery of Antipsychotic Agents Targeting the Central Nervous System. Front. Pharmacol. 2022, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Smith, Q.R.; Liu, X. Brain penetrating peptides and peptide–drug conjugates to overcome the blood–brain barrier and target CNS diseases. Rev. Nanomed. Nanobiotech. 2021, 13, e1695. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Chiu, Y.H.; Li, Y.S.; Lin, E.Y.; Hsieh, D.K.; Lee, C.H.; Huang, M.H.; Chuang, H.M.; Lin, S.Z.; Harn, H.J.; et al. Integration of PEG 400 into a self-nanoemulsifying drug delivery system improves drug loading capacity and nasal mucosa permeability and prolongs the survival of rats with malignant brain tumors. Int. J. Nanomed. 2019, 14, 3601–3613. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, S.; Basavarajappa, G.M.; Karnati, R.K.; Bakir, E.M.; Pund, S. Ion-triggered In Situ gelling nanoemulgel as a platform for nose-to-brain delivery of small lipophilic molecules. Pharmaceutics 2021, 13, 1216. [Google Scholar] [CrossRef] [PubMed]
- Rajpoot, K.; Tekade, M.; Pandey, V.; Nagaraja, S.H.; Youngren-Ortiz, S.R.; Tekade, R.K. Self-Microemulsifying Drug-Delivery System: Ongoing Challenges and Future Ahead; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128145081. [Google Scholar]
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-nano-emulsifying drug-delivery systems: From the development to the current applications and challenges in oral drug delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef]
- Pires, P.C.; Santos, A.O. Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef]
- Froelich, A.; Osmałek, T.; Jadach, B.; Puri, V.; Michniak-Kohn, B. Microemulsion-based media in nose-to-brain drug delivery. Pharmaceutics 2021, 13, 201. [Google Scholar] [CrossRef]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2021, 12, 735–757. [Google Scholar] [CrossRef]
- Kapoor, M.; Cloyd, J.C.; Siegel, R.A. A review of intranasal formulations for the treatment of seizure emergencies. J. Control. Release 2016, 237, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef]
- Oliveira, P.; Fortuna, A.; Alves, G.; Falcão, A. Drug-metabolizing Enzymes and Efflux Transporters in Nasal Epithelium: Influence on the Bioavailability of Intranasally Administered Drugs. Curr. Drug Metab. 2016, 17, 628–647. [Google Scholar] [CrossRef]
- Hampe, A.; Petit, R.J. Cryptic forest refugia on the ‘Roof of the World’. New Phytol. 2010, 185, 5–7. [Google Scholar] [CrossRef]
- Ogren, T.L. The Allergy-Fighting Garden: Stop Asthma and Allergies with Smart Landscaping; Ten Speed Press: Berkeley, CA, USA, 2015; pp. 131–133. [Google Scholar]
- Inatomi, Y.; Murata, H.; Inada, A.; Nakanishi, T.; Lang, F.A.; Murata, J.; Iinuma, M. New glycosides of acetophenone derivatives and phenylpropanoids from Juniperus occidentalis. J. Nat. Med. 2013, 67, 359–368. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Silva, A.M.S. A new 4’,7-epoxy-8,3’-oxyneolignan from the acetone extract of Juniperus brevifolia leaves. Phytochem. Lett. 2010, 3, 126–128. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Hamad, A.M.; Alanazi, M.T.; Alanazi, A.H.; Ali, R.; Foudah, A.I.; Alqarni, M.H. Characterization and hepatoprotective evaluation of sesquiterpenes and diterpenes from the aerial parts of Juniperus sabina L. Saudi Pharm J. 2019, 27, 920–929. [Google Scholar] [CrossRef]
- Fatma, W.; Taufeeq, H.M.; Shaida, W.A.; Rahman, W. Biflavanoids from Juniperus macropoda Boiss and Juniperus phoenicea Linn. (Cupressaceae). Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 1979, 17B, 193–194. [Google Scholar]
- Alqasoumi, S.I.; Abdel-Kader, M.S. Terpenoids from Juniperus procera with hepatoprotective activity. Pak. J. Pharm. Sci. 2012, 25, 315–322. [Google Scholar]
- Alqasoumi, S.I.; Farraj, A.I.; Abdel-Kader, M.S. Study of the hepatoprotective effect of Juniperus phoenicea constituents. Pak. J. Pharm. Sci. 2013, 26, 999–1008. [Google Scholar]
- Moawad, A.; Hifnawy, M. Flavonoids and Biflavonoids of Amentoflavone Class as Potential Psychoactive Drug Leads. J. Complement. Altern. Med. Res. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Shrestha, S.; Park, J.H.; Lee, D.Y.; Cho, J.G.; Seo, W.D.; Kang, H.C.; Baek, N.I.; Yoo, K.-H.; Chung, I.-S.; Jeon, J.-I.; et al. Cytotoxic and neuroprotective biflavonoids from the fruit of Rhus parviflora. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 557–562. [Google Scholar] [CrossRef]
- Kang, S.S.; Lee, J.Y.; Choi, Y.K.; Song, S.S.; Kim, J.S.; Jeon, S.J.; Han, Y.N.; Son, K.H.; Han, B.H. Neuroprotective effects of naturally occurring biflavonoids. Bioorg. Med. Chem. Lett. 2005, 15, 3588–3591. [Google Scholar] [CrossRef]
- Jeong, E.J.; Hwang, L.; Lee, M.; Lee, K.Y.; Ahn, M.-J.; Sung, S.H. Neuroprotective biflavonoids of Chamaecyparis obtusa leaves against glutamate-induced oxidative stress in HT22 hippocampal cells. Food Chem. Toxicol. 2014, 64, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systemic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970. [Google Scholar]
- Ersöz, T.; Harput, Ü.Ş.; Saracoğlu, İ.; Çaliş, İ.; Ogihara, Y. Phenolic Compounds from Scutellaria pontica. Turk. J. Chem. 2002, 26, 16. Available online: https://journals.tubitak.gov.tr/chem/vol26/iss4/16 (accessed on 30 January 2023).
- Wollenweber, E.; Kraut, L.; Mues, R. External Accumulation of Biflavonoids on Gymnosperm Leaves. Z. Nat. C 1998, 53, 946–950. [Google Scholar] [CrossRef]
- Geiger, H.; Seeger, T.; Hahn, H.; Zinsmeister, H.D.; Markham, K.R.; Wong, H. 1HNMR Assignments in Biflavonoid Spectra by Proton-Detected C-H Correlation. Z. Nat. C 1993, 48, 821–826. [Google Scholar] [CrossRef]
- Jo, A.; Yoo, H.J.; Lee, M. Robusta flavone Isolated from Nandina domestica Using Bioactivity-Guided Fractionation Downregulates Inflammatory Mediators. Molecules 2019, 24, 1789. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Sonvico, F.; Clementino, A.; Buttini, F.; Colombo, G.; Pescina, S.; Stanisçuaski Guterres, S.; Raffin Pohlmann, A.; Nicoli, S. Surface-Modified Nanocarriers for Nose-to-Brain Delivery: From Bioadhesion to Targeting. Pharmaceutics 2018, 10, 34. [Google Scholar] [CrossRef]
- Pouton, C.; Porter, C. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef]
- Shahba, A.A.; Mohsin, K.; Alanazi, F.K. The studies of phase equilibria and efficiency assessment for self-emulsifying lipid-based formulations. AAPS Pharm. Sci. Technol. 2012, 13, 522–533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Veronika, B.; Tobias, B.; Brigitte, K.; Werner, W.; Hilke, W. Long-term effects of St. John’s wort and hypericin on monoamine levels in rat hypothalamus and hippocampus. Brain Res. 2002, 930, 21–29. [Google Scholar]
- Oliver, G.; Jie, W.; McGregor, G.P.; Veronika, B. Anxiolytic activity of a phytochemically characterized Passiflora incarnata extract is mediated via the GABAergic system. Planta Med. 2008, 74, 1769–1773. [Google Scholar]
- Abdel-Kader, M.S.; Alanazi, M.T.; Saeedan, A.S.B.; Al-Saikhan, F.I.; Hamad, A.M. Hepatoprotective and nephroprotective activities of Juniperus sabina L. aerial parts. J. Pharm. Pharmacogn. Res. 2017, 5, 29–39. [Google Scholar]
- Shahba, A.A.; Tashish, A.Y.; Alanazi, F.K.; Kazi, M. Combined Self-Nanoemulsifying and Solid Dispersion Systems Showed Enhanced Cinnarizine Release in Hypochlorhydria/Achlorhydria Dissolution Model. Pharmaceutics 2021, 13, 627. [Google Scholar] [CrossRef]
- El-Laithy, H.M.; Basalious, E.B.; El-Hoseiny, B.M.; Adel, M.M. Novel self-nanoemulsifying self-nanosuspension (SNESNS) for enhancing oral bioavailability of diacerein: Simultaneous portal blood absorption and lymphatic delivery. Int. J. Pharm. 2015, 490, 146–154. [Google Scholar] [CrossRef]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Vare and Use of Laboratory Animals, 8th ed.; Institute for Laboratory Animal Research: Washington, DC, USA, 2011. [Google Scholar]
- Marks, D.R.; Tucker, K.; Cavallin, M.A.; Mast, T.G.; Fadool, D.A. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J. Neurosci. 2009, 29, 6734–6751. [Google Scholar] [CrossRef]
- Westin, U.; Piras, E.; Jansson, B.; Bergström, U.; Dahlin, M.; Brittebo, E.; Björk, E. Transfer of morphine along the olfactory pathway to the central nervous system after nasal administration to rodents. Eur. J. Pharm. Sci. 2005, 24, 565–573. [Google Scholar] [CrossRef]
- Erdo, F.; Bors, L.; Farkas, D.; Bajza, A.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain Targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Stevens, J.; Suidgeest, E.; Van Der Graaf, P.; Danhof, M.; De Lange, E. A new minimal-stress freely-moving rat model for preclinical studies on intranasal administration of CNS drugs. Pharm. Res. 2009, 26, 1911–1917. [Google Scholar] [CrossRef][Green Version]
- Wambebe, C.; Gamaniel, K.; Akah, P.; Kapu, S.D.; Samson, A.; Orisadipe, A.T.; Okogun, J.I. Central and uterotonic effects of cycleanine. Indian J. Pharmacol. 1997, 29, S366–S372. [Google Scholar]
- Abada, Y.K.; Nguyen, H.P.; Schreiber, R.; Ellenbroek, B. Assessment of motor function, sensory motor gating and recognition memory in a novel BACHD transgenic rat model for Huntington disease. PLoS ONE 2013, 8, e68584. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Yashiki, T.; Matsuzawa, T.; Mima, H. Absorption of drugs from the nasal mucosa of rat. Int. J. Pharm. 1981, 7, 317–325. [Google Scholar]

| Excipient | Role | Estimated Apparent Solubility |
|---|---|---|
| Miglyol 810 | Oil (medium chain triglycerides) | <2% w/w |
| Capmul MCM | Oil (medium chain monoglycerides | <2% w/w |
| Soybean oil | Oil (long chain triglycerides) | <2% w/w |
| Maisine 35-1 | Oil (long chain monoglycerides) | <2% w/w |
| Caprylic acid c8 | Oil (medium chain free fatty acid) | <2% w/w |
| Oleic acid | Oil (long chain free fatty acid) | <2% w/w |
| PG | Co-solvent | <2% w/w |
| PEG 400 | Co-solvent | <2% w/w |
| Transcutol HP | Co-solvent | <2% w/w |
| DMSO | Co-solvent | 20–24% w/w |
| Imwitor 988 | Co-surfactant | <2% w/w |
| Imwitor 308 | Co-surfactant | <2% w/w |
| Cremophor El | Surfactant | <2% w/w |
| Cremophor RH40 | Surfactant | <2% w/w |
| Tween 85 | Surfactant | <2% w/w |
| HCO-30 | Surfactant | <2% w/w |
| Excipients | Formulations | ||
|---|---|---|---|
| A | B | C | |
| CPF | 0.5 | 0.5 | 0.5 |
| Pluronic F-127 | - | 9.5 | 9.5 |
| DMSO | 99.5 | 90 | 30 |
| Kolliphor El | - | - | 10 |
| Distilled water ad to | - | - | 100 |
| Pharmacokinetic Parameters | CPF Solution | Formula C |
|---|---|---|
| Cmax (ng/mL) | 29.16 ± 3.56 | 422.5 ± 54.71 ** |
| Tmax (h) | 0.5 ± 0.0 | 0.5 ± 0.0 |
| AUC0–12 (ng.h/mL) | 263.02 ± 62.29 | 29.274.87 ± 4.855.87 ** |
| AUC0-∞ (ng.h/mL) | 265.39 ± 61.72 | 29.297.89 ± 4.848.26 ** |
| t1/2 (h) | 2.63 ± 0.35 | 2.7 ± 0.41 |
| K (h−1) | 0.27 ± 0.4 | 0.26 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khafagy, E.-S.; Soliman, G.A.; Shahba, A.A.-W.; Aldawsari, M.F.; Alharthy, K.M.; Abdel-Kader, M.S.; Zaatout, H.H. Brain Targeting by Intranasal Drug Delivery: Effect of Different Formulations of the Biflavone “Cupressuflavone” from Juniperus sabina L. on the Motor Activity of Rats. Molecules 2023, 28, 1354. https://doi.org/10.3390/molecules28031354
Khafagy E-S, Soliman GA, Shahba AA-W, Aldawsari MF, Alharthy KM, Abdel-Kader MS, Zaatout HH. Brain Targeting by Intranasal Drug Delivery: Effect of Different Formulations of the Biflavone “Cupressuflavone” from Juniperus sabina L. on the Motor Activity of Rats. Molecules. 2023; 28(3):1354. https://doi.org/10.3390/molecules28031354
Chicago/Turabian StyleKhafagy, El-Sayed, Gamal A. Soliman, Ahmad Abdul-Wahhab Shahba, Mohammed F. Aldawsari, Khalid M. Alharthy, Maged S. Abdel-Kader, and Hala H. Zaatout. 2023. "Brain Targeting by Intranasal Drug Delivery: Effect of Different Formulations of the Biflavone “Cupressuflavone” from Juniperus sabina L. on the Motor Activity of Rats" Molecules 28, no. 3: 1354. https://doi.org/10.3390/molecules28031354
APA StyleKhafagy, E.-S., Soliman, G. A., Shahba, A. A.-W., Aldawsari, M. F., Alharthy, K. M., Abdel-Kader, M. S., & Zaatout, H. H. (2023). Brain Targeting by Intranasal Drug Delivery: Effect of Different Formulations of the Biflavone “Cupressuflavone” from Juniperus sabina L. on the Motor Activity of Rats. Molecules, 28(3), 1354. https://doi.org/10.3390/molecules28031354







