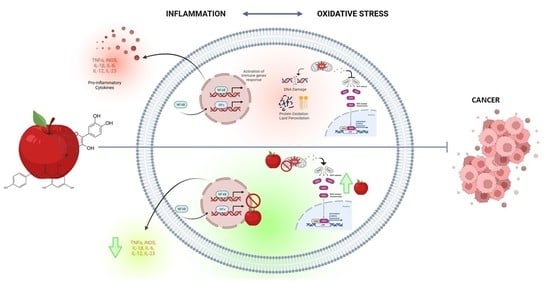
Targeting Nrf2 and NF-κB Signaling Pathways in Cancer Prevention: The Role of Apple Phytochemicals
Abstract
:1. Introduction
2. The Role of Nrf2 in the Cancer Environment
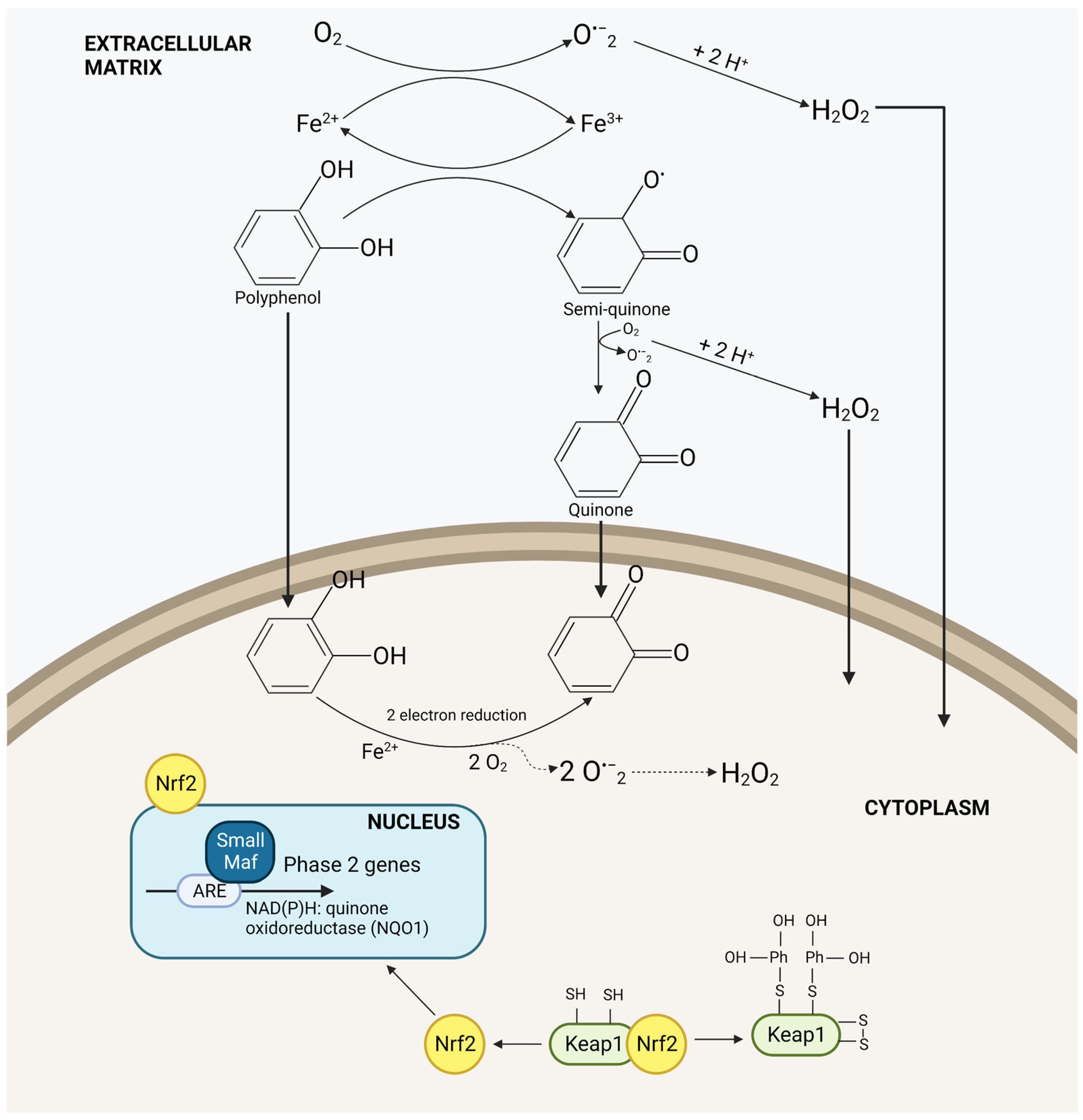
Apple Phytochemicals as Nrf2 Inducers
3. NF-κB Inhibition by Apple Polyphenols Ameliorate Inflammation in Cancer
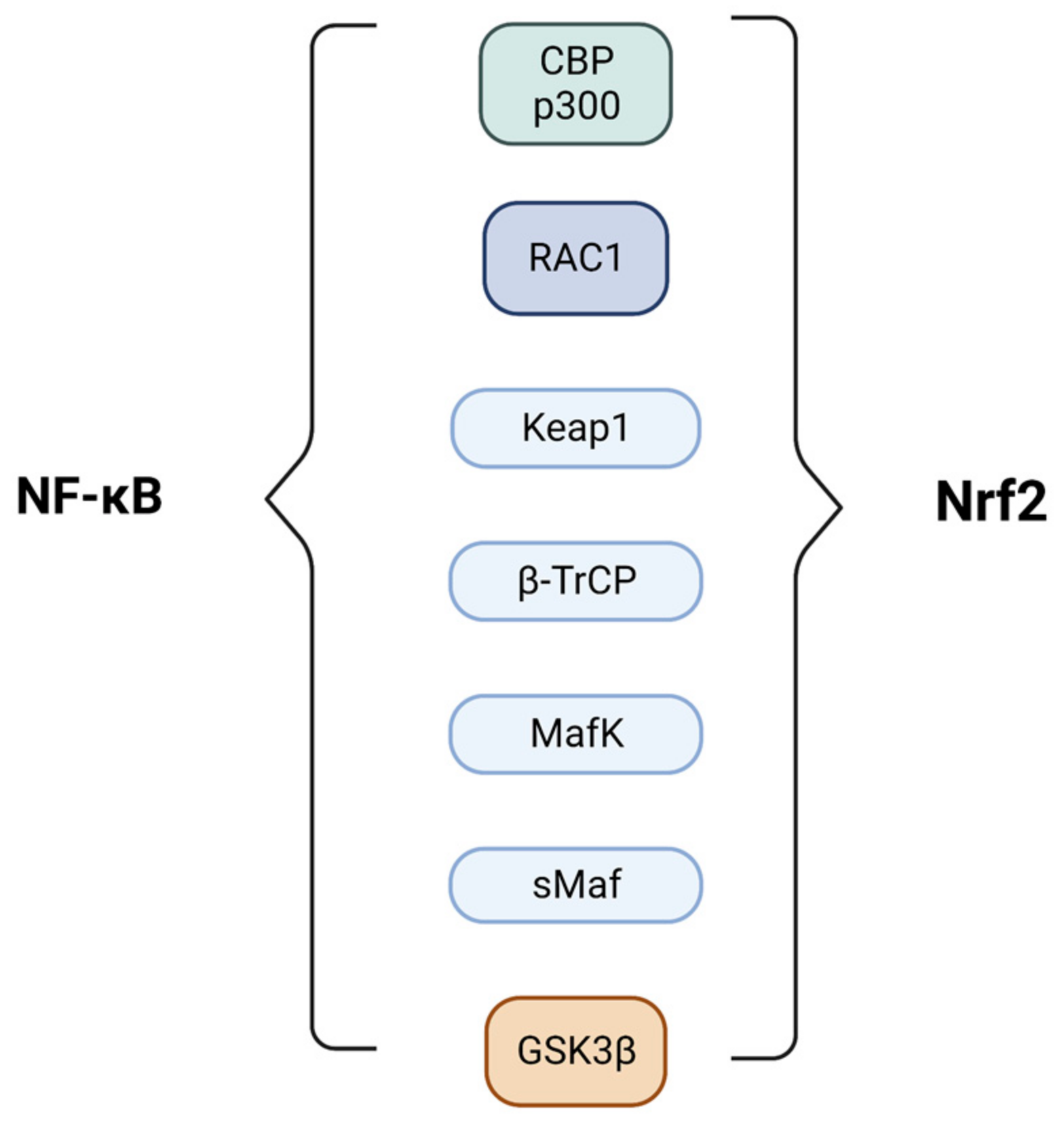
4. Nrf2 and NF-κB Pathways Crosstalk
5. Targeting the Crosstalk between Nrf2 and NF-κB Response Pathways by Synthetic Triterpenoids
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reiss, R.; Johnston, J.; Tucker, K.; De Sesso, J.M.; Keen, C.L. Estimation of Cancer Risks and Benefits Associated with a Potential Increased Consumption of Fruits and Vegetables. Food. Chem. Toxicol. 2012, 50, 4421–4427. [Google Scholar] [CrossRef] [PubMed]
- Basli, A.; Belkacem, N.; Amrani, I. Health Benefits of Phenolic Compounds Against Cancers. In Phenolic Compounds—Biological Activity; Soto-Hernndez, M., Palma-Tenango, M., Garcia-Mateos, M.d.R., Eds.; IntechOpen: London, UK, 2017; ISBN 978-953-51-2959-2. [Google Scholar]
- Davidson, K.T.; Zhu, Z.; Fang, Y. Phytochemicals in the Fight Against Cancer. Pathol. Oncol. Res. 2016, 22, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Shree, T.J.; Poompavai, S.; Begum, S.M.F.M.; Gowrisree, V.; Hemalatha, S.; Sieni, E.; Sundararajan, R. Cancer-Fighting Phytochemicals: Another Look. J. Nanomed. Biother. Discov. 2019, 8, 162. [Google Scholar] [CrossRef]
- Scarpa, E.-S.; Ninfali, P. Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells. Int. J. Mol. Sci. 2015, 16, 15727–15742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollakhalili Meybodi, N.; Mortazavian, A.M.; Bahadori Monfared, A.; Sohrabvandi, S.; Aghaei Meybodi, F. Phytochemicals in Cancer Prevention: A Review of the Evidence. Iran. J. Cancer Prev. 2017, in press. [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef] [Green Version]
- Zubair, H.; Azim, S.; Ahmad, A.; Khan, M.; Patel, G.; Singh, S.; Singh, A. Cancer Chemoprevention by Phytochemicals: Nature’s Healing Touch. Molecules 2017, 22, 395. [Google Scholar] [CrossRef] [Green Version]
- Montané, X.; Kowalczyk, O.; Reig-Vano, B.; Bajek, A.; Roszkowski, K.; Tomczyk, R.; Pawliszak, W.; Giamberini, M.; Mocek-Płóciniak, A.; Tylkowski, B. Current Perspectives of the Applications of Polyphenols and Flavonoids in Cancer Therapy. Molecules 2020, 25, 3342. [Google Scholar] [CrossRef]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus Domestica: A Review on Nutritional Features, Chemical Composition, Traditional and Medicinal Value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef]
- Ferrario, G.; Baron, G.; Gado, F.; Della Vedova, L.; Bombardelli, E.; Carini, M.; D’Amato, A.; Aldini, G.; Altomare, A. Polyphenols from Thinned Young Apples: HPLC-HRMS Profile and Evaluation of Their Anti-Oxidant and Anti-Inflammatory Activities by Proteomic Studies. Antioxidants 2022, 11, 1577. [Google Scholar] [CrossRef]
- Konopacka, D.; Jesionkowska, K.; Kruczyńska, D.; Stehr, R.; Schoorl, F.; Buehler, A.; Egger, S.; Codarin, S.; Hilaire, C.; Höller, I.; et al. Apple and Peach Consumption Habits across European Countries. Appetite 2010, 55, 478–483. [Google Scholar] [CrossRef]
- Nezbedova, L.; McGhie, T.; Christensen, M.; Heyes, J.; Nasef, N.A.; Mehta, S. Onco-Preventive and Chemo-Protective Effects of Apple Bioactive Compounds. Nutrients 2021, 13, 4025. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.-F.; Wu, X.; Liu, R.H. Antioxidant and Antiproliferative Activities of Common Fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How Do Nutritional Antioxidants Really Work: Nucleophilic Tone and Para-Hormesis versus Free Radical Scavenging in Vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.; Rana, A.; Gupta, S.; Bhushan, S. Varietal Influence on Phenolic Constituents and Nutritive Characteristics of Pomace Obtained from Apples Grown in Western Himalayas. J. Food Sci. Technol. 2021, 58, 166–174. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic Anti-Inflammatory Effects and Mechanisms of Combined Phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Rajagopal, C.; Lankadasari, M.B.; Aranjani, J.M.; Harikumar, K.B. Targeting Oncogenic Transcription Factors by Polyphenols: A Novel Approach for Cancer Therapy. Pharmacol. Res. 2018, 130, 273–291. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kim, J.H.; Kannappan, R.; Reuter, S.; Dougherty, P.M.; Aggarwal, B.B. Role of Nuclear Factor-κ B-Mediated Inflammatory Pathways in Cancer-Related Symptoms and Their Regulation by Nutritional Agents. Exp. Biol. Med. 2011, 236, 658–671. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Young, M.R.; Bobe, G.; Colburn, N.H.; Milner, J.A. Bioactive Food Components, Inflammatory Targets, and Cancer Prevention. Cancer Prev. Res. 2009, 2, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Barrera, G.; Cucci, M.A.; Grattarola, M.; Dianzani, C.; Muzio, G.; Pizzimenti, S. Control of Oxidative Stress in Cancer Chemoresistance: Spotlight on Nrf2 Role. Antioxidants 2021, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Zhang, D.D. Non-Canonical Activation of NRF2: New Insights and Its Relevance to Disease. Curr. Pathobiol. Rep. 2017, 5, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, N.F.; Sun, Z.; Chen, W.; Zhang, D.D. Nrf2 and P21 Regulate the Fine Balance between Life and Death by Controlling ROS Levels. Cell Cycle 2009, 8, 3255–3256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tossetta, G.; Marzioni, D. Natural and synthetic compounds in Ovarian Cancer: A focus on NRF2/KEAP1 pathway. Pharmacol. Res. 2022, 183, 106365. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-Y.; Lee, D.-Y.; Chun, K.-S.; Kim, E.-H. The Role of NRF2/KEAP1 Signaling Pathway in Cancer Metabolism. Int. J. Mol. Sci. 2021, 22, 4376. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting Molecular Cross-Talk between Nrf2 and NF-ΚB Response Pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Lu, H.; Bai, Y. Nrf2 in Cancers: A Double-edged Sword. Cancer Med. 2019, 8, 2252–2267. [Google Scholar] [CrossRef]
- Schmidlin, C.J.; Shakya, A.; Dodson, M.; Chapman, E.; Zhang, D.D. The Intricacies of NRF2 Regulation in Cancer. Semin. Cancer Biol. 2021, 76, 110–119. [Google Scholar] [CrossRef]
- Shibata, T.; Ohta, T.; Tong, K.I.; Kokubu, A.; Odogawa, R.; Tsuta, K.; Asamura, H.; Yamamoto, M.; Hirohashi, S. Cancer Related Mutations in NRF2 Impair Its Recognition by Keap1-Cul3 E3 Ligase and Promote Malignancy. Proc. Natl. Acad. Sci. USA 2008, 105, 13568–13573. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.R.; Oh, J.E.; Kim, M.S.; Kang, M.R.; Park, S.W.; Han, J.Y.; Eom, H.S.; Yoo, N.J.; Lee, S.H. Oncogenic NRF2 Mutations in Squamous Cell Carcinomas of Oesophagus and Skin: NRF2 in Squamous Cell Carcinomas. J. Pathol. 2010, 220, 446–451. [Google Scholar] [CrossRef]
- Solis, L.M.; Behrens, C.; Dong, W.; Suraokar, M.; Ozburn, N.C.; Moran, C.A.; Corvalan, A.H.; Biswal, S.; Swisher, S.G.; Bekele, B.N.; et al. Nrf2 and Keap1 Abnormalities in Non–Small Cell Lung Carcinoma and Association with Clinicopathologic Features. Clin. Cancer Res. 2010, 16, 3743–3753. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Chin, Y.E.; Zhang, D.D. Acetylation of Nrf2 by P300/CBP Augments Promoter-Specific DNA Binding of Nrf2 during the Antioxidant Response. Mol. Cell Biol. 2009, 29, 2658–2672. [Google Scholar] [CrossRef] [Green Version]
- Marzioni, D.; Mazzucchelli, R.; Fantone, S.; Tossetta, G. NRF2 modulation in TRAMP mice: An in vivo model of prostate cancer. Mol. Biol. Rep. 2022, 50, 873–881. [Google Scholar] [CrossRef]
- Funes, J.M.; Henderson, S.; Kaufman, R.; Flanagan, J.M.; Robson, M.; Pedley, B.; Moncada, S.; Boshoff, C. Oncogenic Transformation of Mesenchymal Stem Cells Decreases Nrf2 Expression Favoring in Vivo Tumor Growth and Poorer Survival. Mol. Cancer 2014, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Dinkova-Kostova, A.T.; Wang, X.J. Induction of the Keap1/Nrf2/ARE Pathway by Oxidizable Diphenols. Chem. Biol. Interact. 2011, 192, 101–106. [Google Scholar] [CrossRef]
- Potter, G.A.; Patterson, L.H.; Wanogho, E.; Perry, P.J.; Butler, P.C.; Ijaz, T.; Ruparelia, K.C.; Lamb, J.H.; Farmer, P.B.; Stanley, L.A.; et al. The Cancer Preventative Agent Resveratrol Is Converted to the Anticancer Agent Piceatannol by the Cytochrome P450 Enzyme CYP1B1. Br. J. Cancer 2002, 86, 774–778. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Kim, D.-O.; Lee, H.J.; Lee, C.Y. Major Phenolics in Apple and Their Contribution to the Total Antioxidant Capacity. J. Agric. Food Chem. 2003, 51, 6516–6520. [Google Scholar] [CrossRef]
- Soyalan, B.; Minn, J.; Schmitz, H.J.; Schrenk, D.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Apple Juice Intervention Modulates Expression of ARE-Dependent Genes in Rat Colon and Liver. Eur. J. Nutr. 2011, 50, 135–143. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, S.; Patial, V.; Gupta, M.; Bhushan, S.; Padwad, Y. Antioxidant and Hepatoprotective Effect of Polyphenols from Apple Pomace Extract via Apoptosis Inhibition and Nrf2 Activation in Mice. Hum. Exp. Toxicol. 2016, 35, 1264–1275. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Huang, Z.; Chen, D.; He, J.; Zheng, P.; Chen, H.; Luo, J.; Luo, Y.; Yu, B.; et al. Effects of Dietary Apple Polyphenols Supplementation on Hepatic Fat Deposition and Antioxidant Capacity in Finishing Pigs. Animals 2019, 9, 937. [Google Scholar] [CrossRef]
- Huang, T.; Che, Q.; Chen, X.; Chen, D.; Yu, B.; He, J.; Chen, H.; Yan, H.; Zheng, P.; Luo, Y.; et al. Apple Polyphenols Improve Intestinal Antioxidant Capacity and Barrier Function by Activating the Nrf2/Keap1 Signaling Pathway in a Pig Model. J. Agric. Food Chem. 2022, 70, 7576–7585. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuźniak, V.; Paluszczak, J.; Celewicz, L.; Barciszewski, J.; Baer-Dubowska, W. Phloretamide, an Apple Phenolic Compound, Activates the Nrf2/ARE Pathway in Human Hepatocytes. Food Chem. Toxicol. 2013, 51, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Liang, J. Activation of the Nrf2 Defense Pathway Contributes to Neuroprotective Effects of Phloretin on Oxidative Stress Injury after Cerebral Ischemia/Reperfusion in Rats. J. Neurol. Sci. 2015, 351, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Guo, Y.; Xu, L.; Wang, H. Phlorizin Exerts Potent Effects against Aging Induced by d-Galactose in Mice and PC12 Cells. Food Funct. 2021, 12, 2148–2160. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Z.; Liu, D.; Li, X.; Rehman, R.; Wang, H.; Wu, Z. Apple Phlorizin Attenuates Oxidative Stress in Drosophila Melanogaster. J. Food Biochem. 2018, 43, e12744. [Google Scholar] [CrossRef]
- Kim, H.; Ramirez, C.N.; Su, Z.-Y.; Kong, A.-N.T. Epigenetic Modifications of Triterpenoid Ursolic Acid in Activating Nrf2 and Blocking Cellular Transformation of Mouse Epidermal Cells. J. Nutr. Biochem. 2016, 33, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.-R.; Li, J.-Y.; Dong, X.-W.; Tan, Z.-J.; Wu, W.-Z.; Xie, Q.-M.; Yang, Y.-M. Apple Polyphenols Decrease Atherosclerosis and Hepatic Steatosis in ApoE−/−Mice through the ROS/MAPK/NF-ΚB Pathway. Nutrients 2015, 7, 7085–7105. [Google Scholar] [CrossRef] [Green Version]
- Jung, M.; Triebel, S.; Anke, T.; Richling, E.; Erkel, G. Influence of Apple Polyphenols on Inflammatory Gene Expression. Mol. Nutr. Food Res. 2009, 53, 1263–1280. [Google Scholar] [CrossRef]
- Andre, C.M.; Greenwood, J.M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Evers, D.; Perry, N.B.; Laing, W.A. Anti-Inflammatory Procyanidins and Triterpenes in 109 Apple Varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-γ-Valerolactones and Phenylvaleric Acids, the Main Colonic Metabolites of Flavan-3-Ols: Synthesis, Analysis, Bioavailability, and Bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Regazzoni, L.; Fumagalli, L.; Artasensi, A.; Borghi, E.; Ottaviano, E.; Del Bo, C.; Riso, P.; Allegrini, P.; et al. Profiling Vaccinium Macrocarpon Components and Metabolites in Human Urine and the Urine Ex-Vivo Effect on Candida Albicans Adhesion and Biofilm-Formation. Biochem. Pharmacol. 2020, 173, 113726. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-ΚB Activation by Small Molecules as a Therapeutic Strategy. Biochim. Biophys. Acta Gene Regul. Mech. 2010, 1799, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.; Liu, R.H. Effect of Selected Phytochemicals and Apple Extracts on NF-ΚB Activation in Human Breast Cancer MCF-7 Cells. J. Agric. Food Chem. 2007, 55, 3167–3173. [Google Scholar] [CrossRef]
- Fontana, G.; Bruno, M.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Spinella, A.; Rosselli, S. Cytotoxicity of Oleanolic and Ursolic Acid Derivatives toward Hepatocellular Carcinoma and Evaluation of NF-ΚB Involvement. Bioorg. Chem. 2019, 90, 103054. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Zhu, G.; Liu, H.; Chen, J.; Wang, Y.; He, X. Quercetin Inhibits TNF-α Induced HUVECs Apoptosis and Inflammation via Downregulating NF-KB and AP-1 Signaling Pathway in Vitro. Medicine 2020, 99, e22241. [Google Scholar] [CrossRef]
- Kim, S.-H. The Role of TNFα/P53 Pathway in Endometrial Cancer Mouse Model Administered with Apple Seed Extract. Histol. Histopathol. 2022, 37, 169–180. [Google Scholar] [CrossRef]
- Martino, E.; Vuoso, D.C.; D’Angelo, S.; Mele, L.; D’Onofrio, N.; Porcelli, M.; Cacciapuoti, G. Annurca Apple Polyphenol Extract Selectively Kills MDA-MB-231 Cells through ROS Generation, Sustained JNK Activation and Cell Growth and Survival Inhibition. Sci. Rep. 2019, 9, 13045. [Google Scholar] [CrossRef] [Green Version]
- Rios, J.; Recio, M.; Escandell, J.; Andujar, I. Inhibition of Transcription Factors by Plant-Derived Compounds and Their Implications in Inflammation and Cancer. Curr. Pharm. Des. 2009, 15, 1212–1237. [Google Scholar] [CrossRef]
- Ji, C.; Kozak, K.R.; Marnett, L.J. IκB Kinase, a Molecular Target for Inhibition by 4-Hydroxy-2-Nonenal. J. Biol. Chem. 2001, 276, 18223–18228. [Google Scholar] [CrossRef] [Green Version]
- Krajka-Kuźniak, V.; Baer-Dubowska, W. Modulation of Nrf2 and NF-ΚB Signaling Pathways by Naturally Occurring Compounds in Relation to Cancer Prevention and Therapy. Are Combinations Better Than Single Compounds? Int. J. Mol. Sci. 2021, 22, 8223. [Google Scholar] [CrossRef]
- Liu, G.-H.; Qu, J.; Shen, X. NF-ΚB/P65 Antagonizes Nrf2-ARE Pathway by Depriving CBP from Nrf2 and Facilitating Recruitment of HDAC3 to MafK. Biochim. Biophys Acta Mol. Cell Res. 2008, 1783, 713–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-E.; You, D.-J.; Lee, C.; Ahn, C.; Seong, J.Y.; Hwang, J.-I. Suppression of NF-ΚB Signaling by KEAP1 Regulation of IKKβ Activity through Autophagic Degradation and Inhibition of Phosphorylation. Cell. Signal. 2010, 22, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Rojo, A.I.; Chowdhry, S.; McMahon, M.; Hayes, J.D.; Cuadrado, A. SCF/β-TrCP Promotes Glycogen Synthase Kinase 3-Dependent Degradation of the Nrf2 Transcription Factor in a Keap1-Independent Manner. Mol. Cell. Biol. 2011, 31, 1121–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winston, J.T.; Strack, P.; Beer-Romero, P.; Chu, C.Y.; Elledge, S.J.; Harper, J.W. The SCFbeta-TRCP-Ubiquitin Ligase Complex Associates Specifically with Phosphorylated Destruction Motifs in Ikappa Balpha and Beta -Catenin and Stimulates Ikappa Balpha Ubiquitination in Vitro. Genes Dev. 1999, 13, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park-Min, K.-H.; Chen, J.; Hu, X.; Ivashkiv, L.B. Tumor Necrosis Factor Induces GSK3 Kinase–Mediated Cross-Tolerance to Endotoxin in Macrophages. Nat. Immunol. 2011, 12, 607–615. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.-S. The Role of GSK3 in Glucose Homeostasis and the Development of Insulin Resistance. Diabetes Res. Clin. Pract. 2007, 77, S49–S57. [Google Scholar] [CrossRef]
- Ichimura, Y.; Waguri, S.; Sou, Y.; Kageyama, S.; Hasegawa, J.; Ishimura, R.; Saito, T.; Yang, Y.; Kouno, T.; Fukutomi, T.; et al. Phosphorylation of P62 Activates the Keap1-Nrf2 Pathway during Selective Autophagy. Mol. Cell 2013, 51, 618–631. [Google Scholar] [CrossRef] [Green Version]
- Wooten, M.W.; Geetha, T.; Seibenhener, M.L.; Babu, J.R.; Diaz-Meco, M.T.; Moscat, J. The P62 Scaffold Regulates Nerve Growth Factor-Induced NF-ΚB Activation by Influencing TRAF6 Polyubiquitination. J. Biol. Chem. 2005, 280, 35625–35629. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Chen, X.; Huang, Z.; Chen, D.; Yu, J.; Yan, H.; Chen, H.; He, J.; Zheng, P.; Luo, Y.; et al. Apple Polyphenols Improve Intestinal Barrier Function by Enhancing Antioxidant Capacity and Suppressing Inflammation in Weaning Piglets. Anim. Sci. J. 2022, 93, e13747. [Google Scholar] [CrossRef]
- Loboda, A.; Rojczyk-Golebiewska, E.; Bednarczyk-Cwynar, B.; Lucjusz, Z.; Jozkowicz, A.; Dulak, J. Targeting nrf2-mediated gene transcription by triterpenoids and their derivatives. Biomol. Ther. 2012, 20, 499–505. [Google Scholar] [CrossRef]
- Liby, K.; Hock, T.; Yore, M.M.; Suh, N.; Place, A.E.; Risingsong, R.; Williams, C.R.; Royce, D.B.; Honda, T.; Honda, Y.; et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005, 65, 4789–4798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yore, M.M.; Liby, K.T.; Honda, T.; Gribble, G.W.; Sporn, M.B. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol. Cancer Ther. 2006, 5, 232–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liby, K.; Voong, N.; Williams, C.R.; Risingsong, R.; Royce, D.B.; Honda, T.; Gribble, G.W.; Sporn, M.B.; Letterio, J.J. The synthetic triterpenoid CDDO-Imidazolide suppresses STAT phosphorylation and induces apoptosis in myeloma and lung cancer cells. Clin. Cancer Res. 2006, 12, 4288–4293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Waddington, J.C.; Tailor, A.; Lister, A.; Hamlett, J.; Berry, N.; Park, B.K.; Sporn, M.B. CDDO-imidazolide Targets Multiple Amino Acid Residues on the Nrf2 Adaptor, Keap1. J. Med. Chem. 2020, 63, 9965–9976. [Google Scholar] [CrossRef]
- Einbond, L.S. Chapter 11—Black Cohosh: Chemopreventive and Anticancer Potential. In Complementary and Alternative Therapies and the Aging Population; Academic Press: Cambridge, MA, USA, 2009; pp. 193–227. ISBN 9780123742285. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, B.G.; Robinson, J.; Fink, S.; Yan, M.; Sporn, M.B.; Markowitz, S.D.; Letterio, J.J. Synthetic triterpenoid induces 15-PGDH expression and suppresses inflammation-driven colon carcinogenesis. J. Clin. Investig. 2014, 124, 2472–2482. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Kalantar-Zadeh, K. Chapter 13—Inflammation in Chronic Kidney Disease. In Chronic Kidney Disease, Dialysis, and Transplantation, 3rd ed.; Saunders: Philadelphia, PA, USA, 2010; pp. 183–197. ISBN 978-1-4377-0987-2. [Google Scholar]



| Classes | Apple Products/Compounds | In Vitro In Vivo Systems | Findings | Ref. |
|---|---|---|---|---|
| Nrf2 inhibitors | No FDA approved drugs | |||
| Nrf2 inducers | Different type of apple products (juices and smoothie) | Sprague-Dawley rats | Product-dependent increase of Nrf2 at colonic level correlated especially with the intake of the apple product with the highest content of procyanidins | [39] |
| Apple pomace | Mice model of oxidative hepatotoxicity | Dose-dependent Nrf2 induction accompanied by a reduction in liver necrosis | [40] | |
| Apple polyphenols | In vivo pig model | Nrf2 dose dependent induction in pigs’ jejunum, intestinal mucosa and liver | [41,42,43] | |
| -IPEC-J2 cells | Nrf2/Keap1 pathway modulates the effect of apple polyphenols on intestinal antioxidant capacity and tight junction protein expressions (ZO-1, occludin, and claudin-1 ameliorating barrier function. | |||
| Non diphenols compounds | Phloretin | In vivo and in vitro studies. | Nrf2 activation | [44,45,46] |
| Ursolic Acid | In vitro studies | Activation of Nrf2, decreasing the Nrf2 promoter methylation by the negative regulation of DNA methyltransferases and histone deacetylases. | [47] |
| Classes | Apple Product/Compound | In Vitro In Vivo Systems | Findings | Ref. |
|---|---|---|---|---|
| Apple polyphenols acting on NFkB | Apple polyphenols | Animal model of hyperlipidaemia | Improved endothelial dysfunction and plaque formation through the suppression of ROS/MAPK/NF-kB signaling pathway and the reduction in the expression of proinflammatory molecules (CCL-2, ICAM, and VCAM-1) | [48] |
| Apple juice extract and its single major constituent | In vitro studies in human immunorelevant cell lines (DLD-1, T84, MonoMac6, Jurkat) | Inhibition of the expression of proinflammatory genes regulated by the transcription factor NF-kB (TNF-a, IL-1b, CXCL9, CXCL10), and inflammatory enzymes (COX-2, CYP3A4) and transcription factors (STAT1, IRF1) | [49] | |
| Polyphenol from different cultivar | In vitro studies | Cultivars with high levels of procyanidins were the most effective at inhibiting NF-κB activation | [50] | |
| Apple extract | In vitro studies on human breast cancer MCF-7 cells | Inhibition of the activation of NF-κB by inhibiting the proteasomal activity | [54] | |
| Quercetin | In vitro studies on human umbilical vein endothelial cells (HUVECs) | Inhibition of the activation of TNF-a NF-κB signaling pathway | [56] | |
| Apple seed extract | In vivo studies on endometrial cancer mouse model | Apoptosis of cancer cells by downregulating NF-κB | [57] | |
| Polyphenol extract from Annurca apples | In vitro studies on MDA-MB-231 human breast carcinoma cells | ROS generation leading to c-Jun-N-terminal kinase (JNK) activation thus promoting apoptosis and downregulated NF-κB, which is interconnected to JNK by reducing its apoptotic activity | [58] | |
| Synthetic triterpenoids acting on NFkB | Triterpene derivatives of oleanolic and ursolic acid | In vitro studies on tumor cell lines | Involvement of NF-kB in the modulation of their anticancer effects | [55] |
| Classes | Apple Product/Compound | In Vitro In Vivo Systems | Findings | Ref. |
|---|---|---|---|---|
| Apple polyphenols on Nrf2-NFkB crosstalk | Polyphenols from thinned young apples |
|
| [11] |
|
| |||
| Apple polyphenols | Weaning piglets | Nrf2 was upregulated together with HO-1, while NF-kB was downregulated. Improvement of the intestinal villi shape, through jejunal absorption capacity. | [70] | |
| Ursolic acid | In vitro studies | Activity demonstrated on both Nrf2 and NFkB transcription factors. | [47,55] | |
| Synthetic triterpenoids on Nrf2-NFkB crosstalk | CDDO-Im derivative | In vitro studies | It boosts the expression of cytoprotective genes via the KEAP1/NRF2-ARE signaling path by inducing a cis-regulatory element occurring in the 5′ flanking region of genes encoding many cytoprotective enzymes | [76] |
| CDDO-Me (Bardoxolone) | In vitro studies | It activates Nrf2 and suppresses the activity of the pro-oxidant and pro-inflammatory transcription factor NF-κB. | [77] | |
| In vivo studies | Significant anti-inflammatory activity in several animal models of inflammation, including the ischemia-reperfusion model of acute kidney injury, or in the cisplatin-based kidney injury model, and has been shown to suppress the development of colitis-associated cancer (CAC) in mice. | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gado, F.; Ferrario, G.; Della Vedova, L.; Zoanni, B.; Altomare, A.; Carini, M.; Aldini, G.; D’Amato, A.; Baron, G. Targeting Nrf2 and NF-κB Signaling Pathways in Cancer Prevention: The Role of Apple Phytochemicals. Molecules 2023, 28, 1356. https://doi.org/10.3390/molecules28031356
Gado F, Ferrario G, Della Vedova L, Zoanni B, Altomare A, Carini M, Aldini G, D’Amato A, Baron G. Targeting Nrf2 and NF-κB Signaling Pathways in Cancer Prevention: The Role of Apple Phytochemicals. Molecules. 2023; 28(3):1356. https://doi.org/10.3390/molecules28031356
Chicago/Turabian StyleGado, Francesca, Giulio Ferrario, Larissa Della Vedova, Beatrice Zoanni, Alessandra Altomare, Marina Carini, Giancarlo Aldini, Alfonsina D’Amato, and Giovanna Baron. 2023. "Targeting Nrf2 and NF-κB Signaling Pathways in Cancer Prevention: The Role of Apple Phytochemicals" Molecules 28, no. 3: 1356. https://doi.org/10.3390/molecules28031356
APA StyleGado, F., Ferrario, G., Della Vedova, L., Zoanni, B., Altomare, A., Carini, M., Aldini, G., D’Amato, A., & Baron, G. (2023). Targeting Nrf2 and NF-κB Signaling Pathways in Cancer Prevention: The Role of Apple Phytochemicals. Molecules, 28(3), 1356. https://doi.org/10.3390/molecules28031356