Study on Synergistic Anti-Inflammatory Effect of Typical Functional Components of Extracts of Ginkgo Biloba Leaves
Abstract
:1. Introduction
2. Results and Analysis
2.1. The main Functional Components of EGB Inhibit LPS Stimulated Raws264 7 Cell Release of NO
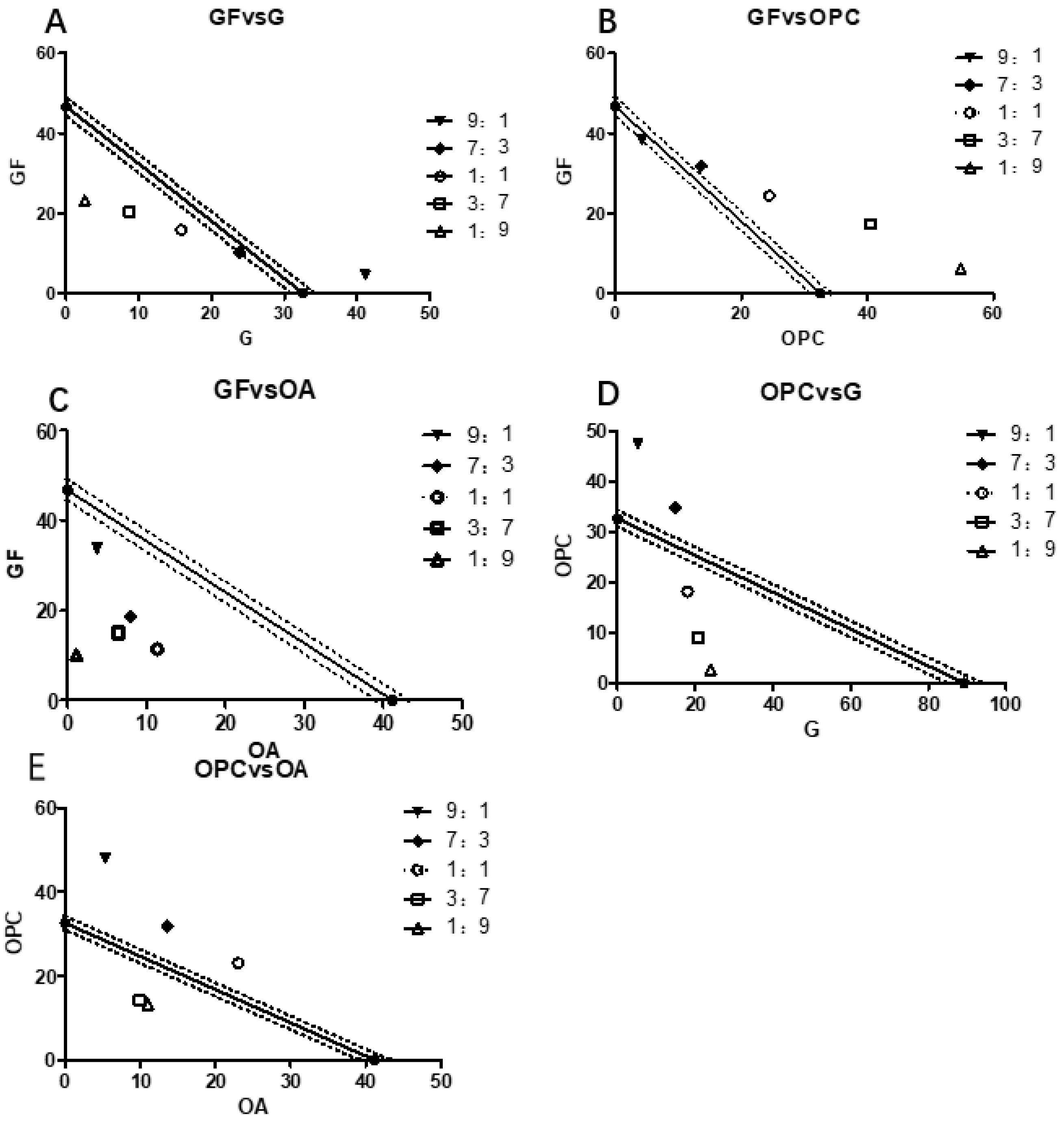
2.2. The Main Efficacy Components of EGB on LPS Stimulated Raws264.7 Synergistic Effect of NO Production Inhibitory Activity
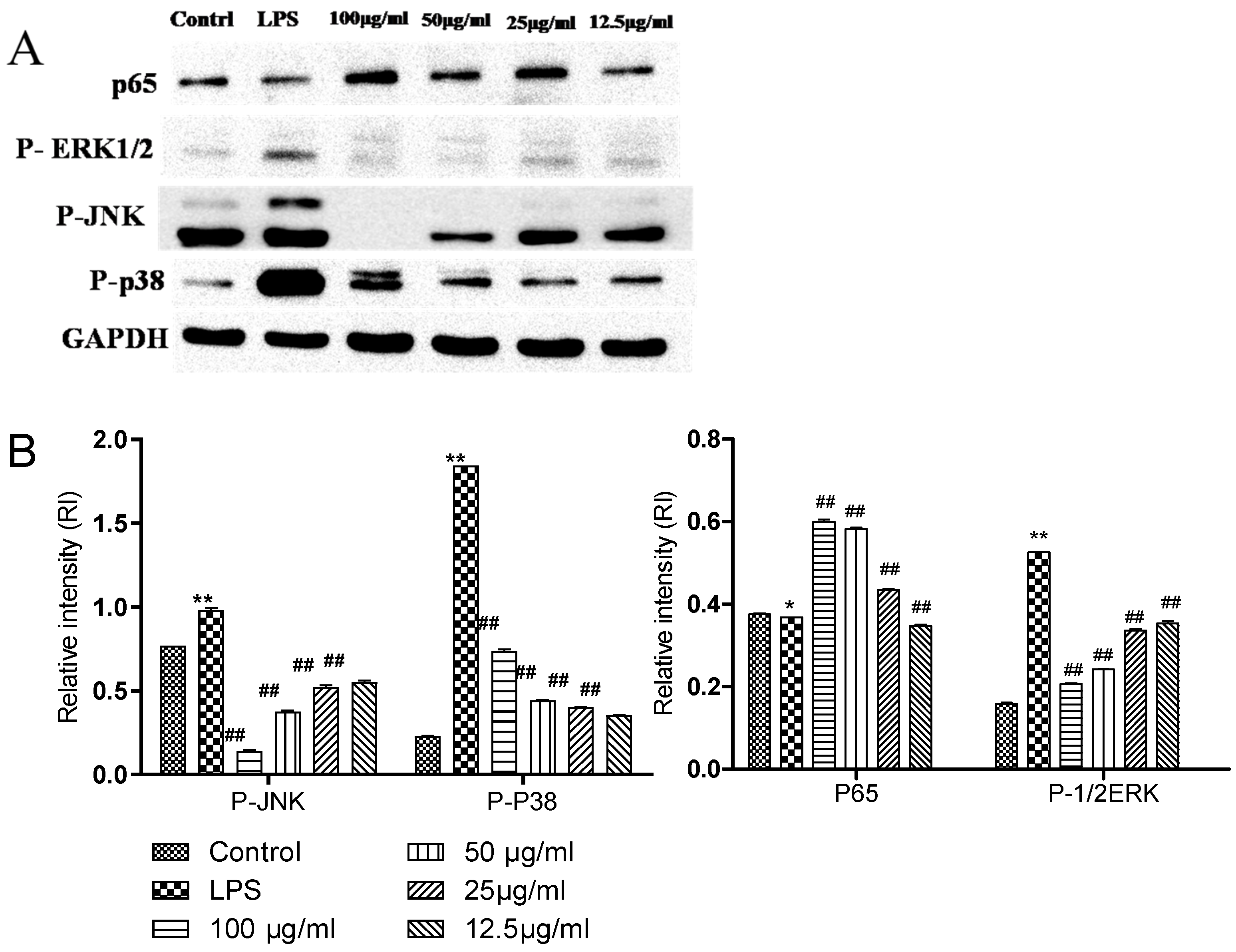
2.3. Comparison of the Effects of Complexes on the Expression of Related Proteins in NFKB and MAPK Pathways
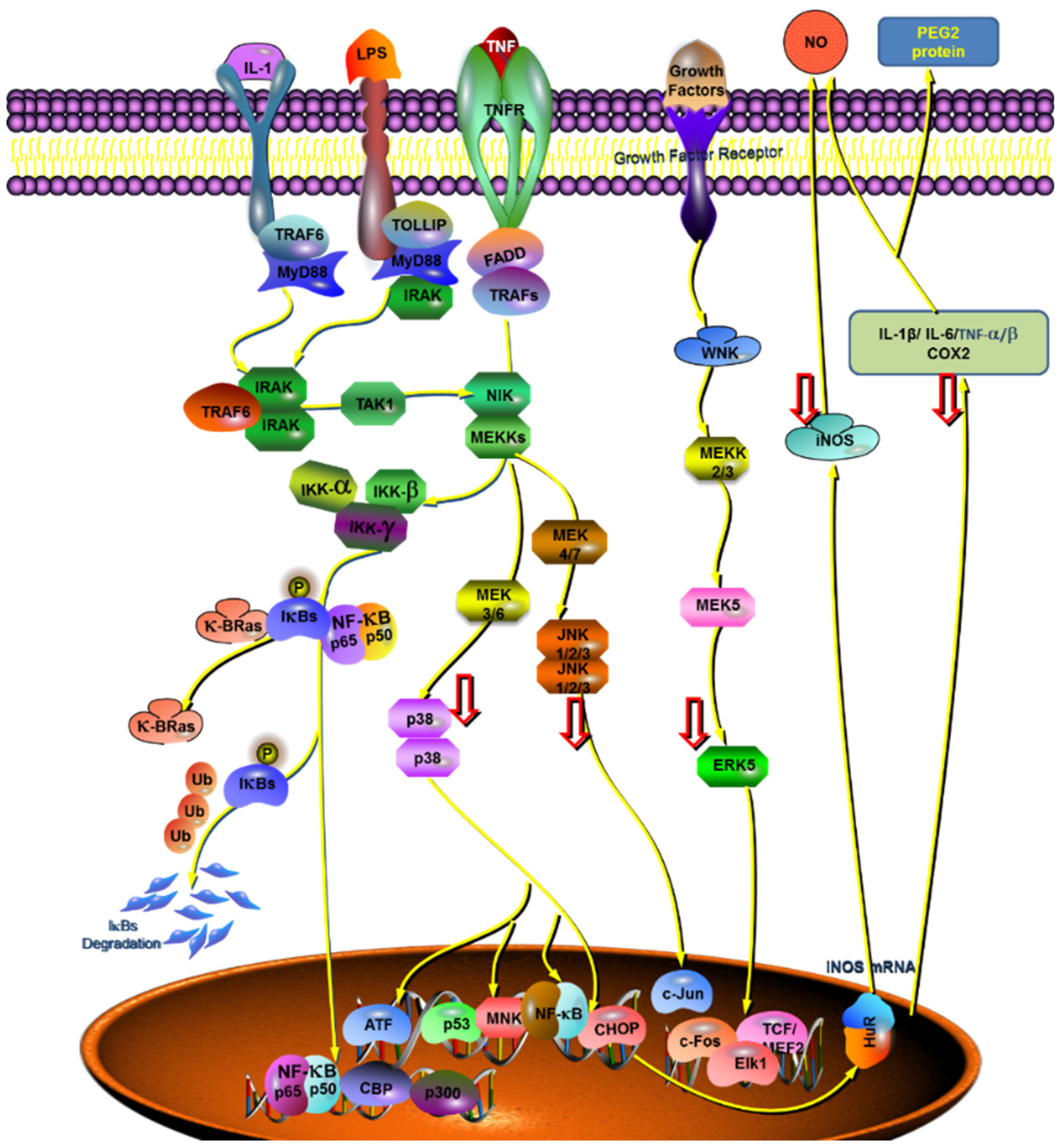
2.4. Effect of the Complex on the Expression of Key Genes in the Anti-Inflammatory Pathway
3. Experimental Method
3.1. Experimental Reagent and Instruments
3.1.1. Experimental Reagents
3.1.2. Cell Lines
3.1.3. Experimental Instruments
3.2. Preparation and HPLC-MS/MS Analysis of Typical Functional Components of EGB
3.3. Isoradiometric Analysis
3.4. Cell passage Culture Method
3.5. Determination of NO Release by Griess Method
3.6. Extraction of Total Cell Protein
3.7. BCA total Protein Quantitative Method
3.8. Western Blotting
3.9. Reverse PCR (RT-PCR)
3.10. Statistical Analysis Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, Y.; Lihu, Z.; Wu, H.; Su, E.; Zhao, L.; Xiao, W. Progress in the Study of Functional Groups and Health Care Efficacy of Ginkgo biloba Leaves. Food Res. Dev. 2020, 41, 6. [Google Scholar]
- Zhang, L.H.; Ting-Ting, W.U.; Zhao, L.G.; Xiao, W.; Zhang, S.H.; Wang, C.X.; Zhao, Y.W. Advances in Studies on Anticancer Activity of Flavonoids from Ginkgo biloba Extract. Chin. Pharm. J. 2019, 54, 444–449. [Google Scholar]
- Gargouri, B.; Carstensen, J.; Bhatia, H.S.; Huell, M.; Dietz, G.P.; Fiebich, B.L. Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cells. Phytomedicine 2018, 44, 45–55. [Google Scholar] [CrossRef]
- Luo, C.; Fan, L.H.; Zhang, H.; Zhao, J.; Li, L.; Zhang, L.; Zhang, H.X.; Ma, M.M. Effects of ginkgo biloba extract on the cognitive function and expression profile of inflammatory factors in a rat model of hemorrhagic stroke. Neuroreport 2018, 29, 1239–1243. [Google Scholar] [CrossRef]
- Wan, W.; Zhang, C.; Danielsen, M.; Li, Q.; Chen, W.; Yuanjin CH, A.N.; Li, Y. EGb761 improves cognitive function and regulates inflammatory responses in the APP/PS1 mouse. Exp. Gerontol. 2016, 81, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Silva, A.C.; Lima, D.D.; Magro, D.; Cruz, J.N. Effects of Ginkgo biloba extract (EGb 761) and repeated swimming on memory, anxiety and motor activity of rats. Rev. Cienc. Farm. Basica Apl. 2010, 31, 149–155. [Google Scholar]
- Cheng, J.-T.; Guo, C.; Cui, W.-J.; Zhang, Q.; Wang, S.-H.; Zhao, Q.-H.; Liu, D.-W.; Zhang, J.; Chen, S.; Chen, C.; et al. Isolation of two rare N-glycosides from Ginkgo biloba and their anti-inflammatory activities. Sci. Rep. 2020, 10, 5994. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Xiao, W.; Wang, Z.; Ding, G.; Zhao, L. Enrichment and Purification of Total Ginkgo Flavonoid O-Glycosides from Ginkgo Biloba Extract with Macroporous Resin and Evaluation of Anti-Inflammation Activities In Vitro. Molecules 2018, 23, 1167. [Google Scholar] [CrossRef]
- Lee, E.-J.; Shin, S.-Y.; Kim, J.-K.; Woo, E.-R.; Kim, Y.-M. Anti-inflammatory Effects of Amentoflavone on Modulation of Signal Pathways in LPS-stimulated RAW264.7 Cells. Bull. Korean Chem. Soc. 2012, 33, 2878–2882. [Google Scholar] [CrossRef]
- Yan, Z.; Dong, X. Abstract 5568: Ginkgo biloba extract kaempferol induces apoptosis in human prostate cancer cells through downregulation of β-Catenin signaling. Cancer Res. 2011, 71 (Suppl. S8), 5568. [Google Scholar]
- Lim, H.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of anti-inflammatory biflavonoid, ginkgetin, on chronic skin inflammation. Biol. Pharm. Bull. 2006, 29, 1046–1049. [Google Scholar] [CrossRef]
- Zhang, L. The anti-inflammatory effects of total flavone of ginkgo biloba and its mechanism. Acta Univ. Med. Nahui 2001, 36, 350–352. [Google Scholar]
- Loggia, R.; Sosa, S.; Tubaro, A.; Bombardelli, E. Anti-Inflammatory Activity of Ginkgo biloba Flavonoids. Planta Med. 1993, 59, A588. [Google Scholar] [CrossRef]
- Li, X.; Huang, L.; Liu, G.; Fan, W.; Li, B.; Liu, R.; Wang, Z.; Fan, Q.; Xiao, W.; Li, Y. Ginkgo diterpene lactones inhibit cerebral ischemia/reperfusion induced inflammatory response in astrocytes via TLR4/NF-κB pathway in rats. J. Ethnopharmacol. 2020, 249, 112365. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Jin, W.; Ao, M.; Zhai, S.; Xu, H.; Yu, L. Evaluation of the anti-inflammatory properties of the active constituents in Ginkgo biloba for the treatment of pulmonary diseases. Food Funct. 2019, 10, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Quispe, C.; Jamaddar, S.; Hossain, R.; Ray, P.; Mondal, M.; Mohamed, Z.A.; Jaafaru, M.S.; Salehi, B.; Islam, M.T.; et al. Therapeutic promises of ginkgolide A: A literature-based review. Biomed. Pharmacother. 2020, 132, 110908. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, Q.; Chen, D.; Yin, Q.; Zhang, W. Effects of Ginkgolide B on the Proliferation and Apoptosis of Cervical Cancer Cells. Curr. Top. Nutraceutical Res. 2020, 18, 227–232. [Google Scholar]
- Ge, Y.; Xu, W.; Zhang, L.; Liu, M. Ginkgolide B attenuates myocardial infarction-induced depression-like behaviors via repressing IL-1β in central nervous system. Int. Immunopharmacol. 2020, 85, 106652. [Google Scholar] [CrossRef]
- Xiong, L.D.; Tang, J.; Li, L.I. Protective effects of bilobalide and ginkgolides B on oxidative damage in human keratinocytes induced by H2O2. China Surfactant Deterg. Cosmet. 2019, 49, 531–536. [Google Scholar]
- Hu, H.; Yan, L.; Xin, Z.; Zhanga, X. Ginkgolide B exerts anti-inflammatory and chondroprotective activity in LPS-induced chondrocytes. Adv. Clin. Exp. Med. 2018, 27, 913–920. [Google Scholar] [CrossRef]
- Chu, X.; Ci, X.; He, J.; Wei, M.; Yang, X.; Ca, O.; Li, H.; Guan, S.; Deng, Y.; Pang, D.; et al. A Novel Anti-Inflammatory Role for Ginkgolide B in Asthma via Inhibition of the ERK/MAPK Signaling Pathway. Molecules 2011, 16, 7634–7648. [Google Scholar] [CrossRef] [PubMed]
- Rui, Z.; Dan, H.; Li, Z.; Shen, C.; Zhang, Y.; Li, J.; Yan, G.; Li, S.; Hu, B.; Li, J. Ginkgolide C Alleviates Myocardial Ischemia/Reperfusion-Induced Inflammatory Injury via Inhibition of CD40-NF-κB Pathway. Front. Pharmacol. 2018, 9, 109. [Google Scholar]
- Zhao, Y.; Chen, Y.; Wang, J.; Zhu, X.; Wang, K.; Li, Y.; Zhou, F. Ginkgolide J protects human synovial cells SW982 via suppression of p38-dependent production of pro-inflammatory mediators. Mol. Med. Rep. 2021, 24, 1–7. [Google Scholar] [CrossRef]
- Yu, W.B.; Wang, Q.; Chen, S.; Cao, L.; Tang, J.; Ma, C.G.; Xiao, W.; Xiao, B.G. The therapeutic potential of ginkgolide K in experimental autoimmune encephalomyelitis via peripheral immunomodulation. Int. Immunopharmacol. 2019, 70, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Zhang, X.; Yin, J.; Ren, S.; Han, Q.; Yu, J.; Li, J.; Yu, J.; Xiao, B.; Ma, C. The neuroprotective effect of ginkgolide K on myelin sheath by inhibiting microglia-mediated inflammatory responses. J. Shanxi Coll. Tradit. Chin. Med. 2019, 20, 250–253. [Google Scholar]
- Meng, C.; Zou, W.; Chen, M.; Cao, L.; Ding, J.; Wei, X.; Gang, H. Ginkgolide K Promotes Angiogenesis in a middle cerebral artery occlusion mouse model via activating JAK2/STAT3 Pathway. Eur. J. Pharmacol. 2018, 833, 221–229. [Google Scholar]
- Zhang, L.H.; Dong, L.I.; Xiao, W.; Wang, Z.Z.; Ding, G.; Zhao, L.G.; University, N.F. Prediction of Anti-inflammatory Mechanism of Ginkgo Folium Extract Based on Molecular Docking and Network Pharmacology. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 192–198. [Google Scholar]
- Zhang, L.; Zhu, C.; Liu, X.; Su, E.; Cao, F.; Zhao, L. Study on Synergistic Antioxidant Effect of Typical Functional Components of Hydroethanolic Leaf Extract from Ginkgo Biloba In Vitro. Molecules 2022, 27, 439. [Google Scholar] [CrossRef]
- Jun, C.; Luyao, C.; Mohan, L.; Fuliang, C.; Linguo, Z.; Erzheng, S. Efficient extraction of proanthocyanidin from Ginkgo biloba leaves employing rationally designed deep eutectic solvent-water mixture and evaluation of the antioxidant activity. J. Pharm. Biomed. Anal. 2018, 158, 317–326. [Google Scholar]
- Cao, J.; Chen, L.; Li, M.; Cao, F.; Zhao, L.; Su, E. Two-phase systems developed with hydrophilic and hydrophobic deep eutectic solvents for simultaneously extracting various bioactive compounds with different polarities. Green Chem. 2018, 20, 1879–1886. [Google Scholar] [CrossRef]
- Cao, J.; Yang, M.; Cao, F.; Wang, J.; Su, E. Well-designed hydrophobic deep eutectic solvents as green and efficient media for the extraction of artemisinin from Artemisia annua leaves. ACS Sustain. Chem. Eng. 2017, 5, 3270–3278. [Google Scholar] [CrossRef]
- Eo, H.J.; Da, S.K.; Park, G.H. Rhamnus crenata leaf extracts exhibit anti-inflammatory activity via modulating the Nrf2/HO-1 and NF-κB/MAPK signaling pathways. Asian Pac. J. Trop. Biomed. 2022, 12, 7. [Google Scholar]
- Kwak, G.-Y.; Han, Y.; Baik, S.; Kong, B.-M.; Yang, D.-C.; Kang, S.-C.; Sukweenadhi, J. Gold Nanoparticles Green-Synthesized by the Suaeda japonica Leaf Extract and Screening of Anti-Inflammatory Activities on RAW 267.4 Macrophages. Coatings 2022, 12, 460. [Google Scholar] [CrossRef]
- Peng, F.; Yin, H.; Du, B.; Niu, K.; Yang, Y.; Wang, S. Anti-inflammatory effect of flavonoids from chestnut flowers in lipopolysaccharide-stimulated RAW 264.7 macrophages and acute lung injury in mice. J. Ethnopharmacol. 2022, 290, 115086. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, C. Inhibition of LPS-induced NO production by taurine chloramine in macrophages is mediated though Ras-ERK-NF-κB. Biochem. Pharmacol. 2005, 70, 1352–1360. [Google Scholar] [CrossRef]
- Pande, V.; Ramos, M.J. Molecular recognition of 15-deoxy-delta(12,14)-prostaglandin J2 by nuclear factor-kappa B and other cellular proteins. Bioorganic Med. Chem. Lett. 2005, 15, 4057–4063. [Google Scholar] [CrossRef] [PubMed]
- Connelly, L.; Jacobs, A.T.; Palacios-Callender, M.; Moncada, S.; Hobbs, A. Macrophage Endothelial Nitric-oxide Synthase Autoregulates Cellular Activation and Pro-inflammatory Protein Expression. J. Biol. Chem. 2003, 278, 26480–26487. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cho, B.J.; Park, T.W.; Park, B.E.; Kim, S.J.; Sim, S.S.; Kim, C.J. Dibenzylbutyrolactone lignans from Forsythia koreana fruits attenuate lipopolysaccharide-induced inducible nitric oxide synthetase and cyclooxygenase-2 expressions through activation of nuclear factor-κb and mitogen-activated protein kinase in RAW264.7 cells. Biol. Pharm. Bull. 2010, 33, 1847–1853. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]

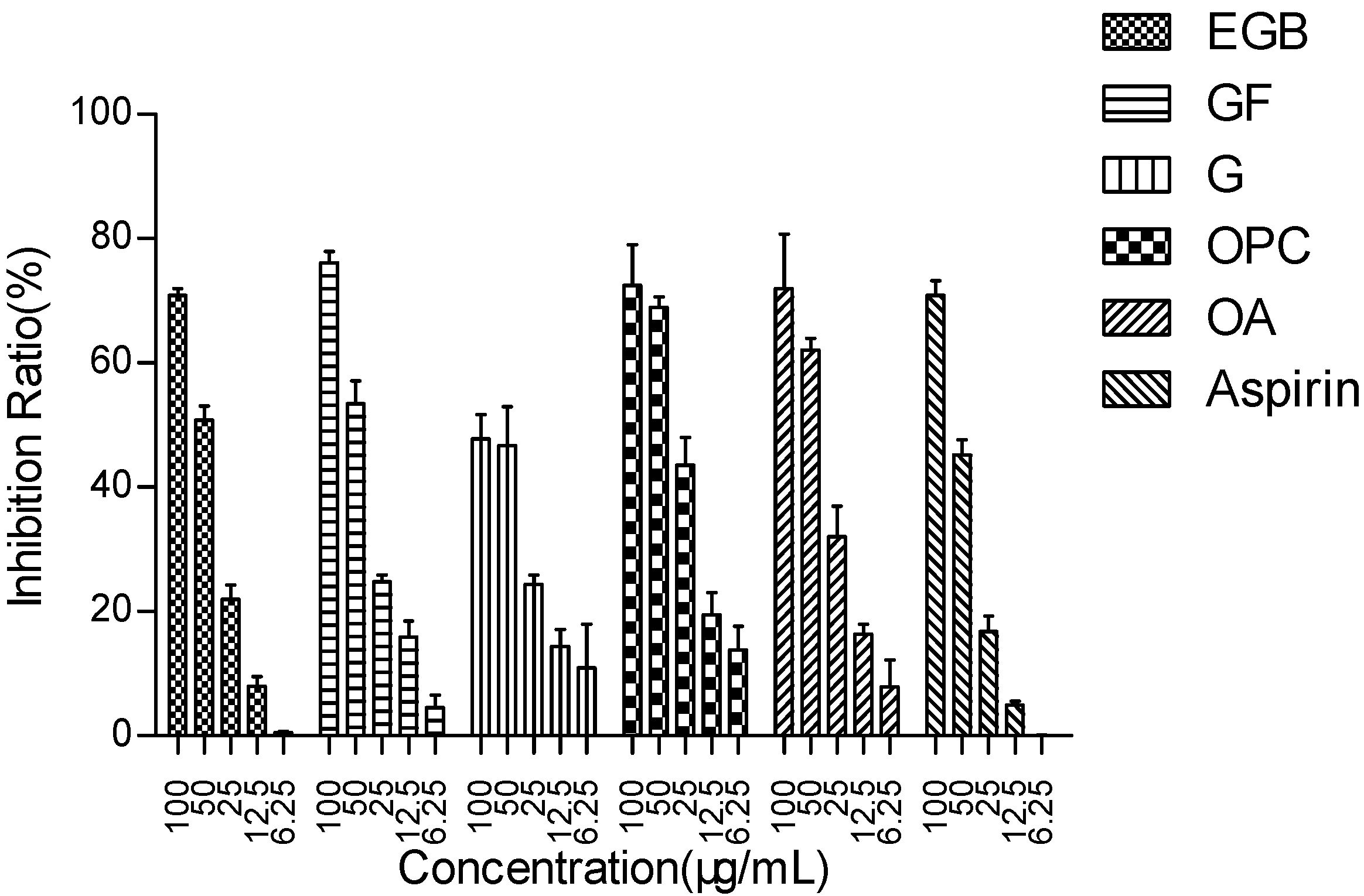


| Components | EGB | GF | G | OPC | OA | Aspirin |
|---|---|---|---|---|---|---|
| IC50 (μg/mL) | 53.15 ± 1.62 | 46.72 ± 1.50 | 89.19 ± 0.80 | 32.56 ± 1.18 | 41.17 ± 1.31 | 58.46 ± 1.81 |
| Class of Complex | Proportion | The IC50 Value of Inhibiting NO Release in Raws264.7 Cells (μg/mL) | |||
|---|---|---|---|---|---|
| Theoretical Values | Measured Values | γ | P (95%) | ||
| GF + G | 9:1 | 81.76 | 45.68 ± 1.21 | 0.56 | 0.008–0.022 |
| 7:3 | 70.08 | 34.09 ± 0.76 | 0.49 | 0.011–0.037 | |
| 1:1 | 61.32 | 31.71 ± 1.03 | 0.52 | 0.032–0.055 | |
| 3:7 | 54.51 | 29.13 ± 0.66 | 0.53 | 0.009–0.017 | |
| 1:9 | 49.06 | 25.82 ± 0.81 | 0.53 | 0.011–0.026 | |
| GF + OPC | 9:1 | 45.00 | 42.59 ± 1.26 | 0.95 | 0.015–0.037 |
| 7:3 | 41.33 | 45.50 ± 1.31 | 1.10 | 0.006–0.018 | |
| 1:1 | 38.38 | 48.84 ± 1.52 | 1.27 | 0.027–0.046 | |
| 3:7 | 35.82 | 57.80 ± 1.18 | 1.61 | 0.041–0.082 | |
| 1:9 | 33.58 | 60.95 ± 2.01 | 1.82 | 0.021–0.035 | |
| GF + OA | 9:1 | 48.67 | 37.49 ± 0.91 | 0.81 | 0.008–0.022 |
| 7:3 | 44.90 | 26.68 ± 1.09 | 0.59 | 0.010–0.024 | |
| 1:1 | 43.77 | 22.71 ± 0.39 | 0.52 | 0.013–0.031 | |
| 3:7 | 42.69 | 21.40 ± 0.72 | 0.48 | 0.008–0.016 | |
| 1:9 | 41.66 | 11.24 ± 0.41 | 0.24 | 0.007–0.025 | |
| OPC + G | 9:1 | 34.77 | 52.62 ± 2.16 | 1.51 | 0.031–0.047 |
| 7:3 | 40.22 | 49.57 ± 1.20 | 1.23 | 0.014–0.026 | |
| 1:1 | 47.70 | 36.10 ± 0.91 | 0.76 | 0.020–0.047 | |
| 3:7 | 58.61 | 29.84 ± 0.63 | 0.51 | 0.009–0.015 | |
| 1:9 | 75.98 | 26.66 ± 0.77 | 0.35 | 0.013–0.028 | |
| OPC + OA | 9:1 | 40.11 | 53.18 ± 2.13 | 1.60 | 0.012–0.026 |
| 7:3 | 38.14 | 45.32 ± 1.45 | 1.30 | 0.031–0.048 | |
| 1:1 | 36.36 | 46.07 ± 1.38 | 1.27 | 0.033–0.080 | |
| 3:7 | 34.74 | 24.16 ± 0.29 | 0.68 | 0.012–0.047 | |
| 1:9 | 33.26 | 24.21 ± 0.41 | 0.67 | 0.013–0.024 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Fang, X.; Sun, J.; Su, E.; Cao, F.; Zhao, L. Study on Synergistic Anti-Inflammatory Effect of Typical Functional Components of Extracts of Ginkgo Biloba Leaves. Molecules 2023, 28, 1377. https://doi.org/10.3390/molecules28031377
Zhang L, Fang X, Sun J, Su E, Cao F, Zhao L. Study on Synergistic Anti-Inflammatory Effect of Typical Functional Components of Extracts of Ginkgo Biloba Leaves. Molecules. 2023; 28(3):1377. https://doi.org/10.3390/molecules28031377
Chicago/Turabian StyleZhang, Lihu, Xianying Fang, Jihu Sun, Erzheng Su, Fuliang Cao, and Linguo Zhao. 2023. "Study on Synergistic Anti-Inflammatory Effect of Typical Functional Components of Extracts of Ginkgo Biloba Leaves" Molecules 28, no. 3: 1377. https://doi.org/10.3390/molecules28031377





