Study on the Effect of Different Viscosity Reducers on Viscosity Reduction and Emulsification with Daqing Crude Oil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Viscosity Reduction Rate
- (1)
- The Effect of Viscosity Reducer Type and Concentration on Viscosity Reduction Rate
- (2)
- The Effect of Oil–Water Ratio on Viscosity Reduction Rate
- (3)
- The Effect of Polymer on Viscosity Reduction Rate
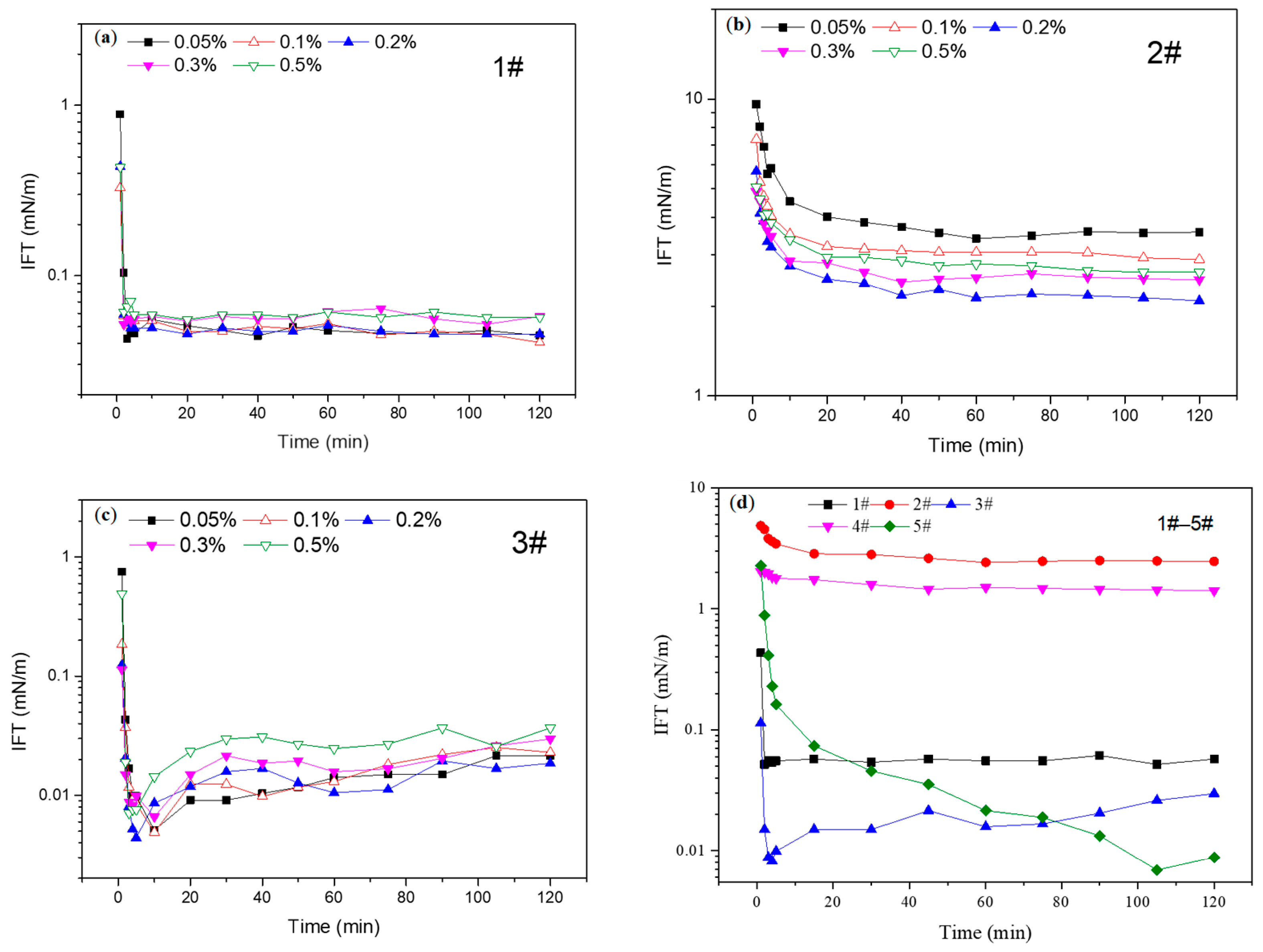
2.2. Interfacial Tension
- (1)
- The Effect of Viscosity Reducer Type and Concentration on Interfacial Tension
- (2)
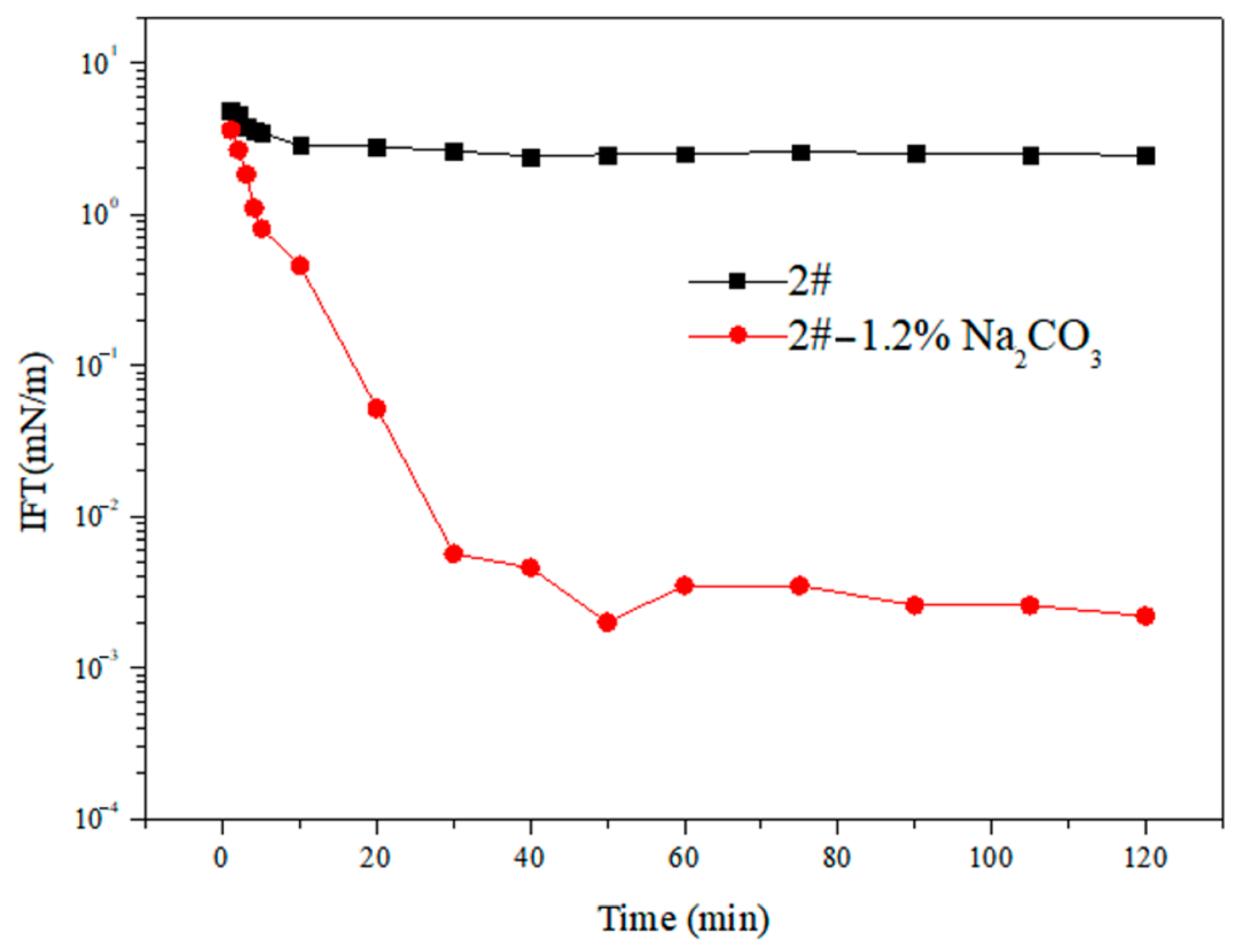
- The Effect of Alkali on Interfacial Tension
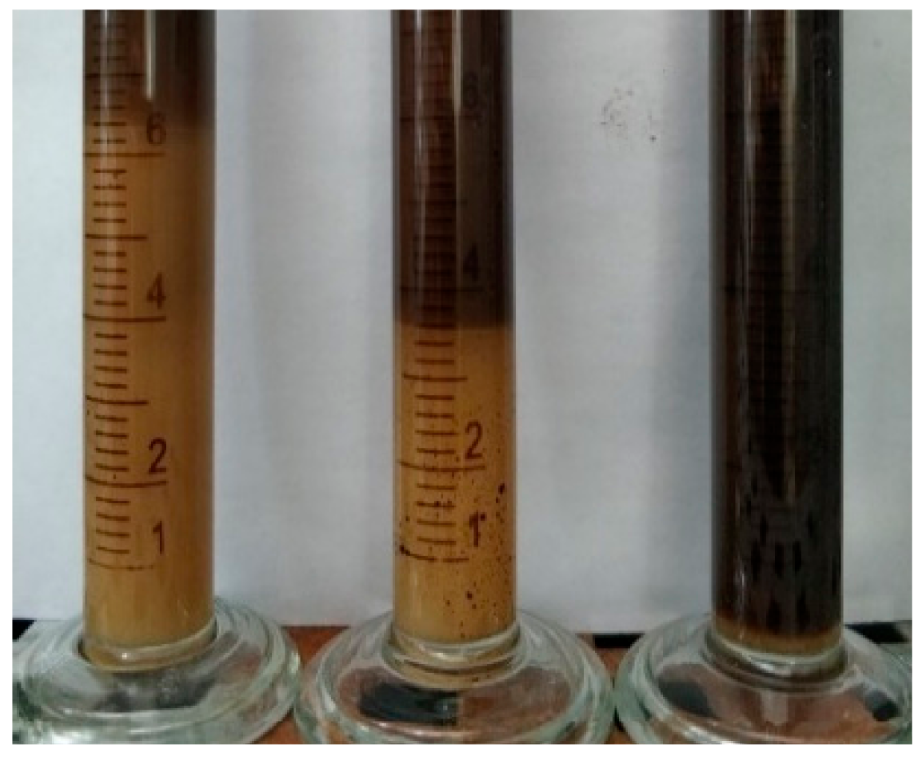
2.3. Water Separation Rate
- (1)
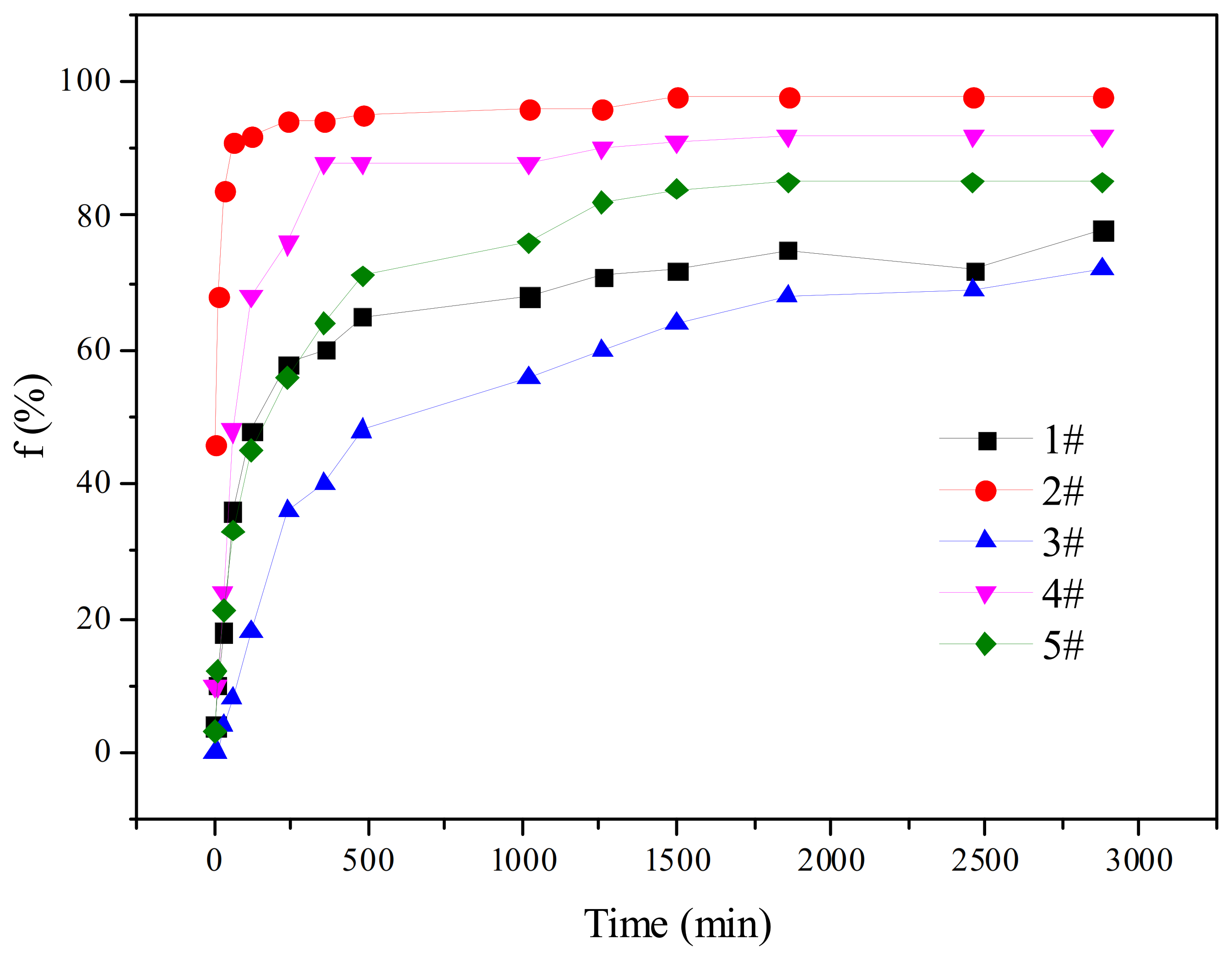
- The Effect of Viscosity Reducer Type on Water Separation Rate
- (2)
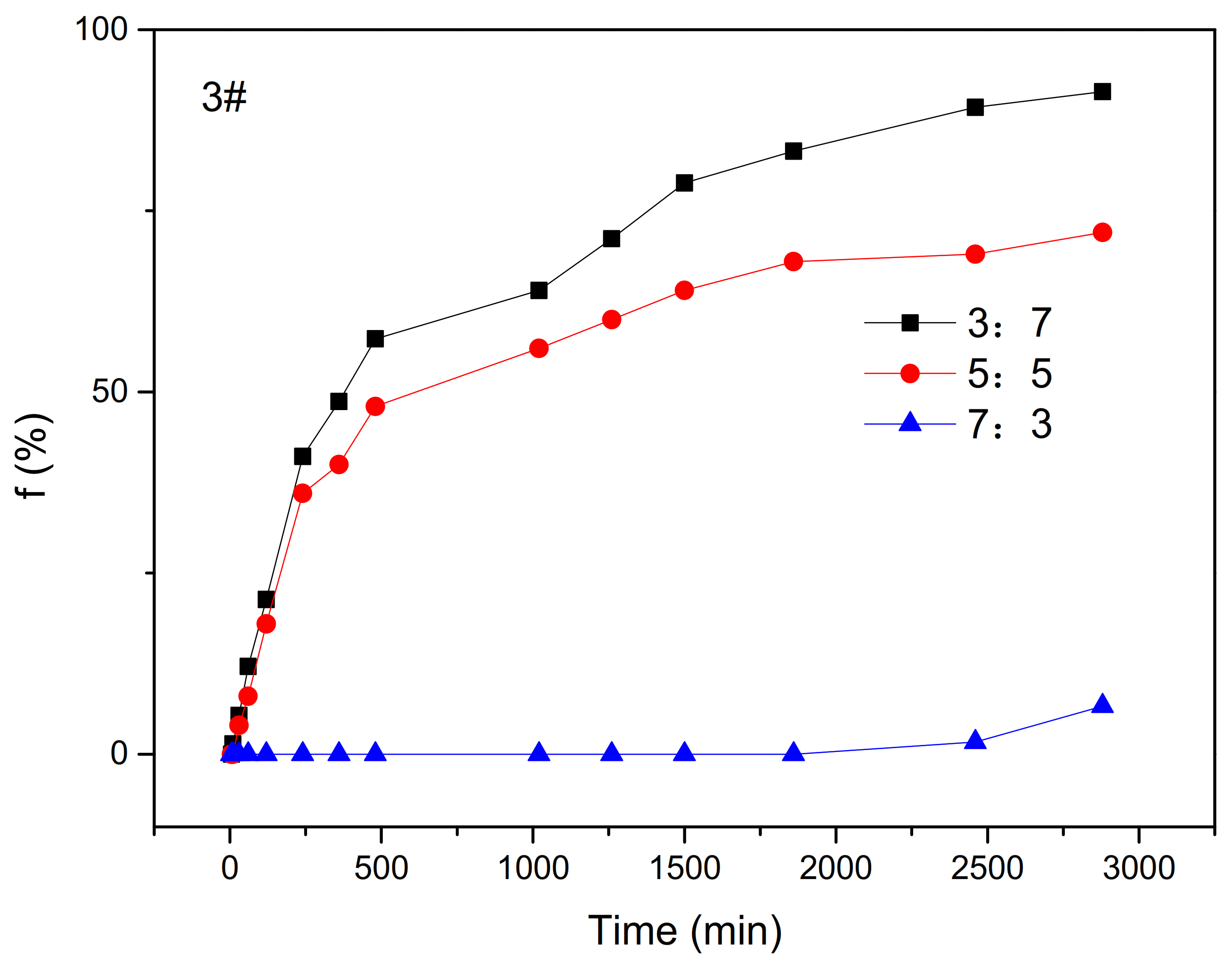
- The Effect of Oil–Water Ratio on Water Separation Rate
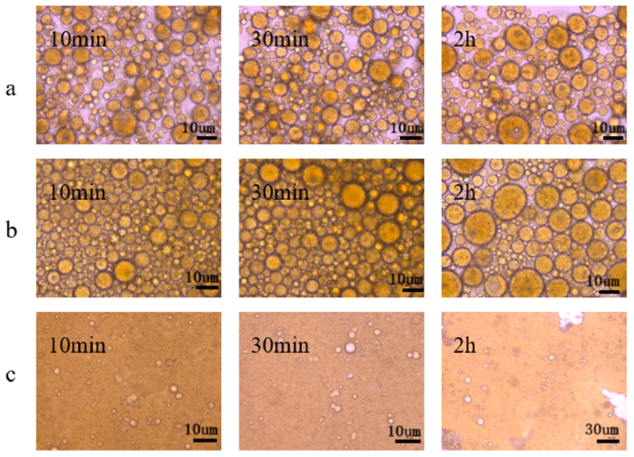
2.4. Microstructure
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Experimental Method
3.2.1. Viscosity Reduction Rate
3.2.2. Interfacial Tension
3.2.3. Water Separation Rate
3.2.4. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Soni, H.P.; Harambe, D.P.; Nagar, A.; Kiranbala. Synthesis of chemical additives and their effect on akholjuni crude oil (Gujarat, India). Indian J. Chem. Technol. 2005, 12, 55–61. [Google Scholar]
- Malkin, A.Y.; Zadymova, N.M.; Skvortsova, Z.N.; Traskine, V.Y.; Kulichikhin, V.G. Formation of concentrated emulsions in heavy oil. Colloid Surf. A 2016, 504, 343–349. [Google Scholar] [CrossRef]
- Hinkle, A.; Shin, E.J.; Liberatore, M.W.; Herring, A.M.; Batzle, M. Correlating the chemical and physical properties of a set of heavy oils from around the world. Fuel 2008, 87, 3065–3070. [Google Scholar] [CrossRef]
- Azimi, A.; Arabkhalaj, A.; Markadeh, R.S.; Ghassemi, H. Fully transient modeling of the heavy fuel oil droplets evaporation. Fuel 2018, 230, 52–63. [Google Scholar] [CrossRef]
- Pfeiffer, J.P.; Saal, R. Asphaltic bitumen as colloid system. J. Phys. Chem. 1940, 44, 139–149. [Google Scholar] [CrossRef]
- Taborda, E.A.; Franco, C.A.; Lopera, S.H.; Alvarado, V.; Cortes, F.B. Effect of nanoparticles/nanofluids on the rheology of heavy crude oil and its mobility on porous media at reservoir conditions. Fuel 2016, 184, 222–232. [Google Scholar] [CrossRef]
- Choi, S.; Choi, S.Q.; Kim, J.-D.; Nho, N.S. Partially oxidized asphaltene as a bitumen viscosity reducer. Energy Fuel. 2017, 31, 9240–9246. [Google Scholar] [CrossRef]
- Santos, I.C.V.M.; Martelloti, R.R.; Oliveira, P.F.; Mansur, C.R.E. Development of microemulsions to reduce the viscocity of crude oil emulsions. Fuel 2017, 210, 684–694. [Google Scholar] [CrossRef]
- Sun, S.; Luo, Y.; Zhou, Y.; Xiao, M.; Zhang, Z.; Hou, J.; Wei, X.; Xu, Q.; Sha, T.; Dong, H.; et al. Application of bacillus spp. in pilot test of microbial huff and puff to improve heavy oil recovery. Energy Fuel 2017, 31, 13724–13732. [Google Scholar] [CrossRef]
- Liu, J.W.; Peng, Y.K.; Zhang, Z.Y.; Zhao, J.Z. Quadripolymers as viscosity reducer for heavy oil. Energy Fuel 2018, 32, 119–124. [Google Scholar]
- Wang, X. Application of chemical flooding in increasing yield of tertiary oil recovery. IOP Conf. Ser. Earth Environ. Sci. 2021, 781, 022066. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Liu, G. Review on new heavy oil viscosity reduction technologies. IOP Conf. Ser. Earth Environ. Sci. 2022, 983, 012059. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, R.Q.; Tang, Y.W.; Zhu, J.F.; Sun, Y.H.; Zhang, G.H. Synthesis of polycarboxylate viscosity reducer and the effect of different chain lengths of polyether on viscosity reduction of heavy oil. Polymers 2022, 14, 3367. [Google Scholar] [CrossRef]
- Xiao, S.S.; Sun, Y.; Wang, S.; Wu, C.; Song, H. Study on polyether carboxylate surfactant as viscosity reducer for heavy oil recovery. Energy Chem. Ind. 2018, 39, 49–54. [Google Scholar]
- Qin, B.P.; Jing, Z. The relationship between the composition and emulsification performance of the condensates with -COOH, -SO3H, -(OCH2CHC)n-groups. Pet. Process. Petrochem. 2002, 33, 32–36. [Google Scholar]
- Zhao, Y.; Zhao, L.; Chang, G.; Chen, H.; Hao, L.; Zhao, N.; Zhao, C.; Geng, C.; Yang, W.; Li, Z. Fabrication of surfactant-biopolymer combined system with dual viscosity reduction and mobility controllability for heavy oil reservoirs. J. Mol. Liq. 2022, 368, 120777. [Google Scholar] [CrossRef]
- Xiong, S.C.; He, Y.; Cui, M.L. Petroleum sulfonates as oil displacement agent and application. Opt. Electron. Mater. Appl. II 2012, 529, 512. [Google Scholar] [CrossRef]
- Yue, X.Y.; Floor, Z.H.; Han, D.; Yuan, S.Y. Application of petroleum sulfonate viscosity reducer in tertiary oil recovery. Prog. Fine Petrochem. Ind. 2005, 2, 48–52. [Google Scholar]
- Xiong, S.; Liu, X.; Liu, W.; He, Y.; Ruan, X. Petroleum sulfonates as oil displacement agents and application to gudao oil field. Petrol. Sci. Technol. 2009, 27, 357–367. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zhou, D.P.; Luo, Y.Y. Oil/water interfacial tension of shengli petroleum sulfonate/alpha-olefin or polyethyleneoxy ether sulfonate binary systeme. Tenside Surfact. Det. 2015, 52, 120–125. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, M.Z.; Yue, X.G.; Hou, J.R. Synergy of alkali and surfactant in emulsification of heavy oil in brine. Colloid Surf. A 2006, 273, 219–228. [Google Scholar] [CrossRef]
- Bai, Y.R.; Xiong, C.M.; Shang, X.S.; Xin, Y.Y. experimental study on ethanolamine/surfactant flooding for enhanced oil recovery. Energy Fuel 2014, 28, 1829–1837. [Google Scholar] [CrossRef]
- Xia, L.X.; Cao, G.Y.; Lu, S.W.; Zhang, L.; Yu, J.Y. Research progress on stability and demulsification of crude oil emulsions. Chem. Res. Appl. 2002, 6, 623–627. [Google Scholar]
- Jirui, H.; Liu, Z.C.; Yue, X.G. Composite system of ultra-low interfacial tension and the actual role of alkali in oil displacement. Pet. Geol. Oilfield Dev. Daqing 2006, 6, 82–86+124. [Google Scholar]
| Viscosity Reducer | Viscosity/mPa·s | Mass Concentration/% | Viscosity Reduction Rate/% |
|---|---|---|---|
| petroleum sulfonate (2#) | 7.8 | 0.05 | 61.57 |
| petroleum sulfonate (2#) | 5.6 | 0.1 | 72.41 |
| petroleum sulfonate (2#) | 5.4 | 0.2 | 73.40 |
| petroleum sulfonate (2#) | 4.7 | 0.3 | 76.85 |
| petroleum sulfonate (2#) | 4.6 | 0.5 | 77.34 |
| carboxylate polyoxyethylene ether (1#) | 11.5 | 0.3 | 43.35 |
| erucamide oxide (3#) | 7.7 | 0.3 | 62.07 |
| alkyl xylene sulfonate (4#) | 17.3 | 0.3 | 14.78 |
| erucic betaine (5#) | 3.2 | 0.3 | 82.24 |
| Oil–Water Ratio | Mass Concentration/% | Viscosity/mPa·s | Viscosity Reduction Rate/% |
|---|---|---|---|
| 3:7 | 0.1 | 1.9 | 90.64 |
| 0.3 | 1.8 | 91.13 | |
| 0.5 | 1.6 | 92.12 | |
| 5:5 | 0.1 | 5.6 | 72.41 |
| 0.3 | 4.7 | 76.85 | |
| 0.5 | 4.6 | 77.34 | |
| 7:3 | 0.1 | 51.9 | −155.67 |
| 0.3 | 46.7 | −130.05 | |
| 0.5 | 43.2 | −112.81 |
| Polymer/ppm | 0 | 1000 | 1500 |
|---|---|---|---|
| Viscosity/mPa·s | 4.7 | 30.1 | 59.1 |
| Viscosity reduction rate/% | 76.85 | −48.28 | −191.13 |
| Ion | CaCl2 | MgCl2·6 H2O | NaCl | NaHCO3 | KCl | Na2SO4 | Na2CO3 | Total Content |
|---|---|---|---|---|---|---|---|---|
| Content/g·L−1 | 0.0282 | 0.0269 | 1.3066 | 1.3412 | 5.5907 | 0.0339 | 0.7995 | 7887.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Zhang, Q.; Zhou, Z.; Sun, L.; Zhou, Y. Study on the Effect of Different Viscosity Reducers on Viscosity Reduction and Emulsification with Daqing Crude Oil. Molecules 2023, 28, 1399. https://doi.org/10.3390/molecules28031399
Zhang F, Zhang Q, Zhou Z, Sun L, Zhou Y. Study on the Effect of Different Viscosity Reducers on Viscosity Reduction and Emulsification with Daqing Crude Oil. Molecules. 2023; 28(3):1399. https://doi.org/10.3390/molecules28031399
Chicago/Turabian StyleZhang, Fan, Qun Zhang, Zhaohui Zhou, Lingling Sun, and Yawen Zhou. 2023. "Study on the Effect of Different Viscosity Reducers on Viscosity Reduction and Emulsification with Daqing Crude Oil" Molecules 28, no. 3: 1399. https://doi.org/10.3390/molecules28031399
APA StyleZhang, F., Zhang, Q., Zhou, Z., Sun, L., & Zhou, Y. (2023). Study on the Effect of Different Viscosity Reducers on Viscosity Reduction and Emulsification with Daqing Crude Oil. Molecules, 28(3), 1399. https://doi.org/10.3390/molecules28031399






