Catalyst-Free Formal Conjugate Addition/Aldol or Mannich Multicomponent Reactions of Mixed Aliphatic Organozinc Reagents, π-Electrophiles and Michael Acceptors
Abstract
:1. Introduction
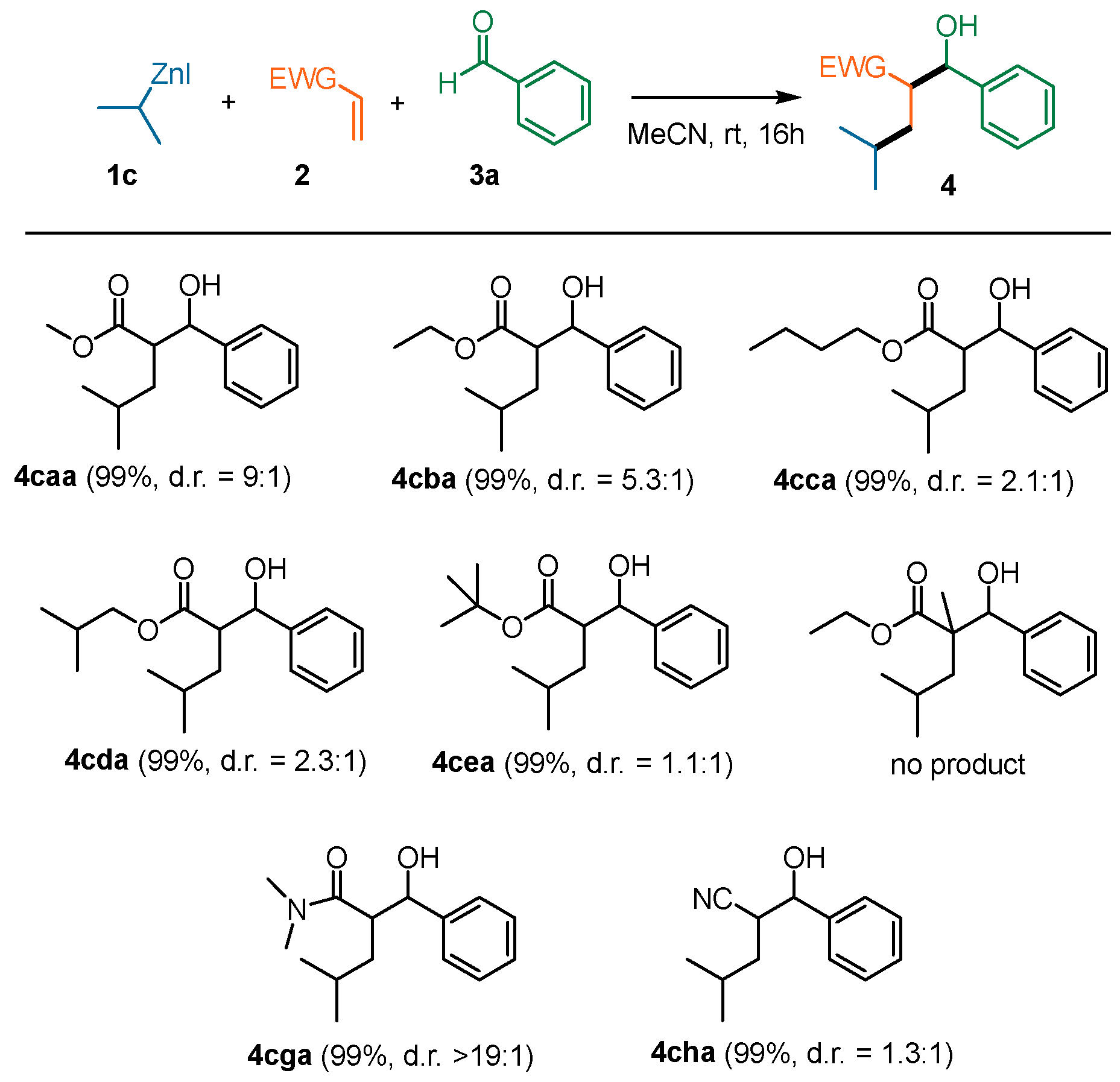
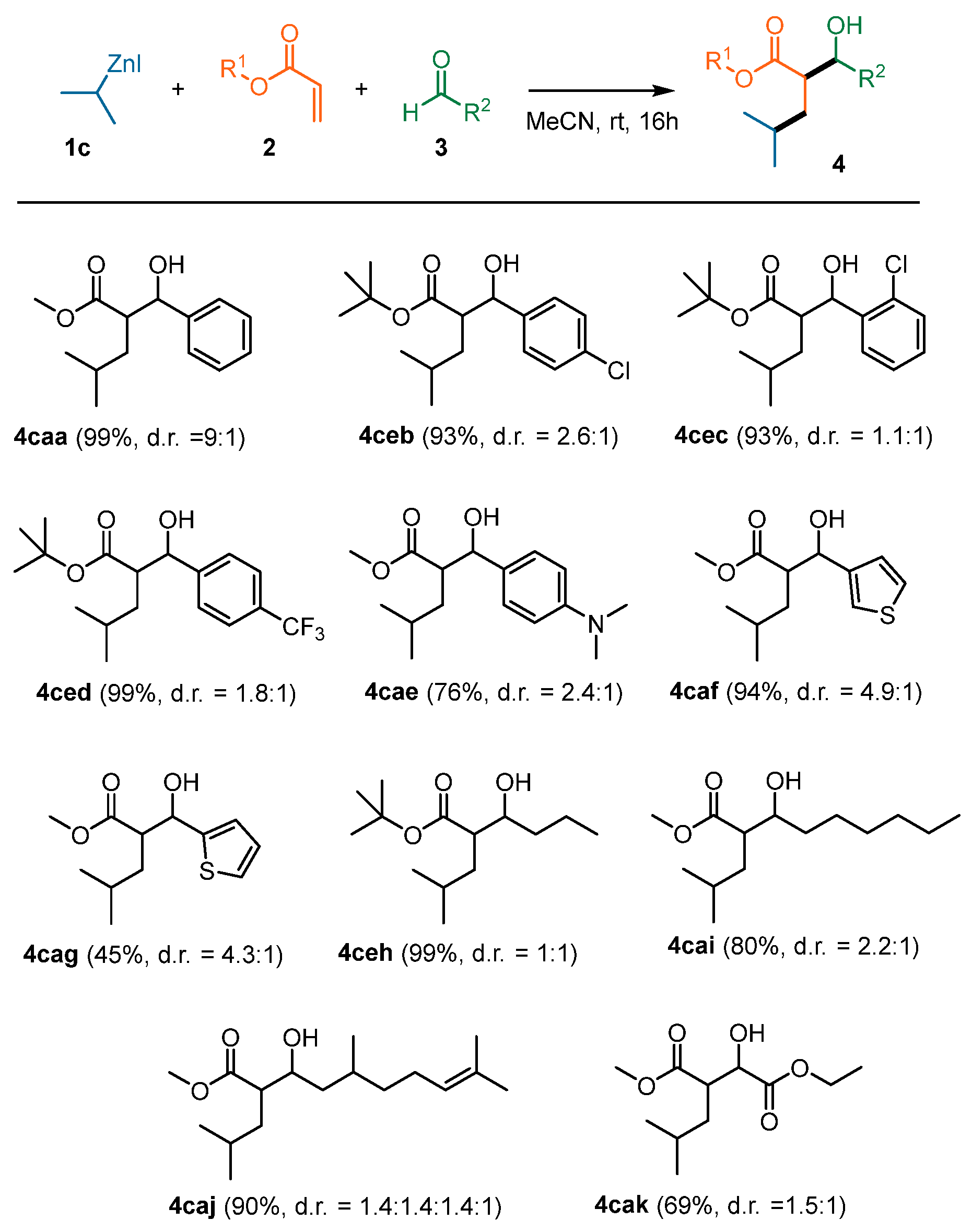
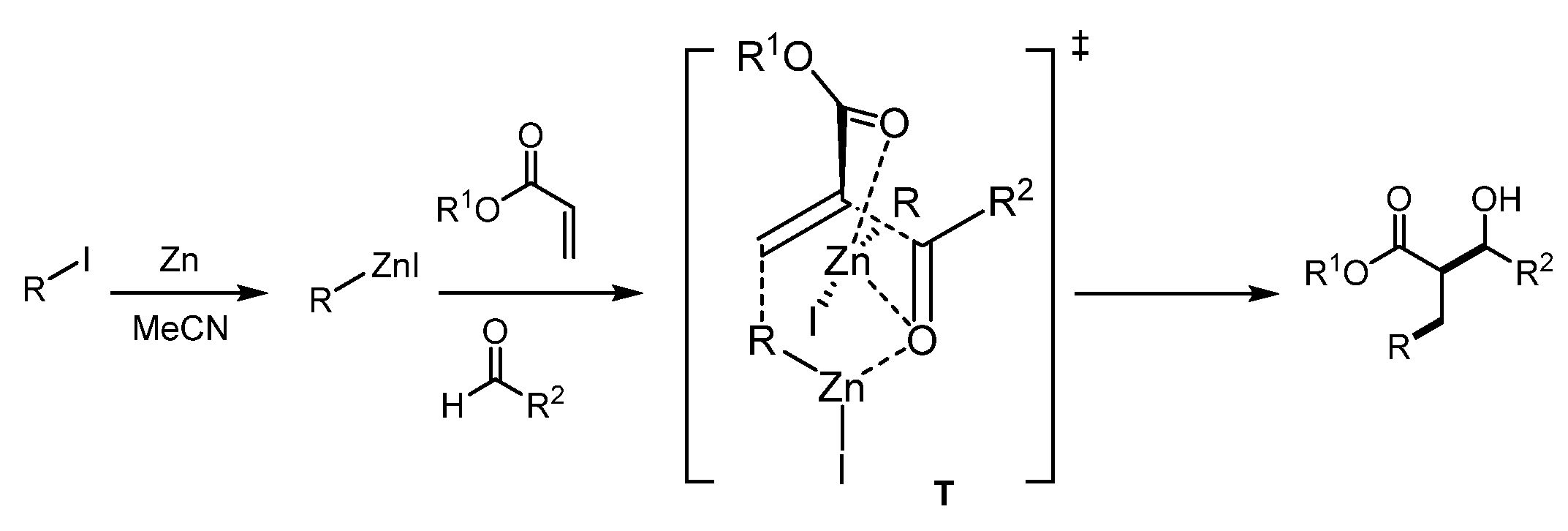
2. Results and Discussion
3. Materials and Methods
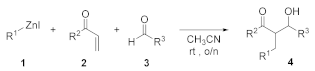
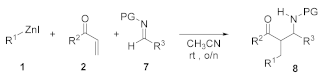
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Guo, H.-C.; Ma, J.-A. Catalytic asymmetric tandem transformations triggered by conjugate additions. Angew. Chem. Int. Ed. 2006, 45, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.J.; Frost, C.G. Tandem and domino catalytic strategies for enantioselective synthesis. Synthesis 2007, 2007, 1–21. [Google Scholar] [CrossRef]
- Galestokova, Z.; Sebesta, R. Domino Reactions Initiated by Enantioselective Cu-Catalyzed Conjugate Addition. Eur. J. Org. Chem. 2012, 2012, 6688–6695. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, Y.O.; Bouffard, J.; Lee, S.-G. Domino Reactions Initiated by Enantioselective Cu-Catalyzed Conjugate Addition. Chem. Soc. Rev. 2015, 44, 2489–2507. [Google Scholar] [CrossRef]
- Feringa, B.L.; Pineschi, M.; Arnold, L.A.; Imbos, R.; de Vries, A.H.M. Highly enantioselective catalytic conjugate addition and tandem conjugate addition–aldol reactions of organozinc reagents. Angew. Chem. Int. Ed. 1997, 36, 2620–2623. [Google Scholar] [CrossRef]
- Alexakis, A.; Trevitt, G.P.; Bernardinelli, G. Tandem enantioselective conjugate addition: Electrophile trapping reactions. application in the formation of syn or anti aldols. J. Am. Chem. Soc. 2001, 123, 4358–4359. [Google Scholar] [CrossRef]
- Aikawa, K.; Okamoto, T.; Mikami, K. Copper (I)-catalyzed asymmetric desymmetrization: Synthesis of five-membered-ring compounds containing all-carbon quaternary stereocenters. J. Am. Chem. Soc. 2012, 134, 10329–10332. [Google Scholar] [CrossRef]
- Howell, G.P.; Fletcher, S.P.; Geurts, K.; ter Horst, B.; Feringa, B.L. Catalytic asymmetric synthesis of acyclic arrays by tandem 1, 4-addition-aldol reactions. J. Am. Chem. Soc. 2006, 128, 14977–14985. [Google Scholar] [CrossRef]
- Mpango, G.B.; Mahalanabis, K.K.; Mahdavi-Damghani, Z.; Snieckus, V. Tandem conjugate addition-α-alkylation of unsaturated amides. Synthetic methodology. Tetrahedron Lett. 1980, 21, 4823–4826. [Google Scholar] [CrossRef]
- Dalai, S.; Limbach, M.; Zhao, L.; Tamm, M.; Sevvana, M.; Sokolov, V.V.; de Meijere, A. Highly diastereoselective sequential Michael-aldol reactions of methyl 2-chloro-2-cyclopropylideneacetate with Grignard reagents and aldehydes. Synthesis 2006, 2006, 471–479. [Google Scholar]
- Garcia, P.; Germain, N.; Woodward, S.; Alexakis, A. Copper-Catalyzed Asymmetric Conjugate Addition to α-Alkylidene Cycloalkanones. Synlett 2015, 26, 901–906. [Google Scholar]
- Marion, F.; Calvet, S.; Courillon, C.; Malacria, M. Highly stereoselective generation of α-pyrones displaying four contiguous stereogenic centers. Tetrahedron Lett. 2002, 43, 3369–3371. [Google Scholar] [CrossRef]
- Galeštoková, Z.; Šebesta, R. Diastereoselective Mannich reaction of chiral enolates formed by enantioselective conjugate addition of Grignard reagents. Eur. J. Org. Chem. 2011, 2011, 7092–7096. [Google Scholar] [CrossRef]
- Šebesta, R.; Bilčík, F.; Fodran, P. Enantioselective One-Pot Conjugate Addition of Grignard Reagents Followed by a Mannich Reaction. Eur. J. Org. Chem. 2010, 2010, 5666–5671. [Google Scholar] [CrossRef]
- Drusan, M.; Lölsberg, W.; Škvorcová, A.; Schmalz, H.-G.; Šebesta, R. TADDOL-Based Phosphane–Phosphite Ligands in Enantioselective Cu-Catalyzed Grignard 1, 4-Additions Followed by Mannich-Type Alkylations. Eur. J. Org. Chem. 2012, 2012, 6285–6290. [Google Scholar] [CrossRef]
- Bazin, S.; Feray, L.; Vanthuyne, N.; Siri, D.; Bertrand, M.P. α, β-Unsaturated diesters: Radical acceptors in dialkylzinc-mediated tandem radical addition/aldol condensation. A straightforward synthesis of rac-nephrosteranic acid. Tetrahedron 2007, 63, 77–85. [Google Scholar] [CrossRef]
- Bazin, S.; Feray, L.; Siri, D.; Naubron, J.-V.; Bertrand, M.P. Tandem radical addition–aldol condensations: Evidence for the formation of zinc enolates in diethylzinc mediated radical additions to N-enoyloxazolidinones. Chem. Commun. 2002, 21, 2506–2507. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Q.; Wang, M.-X. (Eds.) Multicomponent Reaction in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef]
- Sunderhaus, J.D.; Martin, S.F. Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds. Chem. Eur. J. 2009, 15, 1300–1308. [Google Scholar] [CrossRef]
- Biggs-Houck, J.E.; Younai, A.; Shaw, J.T. Recent advances in multicomponent reactions for diversity-oriented synthesis. Curr. Opin. Chem. Biol. 2010, 14, 371–382. [Google Scholar] [CrossRef]
- Ruijter, E.; Scheffelaar, R.; Orru, R.V.A. Multicomponent reaction design in the quest for molecular complexity and diversity. Angew. Chem. Int. Ed. 2011, 50, 6234–6246. [Google Scholar] [CrossRef] [PubMed]
- Dardennes, E.; Labano, S.; Simpkins, N.G.; Wilson, C. Michael addition–electrophilic quenching chemistry of maleimides using dialkylzinc reagents. Tetrahedron Lett. 2007, 48, 6380–6383. [Google Scholar] [CrossRef]
- Li, Z.; Li, R.; Gan, M.; Jiang, L.; Li, Z. Multicomponent reaction design in the quest for molecular complexity and diversity. Tetrahedron Lett. 2015, 56, 5541–5544. [Google Scholar] [CrossRef]
- Brown, M.K.; Degrado, S.J.; Hoveyda, A.H. Highly Enantioselective Cu-Catalyzed Conjugate Additions of Dialkylzinc Reagents to Unsaturated Furanones and Pyranones: Preparation of Air-Stable and Catalytically Active Cu–Peptide Complexes. Angew. Chem. Int. Ed. 2005, 44, 5306–5310. [Google Scholar] [CrossRef] [PubMed]
- Keller, E.; Maurer, J.; Naasz, R.; Schrader, T.; Meetsma, A.; Feringa, B.L. Unexpected enhancement of enantioselectivity in copper (II) catalyzed conjugate addition of diethylzinc to cyclic enones with novel TADDOL phosphorus amidite ligands. Tetrahedron Asymmetry 1998, 9, 2409–2413. [Google Scholar] [CrossRef]
- Kitamura, M.; Miki, T.; Nakano, K.; Noyori, R. Conjugate addition of diorganozincs to α, β-unsaturated ketones catalyzed by a copper (I)-sulfonamide combined system. Tetrahedron Lett. 1996, 37, 5141–5144. [Google Scholar] [CrossRef]
- Arnold, L.A.A.; Naasz, R.; Minnaard, A.J.; Feringa, B.L. Catalytic Enantioselective Synthesis of (−)-Prostaglandin E1 Methyl Ester Based on a Tandem 1, 4-Addition− Aldol Reaction. J. Org. Chem. 2002, 67, 7244–7254. [Google Scholar] [CrossRef]
- Arnold, L.A.; Naasz, R.; Minnaard, A.J.; Feringa, B.L. Catalytic enantioselective synthesis of prostaglandin E1 methyl ester using a tandem 1, 4-addition-aldol reaction to a cyclopenten-3, 5-dione monoacetal. J. Am. Chem. Soc. 2001, 123, 5841–5842. [Google Scholar] [CrossRef]
- Kitamura, M.; Miki, T.; Nakano, K.; Noyori, R. Copper-catalyzed enantioselective conjugate addition of diethylzinc to α, β-unsaturated carbonyl compounds using diphosphonites as chiral ligands. Bull. Soc. Chim. Jpn. 2000, 73, 999–1014. [Google Scholar] [CrossRef]
- Zhao, K.; Loh, T.-P. Asymmetric Conjugate Addition of Grignard Reagents to 3-Silyl Unsaturated Esters for the Facile Preparation of Enantioenriched β-Silylcarbonyl Compounds and Allylic Silanes. Chem. Eur. J. 2014, 20, 16764–16772. [Google Scholar] [CrossRef]
- Arisetti, N.; Reiser, O. Traceless stereoinduction for the enantiopure synthesis of substituted-2-cyclopentenones. Org. Lett. 2015, 17, 94–97. [Google Scholar] [CrossRef]
- Nozaki, K.; Oshima, K.; Utimoto, K. Facile routes to boron enolates. Et3B-mediated Reformatsky type reaction and three components coupling reaction of alkyl iodides, methyl vinyl ketone, and carbonyl compounds. Tetrahedron Lett. 1988, 29, 1041–1044. [Google Scholar] [CrossRef]
- Subburaj, K.; Montgomery, J. A new catalytic conjugate addition/aldol strategy that avoids preformed metalated nucleophiles. J. Am. Chem. Soc. 2003, 125, 11210–11211. [Google Scholar] [CrossRef] [PubMed]
- González-Gómez, J.C.; Foubelo, F.; Yus, M. Tandem enantioselective conjugate addition–Mannich reactions: Efficient multicomponent assembly of dialkylzincs, cyclic enones and chiral N-sulfinimines. Tetrahedron Lett. 2008, 49, 2343–2347. [Google Scholar] [CrossRef]
- Guo, S.; Xie, Y.; Hu, X.; Xia, C.; Huang, H. Diastereo-and Enantioselective Catalytic Tandem Michael Addition/Mannich Reaction: Access to Chiral Isoindolinones and Azetidines with Multiple Stereocenters. Angew. Chem. Int. Ed. 2010, 49, 2728–2731. [Google Scholar] [CrossRef]
- González-Gómez, J.C.; Foubelo, F.; Yus, M. Modular stereocontrolled assembly of R2Zn, cyclic enones and N-tert-butanesulfinyl imines. J. Org. Chem. 2009, 74, 2547–2553. [Google Scholar] [CrossRef]
- Casotti, G.; Ciancaleoni, G.; Lipparini, F.; Nieri, C.; Iuliano, A. Uncatalyzed conjugate addition of organozinc halides to enones in DME: A combined experimental/computational study on the role of the solvent and the reaction mechanism. Chem. Sci. 2020, 11, 257–263. [Google Scholar] [CrossRef]
- Knochel, P.; Singer, R.D. Preparation and reactions of polyfunctional organozinc reagents in organic synthesis. Chem. Rev. 1993, 93, 2117–2188. [Google Scholar] [CrossRef]
- Knochel, P.; Jones, P. Organozinc Reagents, a Practical Approach; Harwood, L.M., Moody, C.J., Eds.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Shono, T.; Nishiguchi, I.; Sasaki, M. Zinc-promoted one-step joining reaction of alkyl halides, activated olefins, and carbonyl compounds. J. Am. Chem. Soc. 1978, 100, 4314–4315. [Google Scholar] [CrossRef]
- Le Gall, E.; Sengmany, S.; Samb, I.; Benakrour, S.; Colin, C.; Pignon, A.; Léonel, E. A multicomponent approach to the synthesis of N-sulfonyl β 2, 3-amino esters. Org. Biomol. Chem. 2014, 12, 3423–3426. [Google Scholar] [CrossRef]
- Paul, J.; Presset, M.; Cantagrel, F.; Le Gall, E.; Leonel, E. Cobalt-Catalyzed Reductive Multicomponent Synthesis of β-Hydroxy-and β-Aminocarbonyl Compounds under Mild Conditions. Chem. Eur. J. 2017, 23, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.; Presset, M.; Le Gall, E.; Léonel, E. Insights into the cobalt-catalyzed three-component coupling of mixed aromatic organozinc species, carbonyl compounds or imines and Michael acceptors: Synthetic and mechanistic aspects. Synthesis 2018, 50, 254–266. [Google Scholar]
- Pinaud, M.; Le Gall, E.; Presset, M. Mixed Aliphatic Organozinc Reagents as Nonstabilized Csp3-Nucleophiles in the Multicomponent Mannich Reaction. J. Org. Chem. 2022, 87, 4961–4964. [Google Scholar] [CrossRef] [PubMed]
- Metzger, A.; Schade, M.A.; Knochel, P. LiCl-mediated preparation of highly functionalized benzylic zinc chlorides. Org. Lett. 2008, 10, 1107–1110. [Google Scholar] [CrossRef]
- González, A.S.; Arrayás, R.G.; Carretero, J.C. Copper (I)-fesulphos Lewis acid catalysts for enantioselective Mannich-type reaction of N-sulfonyl imines. Org. Lett. 2006, 8, 2977–2980. [Google Scholar] [CrossRef]
- Paul, J.; Luong Van My, L.; Behar-Pirès, M.; Guillaume, C.; Léonel, E.; Presset, M.; Le Gall, E. Co (I)-Catalyzed [3 + 2] Annulation of o-Haloaryl Imines with Alkenes for the Synthesis of Indanamines. J. Org. Chem. 2018, 83, 4078–4086. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Qin, Z.-Y.; Huang, Y.-L.; Jin, R.-X.; Lan, Q.; Wang, X.-S. Enantioselective copper-catalyzed cyanation of remote C (sp3)-H bonds enabled by 1, 5-hydrogen atom transfer. Iscience 2019, 21, 490–498. [Google Scholar] [CrossRef]
- Takai, K.; Ueda, T.; Ikeda, N.; Ishiyama, T.; Matsushita, H. Successive Carbon–Carbon Bond Formation by Sequential Generation of Radical and Anionic Species with Manganese and Catalytic Amounts of PbCl2 and Me3SiCl. Bull. Chem. Soc. Jpn. 2003, 76, 347–353. [Google Scholar] [CrossRef]
- Takai, K.; Ueda, T.; Ikeda, N.; Moriwake, T. Sequential Generation and Utilization of Radical and Anionic Species with a Novel Manganese- Lead Reducing Agent. Three-Component Coupling Reactions of Alkyl Iodides, Electron-Deficient Olefins, and Carbonyl Compounds. J. Org. Chem. 1996, 61, 7990–7991. [Google Scholar] [CrossRef]


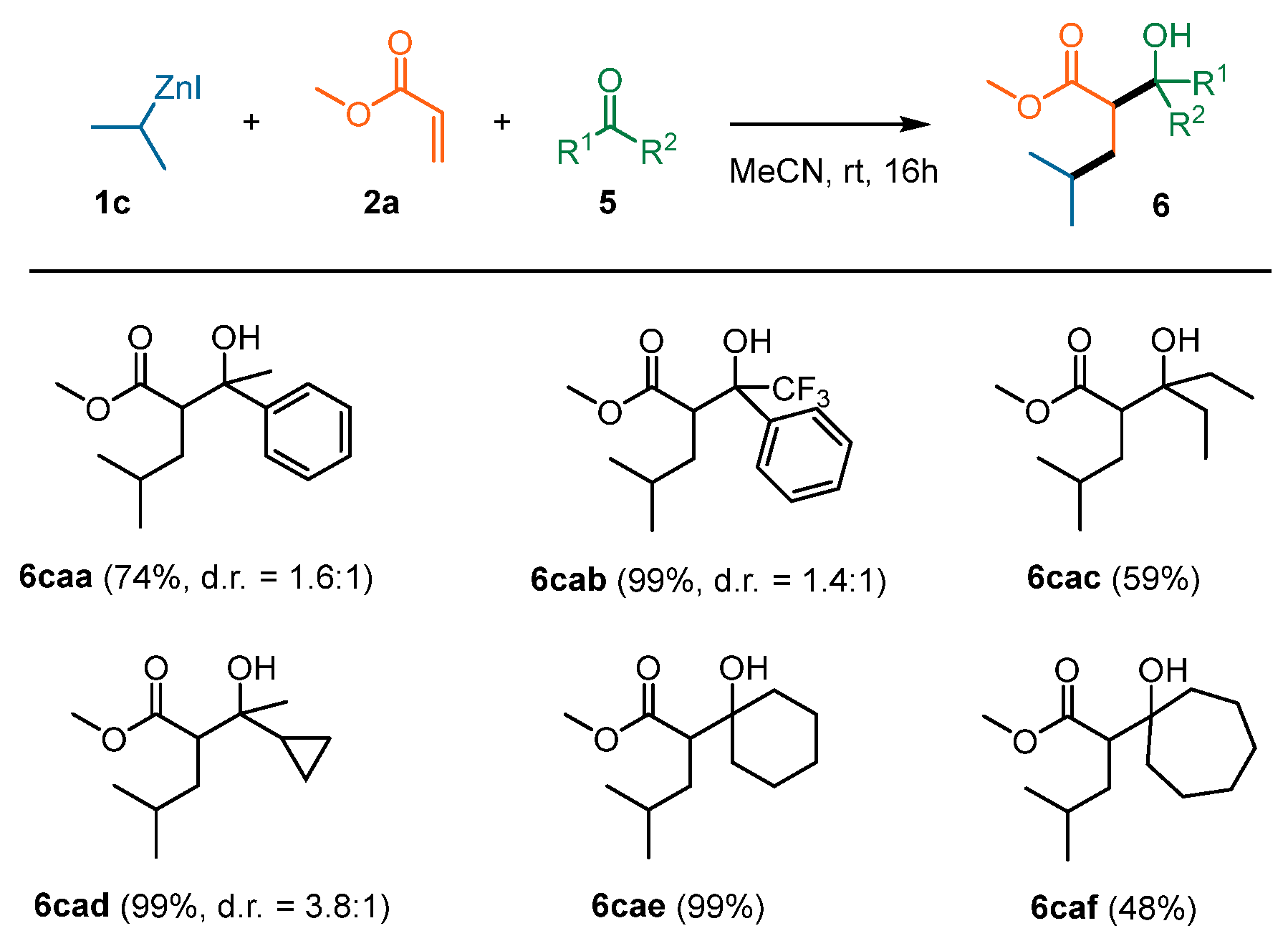
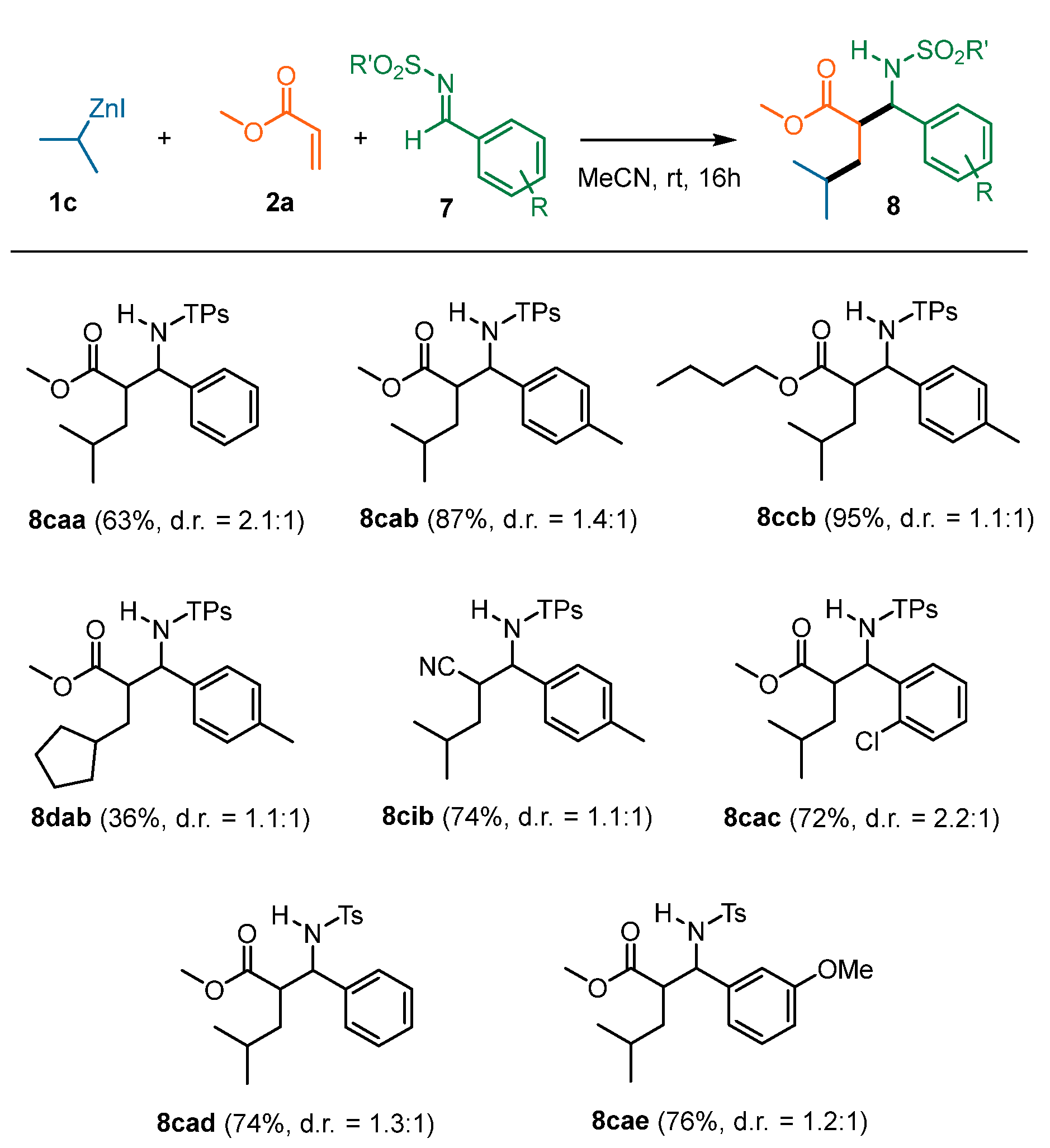
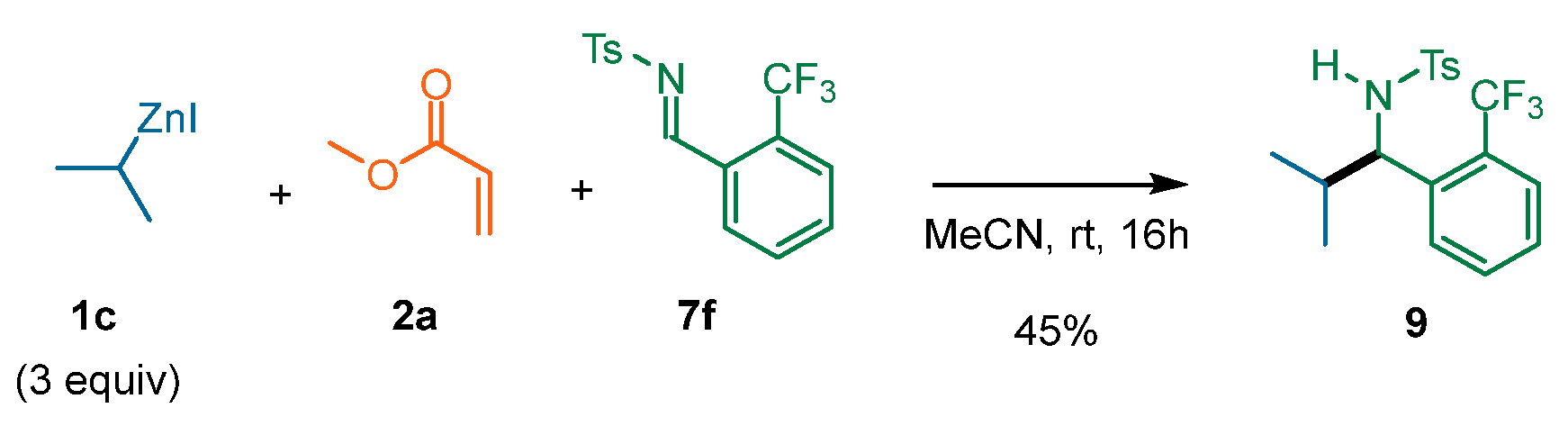
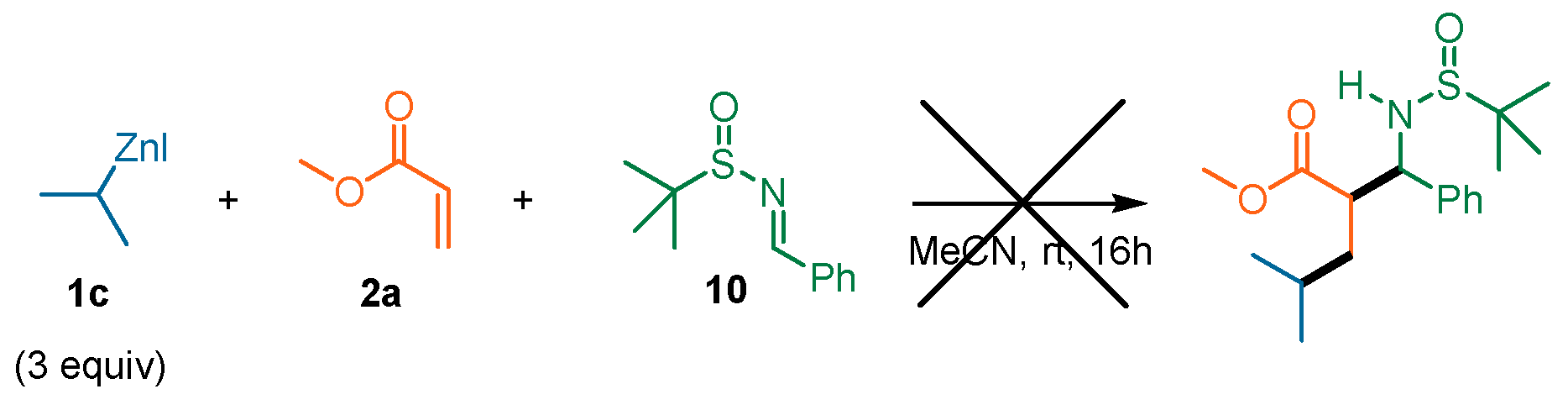
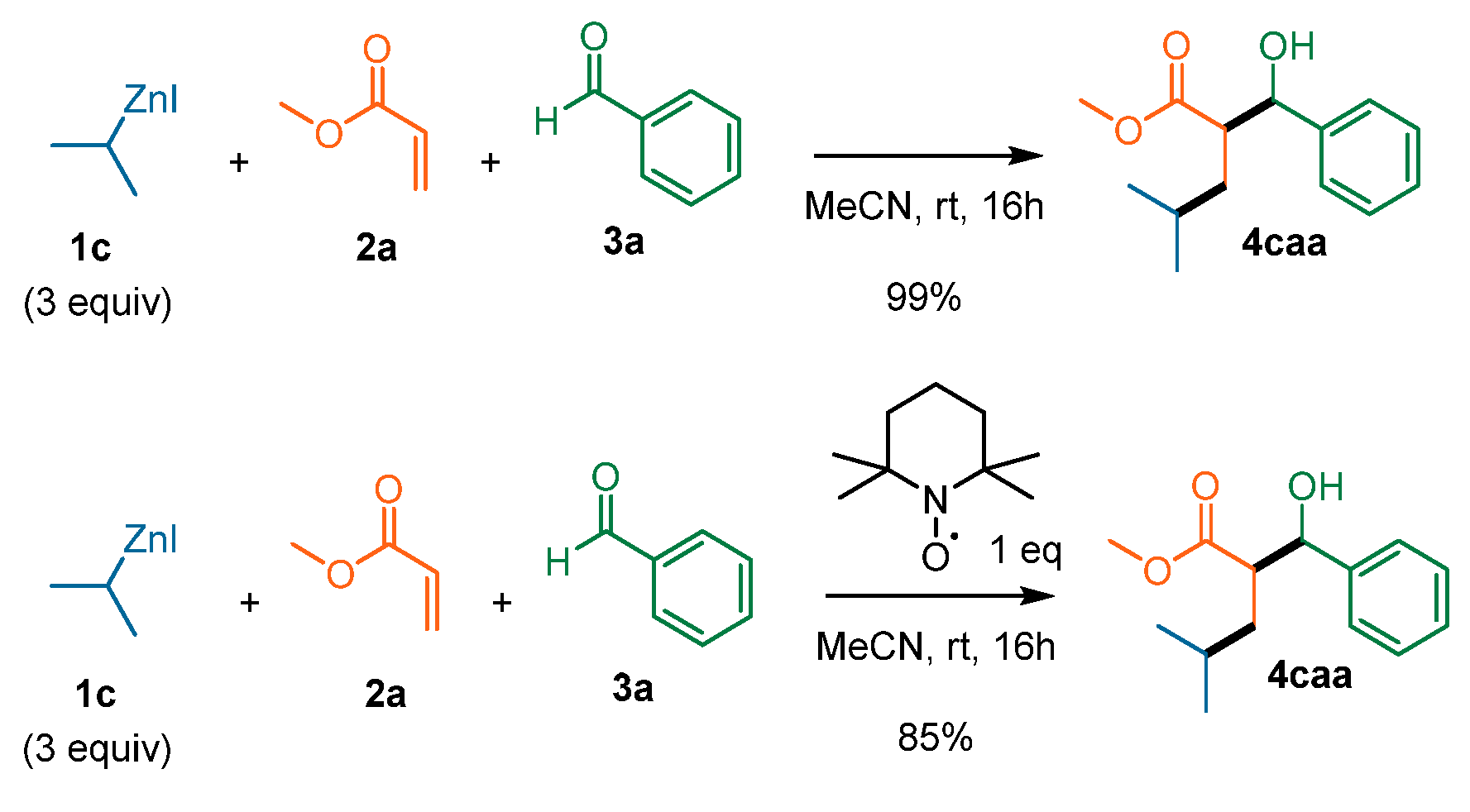
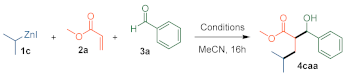
| Entry | T (°C) | Additive | 1c Equiv | 2a Equiv | Yield (%) | d.r. |
|---|---|---|---|---|---|---|
| 1 | 20 | - | 1.5 | 2 | 16% | 6:04 |
| 2 | 20 | CoBr2.bpy2 0.1 eq | 1.5 | 2 | 25% | |
| 3 | 20 | TMSCl 1 eq | 1.5 | 2 | 6% | 9:01 |
| 4 | 80 | - | 1.5 | 2 | 16% | 4:01 |
| 5 | 20 | - | 1.5 | 4 | 18% | 8:02 |
| 6 | 20 | - | 3 | 2 | 68% | 8:02 |
| 7 | 20 | - | 3 | 1 | 99% | 9:01 |
| 8 | −10 | - | 3 | 1 | 27% | 6:04 |
| 9 | 20 | CuI | 3 | 1 | 81% | 8:02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinaud, M.; Presset, M.; Le Gall, E. Catalyst-Free Formal Conjugate Addition/Aldol or Mannich Multicomponent Reactions of Mixed Aliphatic Organozinc Reagents, π-Electrophiles and Michael Acceptors. Molecules 2023, 28, 1401. https://doi.org/10.3390/molecules28031401
Pinaud M, Presset M, Le Gall E. Catalyst-Free Formal Conjugate Addition/Aldol or Mannich Multicomponent Reactions of Mixed Aliphatic Organozinc Reagents, π-Electrophiles and Michael Acceptors. Molecules. 2023; 28(3):1401. https://doi.org/10.3390/molecules28031401
Chicago/Turabian StylePinaud, Marine, Marc Presset, and Erwan Le Gall. 2023. "Catalyst-Free Formal Conjugate Addition/Aldol or Mannich Multicomponent Reactions of Mixed Aliphatic Organozinc Reagents, π-Electrophiles and Michael Acceptors" Molecules 28, no. 3: 1401. https://doi.org/10.3390/molecules28031401
APA StylePinaud, M., Presset, M., & Le Gall, E. (2023). Catalyst-Free Formal Conjugate Addition/Aldol or Mannich Multicomponent Reactions of Mixed Aliphatic Organozinc Reagents, π-Electrophiles and Michael Acceptors. Molecules, 28(3), 1401. https://doi.org/10.3390/molecules28031401







