Abstract
The current study was designed to synthesize, characterize, and screen the molecular and biological activities of different metformin derivatives that possess potent antidiabetic potential with minimal side-effects. Metformin-based derivatives containing the metal complexes Cu II (MCu1–MCu9) and Zn II (MZn1–MZn9) were generated using aromatic aldehydes and ketones in a template process. The novel metal complexes were characterized through elemental analysis, physical state, melting point, physical appearance, Fourier-transform infrared (FTIR) spectroscopy, UV/visible (UV/Vis) spectroscopy, 1H nuclear magnetic resonance (NMR) spectroscopy, and 13C-NMR spectroscopy. Screening for inhibitory activity against the enzymes α-amylase and α-glucosidase, and molecular simulations performed in Schrödinger were used to assess the synthesized derivatives’ biological potential. Met1, Met2, Met3, and Met8 all displayed activities that were on par with the reference in an enzymatic inhibition assay (amylase and glucosidase). The enzyme inhibition assay was corroborated by molecular simulation studies, which also revealed a competitive docking score compared to the gold standard. The Swiss ADME online web server was utilized to compute ADME properties of metformin analogues. Lipinski’s rule of five held true across all derivatives, making it possible to determine the percentage of absorption. Metformin derivatives showed significant antidiabetic activities against both targeted enzymes, and the results of this work suggest that these compounds could serve as lead molecules for future study and development.
Keywords:
copper; zinc; metformin; FTIR; UV; NMR; enzyme inhibition assay; amylase; glucosidase; molecular simulation; docking 1. Introduction
In recent decades, Schiff bases have shown their importance in the industrial and pharmacological fields due to their novel properties. Derivatives of Schiff bases are also important in studying coordination chemistry. Synthesis of novel Schiff bases is of prime interest to scientists in the discovery of medicinal drugs [,]. Metformin is an oral drug used to reduce blood glucose levels in patients suffering from non-insulin-dependent diabetes mellitus (NIDDM) []. It improves insulin sensitivity, thereby lowering the resistance to insulin that is common in NIDDM []. Its history is mainly linked with a herbal plant (Galega officinalis also known as goat’s rue) that is native to European plants and mainly used in lowering blood glucose levels; the plant extract is rich in guanidine. Different derivatives of guanidine (other than metformin) were mainly used between the 1930s as antidiabetic agents but discontinued in the market due to their toxic effects toward vital organs and the invention of insulin []. Therapeutic approaches to control hyperglycemia include increasing the level of insulin or decreasing insulin resistance []. Another pharmacological approach that is implemented in the clinics to manage postprandial hyperglycemia is the inhibition of the α-glucosidase enzyme []. However, some undesirable side-effects are also associated with the prolonged use of these inhibitors such as gastrointestinal side-effects, bloating, and diarrhea. Alternatives with fewer side-effects and cost-effective treatments are desired for the treatment of diabetes [,]. In the early 1940s, metformin was discovered and used as an antimalarial and to treat influenza, although it was observed during testing that it also causes a reduction in blood glucose levels. The antidiabetic use of metformin was first time reported by Jean Steme (a French physician) in 1957 [].
The field of metallo-pharmaceutics has received extensive attention recently. This background has led to the development of a wide range of metal-containing drugs that exhibit notable antibacterial, anticancer, and antidiabetic properties [,,]. Many researchers have synthesized different derivatives of metformin due to the increased glucose-lowering effect among others. Schiff bases are versatile and of a plastic nature; they form the majority of the ligand complexes and show a wide variety of biological effects. Metal complex chemistry containing Schiff-based ligands formed by aldehydes/ketones with amines exhibits significant biological effects, expressing the same features as metalloporphyrin with respect to the electronic and catalytic effects that mimic enzymatic effects. In the past few decades, the catalytic activity of metal complexes has been highlighted []. In the past few years, Schiff base metal complexes of metformin have revealed anticancer effects []. The strongest correlation with diabetes and cancer has been observed in breast, pancreas, and endometrial tumors []. Copper complexes of metformin have been synthesized, showing great levels of antidiabetic and antioxidant defense effects [,]. Various metals such as copper and zinc have been used to form metal complexes with metformin [,]. In this study, we generated different copper and zinc metal complexes with metformin-based derivatives synthesized using an aromatic aldehyde and ketone through a template reaction [].
2. Results
2.1. Characterization of Metabolites
The characterization schemes of all the metformin metabolites and metal complexes are presented in Schemes S1–S3 (Supplementary Materials). While, IR and NMR spectras are shown in Figures S7–S31 (Supplementary Materials).
2.1.1. Characterization of (Met1–Met9)
Metformin; IUPAC name: N,N-dimethyltriimidodicarbonic diamide; M.P. 222–226 °C; molecular formula: C4H11N5; molecular weight: 129.16 g/mol. Elemental analysis for C4H11N5: (calculated) 37.20; H, 8.58; N, 54.22; (found) C, 37.16; H, 8.65; N, 54.28. FT-IR ν (cm−1), 2971 (C–H, s, stretching), 1620 (C=N, m, bending), 1153 (C–N, m, bending), 3368, 3245 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.43 s, (6H, CH3), 1.17 s (1H), 1.19 s (1H), 1.21 s (1H), (NH), 8.15 s, (2H, NH2). 13C-NMR (DMSO, ppm) δ: 45.2 (C1), 45.2 (C2), 161.6 (C3), 150.3 (C4).
Met1; IUPAC name: N′-benzylidene-N,N-dimethyltriimidodicarbonic diamide; yield (65%); M.P. 160–161 °C; molecular formula: C11H15N5; molecular weight: 217.27 g/mol. Elemental analysis for C11H15N5: (calculated) C, 60.81; H, 6.96; N, 32.23; (found) C, 60.75; H, 7.05; N, 32.28. FT-IR ν (cm−1), 2955, 2922, 2854 (C–H, s, stretching), 1627 (C=N, m, bending), 1593, 1463 (CH=CH, w, bending), 1130 (C–N, m, bending), 3372, 3264 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.30 s (6H, CH3), 1.16 s (1H), 1.18 s (1H) 1.19 s (1H), (NH), 8.10 s (1H, CH), 7.87–7.89 d (2H, CH), 7.40–7.44 m, (3H, CH). 13C-NMR (DMSO, ppm) δ: 45.8 (C1,2), 150.4 (C3,4), 148.8 (C5), 131.7 (C6), 128.8 (C7,11), 128.2 (C8,10), 130.7 (C9).
Met2; IUPAC name: N′-[(2-hydroxyphenyl)methylidene]-N,N-dimethyltriimidodicarbonic diamide; yield (61%); M.P. 175–176 °C; molecular formula: C11H15N5O; molecular weight: 233.27 g/mol. Elemental analysis for C11H15N5O: (calculated) C, 56.64; H, 6.48; N, 30.02; (found) C, 56.70; H, 6.41; N, 30.12. FT-IR ν (cm−1), 3066, 2984 (C–H, s, stretching), 1624 (C=N, m, bending), 1593, 1474 (CH=CH, w, bending), 1168 (C–N, m, bending), 1273 (C–O, s, bending), 3344, 3258 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.45 s (6H, CH3), 2.41, 2.51 s (3H, NH), 8.17 s (1H, CH), 7.46–7.47 d (1H, CH), 7.45–7.46 d (2H, CH), 7.87–7.88 t (1H, CH), 5.10 s (1H, OH). 13C-NMR (DMSO, ppm) δ: 45.6 (C1,2), 150.1 (C3), 156.9 (C4), 162.5 (C5), 121.9 (C6), 129.8 (C7), 128.1 (C8), 128.7 (C9), 126.5 (C10), 149.4 (C11).
Met3: IUPAC name: N,N-dimethyl-N′-[(1Z,2E)-3-phenylprop-2-en-1-ylidene]triimidodicarbonic diamide; yield (70%); M.P. 145–146 °C; molecular formula: C13H17N5; molecular weight: 243.31 g/mol. Elemental analysis for C13H17N5: (calculated) C, 64.17; H, 7.04; N, 28.78; (found) C, 64.24; H, 7.00; N, 28.75. FT-IR ν (cm−1), 1645 (C=N, m, bending), 1601, 1488 (CH=CH, w, bending), 1135 (C–N, m, bending), 3312 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.17s (6H, CH3), 3.72 s (3H), 7.86–7.88 d (1H, CH), 6.34–6.35 d (1H, CH), 7.36–7.38 d (1H, CH), 7.40–7.41 d (2H, CH), 7.41–7.43 t (2H, CH), 7.45–7.46 t (1H, CH). 13C-NMR (DMSO, ppm) δ: 46.6 (C1,2), 153.7 (C3), 161.2 (C4), 162.4.7 (C5), 127.9 (C6), 136.0 (C7), 136.3 (C8), 135.8 (C9), 128.9 (C10), 128.3 (C11), 128.6 (C12), 135.7 (C13).
Met4; IUPAC name: N,N-dimethyl-N′-methylidenetriimidodicarbonic diamide; yield (58%); M.P. 186–187 °C; molecular formula: C5H11N5; molecular weight: 141.17 g/mol. Elemental analysis for C5H11N5: (calculated) C, 42.54; H, 7.85; N, 49.61; (found) C, 42.45; H, 7.83; N, 49.70. FT-IR ν (cm−1), 2956, 2871 (C–H, s, stretching), 1651 (C=N, m, bending), 1147 (C–N, m, bending), 3353, 3259 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.44s (6H, CH3), 1.17 s, 7.45 s 7.48 s (3H), 7.88 s (2H, CH2). 13C-NMR (DMSO, ppm) δ: 45.6 (C1,2), 150.3 (C3), 161.4 (C4), 151.0 (C5).
Met5; IUPAC name: N,N-dimethyl-N′-(propan-2-ylidene)triimidodicarbonic diamide; yield (66%); M.P. 152–153 °C; molecular formula: C7H15N5; molecular weight: 169.23 g/mol. Elemental analysis for C7H15N5: (calculated) C, 49.68; H, 8.93; N, 41.38; (found) C, 49.65; H, 8.90; N, 41.44. FT-IR ν (cm−1), 3075 (C–H, s, stretching), 1615 (C=N, m, bending), 1145 (C–N, m, bending), 3291 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.39 s (6H, CH3), 1.19 s, 7.78 s, 7.79 s (3H), 1.15 s, 1.17s (6H, CH3). 13C-NMR (DMSO, ppm) δ: 45.6 (C1,2), 144.9 (C3), 147.7 (C4), 157.2 (C5), 13.1 (C6), 9.6 (C7).
Met6; IUPAC name: N,N-dimethyl-N′-[(1E)-1-phenylethylidene]triimidodicarbonic diamide; yield (68%); M.P. 174–175 °C; molecular formula: C12H17N5; molecular weight: 231.30 g/mol. Elemental analysis for C12H17N5: (calculated) C, 62.31; H, 7.41; N, 30.28; (found) C, 62.31; H, 7.41; N, 30.28. FT-IR ν (cm−1), 2978 (C–H, s, stretching), 1620 (C=N, m, bending), 1601, 1480 (C=C, m, bending), 1146 (C–N, m, bending). 1H-NMR (DMSO, ppm) δ: 1.31 s, 2.39 s (9H, CH3), 6.62 s (3H, NH), 7.12 d (2H, CH), 7.52 m (3H, CH). 13C-NMR (DMSO, ppm) δ: 55.8 (C1), 59.9 (C2), 152.8 (C3), 156.4 (C4), 163.5 (C5), 136.5 (C6), 127.5 (C7,11), 134.8 (C8,10), 136.0 (C9), 32.3 (C12).
Met7; IUPAC name: N′-(diphenylmethylidene)-N,N-dimethyltriimidodicarbonic diamide; yield (74%); M.P. 191–192 °C; molecular formula: C17H19N5; molecular weight: 293.37 g/mol. Elemental analysis for C17H19N5: (calculated) C, 69.60; H, 6.53; N, 23.87; (found) C, 69.55; H, 6.63; N, 23.82. FT-IR ν (cm−1), 3103 (C–H, s, stretching), 1615 (C=N, m, bending), 1585 (C=C, m, bending), 1134 (C–N, m, bending), 3268 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.03 s, 3.05 s, (6H, CH3), 3.09s (3H, NH), 7.33–7.35 d, 7.38–7.42 m, (5H, CH), 7.93–7.94 d, 7.95–7.96 m (5H, CH). 13C-NMR (DMSO, ppm) δ: 45.5 (C1,2), 150.1 (C3), 156.9 (C4), 162.5 (C5), 149.4 (C6), 137.8 (C7,13), 129.8 (C8,14), 128.7 (C9,15), 135.9 (C10,16), 128.3 (C11,17), 129.7 (C12).
Met8; IUPAC name: N′-[(E)-(4-hydroxy-3-methoxyphenyl)methylidene]-N,N-dimethyltriimidodicarbonic diamide; yield (60%); M.P. 170–171 °C; molecular formula: C12H17N5O2; molecular weight: 263.30 g/mol. Elemental analysis for C12H17N5O2: (calculated) C, 54.74; H, 6.51; N, 26.60; (found) C, 54.84; H, 6.45; N, 26.55. FT-IR ν (cm−1), 1680, 1642 (C=N, m, bending), 1590, 1474 (C=C, m, bending), 1138 (C–N, m, bending), 3407, 3321 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 2.15 s, 3.39 s (9H, CH3), 8.12 s(1H, CH), 1.68 s (3H, NH), 6.91 d, 7.12 d, 7.45 s (3H, CH), 4.92 s (1H, OH). 13C-NMR (DMSO, ppm) δ: 55.5 (C1), 54.9 (C2), 156.8 (C3), 159.4 (C4), 163.5 (C5), 127.2 (C6), 117.5 (C7), 145.6 (C8), 146.0 (C9), 112.3 (C10), 119.7 (C11), 45.2 (C12).
Met9; IUPAC name: N′-[(1Z)-2-hydroxy-1,2-diphenylethylidene]-N,N-dimethyltriimidodicarbonic diamide; yield (78%); M.P. 139–140 °C; molecular formula: C18H21N5O; molecular weight: 323.39 g/mol. Elemental analysis for C18H21N5O: (calculated) C, 66.85; H, 6.55; N, 21.66; (found) C, 66.82; H, 6.65; N, 21.61; FT-IR ν (cm−1), 3054, 2922 (C–H, s, stretching), 1647 (C=N, m, bending), 1588, 1481 (C=C, m, bending), 1157 (C–N, m, bending), 3301 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 2.21s (6H, CH3), 1.92 s (3H, NH), 4.5 s (1H, CH), 7.32–7.36 m(5H, CH), 7.82–7.83 d, 7.48–7.49 m (5H, CH), 3.52 3 (1H, OH). 13C-NMR (DMSO, ppm) δ: 56.7(C1), 57.2 (C2), 158.8 (C3), 160.2 (C4), 162.5 (C5), 61.5 (C6), 138.5 (C7), 126.8 (C8,12), 1127.8 (C9,11), 128.3 (C10), 132.3 (C13), 127.5(C14,18), 128.2 (C15,17), 130.5 (C16).
2.1.2. Characterization of (MCu1–MCu9)
MCu1; yield (68%); M.P. 250 °C; molecular formula: C22H40CuN10O9S; molecular weight: 684.23 g/mol. Elemental analysis for C22H40CuN10O9S: (calculated) C, 38.62; H, 5.89; N, 20.47; S, 4.69; (found) C, 38.66; H, 5.93; N, 20.49; S, 4.71. FT-IR ν (cm−1), 2928, (C–H, s, stretching), 1653 (C=N, m, bending), 1601 (CH=CH, w, bending), 1178 (C–N, m, bending), 3372 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.01 s (12H, CH3), 1.18 s (2H), 1.23 s (2H), 1.16 s (2H), (NH), 7.92 s (2H, CH), 7.72–7.73 d (4H, CH), 7.41–7.42 m (6H, CH). 13C-NMR (DMSO, ppm) δ: 45.8 (C1,1′), 45.6 (C2,2′), 150.4 (C3,3′), 154.1 (C4,4′), 148.8 (C5,5′), 131.7 (C6,6′), 128.8 (C7,7′,11,11′), 128.2 (C8,8′,10,10′), 130.7 (C9,9′).
MCu2; yield (70%); M.P. 275–276 °C; molecular formula: C22H40CuN10O11S; molecular weight: 716.22 g/mol. Elemental analysis for C22H40CuN10O11S: (calculated) C, 36.89; H, 5.63; N, 19.56; S, 4.48; (found) C, 36.93; H, 5.60; N, 19.59; S, 4.42. FT-IR ν (cm−1), 3052, 2951 (C–H, s, stretching), 1645 (C=N, m, bending), 1599, 1473 (CH=CH, w, bending), 1164 (C–N), 1275 (C–O, s, bending), 3352 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.41 s (12H, CH3), 2.40, 2.58 s (6H, NH), 8.12 s (2H, CH), 7.44–7.45 d (2H, CH), 7.46–7.47 d (4H, CH), 7.81–7.82 t (2H, CH), 5.13 s (1H, OH). 13C-NMR (DMSO, ppm) δ: 45.5 (C1,1′), 47.6 (C2,2′), 150.2 (C3,3′), 156.6 (C4,4′), 162.3 (C5,5′), 121.9 (C6,6′), 129.8 (C7,7′), 128.3 (C8,8′), 128.8 (C9,9′), 126.8 (C10,10′), 149.3 (C11,11′).
MCu3; yield (65%); M.P. 221–222 °C; molecular formula: C26H44CuN10O9S; molecular weight: 736.30 g/mol. Elemental analysis for C26H44CuN10O9S: (calculated) C, 42.41; H, 6.02; N, 19.02; S, 4.35; (found) C, 42.48; H, 6.07; N, 19.06; S, 4.31. FT-IR ν (cm−1), 3025, 2968 (C–H, s, stretching), 1653 (C=N, m, bending), 1161, 1483 (CH=CH, w, bending), 1170 (C–N, m, bending), 3330 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.11 s (12H, CH3), 3.32 s (6H), 7.81–7.82 d (2H, CH), 6.31–6.32 d (2H, CH), 7.34–7.35 d (2H, CH), 7.42–7.43 d (4H, CH), 7.46–7.47 t (4H, CH), 7.49–7.50 t (2H, CH). 13C-NMR (DMSO, ppm) δ: 46.2 (C1,1′) 45.7 (C2,2′), 153.4 (C3,3′), 161.5 (C4,4′), 162.4.6 (C5,5′), 127.7 (C6,6′), 136.4 (C7,7′), 136.5 (C8,8′), 135.6 (C9,9′), 128.3 (C10,10′), 128.1 (C11,11′), 128.7 (C12,13), 135.6 (C13,13′).
MCu4; yield (55%); M.P. 201–202 °C; molecular formula: C10H32CuN10O9S; molecular weight: 532.03 g/mol. Elemental analysis for C10H32CuN10O9S: (calculated) C, 22.58; H, 6.06; N, 26.33; S, 6.03; (found) C, 22.62; H, 6.10; N, 26.38; S, 6.07. FT-IR ν (cm−1), 2951, 2892 (C–H, s, stretching), 1657 (C=N, m, bending), 1153 (C–N, m, bending), 3353 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.41 s (12H, CH3), 1.11 s, 7.44 s, 7.49 s (6H), 7.84 s (4H, CH2). 13C-NMR (DMSO, ppm) δ: 45.7 (C1,1′), 47.3 (C2,2′), 150.7 (C3,3′), 161.7 (C4,4′), 151.6 (C5,5′).
MCu5; yield (70%); M.P. 252–253 °C; molecular formula: C14H40CuN10O9S; molecular weight: 588.14 g/mol. Elemental analysis for C14H40CuN10O9S: (calculated) C, 28.59; H, 6.86; N, 23.82; S, 5.45; (found) C, 28.65; H, 6.82; N, 23.85; S, 5.42. FT-IR ν (cm−1), 3030 (C–H, s, stretching), 1652 (C=N, m, bending), 1178 (C–N, m, bending), 3335 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.01 s (12H, CH3), 1.21 s, 7.45 s, 7.52 s (6H, NH), 1.11 s, 1.18 s (12H, CH3). 13C-NMR (DMSO, ppm) δ: 45.2 (C1,1′), 47.3 (C2,2′), 148.9 (C3,3′), 152.3 (C4,4′), 158.2 (C5,5′), 17.4 (C6,6′), 12.5 (C7,7′).
MCu6; yield (65%); M.P. 204–205 °C; molecular formula: C24H44CuN10O9S; molecular weight: 712.28 g/mol. Elemental analysis for C24H44CuN10O9S: (calculated) C, 40.47; H, 6.23; N, 19.66; S, 4.50; (found) C, 40.52; H, 6.27; N, 19.62; S, 4.45. FT-IR ν (cm−1), 2952 (C–H, s, stretching), 1636 (C=N, m, bending), 1599, 1473 (C=C, m, bending), 1178 (C–N, m, bending). 1H-NMR (DMSO, ppm) δ: 1.35 s, 2.31 s (18H, CH3), 6.68 s (6H, NH), 7.11–7.12 d (4H, CH), 7.53–7.54 m(6H, CH). 13C NMR (DMSO, ppm) δ: 55.6 (C1,1′), 59.7 (C2,2′), 152.7 (C3,3′), 156.2 (C4,4′), 163.7 (C5,5′), 136.4 (C6,6′), 127.4 (C7,7′), 134.7 (C8,8′), 136.1 (C9,9′), 134.9 (C10,10′), 126.2 (C11,11′), 25.32 (C12,12′).
MCu7; yield (62 %); M.P. 232 °C; molecular formula: C34H48CuN10O9S; molecular weight: 836.42 g/mol. Elemental analysis for C34H48CuN10O9S: (calculated) C, 48.82; H, 5.78; N, 16.75; S, 3.83; (found) C, 48.78; H, 5.81; N, 16.78; S, 3.81. FT-IR ν (cm−1), 3021 (C–H, s, stretching), 1635 (C=N, m, bending), 1598 (C=C, m, bending), 1175 (C–N, m, bending), 3321 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 2.92 s, 3.01 s, 3.11 s, (12H, CH3), 2.77s, 2.81s (6H, NH), 7.35–7.36 d, 7.39–7.40 m (10H, CH), 7.91–7.92 d, 7.97–7.98 m (10H, CH). 13C-NMR (DMSO, ppm) δ: 45.6 (C1,1′), 43.1 (C2,2′), 150.3 (C3,3′), 156.7 (C4,4′), 162.2 (C5,5′), 149.3 (C6,6′), 138.7 (C7,7′), 129.7 (C8,8′), 128.7 (C9,9′) 135.5 (C10,10′), 128.5 (C11,11′), 129.7 (C12,12′), 137.6 (C13,13′), 128.5 (C14, 14′), 127.6 (C15,15′), 136.4 (C16,16′), 128.1 (C17,17′).
MCu8; yield (55%); M.P. 190 °C; molecular formula: C24H44CuN10O13S; molecular weight: 776.28 g/mol. Elemental analysis for C24H44CuN10O13S: (calculated) C, 37.13; H, 5.71; N, 18.04; S, 4.13; (found) C, 37.10; H, 5.75; N, 18.10; S, 4.18. FT-IR ν (cm−1), 3051 (C–H, s, stretching), 1675, 1635 (C=N, m, bending), 1597, 1471 (C=C, m, bending), 1158 (C–N, m, bending), 3331 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 2.11 s, 3.21 s, 2.01 s (18H, CH3), 8.09 s (2H, CH), 1.62 s, 1.52s (6H, NH), 6.91–6.92 d, 7.11–7.12 d, 7.37 s (6H, CH), 4.85 s (2H, OH). 13C-NMR (DMSO, ppm) δ: 55.2 (C1,1′), 54.4 (C2,2′), 156.7 (C3,3′), 159.3 (C4,4′), 163.4 (C5,5′), 127.3 (C6,6′), 119.5 (C7,7′), 145.7 (C8,8′), 146.3 (C9,9′), 112.5 (C10,10′), 119.3 (C11,11′), 45.1 (C12,12′).
MCu9; yield (78%); M.P. 139–140 °C; molecular formula: C36H52CuN10O11S; molecular weight: 896.47 g/mol. Elemental analysis for C36H52CuN10O11S: (calculated) C, 48.23; H, 5.85; N, 15.62; S, 3.58; (found) C, 48.20; H, 5.88; N, 15.68; S, 3.55. FT-IR ν (cm−1), 3051, 2951 (C–H, s, stretching), 1653 (C=N, m, bending), 1598, 1477 (C=C, m, bending), 1177 (C–N, m, bending), 3351 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 2.12 s 1.92 s, 1.85 s, 1.96 s (12H, CH3), 1.25 s, 1.32 s (6H, NH), 4.45 s (2H, CH), 7.31–7.32 m, 7.36–7.37 m (10H, CH), 7.78–7.79 d, 7.45–7.46 m (10H, CH), 3.22 s (2H, OH). 13C-NMR (DMSO, ppm) δ: 56.5 (C1,1′), 57.1 (C2,2′), 158.7 (C3,3′), 160.5 (C4,4′), 162.4 (C5,5′), 61.4 (C6,6′), 138.7 (C7,7′), 126.7 (C8,8′), 127.9 (C9,9′), 128.1 (C10,10′), 127.5 (C11,11′), 126.3 (C12,12′) 132.4 (C13,13′), 127.1 (C14,14′), 128.2 (C15,15′), 130.3 (C16,16′), 128.7 (C17,17′), 126.9 (C18,18′).
2.1.3. Characterization of (MZn1–MZn9)
MZn1; yield (55%); M.P. 250 °C; molecular formula: C22H40N10O9SZn; molecular weight: 686.06 g/mol. Elemental analysis for C22H40N10O9SZn: (calculated) C, 38.51; H, 5.88; N, 20.42; S, 4.67; (found) C, 38.47; H, 5.82; N, 20.47; S, 4.71. FT-IR ν (cm−1), 2951, 2854 (C–H, s, stretching), 1653 (C=N, m, bending), 1598, 1473 (CH=CH, w, bending), 1137 (C–N, m, bending), 3372, (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.20 s (12H, CH3), 1.16 s (2H), 1.18 s (2H) 1.19 s (2H), (NH), 8.01 s, (2H, CH), 7.77–7.78 d, (4H, CH), 7.37–7.38 m, (6H, CH). 13C-NMR (DMSO, ppm) δ: 45.8 (C1,2,1′,2′), 150.4 (C3,4,3′,4′), 148.8 (C5,5′), 131.7 (C6,6′), 128.8 (C7,11,7′,11′), 128.2 (C8,10,8′,10′), 130.7 (C9,9′).
MZn2; yield (64%); M.P. 272 °C; molecular formula: C22H40N10O11SZn; molecular weight: 718.06 g/mol. Elemental analysis for C22H40N10O11SZn: (calculated) C, 36.80; H, 5.61; N, 19.51; S, 4.47; (found) C, 36.85; H, 5.64; N, 19.45; S, 4.46. FT-IR ν (cm−1), 3066 (C–H, s, stretching), 1680 (C=N, m, bending), 1603, 1473 (CH=CH, w, bending), 1165 (C–N, m, bending), 1275 (C–O, s, bending), 3344 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.42s (12H, CH3), 2.43, 2.62 s (6H, NH), 7.92 s (2H, CH), 7.41–7.42 d (2H, CH), 7.44–7.45 d (4H, CH), 7.82–7.83 t (2H, CH), 5.12 s (2H, OH). 13C-NMR (DMSO, ppm) δ: 45.6 (C1,1′), 47.3 (C2, 2′), 150.0 (C3,3′), 156.7 (C4,4′), 162.4 (C5,5′), 121.8 (C6,6′), 129.7 (C7,7′), 128.0 (C8,8′), 128.8 (C9,9′), 126.7 (C10,10′), 149.4 (C11,11′).
MZn3; yield (50%); M.P. 246 °C; molecular formula: C26H44N10O9SZn; molecular weight: 738.13 g/mol. Elemental analysis for C26H44N10O9SZn: (calculated) C, 42.31; H, 6.01; N, 18.98; S, 4.34; (found) C, 42.31; H, 6.01; N, 18.98; S, 4.34. FT-IR ν (cm−1), 2925 (C–H, s, stretching), 1645 (C=N, m, bending), 1602, 1488 (CH=CH, w, bending), 1178 (C–N, m, bending), 3366 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.11 s (12H, CH3), 3.70 s (6H), 7.81–7.82 d (2H, CH), 6.31–6.32 d (2H, CH), 7.32–7.33 d (2H, CH), 7.39–7.41 d (4H, CH), 7.43–7.44 t (4H, CH), 7.47–7.48 t (2H, CH). 13C-NMR (DMSO, ppm) δ: 46.6 (C1,1′), 53.4 (C2,2′), 153.6 (C3,3′), 161.4 (C4,4′), 162.5 (C5,5′), 127.8 (C6,6′), 136.0 (C7,7′), 136.5 (C8,8′), 135.7 (C9,9′), 128.8 (C10,10′), 128.4 (C11,11′), 128.7 (C12,12′), 135.3 (C13,13′).
MZn4; yield (58%); M.P. 210–211 °C; molecular formula: C10H32N10O9SZn; molecular weight: 533.87 g/mol. Elemental analysis for C10H32N10O9SZn: (calculated) C, 22.50; H, 6.04; N, 26.24; S, 6.01; (found) C, 22.55; H, 6.00; N, 26.21; S, 6.07. FT-IR ν (cm−1), 2961, 2889 (C–H, s, stretching), 1653 (C=N, m, bending), 1178 (C–N, m, bending), 3353 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 3.41 s (12H, CH3), 1.11 s, 7.51 s, 7.45 s (6H), 7.72 s (4H, CH2). 13C-NMR (DMSO, ppm) δ: 45.6 (C1,1′) 46.7 (C2,2′), 154.3 (C3,3′), 161.7 (C4,4′), 151.4 (C5,5′).
MZn5; yield (66%); M.P. 152–153 °C; molecular formula: C14H40N10O9SZn; molecular weight: 589.97 g/mol. Elemental analysis for C14H40N10O9SZn: (calculated) C, 28.50; H, 6.83; N, 23.74; S, 5.43; (found) C, 28.55; H, 6.80; N, 23.70; S, 5.46. FT-IR ν (cm−1), 3001 (C–H, s, stretching), 1645 (C=N, m, bending), 1235 (C–N, m, bending), 3271 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 2.71 s (12H, CH3), 1.51 s, 7.58 s 7.63 s (6H, NH), 1.25 s, 1.11s (12H, CH3). 13C-NMR (DMSO, ppm) δ: 45.2 (C1,1′), 47.6 (C2,2′), 151.2 (C3,3′), 149.7 (C4,4′), 158.1 (C5,5′), 25.6 (C6,6′), 12.6 (C7,7′).
MZn6; yield (74%); M.P. 211–212 °C; molecular formula: C24H44N10O9SZn; molecular weight: 714.11 g/mol. Elemental analysis for C24H44N10O9SZn: (calculated) C, 40.37; H, 6.21; N, 19.61; S, 4.49; (found) C, 40.41; H, 6.18; N, 19.63; S, 4.53. FT-IR ν (cm−1), 2952 (C–H, s, stretching), 1635 (C=N, m, bending), 1603, 1476 (C=C, m, bending), 1178 (C–N, m, bending). 1H-NMR (DMSO, ppm) δ: 1.30 s, 2.35 s (18H, CH3), 6.61 s (6H, NH), 7.11–7.12 d (4H, CH), 7.50–7.51 m (6H, CH). 13C-NMR (DMSO, ppm) δ: 55.6 (C1,1′), 59.6 (C2,2′), 152.5 (C3,3′), 156.2 (C4,4′), 163.4 (C5,5′), 136.4 (C6,6′), 127.5(C7,7′), 134.6 (C8,8′), 136.3 (C9,9′), 134.5 (10,10′) 127.3 (C11,11′), 25.3 (C12,12′).
MZn7; yield (64%); M.P. 237 °C; molecular formula: C34H48N10O9SZn; molecular weight: 838.25 g/mol. Elemental analysis for C34H48N10O9SZn: (calculated) C, 48.72; H, 5.77; N, 16.71; S, 3.83; (found) C, 48.70; H, 5.79; N, 16.75; S, 3.80. FT-IR ν (cm−1), 3006 (C–H, s, stretching), 1657 (C=N, m, bending), 1601, 1473 (C=C, m, bending), 1165 (C–N, m, bending), 3303 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 2.85 s, 3.05 s, 3.17 s, (12H, CH3), 2.81 s, 2.85 s (6H, NH), 7.41–7.42 d, 7.45–7.46 m (10H, CH), 7.81–7.82 d, 7.95–7.96 m (10H, CH). 13C-NMR (DMSO, ppm) δ: 45.5 (C1,1′), 43.3 (C2,2′), 151.3 (C3,3′), 156.9 (C4,4′), 162.3 (C5,5′), 149.7 (C6,6′), 138.9 (C7,7′), 129.8 (C8,8′), 128.9 (C9,9′) 135.7 (C10,10′), 128.7 (C11,11′), 129.9 (C12,12′), 137.8 (C13,13′), 128.3 (C14,14′), 127.9 (C15,15′), 136.1 (C16,16′), 128.0 (C17,17′).
MZn8; yield (67%); M.P. 197 °C; molecular formula: C24H44N10O13SZn; molecular weight: 778.11 g/mol. Elemental analysis for C24H44N10O13SZn: (calculated) C, 37.05; H, 5.70; N, 18.00; S, 4.12; (found) C, 37.10; H, 5.65; N, 17.95; S, 4.15. FT-IR ν (cm−1), 3051 (C–H, s, stretching), 1681, (C=N, m, bending), 1601, 1473 (C=C, m, bending), 1173 (C–N, m, bending), 3291 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 2.10 s, 3.22 s, 2.15 s, (18H, CH3), 8.01 s (2H, CH), 1.61 s, 1.51 s, (6H, NH), 6.90–6.91 d, 7.10–7.11 d, 7.35 s (6H, CH), 4.83 s, (2H, OH). 13C-NMR (DMSO, ppm) δ: 55.4 (C1,1′), 54.3 (C2,2′), 156.6 (C3,3′), 159.5 (C4,4′), 163.1 (C5,5′), 127.2 (C6,6′), 119.1 (C7,7′), 145.5 (C8,8′), 146.1 (C9,9′), 112.4 (C10,10′), 119.7 (C11,11′), 45.5 (C12,12′).
MZn9; yield (77%); M.P. 240 °C; molecular formula: C36H52N10O11SZn; molecular weight: 898.30 g/mol. Elemental analysis for C36H52N10O11SZn: (calculated) C, 48.13; H, 5.83; N, 15.59; S, 3.57; (found) C, 48.17; H, 5.88; N, 15.62; S, 3.51. FT-IR ν (cm−1), 3021, 2971 (C–H, s, stretching), 1657 (C=N, m, bending), 1605, 1474 (C=C, m, bending), 1157 (C–N, m, bending), 3301 (N–H, s, stretching). 1H-NMR (DMSO, ppm) δ: 2.11 s 1.90 s, 1.82 s, 1.98 s, (12H, CH3), 1.22 s, 1.31 s, (6H, NH), 4.41 s (2H, CH), 7.30–7.31 m, 7.34–7.35 m (10H, CH), 7.76–7.77 d, 7.42–7.43 m (10H, CH), 3.20 s, (2H, OH). 13C-NMR (DMSO, ppm) δ: 56.3 (C1,1′), 57.0 (C2,2′), 158.5 (C3,3′), 160.4 (C4,4′), 162.3 (C5,5′), 61.3 (C6,6′), 138.4 (C7,7′), 126.5 (C8,8′), 127.7 (C9,9′), 128.5 (C10,10′), 127.7 (C11,11′), 126.1 (C12,12′) 132.9 (C13,13′), 127.0 (C14,14′), 128.1 (C15,15′), 130.7 (C16,16′), 128.9 (C17,17′), 126.7 (C18,18′).
2.2. In Silico ADME Studies
Swiss ADME can help to determine different physicochemical properties of novel tested compounds such as molecular weight, hydrogen bond donors (HBDs), hydrogen bond acceptors (HBAs), partition coefficient (log P). and solvent-accessible surface area within the desired range of acceptance, with the results depicted in Table 1, Table 2 and Table 3 and represented in Figure 1, Figure 2 and Figure 3. The detailed ADME predictions are shown in Table S1 (Supplementary Materials).

Table 1.
Swiss ADME of Met1–Met9.

Table 2.
Swiss ADME of MCu1–MCu9.

Table 3.
Swiss ADME of MZn1–MZn9.
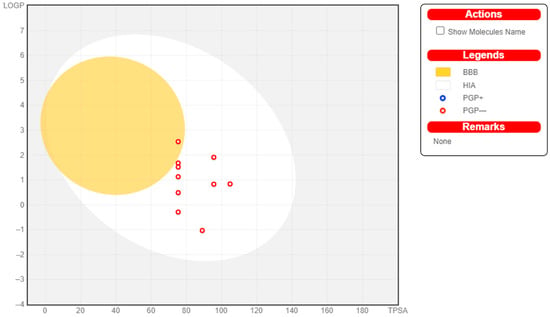
Figure 1.
Swiss ADME Analysis of Met1–Met9 (egg plot).
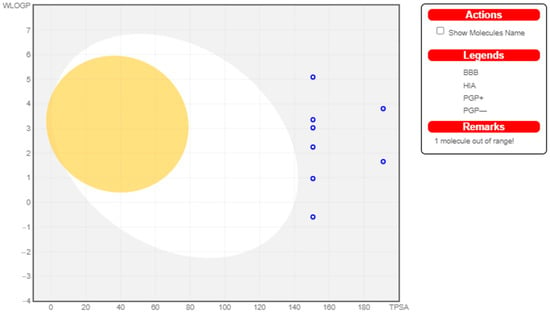
Figure 2.
Swiss ADME Analysis of MCu1–MCu9.
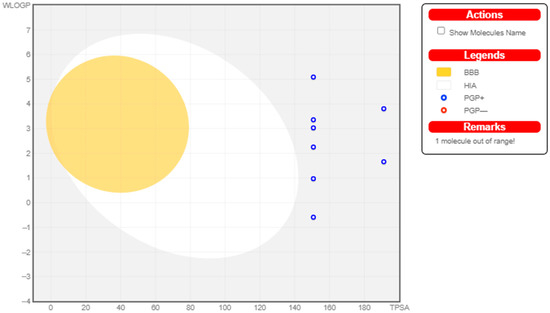
Figure 3.
Swiss ADME Analysis of MZn1–MZn9.
2.3. Enzyme Inhibition Assays
2.3.1. α-Glucosidase
The α-glucosidase enzyme inhibition potential of metformin derivatives is presented in Table 4, Table 5 and Table 6. The graphical depiction of the results is shown in Figures S1–S3 (Supplementary Materials). Metabolites Met8 (IC50 562.64 µg/mL), MCu1 (IC50 421.7 µg/mL), and MZn8 (IC50 511.4 µg/mL) expressed the highest α-glucosidase inhibition activities. Furthermore, Table S3 (Supplementary Materials) shows 2D structures of all metformin derivatives with respect to glucosidase enzyme.

Table 4.
α-Glucosidase activity of Met1–Met9.

Table 5.
α-Glucosidase activity of MCu1–MCu9.

Table 6.
α-Glucosidase activity of MZn1–MZn9.
2.3.2. α-Amylase Assay
The inhibitory potential along with the IC50 values of metformin derivatives against the α-amylase enzyme is displayed in Table 7, Table 8 and Table 9. The maximum percentage inhibition against α-amylase was displayed by Met8 (IC50 752.376 µg/mL), MCu8 (IC50 712.6 µg/mL), and MZn2 (IC50 484.9 µg/mL). The graphical representation of the results is shown in Figures S4–S6 (Supplementary Materials). Table S2 (Supplementary Materials) depicts 2D structures of all Metformin derivatives.

Table 7.
α-Amylase activity of Met1–Met9.

Table 8.
α-Amylase activity of MCu1–MCu9.

Table 9.
α-Amylase activity results of MZn1–MZn9.
2.4. Molecular Docking Studies
A total of 27 ligands were docked to the enzymes. The interactions of the receptors α-amylase and α-glucosidase with the ligands are shown in Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8, while the binding affinities and binding forces of the ligands and enzyme are reported in Table 10 and Table 11.
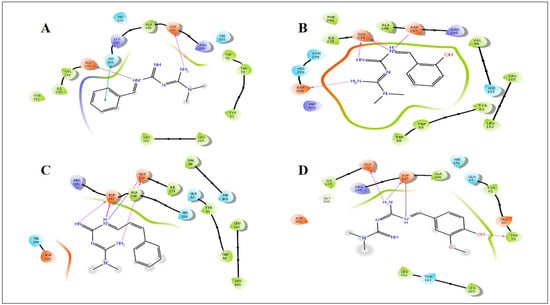
Figure 4.
Induced fit docking study of the best pose generated on α-amylase (5E0F): (A) Met1 ligand-binding site; (B) Met2 ligand-binding site; (C) Met3 ligand-binding site; (D) Met8 ligand-binding site.
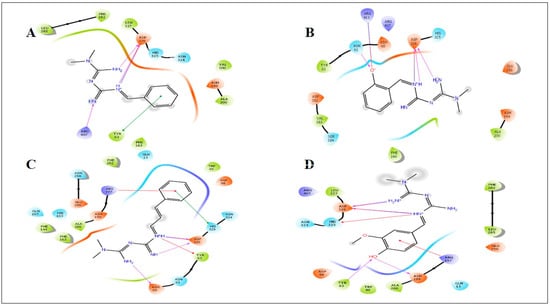
Figure 5.
Induced fit docking study of the best pose generated on α-glucosidase (2ZEO): (A) Met1 ligand-binding site; (B) Met2 ligand-binding site; (C) Met3 ligand-binding site; (D) Met8 ligand-binding site.
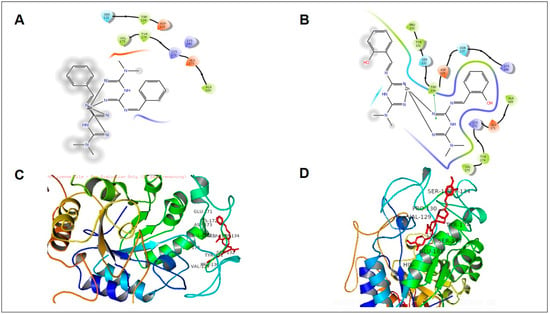
Figure 6.
Induced fit docking study of the best pose generated on α-amylase: (A) MZn1 ligand-binding site; (B) MZn2 ligand-binding site; (C) MZn3 ligand-binding site; (D) MZn8 ligand-binding site.
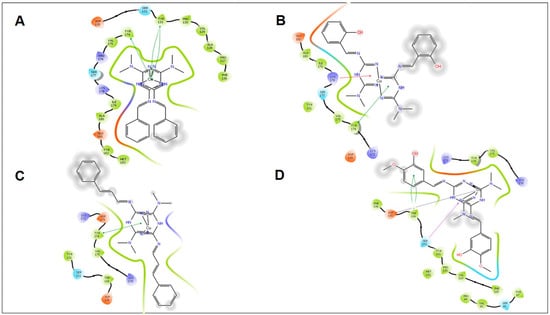
Figure 7.
Induced fit docking study of the best pose generated on α-amylase: (A) MCu1 ligand-binding site; (B) MCu2 ligand-binding site; (C) MCu3 ligand-binding site; (D) MCu8 ligand-binding site.
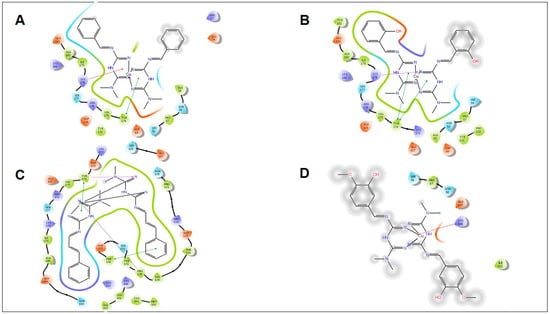
Figure 8.
Induced fit docking study of the best pose generated on α-glucosidase: (A) MCu1 ligand-binding site; (B) MCu2 ligand-binding site; (C) MCu3 ligand-binding site; (D) MCu8 ligand-binding site.

Table 10.
Docking scores of metformin derivatives.

Table 11.
Docking scores of metformin metal complex derivatives.
2.5. Three-Dimensional Coordination Geometries
The 3D coordination geometries and poses were established to show the 3D coordination of all metformin derivatives and metal complexes with respect to different interaction sites (Table S4, Supplementary Materials).
3. Discussion
Most drugs fail at the clinical trial stage or are withdrawn from the market due to the negative properties of ADME (absorption, distribution, metabolism, and excretion) and unavoidable side-effects. The in silico prediction of ADME properties can reduce the effort and time needed by investigators to make models for every new derivative in the development of a lead compound []. Swiss ADME can help to determine the different physicochemical properties of novel tested compounds such as molecular weight, hydrogen bond donors (HBDs), hydrogen bond acceptors (HBAs), partition coefficient (log P), and solvent-accessible surface area within a desired range of acceptance. All novel derivatives of metformin had the ability to cross the gastrointestinal layer, and only one derivative (MET-7) could cross the blood–brain barrier (BBB). All novel derivatives exhibited log S under the predicted value (−6.5 to 0.5). All derivatives followed Lipinski’s rule of five and were freely soluble in aqueous media. Therefore, according to the overall physicochemical prediction, most derivatives possessed drug-likeness behavior. No derivatives of copper and zinc could cross the blood–brain barrier, and they had low affinity for gastrointestinal absorption.
All derivatives showed a positive effect of enzyme inhibition on amylase and glucosidase. Met8 showed good enzyme inhibition when compared to the parent drug, as well as the standard. Met1, Met2, and Met3 showed comparable activity against glucosidase and amylase. The metal complexes with copper showed a linear graph, whereas zinc derivatives showed abrupt inhibition values.
Docking studies were performed to evaluate the binding interaction and binding energy between the receptors and small organic molecules. Docking results were further characterized with biological studies. All novel synthesized derivatives were simulated with the active pockets of α-glucosidase and α-amylase. After evaluating the docking scores (Table 7 and Table 8), only four active compounds (Met1, Met2, Met3, and Met8) were selected for deep insight into different interactions.
All synthetic derivatives were analyzed by molecular simulation studies to identify binding interactions in the active pocket site of the enzyme. The radiographic structures of human pancreatic α-amylase (PDB: 5EOF) were selected as models for this purpose. The molecular docking results showed that several interactions were available between the derivatives and active pocket residues. The top four active derivatives were Met1, Met2, Met3, and Met8, showing multiple additional hydrogen bond hydrophobic interactions with residues Tyr62, Leu165, Lys200, and Ile235. In addition to the hydrophobic interactions, multiple hydrogen bonds were also observed. The metal bond order was considered as zero order []. Therefore, the force field method was able to adequately handle the coordinate bonds of metal complexes. All derivatives had a nitrogen moiety to build a hydrogen bond with residues Asp197, Glu233, and Asp300. However, an additional hydrogen bond was formed by the hydroxyl group of the derivatives with Trp59. Additional interactions such as salt bridges were seen in derivatives of positively charged nitrogen atoms on residues Asp197 and Glu233. Derivative Met1 exhibited pi stacking with the phenyl group of His201. Zinc complex derivatives showed different types of interactions in the pocket site such as hydrophobic interactions, pi–pi stacking, positive hydrogen bond, and metal bonding. MZn1, MZn2, MZn3, and MZn8 exhibited hydrophobic interactions with Val175, Ala169, and Trp134. MZn2 established a positive hydrogen bond with Trp134. Copper metal derivatives exhibited a positive hydrophobic interaction with copper metal, with the benzene ring being mainly responsible for the hydrophobic interactions. MCu1, MCu2, MCu3, and MCu8 established hydrogen bonds with copper via the active pocket residues Tyr131 and Tyr174. MCu8 exhibited a strong metal bond with copper metal and with residue Asp135.
The simulated binding of compounds resulted in several polar and nonpolar contacts with our target protein (2ZEO). Although the compounds were present in the active pocket site, some minor changes were observed. The nitrogen groups formed hydrogen bonds with active residue Asn61, Tyr63, Asp199, and Asp326, although the active pocket featured a few hydrophobic residues, e.g., Trp49, Val100, Asp199, Ala200, Leu285, and Val383. The novel derivatives established pi–pi stacking of Met1 and Met3 with the phenyl rings of residues Tyr63 and His325, as well as salt bridges between the positively charged nitrogen moieties present in the structure via residue Asp326, while Met2 formed a salt bridge with Arg411 of the hydroxyl group present in the structure.
In vitro studies showed that the Met8 derivative exhibited both amylase and glucosidase activity, while the docking studies revealed that derivative Met2 showed the highest docking scores for both enzymes. The α-glucosidase activity of derivative Met8 showed good activity in both in vitro and in silico docking studies. The metal complex with copper showed better activity than zinc complexes. The α-amylase activity results and docking studies revealed that MZn8 and MCu8 showed good activity.
4. Materials and Methods
4.1. Synthesis of Metformin Schiff Base
Metformin was dissolved in 10 mL of methanol, and the methanolic solution was reacted with aromatic aldehyde/ketone added dropwise. Drops of acetic acid were added, and the mixture was refluxed with continuous stirring over 4 h. Synthesized products were recrystallized by washing with methanol. Scheme 1 represents copper and zinc metal complexes with functional groups depicted in Table 12.
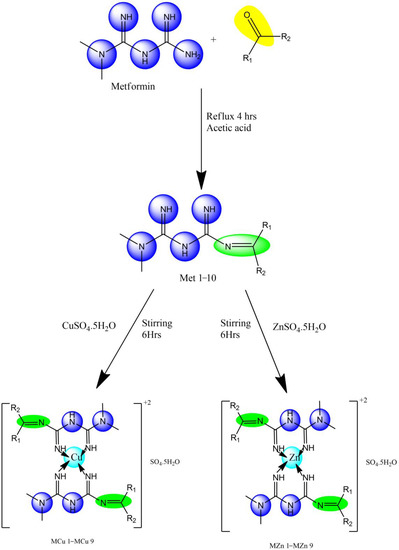
Scheme 1.
Synthesis of metformin Schiff base with copper (MCu1–MCu9) and Zinc (MZn1–MZn9) complexes.

Table 12.
Copper and Zinc metal complexes with functional groups.
4.2. Copper Complexes of Metformin Derivatives (Met1–Met9)
The complexes were prepared via the reaction of CuSO4·5H2O and ligands (Met1–Met9) in methanol. The methanolic solution (10 mL) of CuSO4·5H2O (0.748 g; 3 mmol) was added dropwise to the methanolic solution (20 mL) of ligand (6 mmol) with constant stirring for 6 h. The solution was then concentrated to 15 mL volume and left to stand for 48 h. The precipitate was filtered with filter paper, and then dried in a desiccator for 24 h.
4.3. Zinc Complexes of Metformin Derivatives (Met1–Met9)
The complexes were prepared via the reaction of ZnSO4·5H2O and ligands (Met1–Met9) in methanol. Methanolic solution (10 mL) of ZnSO4·5H2O (0.748 g; 3 mmol) was added dropwise to the methanolic solution (20 mL) of ligand (6 mmol) with constant stirring for 6 h. The solution was then concentrated to 15 mL volume and left to stand for 48 h. The precipitate was filtered with filter paper, and then dried in a desiccator for 24 h.
4.4. In Silico ADME Prediction
Swiss ADME, an online ADME prediction tool, was applied in the present study to predict the drug likeness and physicochemical properties of novel derivatives of metformin []. The structure of derivatives was translated into canonical SMILES format and then submitted to the Swiss ADME tool, which provides free access to predict different properties of compounds, e.g., drug likeness, Lipinski’s rule of five, gastrointestinal absorption, blood–brain barrier, and total polar surface area [].
4.5. Enzyme Inhibition Analysis
4.5.1. α-Amylase
All derivatives of metformin were analyzed for serum amylase inhibitory activity using the Yukihoko Hara method []. Three test tubes were labeled as blank (B), test (T), and control (C). Then, 2.5 mL of phosphate buffer (pH 6.8) was added to each test tube. Next, 1 mL of starch and 1 mL of 2 N sodium chloride solution were added to each test tube. The test tubes were incubated at 37 °C for 10 min. After incubation, 0.5 mL of derivatives was added to test tube T, followed by 0.2 mL of enzyme (α-amylase). All contents were mixed well, and then the tube was incubated at 37 °C for 10 min. In the control test tube, normal saline (2 N), starch solution (1 mL), and enzyme solution (0.2 mL) were added before incubating at 37 °C for 10 min. In the blank test tube, only 5.7 mL of distilled water was added. Dinitro-salicylic acid (0.2 mL) was added to all test tubes. The contents were mixed well, and the test tubes were kept in a boiling water bath for 15 min. The intensity of reddish orange was read at 540 nm. The percentage inhibitory action of serum amylase was calculated using the following formula:
Percentage inhibition = OD of control − OD of test tube/OD of control × 100.
4.5.2. α-Glucosidase
The α-glucosidase-inhibitory activity of all derivatives of metformin was tested using the method previously described []. Three test tubes were marked as test (T), blank (B), and control (C). Then, 200 µL of substrate (starch) and enzyme (α-glucosidase) was added to test tubes T and C. The contents were mixed well, and then the test tubes were incubated at 37 °C for 30 min. After incubation, trichloroacetic acid was added to all test tubes, which were then centrifuged for 10 min. Free glucose was available after the reaction with anthrone reagent (1.5 mL). The contents in the test tubes were boiled for 15 min, and the absorbance was read at a wavelength at 640 nm. The percentage inhibition was calculated using the following formula:
Percentage inhibition = OD of control − OD of test tube/OD of control × 100.
4.6. Molecular Docking
Docking studies of novel derivatives of metformin were performed using the Glide module of Schrodinger software 4.6 [,]. The selected target proteins (amylase and glucosidase) were downloaded from the Protein Data Bank (RCSB) []. Novel derivatives of metformin were drawn using Chemdraw12.0 PerkinElmer software.
4.6.1. Ligand Preparation
All ligands used in the docking studies were drawn in ChemDraw 12.0, and the structure was optimized for bond and structure alignment. Then, the structure was imported into the Schrodinger software workspace for energy minimization using the OPLS4 (version 9.03) (Optimized Potentials for Ligand Simulations) (44) force field in LigPrep wizard []. Maestro (12.8) was then used to hydrogens, assign bond orders, and convert the 2D ligand structure to 3D.
4.6.2. Protein Preparation
The Protein preparation wizard in Maestro was used to minimize the selected proteins from RCSB. All nonprotein molecules were removed without interfering with the already attached ligand, which became the basis for the grid box generation. As a first step, charges were equally distributed, and polar hydrogens were added using Epik at pH 7.0 ± 2.0. The heat state was generated, and the protein structure was refined. Water molecules were removed before the protein was subjected to docking analyses.
4.6.3. Receptor Grid Generation
The receptor grid was generated using the co-crystallized ligand. The ligand center was selected for grid box creation, and the van der Waals radius for the receptor was scaled at 1.00 Å with a partial charge of 0.25. For α-amylase (5E0F), the grid box coordinates were x −7.20, y 5.69, z −23.42 with a box size of 20 Å. For α-glucosidase (2ZE0), the grid box coordinates were x 10.39, y 4.11, z 16.74 with a box size of 20 Å.
4.6.4. Docking and Analysis
Docking studies were carried out using the Glide wizard with the proteins and ligands prepared as described above. In the final step of docking, in extra precision mode, the RMSD (root-mean-square deviation) value was restrained within 0.46 Å and the atomic charge was restrained within 0.15 for the receptor and all derivatives. When the docking process was complete, the interactions were observed using XP visualizer and Pymol (1.5.7).
5. Conclusions
This study focused on the synthesis, characterization, design, and screening of many metformin derivatives with the potential to effectively treat diabetes while causing only a small number of adverse effects. It was determined whether any of the compounds had the ability to inhibit the enzymes α-amylase and α-glucosidase. Only derivatives 1, 2, 3, and 8 exhibited any significant activity during the amylase and glucosidase tests. Each derivative included a benzene ring with a replacement. The presence of a substituted benzene ring in the meta position, a hydroxyl group, and an ether linkage in the para position provided derivative 8 with the maximum activity. This was also due to the presence of a hydroxyl group. When two benzene rings were included, a reduction in activity was observed, e.g., in derivatives 7 and 9. Docking experiments were conducted on all compounds against the human pancreatic amylase and glucosidase enzymes, and the results showed a variety of interactions, including hydrogen bonds, pi–pi stacking, and salt bridges. Accordingly, we were able to identify novel metformin derivatives with the potential to serve as lead compounds in subsequent studies for the development of effective inhibitors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031406/s1, Schemes S1–S3: The characterization schemes of all the metformin metabolites and metal complexes; Table S1: ADME predictions of Metformin metabolites and metal complexes; Table S2: 2D structures of all Metformin derivatives with respect to amylase enzyme; Table S3: 2D structures of all Metformin derivatives with respect to glucosidase enzyme; Table S4: 3D pose and geometries of all the metformin derivatives; Figure S1: Graphical representation of α-glucosidase activity of Met-1–Met-9; Figure S2: Graphical representation of α-glucosidase activity of MCu1–MCu9; Figure S3: Graphical representation of α-glucosidase activity of MZn1–MZn9; Figure S4: Graphical representation of α-amylase activity of Met1–Met9; Figure S5: Graphical representation of α-amylase activity of MCu1–MCu9; Figure S6: Graphical representation of α-amylase activity of MZn1-MZn9; Figures S7–S31: IR and NMR spectras of all the metformin metabolites and metal complexes.
Author Contributions
Writing—original draft, data curation, and formal analysis, J.A., I.A. and M.A.K.; conceptualization, supervision, and investigation, U.K., M.A.K., S.K., M.O.G. and I.A.; conceptualization, methodology, and project administration, S.K. and M.A.K.; writing—review and editing, validation, resources, and project administration, M.E.K., I.P. and M.A.A.; data curation, methodology, software, and formal analysis, G.M.A., A.E.A., A.A.S., M.M.A.-D. and K.u.R.K.; investigation, U.K.; resources, M.A.K. and M.M.A.-D.; data curation, U.K., M.A.K., M.O.G. and I.A.; writing—original draft preparation, J.A., M.A.K., U.K. and I.A.; writing—review and editing, J.A. and M.A.K.; writing—review and editing, validation, resources, and project administration, G.M.A., A.E.A., A.A.S. and M.M.A.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Princess Nourah bint Abdulrahman University Researchers Supporting Project (PNURSP2023R30), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff bases: A versatile pharmacophore. J. Catal. 2013, 2013, 893512. [Google Scholar] [CrossRef]
- Qin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff bases: A short survey on an evergreen chemistry tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Biguanides and NIDDM. Diabetes Care 1992, 15, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.B.; Pilcher, C.C.; Fergusson, D.M.; Stewart, A.W. A population based strategy to prevent insulin-dependent diabetes using nicotinamide. J. Pediatr. Endocrinol. Metab. 1996, 9, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Asogwa, K.K.; Mebarek-Oudina, F.; Animasaun, I. Comparative investigation of water-based Al2O3 nanoparticles through water-based CuO nanoparticles over an exponentially accelerated radiative Riga plate surface via heat transport. Arab. J. Sci. Eng. 2022, 47, 8721–8738. [Google Scholar] [CrossRef]
- Alam, F.; Islam, A.; Khalil, I.; Gan, S.H. Metabolic control of type 2 diabetes by targeting the GLUT4 glucose transporter: Intervention approaches. Curr. Pharm. Des. 2016, 22, 3034–3049. [Google Scholar] [CrossRef]
- Elasy, T.A.; Griffin, M. Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and American Diabetes Association: Response to Nesto. Diabetes Care 2004, 27, 2096. [Google Scholar] [CrossRef]
- Campbell, R.K.; White, J.R., Jr.; Saulie, B.A. Metformin: A new oral biguanide. Clin. Ther. 1996, 18, 360–371. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014, 11, 1185. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Mehanna, A. Antidiabetic agents: Past, present and future. Future Med. Chem. 2013, 5, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Piscioneri, A.; Salerno, S.; Drioli, E.; De Bartolo, L. Biohybrid membrane systems for testing molecules and stem cell therapy in neuronal tissue engineering. Curr. Pharm. Des. 2017, 23, 3858–3870. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Kaushik, N.K.; Verma, A.K.; Hundal, G.; Gupta, R. Synthesis, structure and biological activity of copper (II) complexes of 4-(2-pyridylmethyl)-1, 7-dimethyl-1, 4, 7-triazonane-2, 6-dione and 4-(2-pyridylethyl)-1, 7-dimethyl-1, 4, 7-triazonane-2, 6-dione. Eur. J. Med. Chem. 2009, 44, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Mitra, A. Synthesis, characterization and reactivities of Schiff-base complexes of ruthenium (III). J. Coord. Chem. 2004, 57, 175–182. [Google Scholar] [CrossRef]
- Koh, M.; Lee, J.-C.; Min, C.; Moon, A. A novel metformin derivative, HL010183, inhibits proliferation and invasion of triple-negative breast cancer cells. Bioorg. Med. Chem. 2013, 21, 2305–2313. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic extract of propolis (EEP) enhances the apoptosis-inducing potential of TRAIL in cancer cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef]
- Danish, M.; Raza, M.A.; Khalid, H.; Iftikhar, U.; Arshad, M.N. New metal complexes of sulfonamide: Synthesis, characterization, in-vitro anticancer, anticholinesterase, antioxidant, and antibacterial studies. Appl. Organomet. Chem. 2021, 35, e6033. [Google Scholar] [CrossRef]
- El-Gammal, O.A.; Mohamed, F.S.; Rezk, G.N.; El-Bindary, A.A. Structural characterization and biological activity of a new metal complexes based of Schiff base. J. Mol. Liq. 2021, 330, 115522. [Google Scholar] [CrossRef]
- El-Megharbel, S. Synthesis, characterization and antidiabetic activity of chromium (III) metformin complex. J. Microb. Biochem. Technol 2015, 7, 65–75. [Google Scholar]
- Tanaka, A.; Kaneto, H.; Miyatsuka, T.; Yamamoto, K.; Yoshiuchi, K.; Yamasaki, Y.; Shimomura, I.; Matsuoka, T.-A.; Matsuhisa, M. Role of copper ion in the pathogenesis of type 2 diabetes. Endocr. J. 2009, 56, 699–706. [Google Scholar] [CrossRef]
- Alaysuy, O.; Abumelha, H.M.; Alsoliemy, A.; Alharbi, A.; Alatawi, N.M.; Osman, H.E.M.; Zaky, R.; El-Metwaly, N.M. Elucidating of new hydrazide-based complexes derived from Pd (II), Cu (II) and Cd (II) ions: Studies concerning spectral, DFT, Hirshfeld-crystal, biological screening beside Swiss-ADME verification. J. Mol. Struct. 2022, 1259, 132748. [Google Scholar] [CrossRef]
- Ashok, P.; Sharma, H.; Lathiya, H.; Chander, S.; Murugesan, S. In-silico design and study of novel piperazinyl β-carbolines as inhibitor of HIV-1 reverse transcriptase. Med. Chem. Res. 2015, 24, 513–522. [Google Scholar] [CrossRef]
- Popova, E.A.; Protas, A.V.; Mukhametshina, A.V.; Ovsepyan, G.K.; Suezov, R.V.; Eremin, A.V.; Stepchenkova, E.I.; Tarakhovskaya, E.R.; Fonin, A.V.; Starova, G.L.; et al. Synthesis, biological evaluation and molecular docking studies on the DNA and BSA binding interactions of palladium (II) and platinum (II) complexes featuring amides of tetrazol-1-yl-and tetrazol-5-ylacetic acids. Polyhedron 2019, 158, 36–46. [Google Scholar] [CrossRef]
- Hadanu, R.; Mastjeh, S.; Mustofa, M.; Sholikhah, E.N.; Wijayanti, M.A.; Tahir, I. Quantitave structure-activity relationship analysis (QSAR) of antimalarial 1, 10-phenanthroline derivatives compounds. Indones. J. Chem. 2010, 7, 72–77. [Google Scholar] [CrossRef]
- Ibrahim, Z.Y.U.; Uzairu, A.; Shallangwa, G.A.; Abechi, S.E. Application of QSAR method in the design of enhanced antimalarial derivatives of Azetidine-2-carbonitriles, their molecular docking, drug-likeness, and SwissADME properties. Iran. J. Pharm. Res. IJPR 2021, 20, 254. [Google Scholar] [PubMed]
- Malathi, V.; Devi, S.S.; Revathi, K. Anti Diabetic Activity By The in vitro alpha amylase and alpha-glucosidase inhibitory activity of catharanthus roseus. Bioscan 2010, 5, 655–659. [Google Scholar]
- Li, Y.; Peng, G.; Li, Q.; Wen, S.; Huang, T.H.-W.; Roufogalis, B.D.; Yamahara, J. Salacia oblonga improves cardiac fibrosis and inhibits postprandial hyperglycemia in obese Zucker rats. Life Sci. 2004, 75, 1735–1746. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Release, S. 3: Maestro; Schrödinger, LLC: New York, NY, USA, 2019. [Google Scholar]
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Di Costanzo, L.; Christie, C.; Dalenberg, K.; Duarte, J.M.; Dutta, S.; et al. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucl. Acids Res. 2019, 47, D464–D474. [Google Scholar] [CrossRef]
- Release, S. 4: LigPrep; Schrödinger, LLC: New York, NY, USA, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).