Towards Antibiotic Synthesis in Continuous-Flow Processes
Abstract
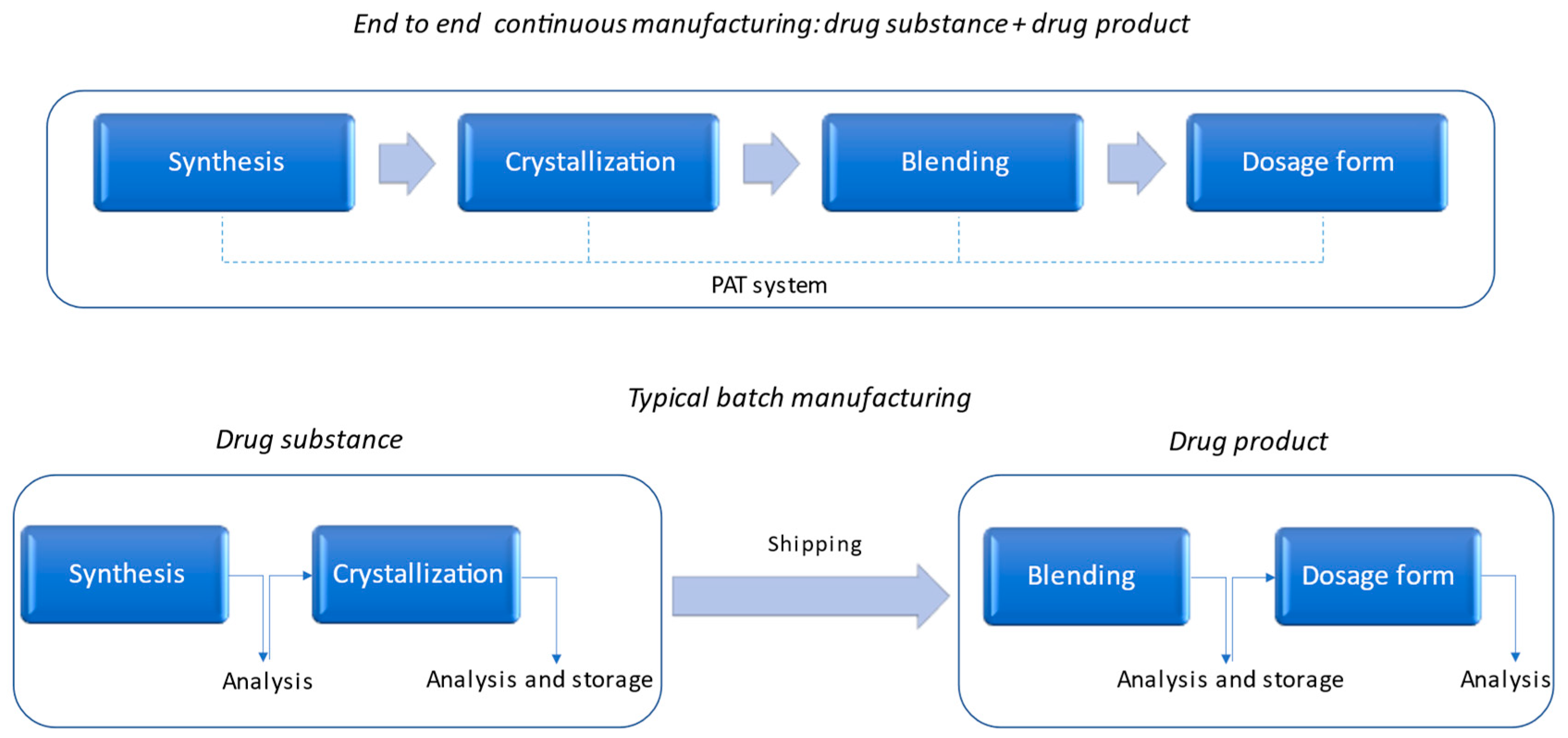
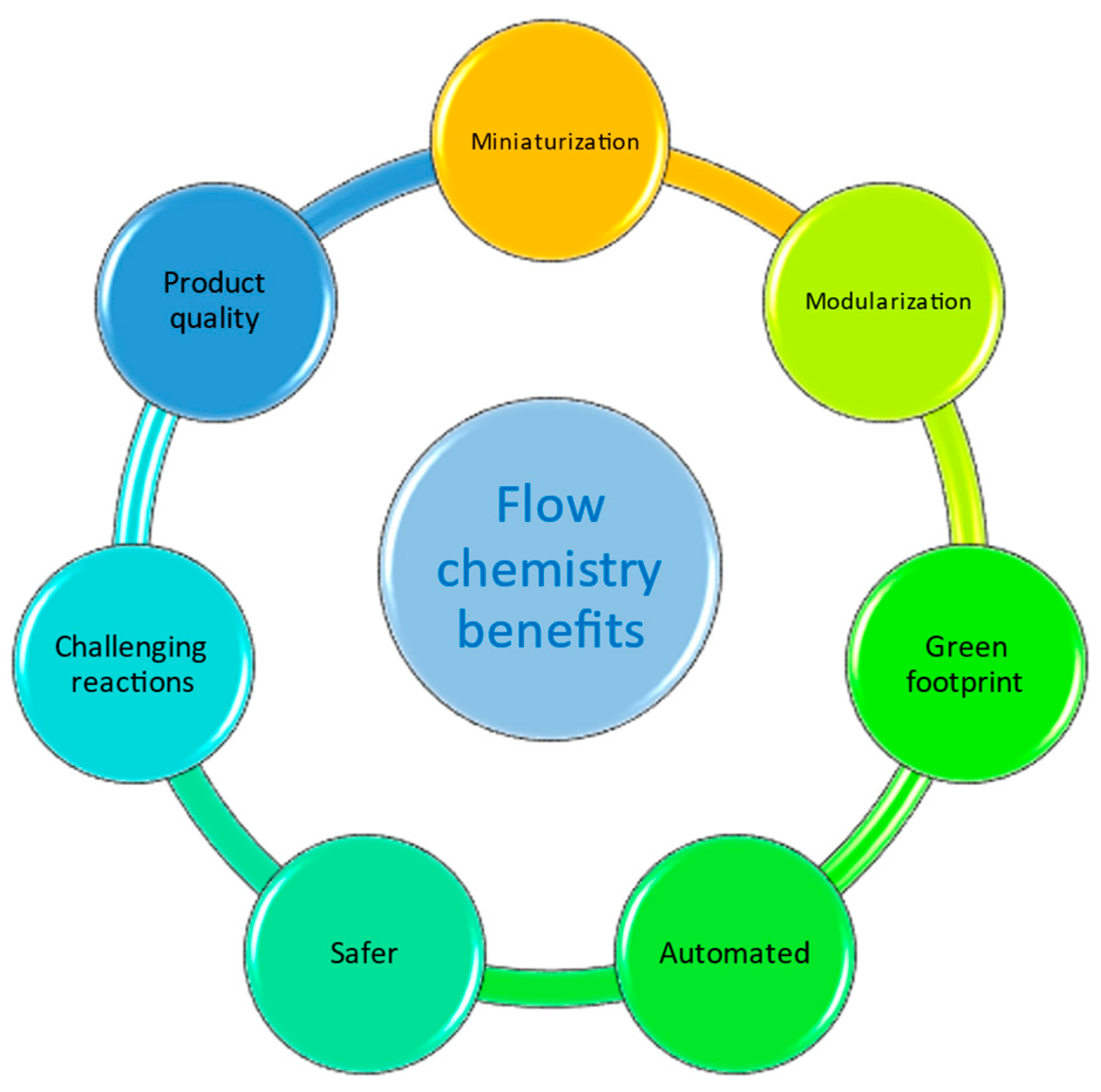
1. Introduction
2. Discussion
3. Antibiotic Synthesis in Flow Mode
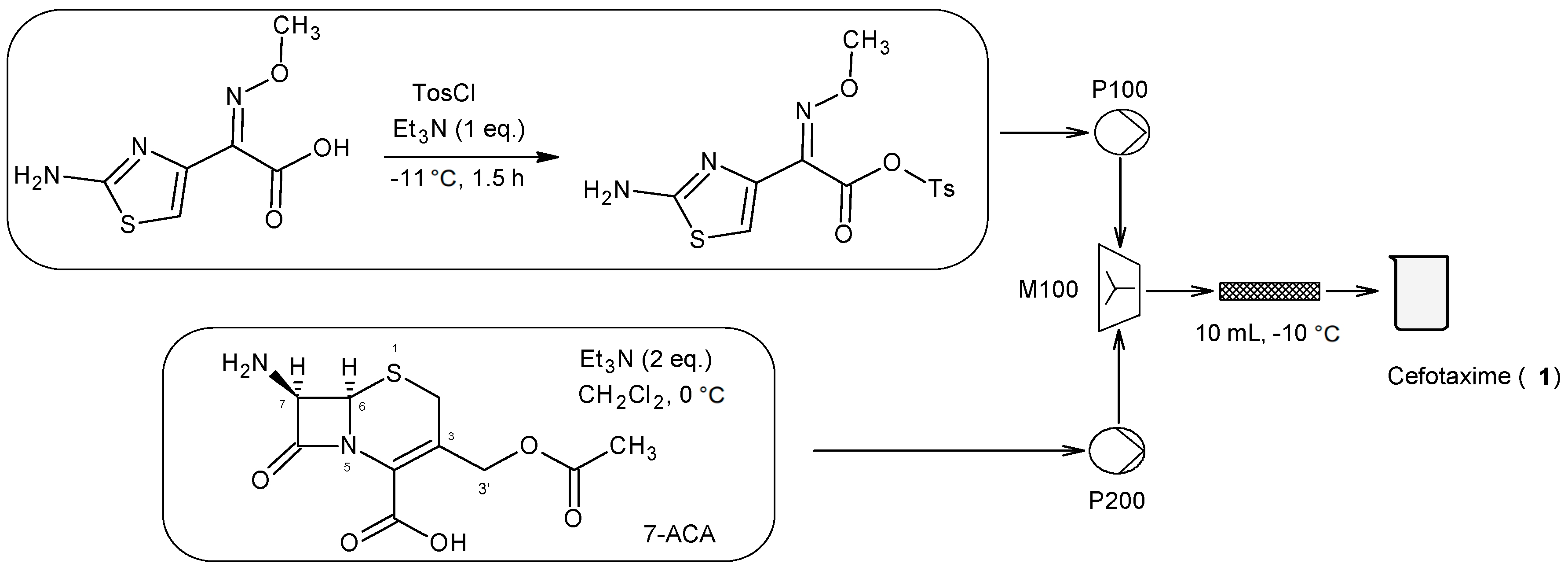
3.1. Cefotaxime
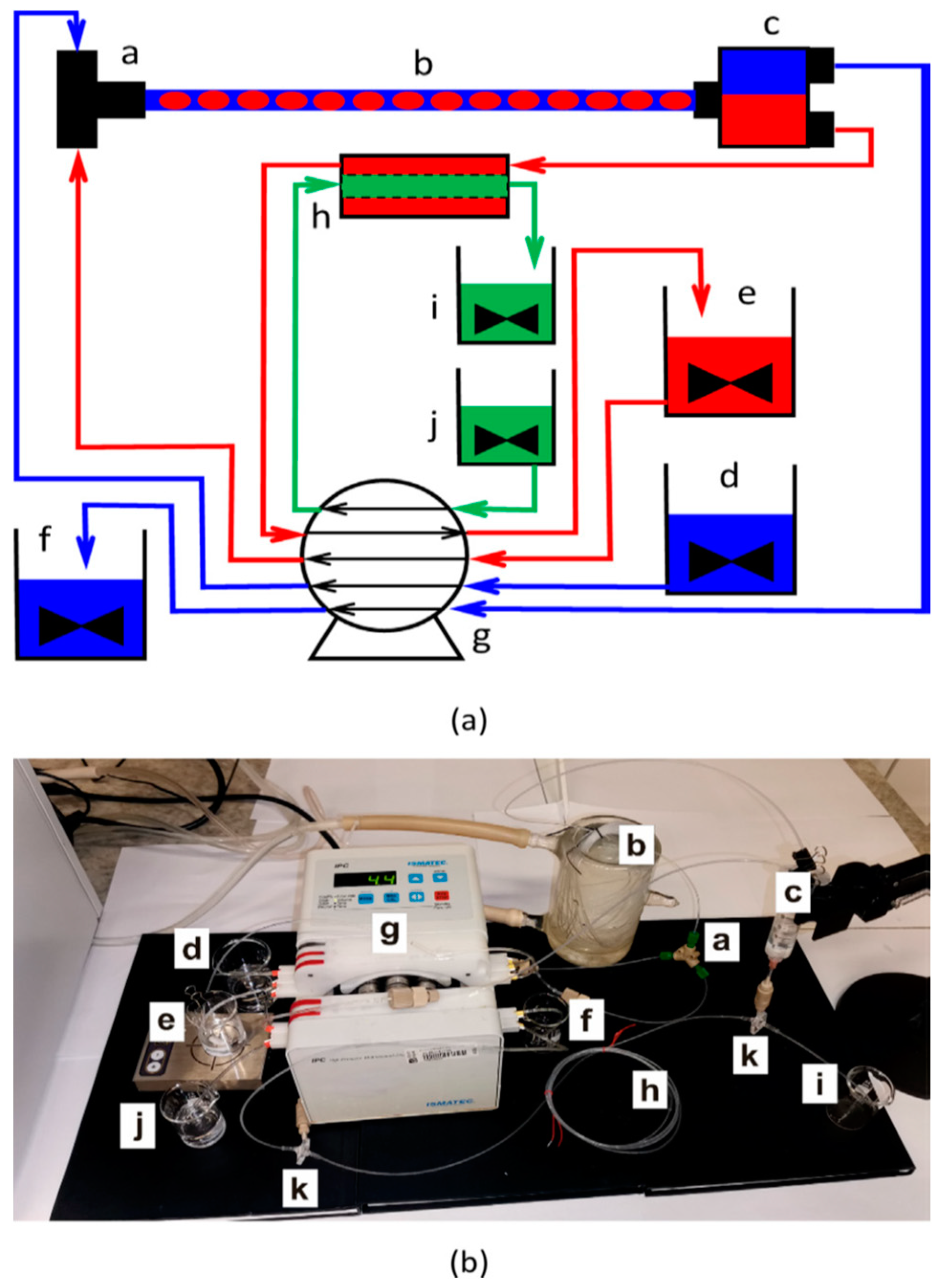
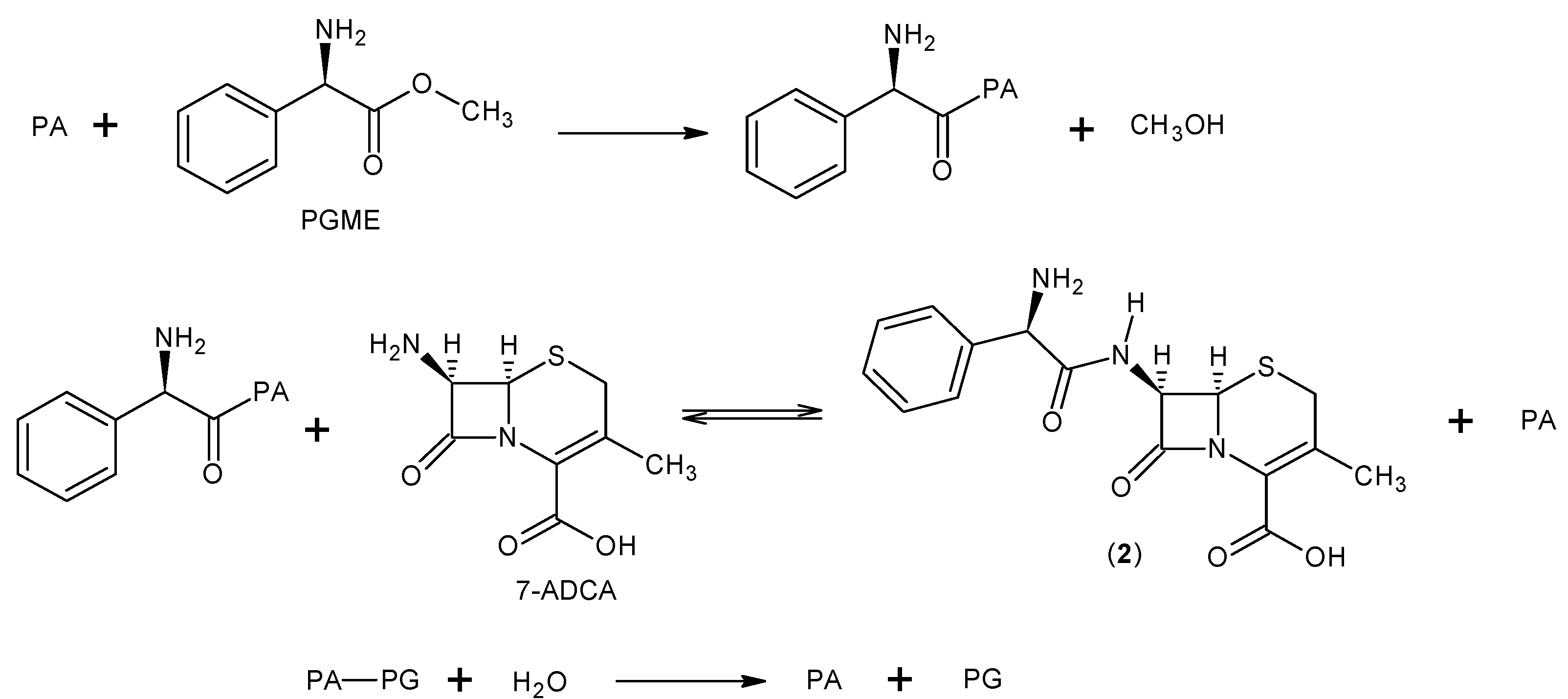
3.2. Cephalexin
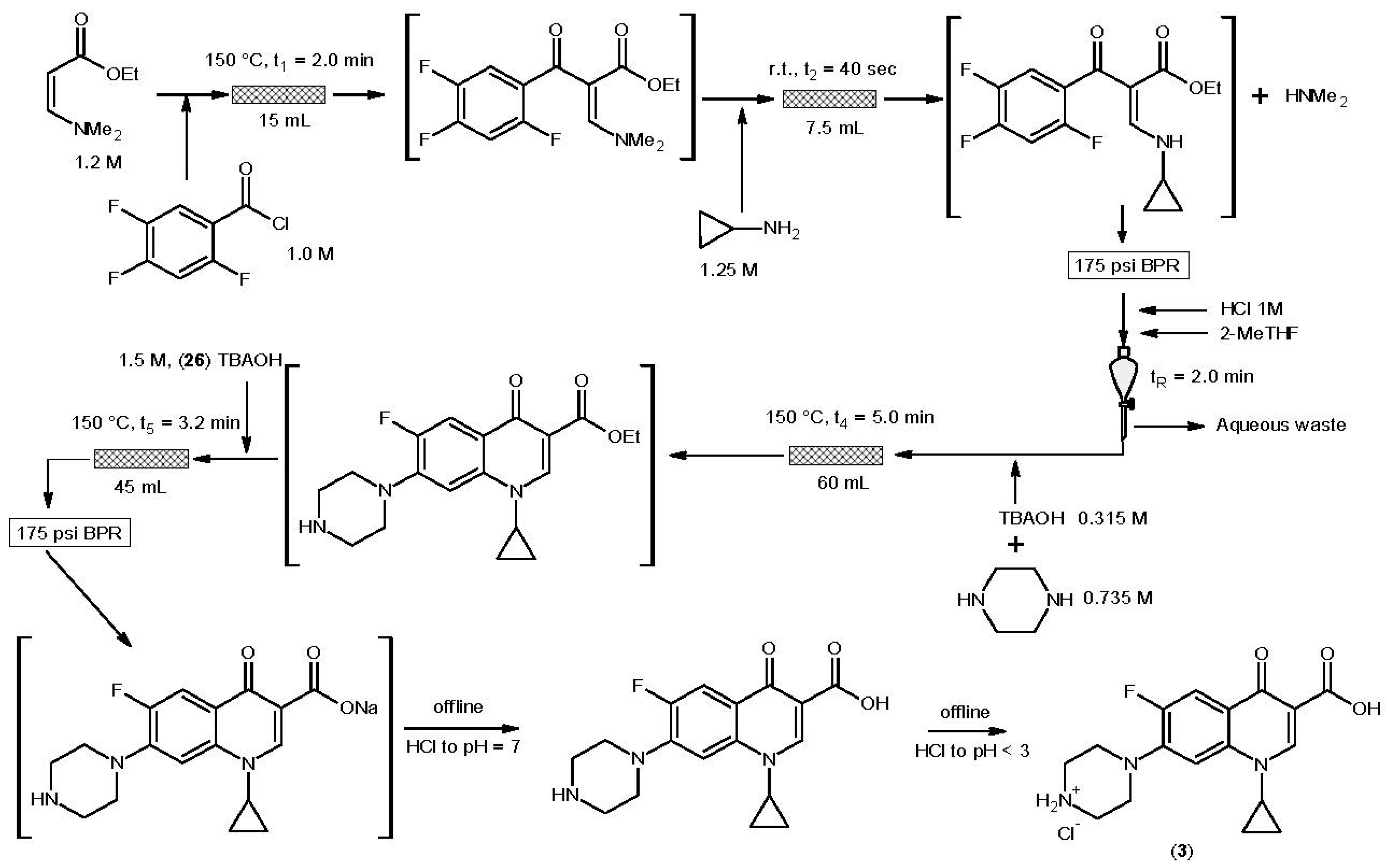
3.3. Ciprofloxacin Hydrochloride
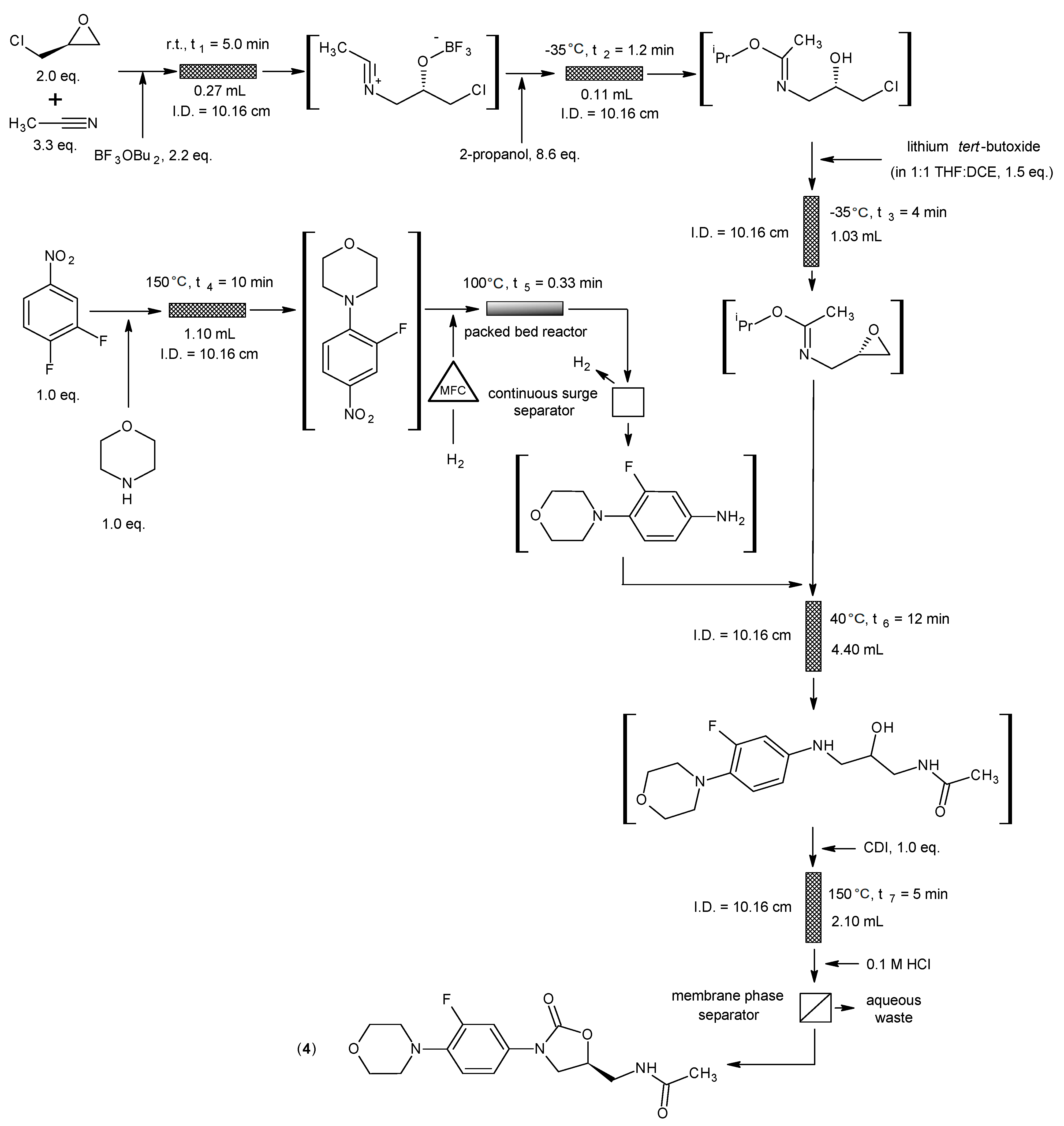
3.4. Linezolid
3.5. Tazobactam
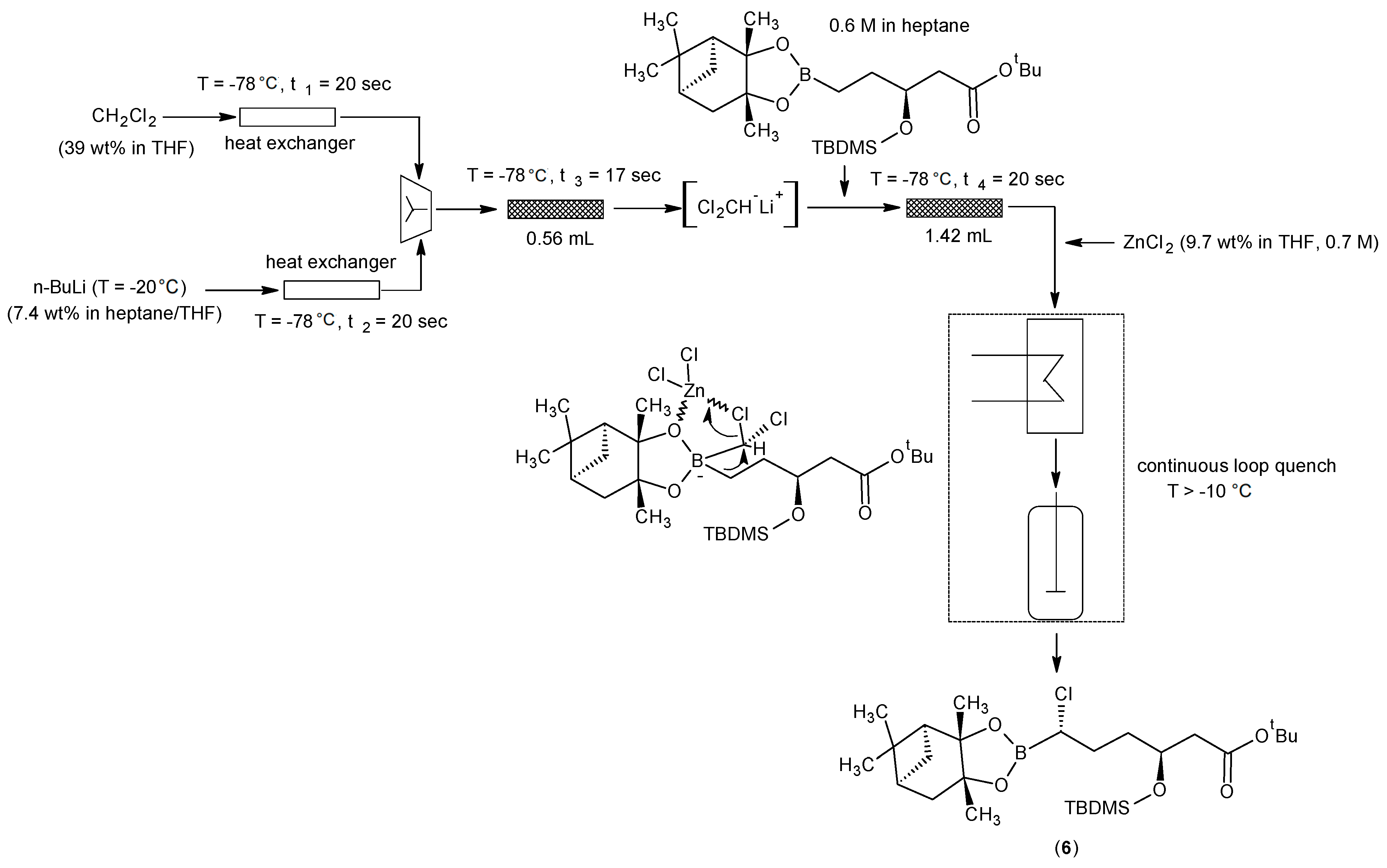
3.6. Key Vaborbactam Intermediate
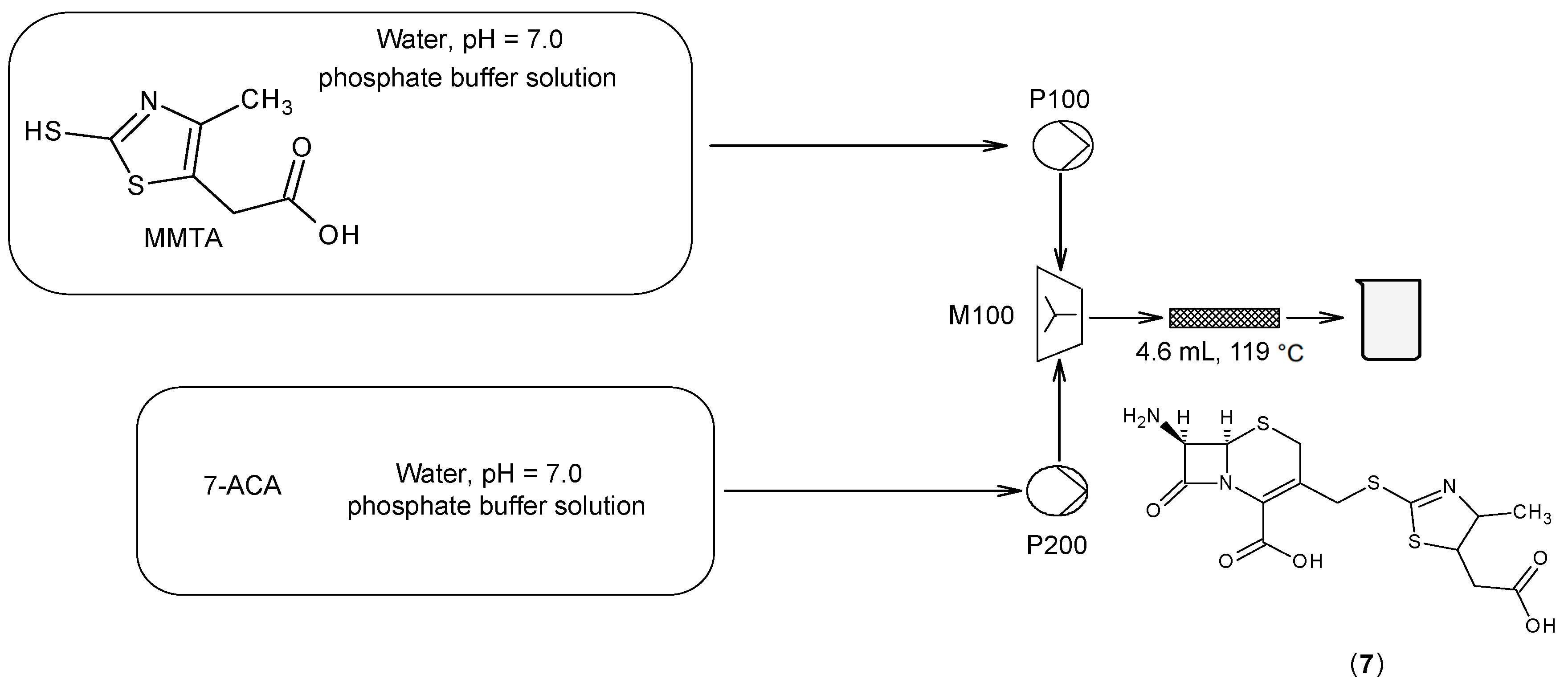
3.7. Key Cefodizime Intermediate
4. Antibiotic Building Blocks in Flow Mode
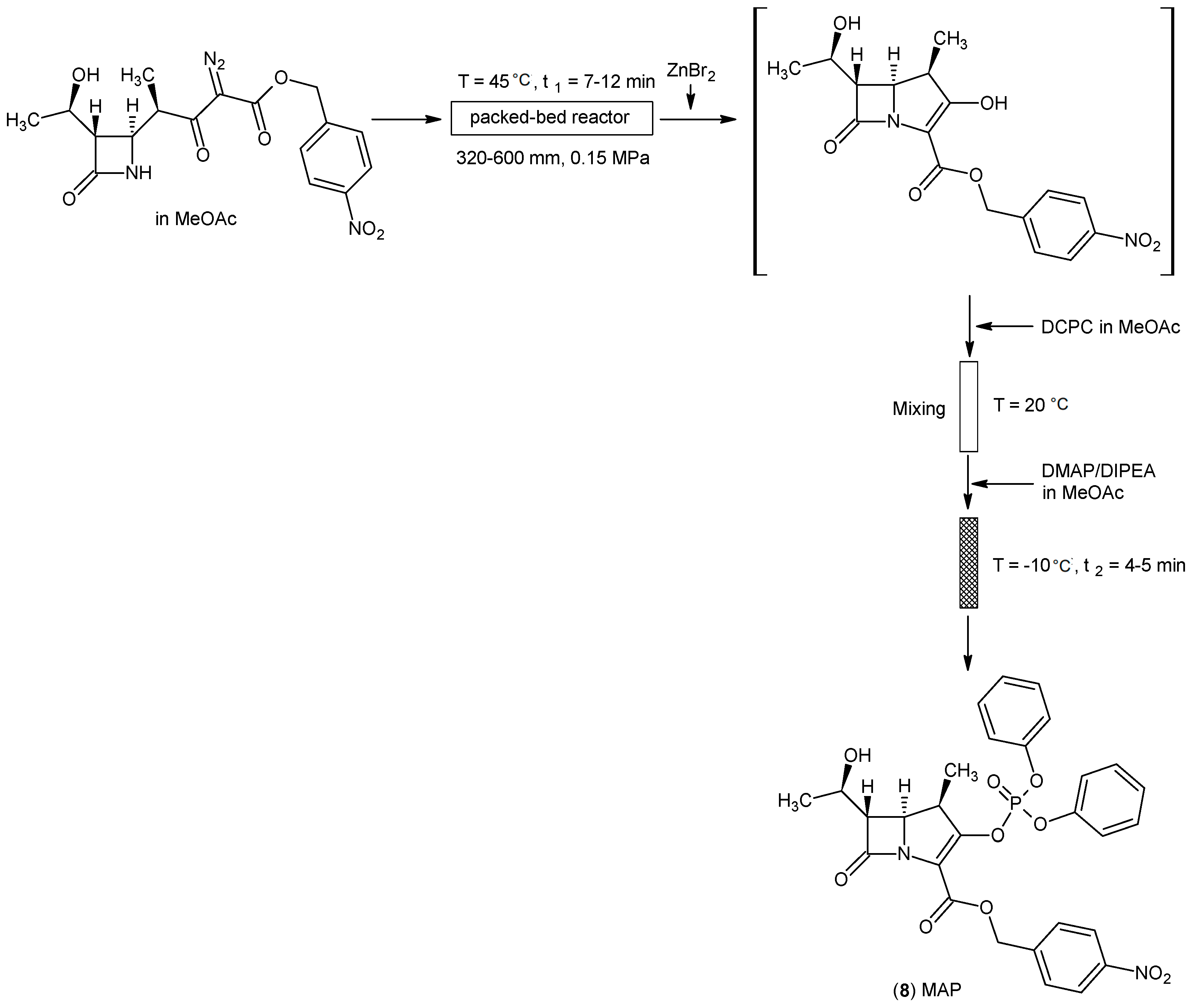
4.1. β-Methyl Vinyl Phosphate (MAP) as a Building Block for β-Methyl Carbapenem
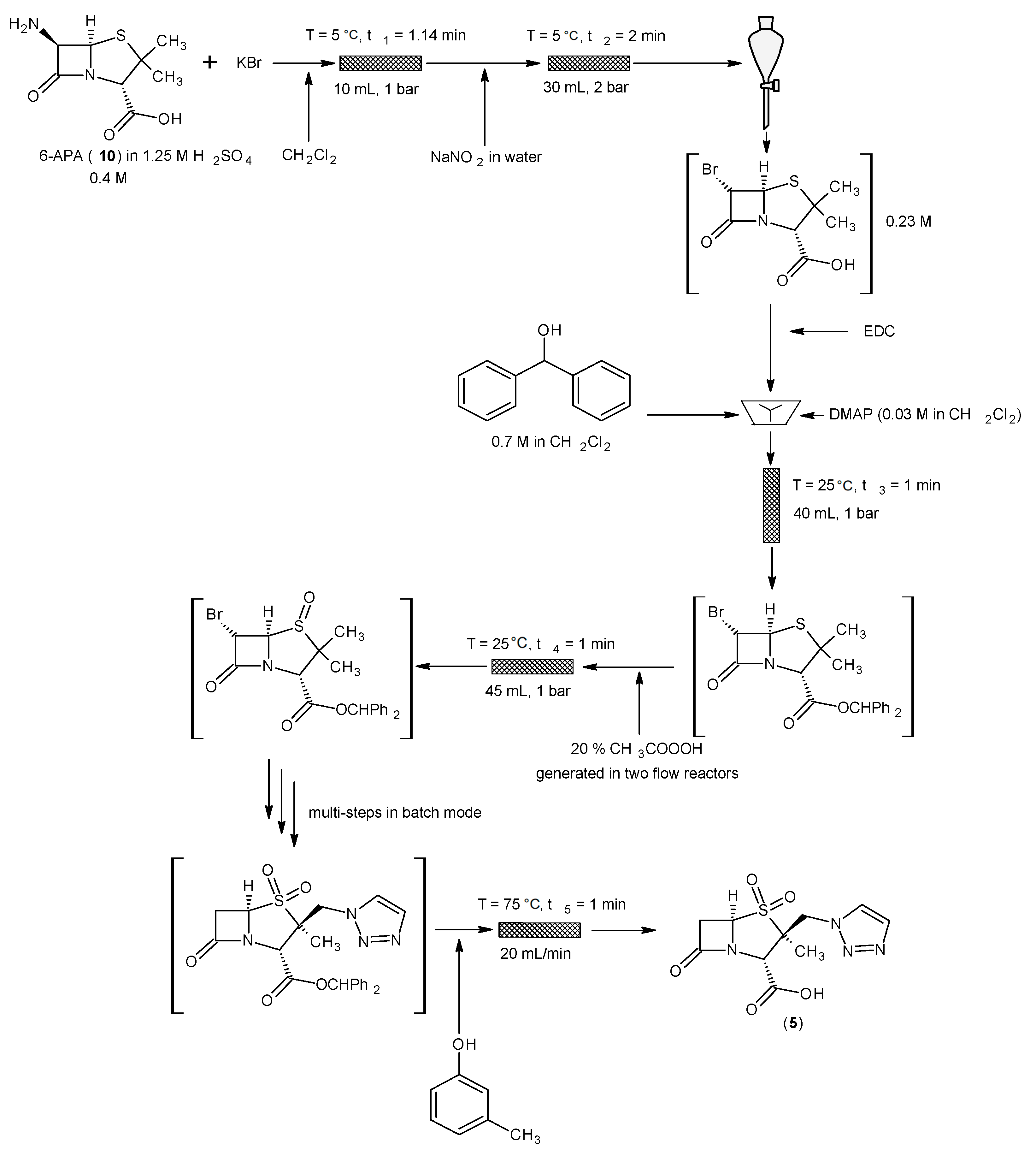
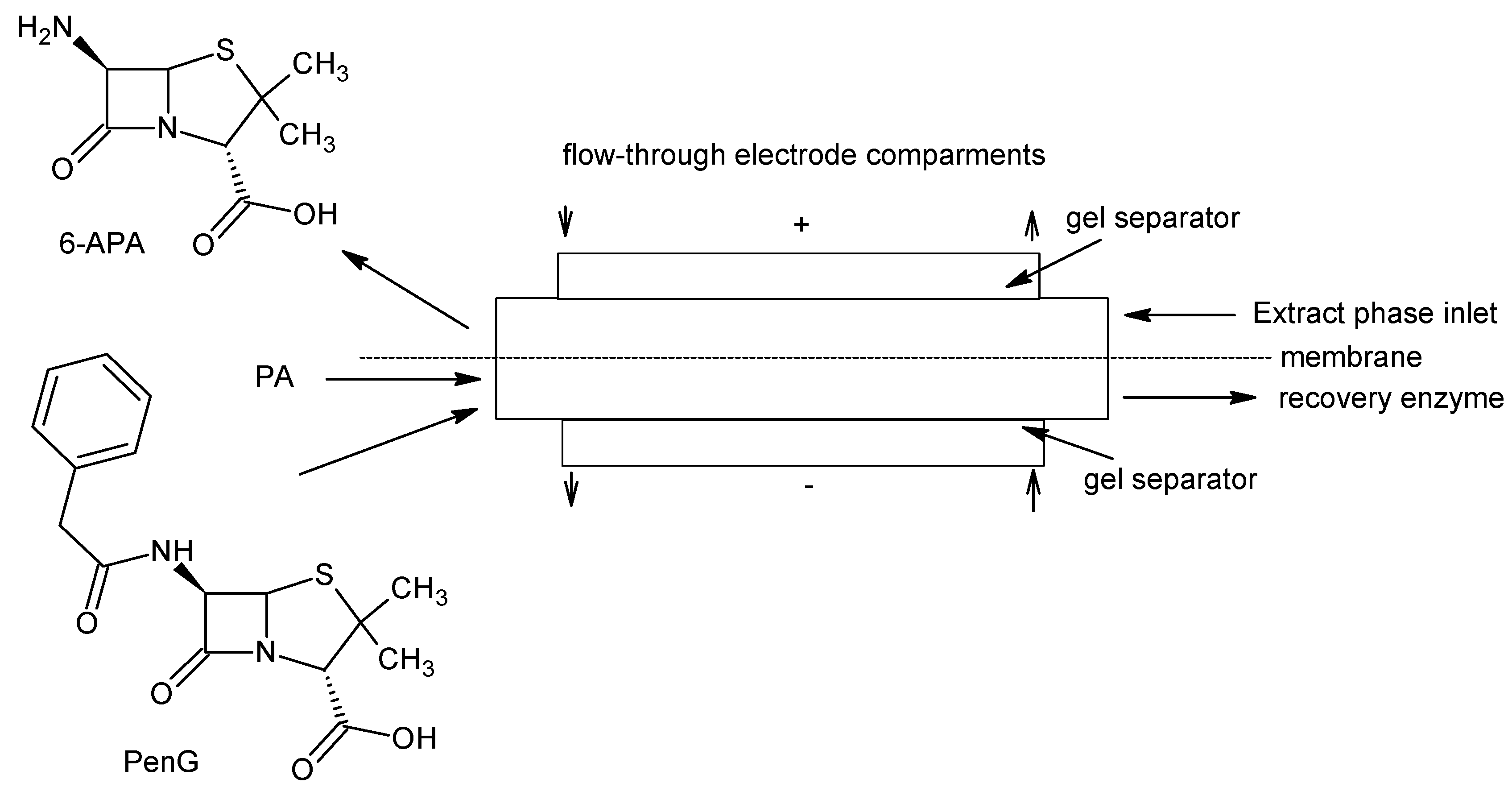
4.2. 6-Aminopenicillanic Acid (6-APA)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Hanna, E.; Remuzat, C.; Auquier, P.; Toumi, M. Advanced therapy medicinal products: Current and future perspectives. J. Mark. Access Health Policy 2016, 4, 31036. [Google Scholar] [CrossRef] [PubMed]
- Ginn, S.L.; Alexander, I.E.; Edelstein, M.L.; Abedi, M.R.; Wixon, J. Gene therapy clinical trials worldwide to 2012—An update. J. Gene Med. 2013, 15, 65–77. [Google Scholar] [CrossRef]
- Bisson, I.; green, E.; Sharpe, M.; Herbert, C.; Hyllner, J.; Mount, N. Landscape of current and emerging cell therapy clinical trials in the UK: Current status, comparison to global trends and future perspectives. Regen. Med. 2015, 10, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.T.; Hessel, V. Micro reactor and flow chemistry for industrial applications in drug discovery and development. Green Process. Synth. 2012, 1, 149–167. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R. The synthesis of active pharmaceuticals ingredients (APIs) using continuous flow chemistry. Beilstein J. Org. Chem. 2015, 11, 1194–1219. [Google Scholar] [CrossRef]
- Baumann, M.; Moody, T.S.; Smyth, M.; Wharry, S. A perspective on continuous flow chemistry in the pharmaceutical industry. Org. Process Res. Dev. 2020, 24, 1802–1813. [Google Scholar] [CrossRef]
- Burange, A.S.; Osman, S.M.; Luque, R. Understanding flow chemistry for the production of active pharmaceutical ingredients. iScience 2022, 25, 103892. [Google Scholar] [CrossRef]
- De Souza, J.M.; Galaverna, R.; De Souza, A.A.N.; Brocksom, T.J.; Pastre, J.C.; De Souza, R.O.M.A.; De Oliveira, K.T. Impact of continuous flow chemistry in the synthesis of natural products and active pharmaceutical ingredients. An. Acad. Bras. Ciệnc. 2018, 90, 1131–1174. [Google Scholar] [CrossRef]
- Glasnov, T.N.; Kappe, C.O. Continuous-flow syntheses of heterocycles. J. Heterocycl. Chem. 2011, 48, 11–30. [Google Scholar] [CrossRef]
- Lee, S.L.; O’Connor, T.F.; Yang, X.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Moore, C.M.V.; Yu, L.X.; Woodcock, J. Modernizing pharmaceutical manufacturing: From batch to continuous production. J. Pharm. Innov. 2015, 10, 191–199. [Google Scholar] [CrossRef]
- Hughes, D.L. Applications of flow chemistry in the pharmaceutical industry-highlights of the recent patent literature. Org. Process Res. Dev. 2020, 24, 1850–1860. [Google Scholar] [CrossRef]
- Koenig, S.G.; Sneddon, H.F. Recent advances in flow chemistry in the pharmaceutical industry. Green Chem. 2017, 19, 1418–1419. [Google Scholar] [CrossRef]
- Navarro, P.M.L.; Lanari, D. Flow synthesis of biologically-relevant compound libraries. Molecules 2020, 25, 909. [Google Scholar] [CrossRef]
- O’Beirne, C.; Piatek, M.E.; Fossen, J.; Müller-Bunz, H.; Andes, D.R.; Kavanagh, K.; Patil, S.A.; Baumann, M.; Tacke, M. Continuous flow synthesis and antimicrobial evaluation of NHC* silver carboxylate derivates of SBC3 in vitro and in vivo. Metallomics 2021, 13, mfaa011. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s guide to flow chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Porta, R.; Benaglia, M.; Puglisi, A. Flow chemistry: Recent developments in the synthesis of pharmaceutical products. Org. Process Res. Dev. 2016, 20, 2–25. [Google Scholar] [CrossRef]
- Santi, M.; Sancineto, L.; Nascimento, V.; Azeredo, J.B.; Orozco, E.V.M.; Andrade, L.H.; Gröger, H.; Santi, C. Flow biocatalysis: A challenging alternative for the synthesis of APIs and natural compounds. Int. J. Mol. Sci. 2021, 22, 990. [Google Scholar] [CrossRef]
- Ferlin, F.; Lanari, D.; Vaccaro, L. Sustainable flow synthesis of active pharmaceutical ingredients. Green Chem. 2020, 22, 5937–5955. [Google Scholar] [CrossRef]
- Martina, K.; Cravotto, G.; Varma, R.S. Impact of microwaves on organic synthesis and strategies toward flow processes and scaling up. J. Org. Chem. 2021, 86, 13857–13872. [Google Scholar] [CrossRef]
- Cintas, P.; Tagliapietra, S.; Caporaso, M.; Tabasso, S.; Cravotto, G. Enabling technologies built on a sonochemical platform: Challenges and opportunities. Ultrason. Sonochem. 2015, 25, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Bonrath, W.; Tagliapietra, S.; Speranza, C.; Calcio Gaudino, E.; Barge, A. Intensification of organic reactions with hybrid flow reactors. Chem. Eng. Process. Process Intensif. 2010, 49, 930–935. [Google Scholar] [CrossRef]
- Metro, T.X.; Martinez, J.; Lamaty, F. 1,1’-Carbonyldiimidazole and mechanochemistry: A shining green combination. ACS Sustain. Chem. Eng. 2017, 5, 9599–9602. [Google Scholar] [CrossRef]
- May, S.A. Flow chemistry, continuous processing, and continuous manufacturing: A pharmaceutical perspective. J. Flow Chem. 2017, 7, 137–145. [Google Scholar] [CrossRef]
- Stuart, J.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friŝčič, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Green chemistry in the fine chemicals and pharmaceutical industries. Org. Process Res. Dev. 2013, 17, 1479–1484. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; Oliva Gonzalez, C.M.; Mendez, Y.P.; Lopez, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef]
- Marques, C.A.; Machado, A.A.S.C. Environmental sustainability: Implications and limitations to green chemistry. Found. Chem. 2013, 16, 125–147. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Green Chemistry. Available online: https://www.epa.gov/greenchemistry (accessed on 20 October 2022).
- European Environment Agency. Available online: https://www.eea.europa.eu (accessed on 20 October 2022).
- Makone, S.S.M.; Niwadange, S.N. Green chemistry alternatives for sustainable development in organic synthesis. Int. Adv. Res. J. Sci. Eng. Technol. 2016, 3, 113–115. [Google Scholar] [CrossRef]
- Rossen, K. Greening organic chemistry with process chemistry. J. Org. Chem. 2019, 84, 4580–4582. [Google Scholar] [CrossRef] [PubMed]
- ACS Green Chemistry Institute. Available online: https://www.acsgcipr.org (accessed on 20 October 2022).
- Unterlass, M.M. Green synthesis of inorganic-organic hybrid materials: State of the art and future perspectives. Eur. J. Inorg. Chem. 2016, 8, 1135–1156. [Google Scholar] [CrossRef]
- Jun-Li, C.; Trost, B.M. Green chemistry for chemical synthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 13197–13202. [Google Scholar] [CrossRef]
- Mishra, M.; Sharma, M.; Dubey, R.; Kumari, P.; Ranjan, V.; Pandey, J. Green synthesis interventions of pharmaceutical industries for sustainable development. Curr. Res. Green Sustain. Dev. 2021, 4, 100174. [Google Scholar] [CrossRef]
- EvaluatePharma. Available online: https://info.evaluate.com/rs/607-YGS-364/images/EvaluatePharma_World_Preview_2019.pdf (accessed on 25 October 2022).
- Constable, D.J.C.; Dunn, P.J.; Hayler, J.D.; Humphrey, G.R.; Leazer, J.L., Jr.; Linderman, R.J.; Lorenz, K.; Manley, J.; Pearlman, B.A.; Wells, A.; et al. Key green chemistry research areas-a perspective from pharmaceutical manufacturers. Green Chem. 2007, 9, 411–420. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.; Poechlauer, P.; Broxterman, Q.B.; Yang, B.S.; Ende, D.; Baird, J.; Bertsch, C.; Hannah, R.E.; Dell’Orco, P.; Noorman, H.; et al. Key green engineering research areas for sustainable manufacturing: A perspective from pharmaceutical and fine chemicals manufacturers. Org. Process Res. Dev. 2011, 15, 900–911. [Google Scholar] [CrossRef]
- Bryan, M.C.; Dunn, P.J.; Entwistle, D.; Gallou, F.; Koenig, S.G.; Hayler, J.D.; Hickey, M.R.; Hughes, S.; Kopach, M.E.; Moine, G.; et al. Key green chemistry research areas from a pharmaceutical manufacturers’ perspective revisited. Green Chem. 2018, 20, 5082–5103. [Google Scholar] [CrossRef]
- Quality Considerations for Continuous Manufacturing. Guidance for Industry. Available online: https://www.fda.gov/media/121314/download (accessed on 24 October 2022).
- ICH Guideline Q13 on Continuous Manufacturing of Drug Substances and Drug Products. Available online: https://www.ema.europa.eu/en/ich-guideline-q13-continuous-manufacturing-drug-substances-drug-products (accessed on 24 October 2022).
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-flow technology-A tool for the safe manufacturing of active pharmaceutical ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. [Google Scholar] [CrossRef]
- Fülöp, Z.; Szemesi, P.; Bana, P.; Éles, J.; Greiner, I. Evolution of flow-oriented design strategies in the continuous preparation of pharmaceuticals. React. Chem. Eng. 2020, 5, 1527–1555. [Google Scholar] [CrossRef]
- Baumann, M.; Moody, T.S.; Smyth, M.; Wharry, S. Overcoming the hurdles and challenges associated with developing continuous industrial processes. Eur. J. Org. Chem. 2020, 2020, 7398–7406. [Google Scholar] [CrossRef]
- Bogdan, A.R.; Dombrowski, A.W. Emerging trends in flow chemistry and applications to the pharmaceutical industry. J. Med. Chem. 2019, 62, 6422–6468. [Google Scholar] [CrossRef] [PubMed]
- Bana, P.; Örk ényi, R.; Lövei, K.; Lakó, Á.; Túrós, G.I.; Éles, J.; Faigl, F.; Greiner, I. The Route from problem to solution in multistep continuous flow synthesis of pharmaceutical compounds. Bioorg. Med. Chem. 2017, 25, 6180–6189. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Ania, D.; Rutjes, F.P.J.T. Continuous-flow chemistry in chemical education. J. Flow Chem. 2017, 7, 157–158. [Google Scholar] [CrossRef]
- Leibfarth, F.A.; Russell, M.G.; Langley, D.M.; Seo, H.; Kelly, L.P.; Carney, D.W.; Sello, J.K.; Jamison, T.F. Continuous-flow chemistry in undergraduate education: Sustainable conversion of reclaimed vegetable oil into biodiesel. J. Chem. Educ. 2018, 95, 1371–1375. [Google Scholar] [CrossRef]
- Britton, J.; Jamison, T.F. The assembly and use of continuous flow systems for chemical synthesis. Nat. Protoc. 2017, 12, 2423–2446. [Google Scholar] [CrossRef]
- End-to-End: Can Pharma Finally Make the Dream of Continuous Manufacturing a Reality? Available online: https://www.fiercepharma.com/manufacturing/end-to-end-how-pharma-making-dream-continuous-manufacturing-a-reality (accessed on 10 November 2022).
- Bonner, A.; Loftus, A.; Padgham, A.C.; Baumann, M. Forgotten and forbidden chemical reactions revitalized through continuous flow technology. Org. Biomol. Chem. 2021, 19, 7737–7753. [Google Scholar] [CrossRef]
- Razzaq, T.; Kappe, C.O. Continuous flow organic synthesis under high-temperature/pressure conditions. Chem. Asian J. 2010, 5, 1274–1289. [Google Scholar] [CrossRef]
- Bogdan, A.R.; Charaschanya, M.; Dombrowski, A.W.; Wang, Y.; Djuric, S.W. High-temperature boc deprotection in flow and its application in multistep reaction sequences. Org. Lett. 2016, 18, 1732–1735. [Google Scholar] [CrossRef]
- Newby, J.A.; Blaylock, D.W.; Witt, P.M.; Pastre, J.C.; Zacharova, M.K.; Ley, S.V.; Browne, D.L. Design and application of a low-temperature continuous flow chemistry platform. Org. Process Res. Dev. 2014, 18, 1211–1220. [Google Scholar] [CrossRef]
- Donnelly, K.; Bauman, M. A continuous flow synthesis of [1.1.1] propellane and bicyclo [1.1.1] pentane derivates. Chem. Commun. 2021, 57, 2871–2874. [Google Scholar] [CrossRef]
- Tsoung, J.; Bogdan, A.R.; Kantor, S.; Wang, Y.; Charaschanya, M.; Djuric, S.W. Synthesis of fused pyrimidinone and quinolone derivates in an automated high-temperature and high-pressure flow reactor. J. Org. Chem. 2017, 82, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Gutman, B. The development of high-temperature/high pressure flow chemistry—A tribute to the pioneering studies of Jürgen O. Metzger. J. Flow Chem. 2017, 7, 1–3. [Google Scholar] [CrossRef]
- Tsoung, J.; Wang, Y.; Djuric, S.W. Expedient Diels-Alder cycloadditions with ortho-quinodimethanes in a high temperature/pressure flow reactor. React. Chem. Eng. 2017, 2, 458–461. [Google Scholar] [CrossRef]
- Movsisyan, M.; Delbeke, E.I.P.; Berton, J.K.E.T.; Battilocchio, C.; Ley, S.V.; Stevens, C.V. Taming hazardous chemistry by continuous flow technology. Chem. Soc. Rev. 2016, 45, 4892–4928. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.; Bracken, C.; Baumann, M. Continuous flow photochemistry for the preparation of bioactive molecules. Molecules 2020, 25, 356. [Google Scholar] [CrossRef]
- Donnelly, K.; Baumann, M. Scalability of photochemical reactions in continuous mode. J. Flow Chem. 2021, 11, 223–241. [Google Scholar] [CrossRef]
- Atobe, M.; Tateno, H.; Matsumura, Y. Applications of flow microreactors in electrosynthetic processes. Chem. Rev. 2018, 118, 4541–4572. [Google Scholar] [CrossRef]
- Pletcher, D.; Green, R.A.; Brown, R.C.D. Flow electrolysis cells for the synthetic organic chemistry laboratory. Chem. Rev. 2018, 118, 4573–4591. [Google Scholar] [CrossRef]
- Buglioni, L.; Raymenants, F.; Slattery, A.; Zondag, S.D.A.; Noël, T. Technological innovations in photochemistry for organic synthesis: Flow chemistry, high-throughput experimentation, scale-up, and photoelectrochemistry. Chem. Rev. 2022, 122, 2752–2906. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Why flow means green-evaluating the merits of continuous processing in the context of sustainability. Curr. Opin. Green Sustain. Chem. 2017, 2, 258–280. [Google Scholar] [CrossRef]
- Kockmann, N.; Thenèe, P.; Fleischer-Trebes, C.; Laudadio, G.; Noël, T. Safety assessment in development and operation of modular continuous-flow processes. React. Chem. Eng. 2017, 2, 258–280. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Lemos, V.A.; De Oliveira, D.M.; Novaes, C.G.; Barreto, J.A.; Alves, J.P.S.; Da Mata Cerqueira, U.M.F.; Dos Santos, Q.O.; Araùjo, S.A. Automation of continuous flow analysis systems—A review. Microchem. J. 2020, 155, 104731. [Google Scholar] [CrossRef]
- Ley, S.V.; Fitzpatrick, D.E.; Myers, R.M.; Battilocchio, C.; Ingham, R.J. Machine-assisted organic synthesis. Angew. Chem. Int. Ed. 2015, 54, 10122–10136. [Google Scholar] [CrossRef] [PubMed]
- Weeranoppanant, N.; Adamo, A. In-line purification: A key component to facilitate drug synthesis and process development in medicinal chemistry. ACS Med. Chem. Lett. 2020, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gioiello, A.; Piccinino, A.; Lozza, A.M.; Cerra, B. The medicinal chemistry in the era of machines and automation: Recent advances in continuous flow technology. J. Med. Chem. 2020, 63, 6624–6647. [Google Scholar] [CrossRef]
- Baumann, M.; Moody, T.S.; Smyth, M.; Wharry, S. Evaluating the green credentials of flow chemistry towards industrial applications. Synthesis 2021, 53, 3963–3976. [Google Scholar] [CrossRef]
- Ley, S.V. On being green: Can flow chemistry help? Chem. Rec. 2012, 12, 378–390. [Google Scholar] [CrossRef]
- Comito, M.; Monguzzi, R.; Tagliapietra, S.; Palmisano, G.; Cravotto, G. Cefonicid benzathine salt: A convenient, lean, and high-performance protocol to make an old cephalosporin shine. Antibiotics 2022, 11, 1095. [Google Scholar] [CrossRef]
- Comito, M.; Monguzzi, R.; Tagliapietra, S.; Palmisano, G.; Cravotto, G. Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature. Green Process. Synth. 2022, 11, 96–105. [Google Scholar] [CrossRef]
- Hughes, D.L. Patent review of manufacturing routes to fifth-generation cephalosporin drugs. Part 1, ceftolozane. Org. Process. Res. Dev. 2017, 21, 430–443. [Google Scholar] [CrossRef]
- Hughes, D.L. Patent review of manufacturing routes to fifth-generation cephalosporin drugs. Part 2, ceftaroline fosamil and ceftobiprole medocaril. Org. Process. Res. Dev. 2017, 21, 800–815. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, present, and future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef] [PubMed]
- Peilleron, L.; Cariou, K. Synthetic approaches towards avibactam and other diazabicyclooctane β-lactamaseinhibitors. Org. Biomol. Chem. 2020, 18, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Pieper, M.; Kumpert, M.; König, B.; Schleich, H.; Bayer, T.; Gröger, H. Process development of synthesizing the cephalosporin antibiotic cefotaxime in batch mode and flow mode. Org. Process. Res. Dev. 2018, 22, 947–954. [Google Scholar] [CrossRef]
- Vobeckà, L.; Tichà, L.; Atanasova, A.; Slouka, Z.; Hasal, P.; Přibyl, M. Enzyme synthesis of cephalexin in continuous-flow microfluidic device in ATPS environment. Chem. Eng. J. 2020, 396, 125236. [Google Scholar] [CrossRef]
- Lin, H.; Dai, C.; Jamison, T.; Jensen, K.F. A rapid total synthesis of ciprofloxacin hydrochloride in continuous flow. Angew. Chem. Int. Ed. 2017, 56, 8870–8873. [Google Scholar] [CrossRef]
- Armstrong, C.; Miyai, Y.; Formosa, A.; Thomas, D.; Chen, E.; Hart, T.; Schultz, V.; Desai, B.K.; Cai, A.Y.; Almasay, A.; et al. On-demand continuous manufacturing of ciprofloxacin in portable plug-and-play factories: Development of a highly efficient synthesis for ciprofloxacin. Org. Process. Res. Dev. 2021, 25, 1524–1533. [Google Scholar] [CrossRef]
- Russell, M.G.; Jamison, T.F. Seven-step continuous flow synthesis of linezolid without intermediate purification. Angew. Chem. Int. Ed. 2019, 58, 7678–7681. [Google Scholar] [CrossRef]
- Zhou, S.; Xin, Y.; Wang, J.; Wu, C.; Sun, T. Application of continuous flow in tazobactam synthesis. Org. Process Res. Dev. 2021, 25, 1648–1657. [Google Scholar] [CrossRef]
- Stueckler, C.; Hermsen, P.; Ritzen, B.; Vasiloiu, M.; Poechlauer, P.; Steinhofer, S.; Pelz, A.; Zinganell, C.; Felfer, U.; Boyer, S.; et al. Development of a continuous flow process for a matteson reaction: From lab scale to full-scale production of a pharmaceutical intermediate. Org. Process Res. Dev. 2019, 23, 1069–1077. [Google Scholar] [CrossRef]
- Wirth, V.; Volkmar, J.; Bayer, T. Synthesis of the antibiotic precursor thiazolyl-7-aminocephalosporanic acid in a microstructured flow system. Chem. Eng. Technol. 2019, 42, 2035–2043. [Google Scholar] [CrossRef]
- Gage, J.R.; Chen, F.; Dong, C.; Gonzalez, M.A.; Jiang, Y.; Luo, Y.; McLaws, M.D.; Tao, J. Semicontinuous process for GMP manufacture of a carbapenem intermediate via carbene insertion using an immobilized rhodium catalyst. Org. Process Res. Dev. 2020, 24, 2025–2033. [Google Scholar] [CrossRef]
- Vobeckà, L.; Romanov, A.; Slouka, Z.; Hasal, P.; Pṙibyl, M. Optimization of aqueous two-phase systems for the production of 6-aminopenicillanic acid in integrated microfluidic reactors-separators. New Biotechnol. 2018, 47, 73–79. [Google Scholar] [CrossRef]
- Todd, P.A.; Brogden, R.N. Cefotaxime. An update of its pharmacology and therapeutic use. Drugs 1990, 40, 608–651. [Google Scholar] [CrossRef] [PubMed]
- PLoSker, G.L.; Foster, R.H.; Benfield, P. Cefotaxime. A pharmacoeconomic review of its use in the treatment of infections. PharmacoEconomics 1998, 13, 91–106. [Google Scholar] [CrossRef]
- Carmine, A.A.; Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Cefotaxime. A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs 1983, 25, 223–289. [Google Scholar] [CrossRef] [PubMed]
- LeFrock, J.L.; Prince, R.A.; Leff, R.D. Mechanism of action, antimicrobial activity, pharmacology, adverse effects, and clinical efficacy of cefotaxime. Pharmacotherapy 1982, 2, 174–184. [Google Scholar] [CrossRef]
- Gonik, B.; Cotton, D.B.; Feldman, S.; Cleary, T.G.; Pickering, L.K. Pharmacokinetics of cefotaxime in the postpartum patient. Am. J. Perinatol. 1985, 2, 114–117. [Google Scholar] [CrossRef]
- Sader, H.S.; Jones, R.N. Cefotaxime is extensively used for surgical prophylaxis. Am. J. Surg. 1992, 164, 28S–38S. [Google Scholar] [CrossRef]
- WHO Model List of Essential Medicines. Available online: https://en.wikipedia.org/wiki/WHO_Model_List_of_Essential_Medicines (accessed on 22 November 2022).
- Fanelli, F.; Parisi, G.; Degennaro, L.; Luisi, R. Contribution of microreactor technology and flow chemistry to the development of green and sustainable synthesis. Beilstein J. Org. Chem. 2017, 13, 520–542. [Google Scholar] [CrossRef] [PubMed]
- Hessel, W.; Löwe, H. Organic synthesis with microstructured reactors. Chem. Eng. Technol. 2005, 28, 267–284. [Google Scholar] [CrossRef]
- Hessel, V.; Noël, T. Micro process technology, 1. Introduction. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar] [CrossRef]
- The Top 300 of 2020. Available online: https://clincal.com/DrugStats/Top300Drugs.aspx (accessed on 30 November 2022).
- Cephalexin. Drug Usage Statistics. Available online: https://clincal.com/DrugsStats/Drugs/Cephalexin (accessed on 30 November 2022).
- Bailey, A.; Walker, A.; Hadley, A.; James, D.G. Cephalexin. A new oral antibiotic. Postgrad. Med. J. 1970, 46, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Gwee, A.; Autmizguine, J.; Curtis, N.; Duffull, S.B. Twice-and thrice-daily cephalexin dosing for Staphyloccus aureus infections in children. Pediatr. Infect. Dis. J. 2020, 39, 519–522. [Google Scholar] [CrossRef]
- Valent, A.M.; DeArmond, C.; Houston, j.M.; Reddy, S.; Masters, H.R.; Gold, A.; Boldt, M.; DeFranco, M.; Evans, A.T.; Warshak, C.R. Effect of post-cesarean delivery oral cephalexin and metronidazole on surgical site infection among obese women: A randomized clinical trial. JAMA 2017, 318, 1026–1034. [Google Scholar] [CrossRef]
- Gill, C.L. Cephalexin. J. Am. Dent. Assoc. 1980, 100, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Speight, T.M.; Brogden, R.N.; Avery, G.S. Cephalexin: A review of its antibacterial, pharmacological and therapeutic properties. Drugs 1972, 3, 9–78. [Google Scholar] [CrossRef]
- Morin, R.B.; Jackson, B.G. Certain 3-Methyl-Cephalosporin Compounds. U.S. Patent 3,507,861, 21 April 1970. [Google Scholar]
- Sharma, P.C.; Jain, A.; Jain, S.; Pahwa, R.; Yar, M.S. Ciprofloxacin: Review on developments in synthetic, analytical, and medicinal aspects. J. Enzyme Inhib. Med. Chem. 2010, 25, 577–589. [Google Scholar] [CrossRef]
- Meyerhoff, A.; Albrecht, R.; Meyer, J.M.; Dionne, P.; Higgins, K.; Murphy, D. US Food and Drug Administration approval of ciprofloxacin hydrochloride for management of postexposure inhalational anthrax. Clin. Infect. Dis. 2004, 39, 303–308. [Google Scholar] [CrossRef]
- Campoli-Richards, D.M.; Monk, J.P.; Price, A.; Benfield, P.; Todd, P.A.; Ward, A. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1988, 35, 373–447. [Google Scholar] [CrossRef]
- Apangu, T.; Griffith, K.; Abaru, J.; Candini, G.; Apio, H.; Okoth, F.; Okello, R.; Kaggwa, J.; Acayo, S.; Ezama, G.; et al. Successful treatment of human plague with oral ciprofloxacin. Emerg. Infect. Dis. 2017, 23, 553–555. [Google Scholar] [CrossRef]
- Zhang, H.L.; Tan, M.; Qiu, A.M.; Tao, Z.; Wang, C.H. Antibiotics for treatment of acute exacerbation of chronic obstructive pulmonary disease: A network meta-analysis. BMC Pulm. Med. 2017, 17, 196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Liu, X.; Zhang, S.; Pan, B.; Liu, M.L. Ciprofloxacin derivates and their antibacterial activities. Eur. J. Med. Chem. 2018, 146, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Terp, D.K.; Ryback, M.J. Ciprofloxacin. Drug Intell. Clin. Pharm. 1987, 21, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Schwalbe, T.; Kadzimirsz, D.; Jas, G. Synthesis of library of ciprofloxacin analogues by means of sequential organic synthesis in microreactors. QSAR Comb. Sci. 2005, 24, 758–768. [Google Scholar] [CrossRef]
- Adamo, A.; Beingessner, R.L.; Behnam, M.; Chen, J.; Jamison, T.F.; Jensen, K.F.; Monbaliu, J.C.; Myerson, A.S.; Revalor, E.M.; Snead, D.R.; et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 2016, 352, 61–67. [Google Scholar] [CrossRef]
- Rogers, L.; Briggs, N.; Acherman, R.; Adamo, A.; Azad, M.; Brancazio, D.; Capellades, G.; Hammersmith, G.; Hart, T.; Imbrogno, J.; et al. Continuous production of five active pharmaceutical ingredients in flexible plug-and-play modules: A demonstration campaign. Org. Process. Res. Dev. 2020, 24, 2183–2196. [Google Scholar] [CrossRef]
- Zhang, P.; Weeranoppanant, N.; Thomas, D.A.; Tahara, K.; Stelzer, T.; Russell, M.G.; O’Mahony, M.; Myerson, A.S.; Lin, H.; Kelly, L.P.; et al. Advanced continuous flow platform for on-demand pharmaceutical manufacturing. Chem. Eur. J. 2018, 24, 1–10. [Google Scholar] [CrossRef]
- Capellades, G.; Neurohr, C.; Briggs, N.; Rapp, K.; Hammersmith, G.; Brancazio, D.; Derksen, B.; Myerson, A.S. On-demand continuous manufacturing of ciprofloxacin in portable plug-and-play factories: Implementation and in situ control of downstream production. Org. Process. Res. Dev. 2021, 25, 1534–1546. [Google Scholar] [CrossRef]
- Perry, C.M.; Jarvis, B. Linezolid: A review of its use in the management of serious gram-positive infections. Drugs 2001, 61, 525–551. [Google Scholar] [CrossRef]
- Hashemian, S.M.R.; Farhadi, T.; Ganjparvar, M. Linezolid: A review of its property, function, and use in critical care. Drug Des. Dev. Ther. 2018, 12, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.H. Efficacy of linezolid versus comparator therapies in gram-positive infections. J. Antimicrob. Chemother. 2003, 51, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M. Linezolid in vitro: Mechanism and antibacterial spectrum. J. Antimicrob. Chemother. 2003, 51, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Clemett, D.; Markham, A. Linezolid. Drugs 2000, 59, 815–827. [Google Scholar] [CrossRef]
- Chien, J.W.; Kucia, M.L.; Salata, R.A. Use of linezolid, an oxazolidinone, in the treatment of multidrug-resistant gram-positive bacterial infections. Clin. Infect. Dis. 2000, 30, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.Y.; Qin, S.S.; Chu, W.C.; Yang, Y.; Cui, D.Y.; Hua, Y.G.; Yang, Q.Q.; Zhang, E. Synthesis and antibacterial bioactivities of cationic deacetyl linezolid amphiphiles. Eur. J. Med. Chem. 2018, 155, 925–945. [Google Scholar] [CrossRef] [PubMed]
- Brickner, S.J.; Hutchinson, D.K.; Barbachyn, M.R.; Manninen, P.R.; Ulanowicz, D.A.; Garmon, S.A.; Grega, K.C.; Hendges, S.K.; Toops, D.S.; Ford, C.W.; et al. Synthesis of antibacterial activity of U-100592 and U-100766, two oxazolidinone antibacterial agents for the potential treatment of multidrug-resistant gram-positive bacterial infections. J. Med. Chem. 1996, 39, 673–679. [Google Scholar] [CrossRef]
- Ramgren, S.D.; Silberstein, A.L.; Yang, Y.; Garg, N.K. Nickel-catalyzed amination of aryl sulfamates. Angew. Chem. Int. Ed. Engl. 2011, 50, 2171–2173. [Google Scholar] [CrossRef]
- Perrault, W.R.; Pearlman, B.A.; Godrej, D.B.; Jeganathan, A.; Yamagata, K.; Chen, J.J.; Lu, C.V.; Herrinton, P.M.; Gadwood, R.C.; Chan, L.; et al. The synthesis of N-aryl-5(S)-aminomethyl-2-oxazolidinone antibacterials and derivates in one step from aryl carbamates. Org. Process Res. Dev. 2003, 7, 533–546. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Kadima, T.A.; Weiner, J.H. Mechanism of suppression of piperacillin resistance in enterobacteria by tazobactam. Antimicrob. Agents Chemother. 1997, 41, 2177–2183. [Google Scholar] [CrossRef]
- Perry, C.M.; Markham, A. Piperacillin/tazobactam: An update review of its use in the treatment of bacterial infections. Drugs 1999, 57, 805–843. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rasmussen, B.A.; Shlaes, D.M. Class A beta-lactamases-enzyme-inhibitor interactions and resistance. Pharmacol. Ther. 1999, 83, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Lizza, B.D.; Betthauser, K.D.; Ritchie, D.J.; Micek, S.T.; Kollef, M.H. New perspectives on antimicrobial agents: Ceftolozane-tazobactam. Antimicrob. Agents Chemother. 2021, 65, e02318-20. [Google Scholar] [CrossRef] [PubMed]
- Hecker, S.J.; Reddy, K.R.; Totrov, M.; Hirst, G.C.; Lomovskaya, O.; Griffith, D.C.; King, P.; Tsivkovski, R.; Sun, D.; Sabet, M.; et al. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs. class a serine carbapenemases. J. Med. Chem. 2015, 58, 3682–3692. [Google Scholar] [CrossRef] [PubMed]
- Novelli, A.; Del Giacomo, P.; Rossolini, G.M.; Tumbarello, M. Meropenem/vaborbactam: A next generation β-lactam β-lactamase inhibitor combination. Expert Rev. Anti-Infect. Ther. 2020, 18, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, T.; Weinstein, M.P. Microbiology of meropenem-vaborbactam: A novel carbapenem beta-lactamase inhibitor combination for carbapenem-resistant enterobacterales infections. Infect. Dis. Ther. 2020, 9, 757–767. [Google Scholar] [CrossRef]
- FDA Approves New Antibacterial Drug. Available online: https://fda.gov/news-events/press-announcements/fda-approves-new-antibacterial-drug (accessed on 7 December 2022).
- Lee, Y.R.; Baker, N.T. Meropenem-vaborbactam: A carbapenem and beta-lactamase inhibitor with activity against carbapenem-resistant Enterobacteriaceae. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.C.; Zmarlicka, M.T.; Shaeer, K.M.; Pardo, J. Meropenem/vaborbactam, the first carbapenem/β-lactamase inhibitor combination. Ann. Pharmacother. 2018, 52, 769–779. [Google Scholar] [CrossRef]
- Burgos, R.M.; Biagi, M.J.; Rodvold, K.A.; Danziger, L.H. Pharmacokinetic evaluation of meropenem and vaborbactam for the treatment of urinary tract infection. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1007–1021. [Google Scholar] [CrossRef]
- Felfer, U.; Stueckler, C.; Steinhofer, S.; Pelz, A.; Hanacek, M.; Pabst, T.H.; Winkler, G.; Poechlauer, P.; Ritzen, B.; Gold-Bach, M. Apparatus and Continuous Flow Process for Production of Boronic Acid Derivates. WO 2016/100043, 23 June 2016. [Google Scholar]
- Barradell, L.B.; Brogden, R.N. Cefodizime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1992, 44, 800–834. [Google Scholar] [CrossRef] [PubMed]
- Brockmeier, D.; Dagrosa, E.E. Pharmacokinetic profile of cefodizime. Infection 1992, 20, S14–S17. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, G.; Ye, J.; Wan, F.; Qian, Y. Immunomodulating effects of cefodizime on Klebsiella pneumoniae-stimulated neutrophils. Immunobiology 2004, 209, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S.; Garau, J.; Lode, H.; Rolston, K.V.; Wilson, S.E.; Quinn, J.P. Carbapenems in clinical practice: A guide to their use in serious infection. Int. J. Antimicrob. Agents 1999, 11, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, F.R.; Doyle, F.P.; Nayler, J.H.C.; Rolinson, G.N. Synthesis of penicillin: 6-aminopenicillanic acid in penicillin fermentations. Nature 1959, 183, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Doyle, F.P.; Nayler, J.H.C.; Rolinson, G.N. Recovery of Solid 6-Aminipenicillanic Acid. U.S. Patent 2,941,995, 22 July 1958. [Google Scholar]

| Name | Structure | Classification | Ref. |
|---|---|---|---|
| Drug Substance | |||
| Cefotaxime (1) | 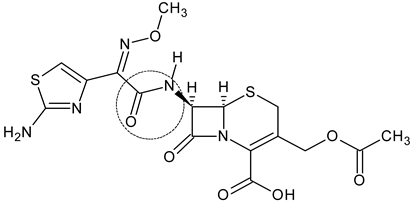 | Third-generation cephalosporin | [82] |
| Cephalexin (2) | 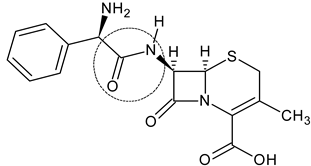 | First-generation cephalosporin | [83] |
| Ciprofloxacin hydrochloride a (3) | 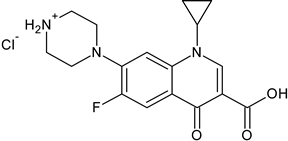 | Fluoroquinolone | [84,85] |
| Linezolid a (4) | 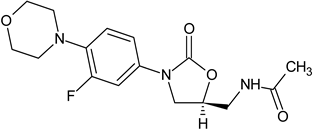 | Oxazolidinone | [86] |
| Tazobactam a,b (5) | 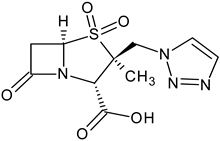 | β-lactamase inhibitor | [87] |
| Drug Key Intermediates | |||
| Vaborbactam intermediate (6) | 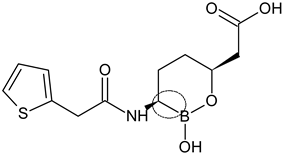 | Non-β-lactam β-lactamase inhibitor | [88] |
| Cefodizime intermediate (7) | 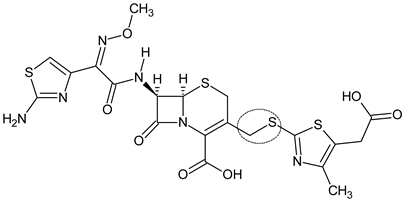 | Third-generation cephalosporin | [89] |
| Building Blocks | |||
| β-methyl vinyl phosphate (MAP) (8) | 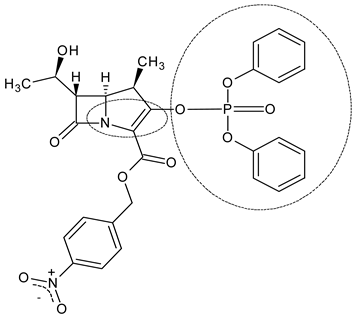 | β-methyl carbapenems | [90] |
| 6-aminopenicillanic acid | 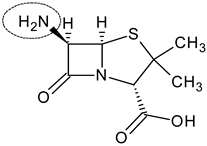 | Penicillins, β-lactamase inhibitor | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comito, M.; Monguzzi, R.; Tagliapietra, S.; Palmisano, G.; Cravotto, G. Towards Antibiotic Synthesis in Continuous-Flow Processes. Molecules 2023, 28, 1421. https://doi.org/10.3390/molecules28031421
Comito M, Monguzzi R, Tagliapietra S, Palmisano G, Cravotto G. Towards Antibiotic Synthesis in Continuous-Flow Processes. Molecules. 2023; 28(3):1421. https://doi.org/10.3390/molecules28031421
Chicago/Turabian StyleComito, Marziale, Riccardo Monguzzi, Silvia Tagliapietra, Giovanni Palmisano, and Giancarlo Cravotto. 2023. "Towards Antibiotic Synthesis in Continuous-Flow Processes" Molecules 28, no. 3: 1421. https://doi.org/10.3390/molecules28031421
APA StyleComito, M., Monguzzi, R., Tagliapietra, S., Palmisano, G., & Cravotto, G. (2023). Towards Antibiotic Synthesis in Continuous-Flow Processes. Molecules, 28(3), 1421. https://doi.org/10.3390/molecules28031421







