Toxicity of the 3,4-Methylenedioxymethamphetamine and Its Enantiomers to Daphnia magna after Isolation by Semipreparative Chromatography
Abstract
1. Introduction
2. Results and Discussion
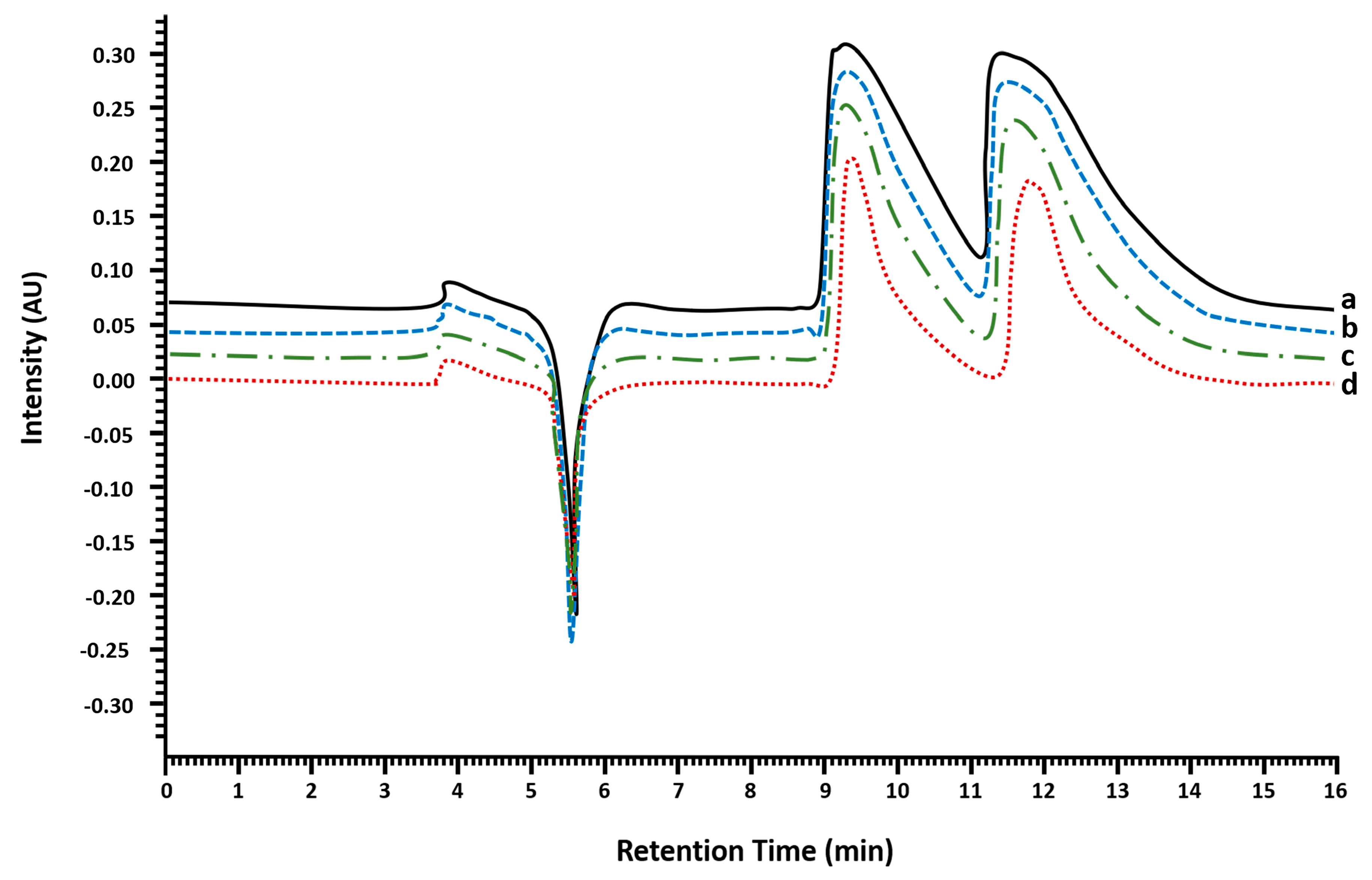
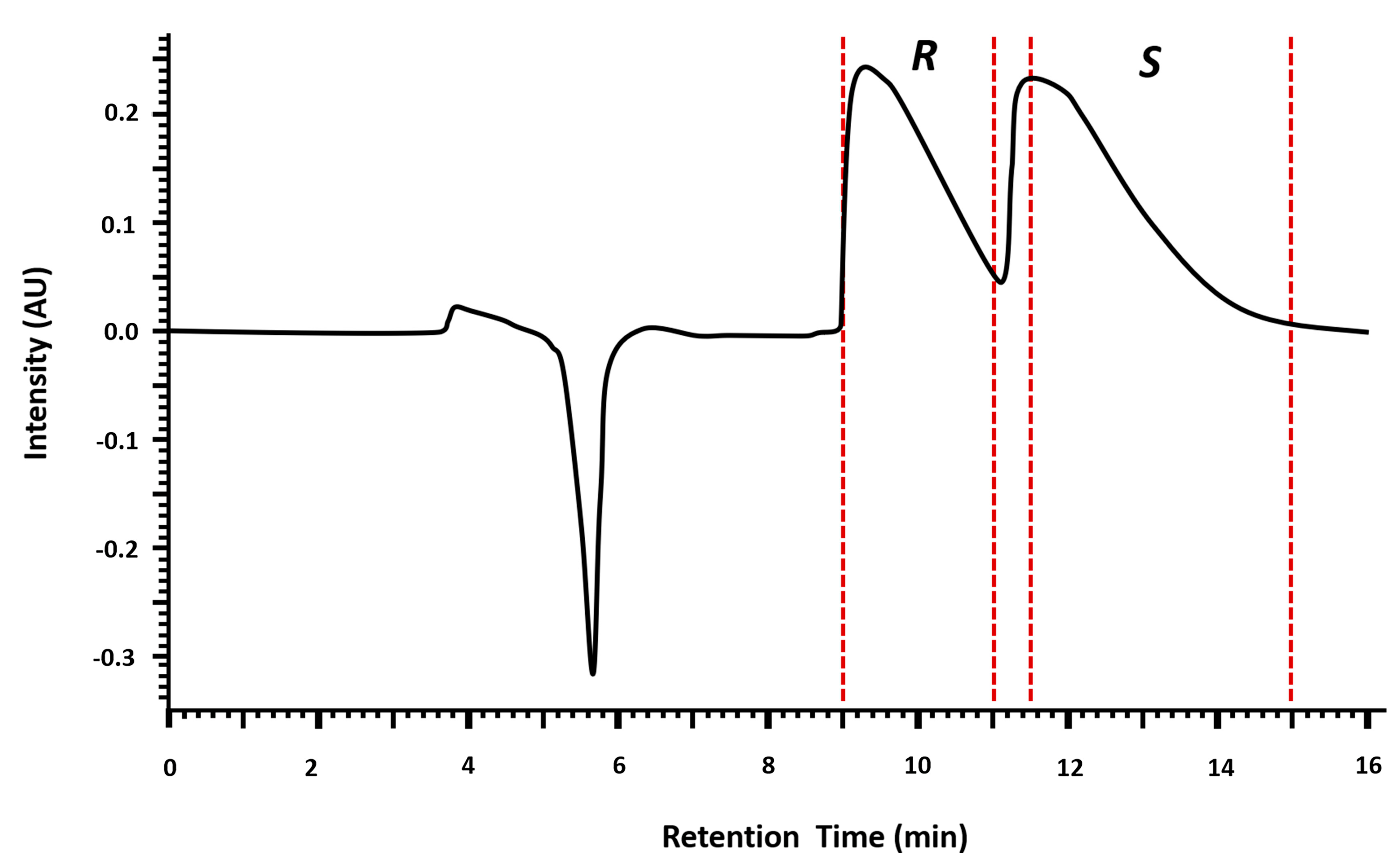
2.1. Multimilligram Enantioresolution of MDMA
2.1.1. Injection Volume Optimization and Enantioseparation
2.1.2. Enantiomeric Purity and Recovery of the Enantiomers
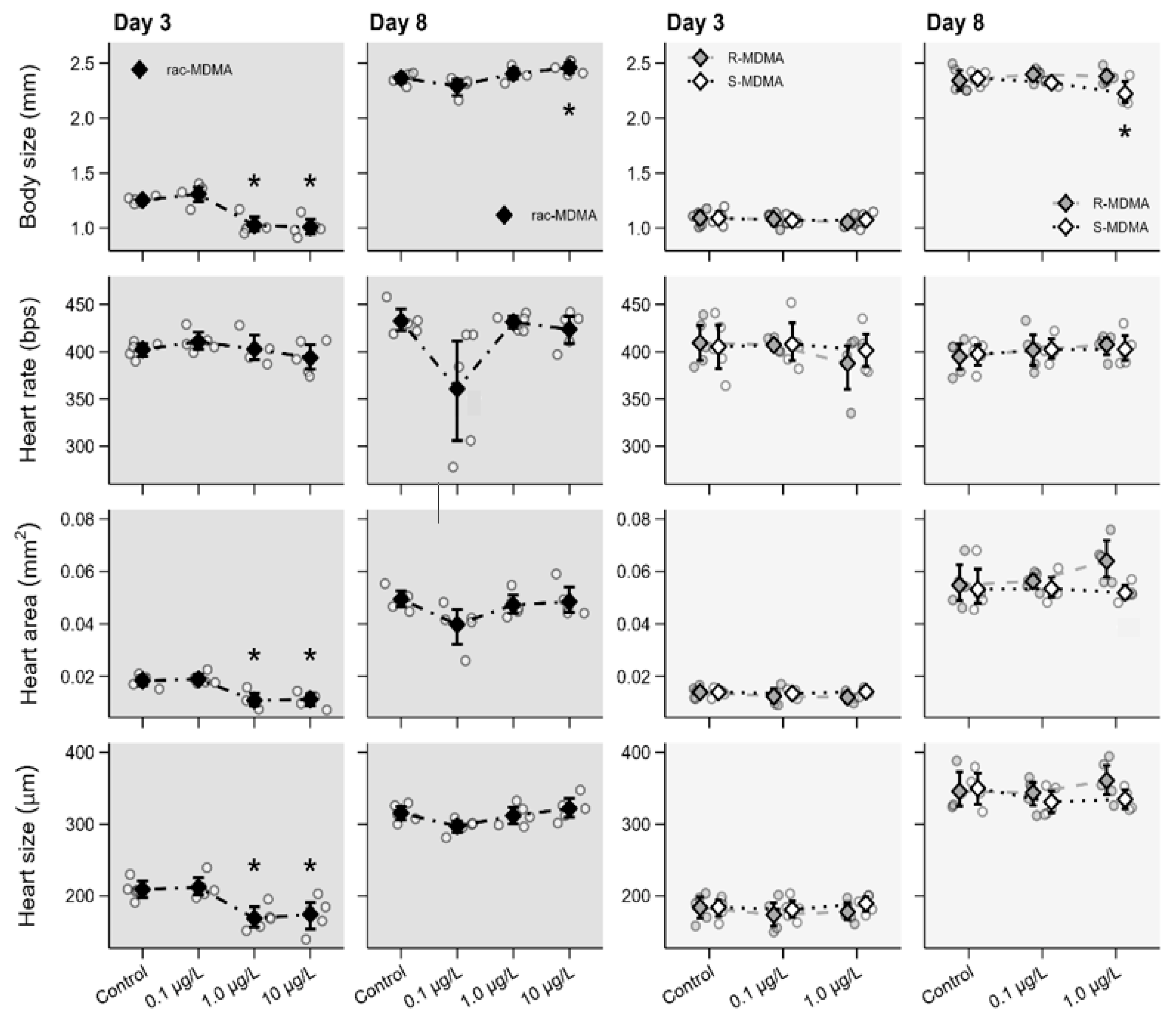
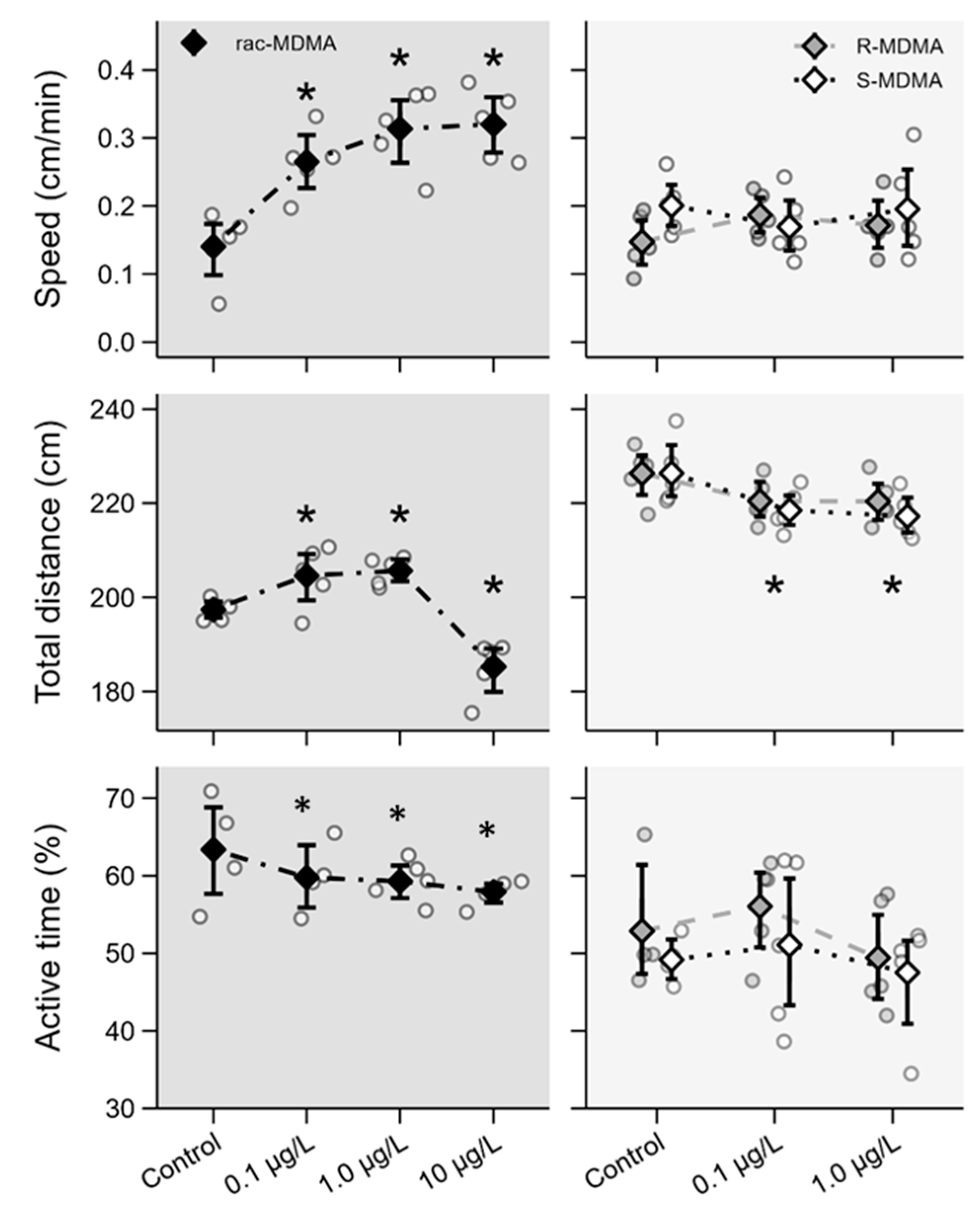
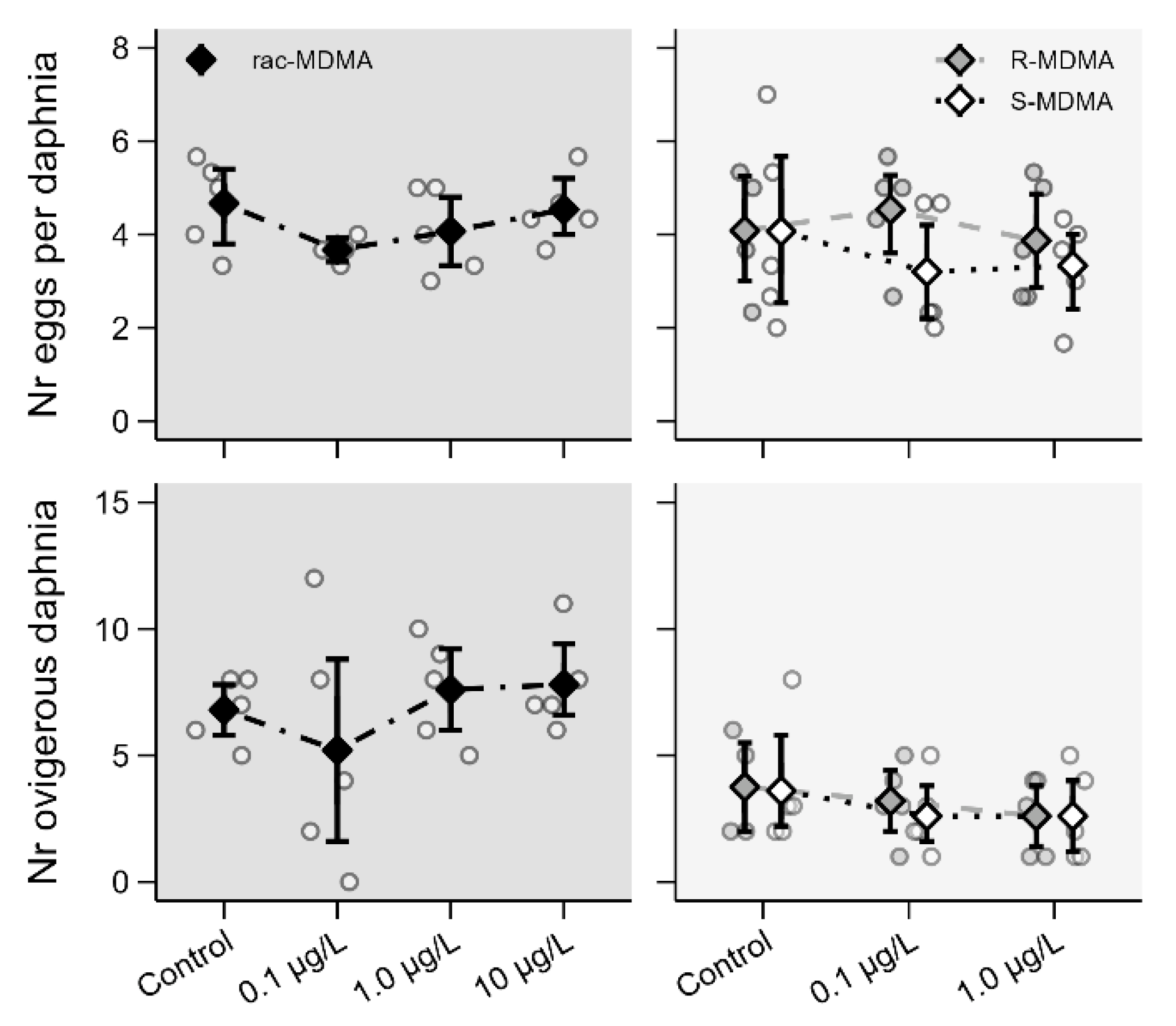
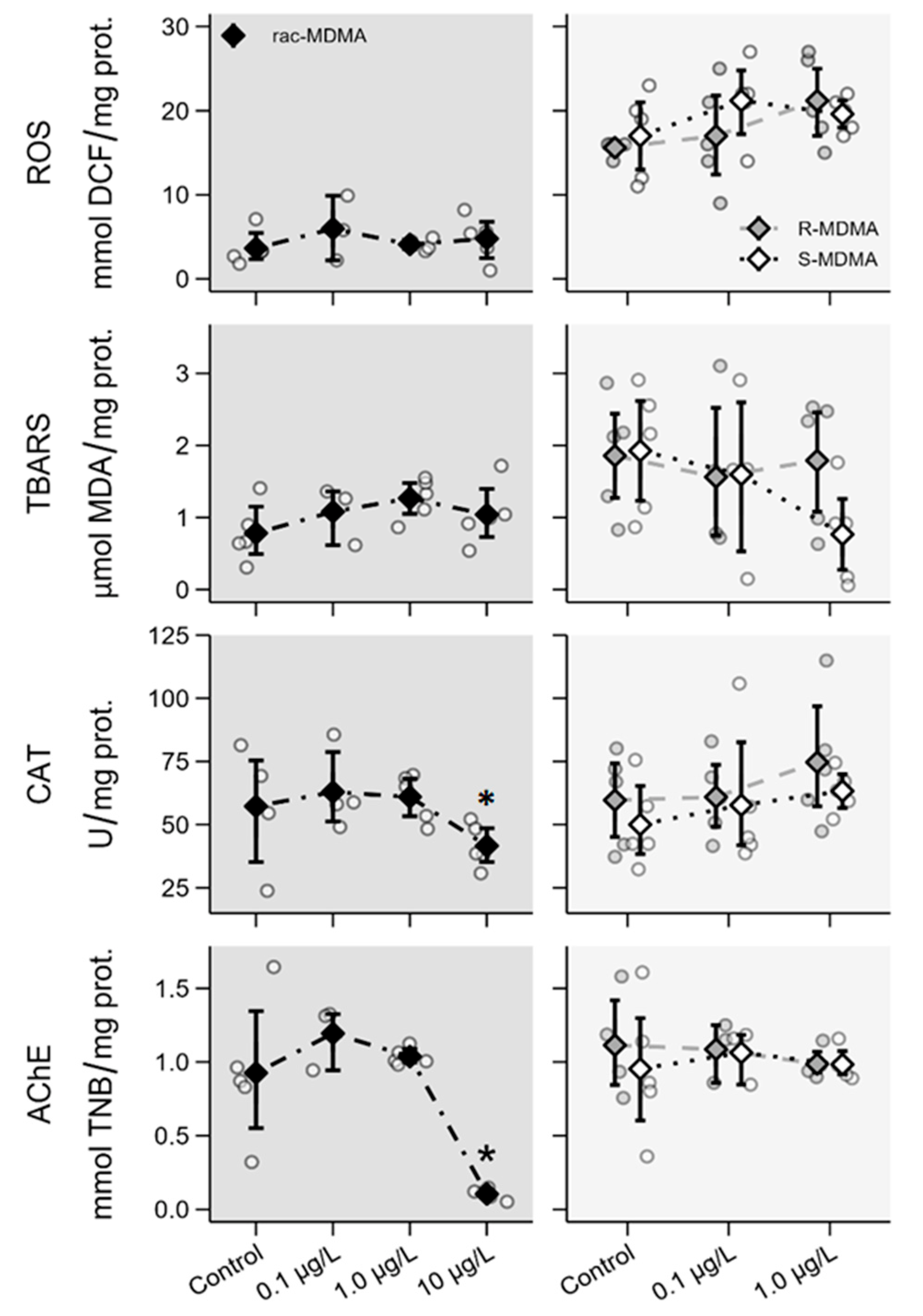
2.2. Ecotoxicity Assays
2.2.1. Morphophysiological Parameters
2.2.2. Swimming Behaviour
2.2.3. Reproductive Parameters
2.2.4. Biochemical Parameters
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Equipment and Chromatographic Conditions
3.3. Chemical Analysis of MDMA in Culture Media
3.4. Daphnia Magna Cultures
3.5. Ecotoxicity Assay: Experimental Design and Procedures
3.6. Ecotoxicity Assay: Biomarker Quantification
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ribeiro, C.; Santos, C.; Gonçalves, V.; Ramos, A.; Afonso, C.; Tiritan, M.E. Chiral Drug Analysis in Forensic Chemistry: An Overview. Molecules 2018, 23, 262. [Google Scholar] [CrossRef] [PubMed]
- Langa, I.; Tiritan, M.E.; Silva, D.; Ribeiro, C. Gas Chromatography Multiresidue Method for Enantiomeric Fraction Determination of Psychoactive Substances in Effluents and River Surface Waters. Chemosensors 2021, 9, 224. [Google Scholar] [CrossRef]
- EMCDDA. European Drug Report 2020: Trends and Developments; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2020. [Google Scholar]
- EMCDDA. European Drug Report 2021: Trends and Developments; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2021. [Google Scholar]
- Fallon, J.K.; Kicman, A.T.; Henry, J.A.; Milligan, P.J.; Cowan, D.A.; Hutt, A.J. Stereospecific analysis and enantiomeric disposition of 3, 4-methylenedioxymethamphetamine (Ecstasy) in humans. Clin. Chem. 1999, 45, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Losacker, M.; Roehrich, J.; Hess, C. Enantioselective determination of plasma protein binding of common amphetamine-type stimulants. J. Pharm. Biomed. Anal. 2021, 205, 114317. [Google Scholar] [CrossRef]
- Feduccia, A.A.; Mithoefer, M.C. MDMA-assisted psychotherapy for PTSD: Are memory reconsolidation and fear extinction underlying mechanisms? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84 Pt A, 221–228. [Google Scholar] [CrossRef]
- Sessa, B. MDMA and PTSD treatment: “PTSD: From novel pathophysiology to innovative therapeutics”. Neurosci. Lett. 2017, 649, 176–180. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora, G.M.; Garas, W.; Paleos, C.; Gorman, I.; et al. MDMA-assisted therapy for severe PTSD: A randomized, double-blind, placebo-controlled phase 3 study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef]
- Evans, S.E.; Davies, P.; Lubben, A.; Kasprzyk-Hordern, B. Determination of chiral pharmaceuticals and illicit drugs in wastewater and sludge using microwave assisted extraction, solid-phase extraction and chiral liquid chromatography coupled with tandem mass spectrometry. Anal. Chim. Acta 2015, 882, 112–126. [Google Scholar] [CrossRef]
- Barenys, M.; Álvarez, S.; Santamaria, A.; Teixidó, E.; Gómez-Catalán, J. Developmental exposure to MDMA (ecstasy) in zebrafish embryos reproduces the neurotoxicity adverse outcome ‘lower motor activity’ described in humans. NeuroToxicology 2022, 88, 116–123. [Google Scholar] [CrossRef]
- Ribeiro, O.; Félix, L.; Ribeiro, C.; Castro, B.; Tiritan, M.E.; Monteiro, S.M.; Carrola, J.S. Enantioselective Ecotoxicity of Venlafaxine in Aquatic Organisms: Daphnia and Zebrafish. Environ. Toxicol. Chem. 2022, 41, 1851–1864. [Google Scholar] [CrossRef]
- Pérez-Pereira, A.; Ribeiro, C.; Teles, F.; Gonçalves, R.; Gonçalves, V.; Pereira, J.A.; Carrola, J.S.; Pires, C.A.; Tiritan, M.E. Ketamine and norketamine: Enantioresolution and enantioselective aquatic ecotoxicity studies. Environ. Toxicol. Chem. 2020, 41, 569–579. [Google Scholar] [CrossRef]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Stimulatory Drugs of Abuse in Surface Waters and Their Removal in a Conventional Drinking Water Treatment Plant. Environ. Sci. Technol. 2008, 42, 6809–6816. [Google Scholar] [CrossRef]
- Langa, I.; Gonçalves, R.; Tiritan, M.E.; Ribeiro, C. Wastewater analysis of psychoactive drugs: Non-enantioselective vs enantioselective methods for estimation of consumption. Forensic Sci. Int. 2021, 325, 110873. [Google Scholar] [CrossRef]
- Masteling, R.P.; Castro, B.B.; Antunes, S.C.; Nunes, B. Whole-organism and biomarker endpoints in Daphnia magna show uncoupling of oxidative stress and endocrine disruption in phenolic derivatives. Ecotoxicol. Environ. Saf. 2016, 134 Pt 1, 64–71. [Google Scholar] [CrossRef]
- Castro, B.B.; Freches, A.R.; Rodrigues, M.; Nunes, B.; Antunes, S.C. Transgenerational Effects of Toxicants: An Extension of the Daphnia 21-day Chronic Assay? Arch. Environ. Contam. Toxicol. 2018, 74, 616–626. [Google Scholar] [CrossRef]
- Antunes, S.; Castro, B.; Porto, C.U.d.; Minho, C.U.d. Pulgas-de-água (Daphnia spp.). Rev. Ciência Elem. 2017, 5, 50. [Google Scholar] [CrossRef]
- Ribeiro, O.M.; Pinto, M.Q.; Ribeiro, C.; Tiritan, M.E.; Carrola, J.S. A dáfnia como sensor da ecotoxidade. Rev. Ciência Elem. 2021, 9, 44. [Google Scholar] [CrossRef]
- Gonçalves, R.; Ribeiro, C.; Cravo, S.; Cunha, S.C.; Pereira, J.A.; Fernandes, J.O.; Afonso, C.; Tiritan, M.E. Multi-residue method for enantioseparation of psychoactive substances and beta blockers by gas chromatography–mass spectrometry. J. Chromatogr. B 2019, 1125, 121731. [Google Scholar] [CrossRef]
- Lari, E.; Jeong, T.; Labine, L.M.; Simpson, M.J. Metabolomic analysis predicted changes in growth rate in Daphnia magna exposed to acetaminophen. Aquat. Toxicol. 2022, 249, 106233. [Google Scholar] [CrossRef]
- De Felice, B.; Salgueiro-González, N.; Castiglioni, S.; Saino, N.; Parolini, M. Biochemical and behavioral effects induced by cocaine exposure to Daphnia magna. Sci. Total Environ. 2019, 689, 141–148. [Google Scholar] [CrossRef]
- Stewart, A.; Riehl, R.; Wong, K.; Green, J.; Cosgrove, J.; Vollmer, K.; Kyzar, E.; Hart, P.; Allain, A.; Cachat, J.; et al. Behavioral effects of MDMA (‘ecstasy’) on adult zebrafish. Behav. Pharmacol. 2011, 22, 275–280. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Mondellini, S.; Salgueiro-González, N.; Castiglioni, S.; Parolini, M. Methamphetamine exposure modulated oxidative status and altered the reproductive output in Daphnia magna. Sci. Total Environ. 2020, 721, 137728. [Google Scholar] [CrossRef]
- Parolini, M.; Bini, L.; Magni, S.; Rizzo, A.; Ghilardi, A.; Landi, C.; Armini, A.; Del Giacco, L.; Binelli, A. Exposure to cocaine and its main metabolites altered the protein profile of zebrafish embryos. Environ. Pollut. 2018, 232, 603–614. [Google Scholar] [CrossRef]
- Félix, L.M.; Serafim, C.; Martins, M.J.; Valentim, A.M.; Antunes, L.M.; Matos, M.; Coimbra, A.M. Morphological and behavioral responses of zebrafish after 24h of ketamine embryonic exposure. Toxicol. Appl. Pharmacol. 2017, 321, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Félix, L.M.; Vidal, A.M.; Serafim, C.; Valentim, A.M.; Antunes, L.M.; Campos, S.; Matos, M.; Monteiro, S.M.; Coimbra, A.M. Ketamine-induced oxidative stress at different developmental stages of zebrafish (Danio rerio) embryos. RSC Adv. 2016, 6, 61254–61266. [Google Scholar] [CrossRef]
- Félix, L.M.; Vidal, A.M.; Serafim, C.; Valentim, A.M.; Antunes, L.M.; Monteiro, S.M.; Matos, M.; Coimbra, A.M. Ketamine induction of p53-dependent apoptosis and oxidative stress in zebrafish (Danio rerio) embryos. Chemosphere 2018, 201, 730–739. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, G.; Rubio-Escalante, F.J.; Noreña-Barroso, E.; Escalante-Herrera, K.S.; Schlenk, D. Impacts of oxidative stress on acetylcholinesterase transcription, and activity in embryos of zebrafish (Danio rerio) following Chlorpyrifos exposure. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2015, 172–173, 19–25. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In CRC Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Lanzarin, G.A.B.; Félix, L.M.; Santos, D.; Venâncio, C.A.S.; Monteiro, S.M. Dose-dependent effects of a glyphosate commercial formulation—Roundup® UltraMax—On the early zebrafish embryogenesis. Chemosphere 2019, 223, 514–522. [Google Scholar] [CrossRef]
- Deng, J.; Yu, L.; Liu, C.; Yu, K.; Shi, X.; Yeung, L.W.; Lam, P.K.; Wu, R.S.; Zhou, B. Hexabromocyclododecane-induced developmental toxicity and apoptosis in zebrafish embryos. Aquat. Toxicol. 2009, 93, 29–36. [Google Scholar] [CrossRef]
- Gartaganis, S.P.; Patsoukis, N.E.; Nikolopoulos, D.K.; Georgiou, C.D. Evidence for oxidative stress in lens epithelial cells in pseudoexfoliation syndrome. Eye 2007, 21, 1406–1411. [Google Scholar] [CrossRef]
- Szöcs, E.; Schäfer, R.B. Ecotoxicology is not normal. Environ. Sci. Pollut. Res. 2015, 22, 13990–13999. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- The Jamovi Project, Jamovi (Version 2.2.5) [Computer Software]. 2021. Available online: https://www.jamovi.org (accessed on 29 December 2022).
- R Core Team. R: A Language and Environment for Statistical Computing [Computer Software]; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R [Computer Software]; RStudio, PBC: Boston, MA, USA, 2022. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wilke, C. Cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’ [Computer Software]; R Package Version 1.1.1. 2020. Available online: https://CRAN.R-project.org/package=cowplot (accessed on 20 December 2022).
- Steinmetz, Z. Envalysis: Miscellaneous Functions for Environmental Analyses [Computer Software]; R Package Version 0.5.1. 2021. Available online: https://CRAN.R-project.org/package=envalysis (accessed on 20 December 2022).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.R.; Gonçalves, V.M.F.; Castro, B.B.; Carrola, J.S.; Langa, I.; Pereira, A.; Carvalho, A.R.; Tiritan, M.E.; Ribeiro, C. Toxicity of the 3,4-Methylenedioxymethamphetamine and Its Enantiomers to Daphnia magna after Isolation by Semipreparative Chromatography. Molecules 2023, 28, 1457. https://doi.org/10.3390/molecules28031457
Costa AR, Gonçalves VMF, Castro BB, Carrola JS, Langa I, Pereira A, Carvalho AR, Tiritan ME, Ribeiro C. Toxicity of the 3,4-Methylenedioxymethamphetamine and Its Enantiomers to Daphnia magna after Isolation by Semipreparative Chromatography. Molecules. 2023; 28(3):1457. https://doi.org/10.3390/molecules28031457
Chicago/Turabian StyleCosta, Ana Rita, Virgínia M. F. Gonçalves, Bruno B. Castro, João Soares Carrola, Ivan Langa, Ariana Pereira, Ana Rita Carvalho, Maria Elizabeth Tiritan, and Cláudia Ribeiro. 2023. "Toxicity of the 3,4-Methylenedioxymethamphetamine and Its Enantiomers to Daphnia magna after Isolation by Semipreparative Chromatography" Molecules 28, no. 3: 1457. https://doi.org/10.3390/molecules28031457
APA StyleCosta, A. R., Gonçalves, V. M. F., Castro, B. B., Carrola, J. S., Langa, I., Pereira, A., Carvalho, A. R., Tiritan, M. E., & Ribeiro, C. (2023). Toxicity of the 3,4-Methylenedioxymethamphetamine and Its Enantiomers to Daphnia magna after Isolation by Semipreparative Chromatography. Molecules, 28(3), 1457. https://doi.org/10.3390/molecules28031457










