Methyl Jasmonate and Nanoparticles Doped with Methyl Jasmonate affect the Cell Wall Composition of Monastrell Grape Skins
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characteristics of the Grapes
2.2. Isolation of Cell Wall Material (CWM)
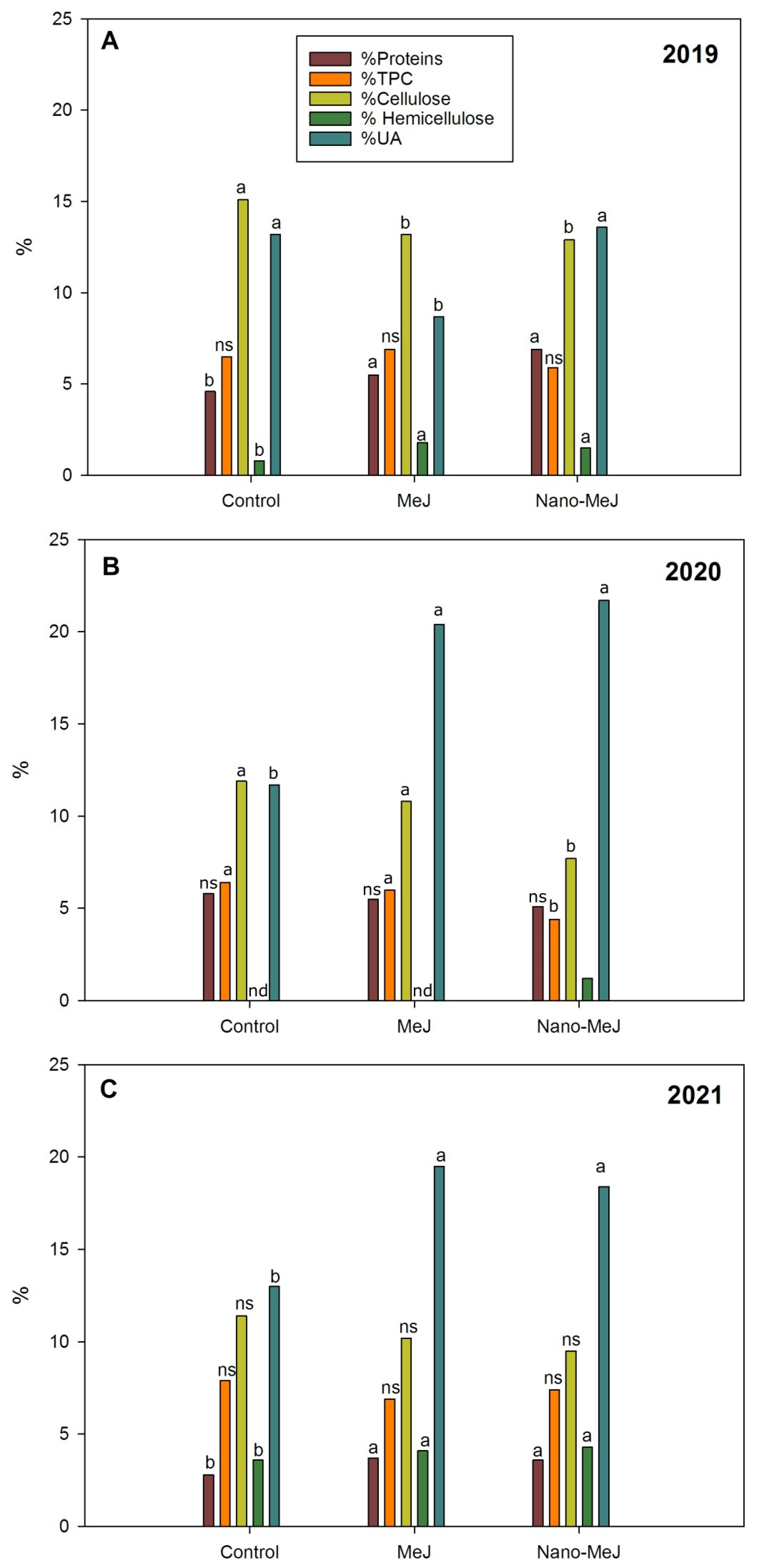
2.3. Analysis of Grape Skin Cell Wall Composition
2.3.1. Proteins
2.3.2. Total Phenolic Compounds (TPC)
2.3.3. Carbohydrate Composition (Uronic Acids, Cellulosic Glucose, and Non-Cellulosic Glucose)
2.3.4. Multivariable Factorial Analysis
2.4. Wine Anthocyanins and Wine Spectrophotometric Variables
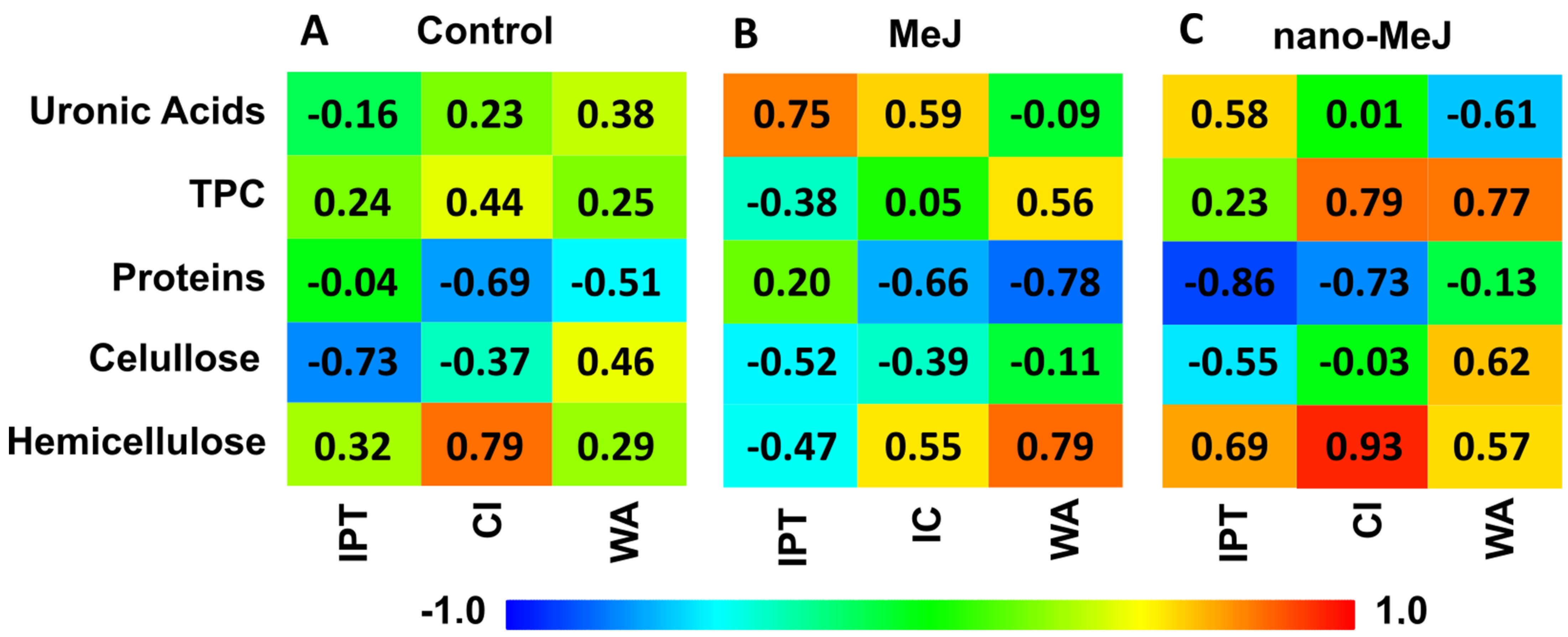
2.5. Pearson Correlation Study
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Nanoparticles Doped with MeJ (Nano-MeJ)
3.3. Vegetal Material and Open Field Treatments
3.4. Physicochemical Characterisation of Grapes
3.5. Isolation of Cell Wall Material (CWM)
3.6. Analysis of the Composition of Grape Skin Cell Wall
3.6.1. Proteins and Total Phenolic Compounds
3.6.2. Uronic Acids and Glucose
3.7. Vinifications
3.8. Spectrophotometric Variables in Wines
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ortega-Regules, A.; Romero-Cascales, I.; Ros-García, J.M.; López-Roca, J.M.; Gómez-Plaza, E. A first approach towards the relationship between grape skin cell-wall composition and anthocyanin extractability. Anal. Chim. Acta 2006, 563, 26–32. [Google Scholar] [CrossRef]
- Garrido-Bañuelos, G.; Buica, A.; du Toit, W. Relationship between anthocyanins, proanthocyanidins, and cell wall polysaccharides in grapes and red wines. A current state-of-art review. Crit. Rev. Food Sci. Nutr. 2021, 62, 7743–7759. [Google Scholar] [CrossRef]
- Vidal, S.; Williams, P.; O’Neill, M.A.; Pellerin, P. Polysaccharides from grape berry cell walls. Part I: Tissue distribution and structural characterization of the pectic polysaccharides. Carbohydr. Polym. 2001, 45, 315–323. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M. The composition of cell walls from grape marcs is affected by grape origin and enological technique. Food Chem. 2015, 167, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Regules, A.; Ros-García, J.; Osé, M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Changes in skin cell wall composition during the maturation of four premium wine grape varieties. J. Sci. Food Agric. 2008, 88, 420–428. [Google Scholar] [CrossRef]
- Garrido-Bañuelos, G.; Buica, A.; Schückel, J.; Zietsman, A.J.J.; Willats, W.G.T.; Moore, J.P.; Du Toit, W.J. Investigating the relationship between grape cell wall polysaccharide composition and the extractability of phenolic compounds into Shiraz wines. Part I Vintage and ripeness effects. Food Chem. 2019, 278, 36–46. [Google Scholar] [CrossRef]
- Castro-López, L.D.R.; Gómez-Plaza, E.; Ortega-Regules, A.; Lozada, D.; Bautista-Ortín, A.B. Role of cell wall deconstructing enzymes in the proanthocyanidin-cell wall adsorption-desorption phenomena. Food Chem. 2016, 196, 526–532. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; Beaver, J.W.; Lerno, L.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.E.; Oberholster, A. Impact of temperature, ethanol and cell wall material composition on cell wall-anthocyanin interactions. Molecules 2019, 24, 3350. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Fernández-Fernández, J.I.; Portu, J.; Garde-Cerdán, T. Methyl jasmonate: Effect on proanthocyanidin content in Monastrell and Tempranillo grapes and wines. Eur. Food Res. Technol. 2017, 244, 611–621. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Improving grape phenolic content and wine chromatic characteristics through the use of two different elicitors: Methyl jasmonate versus benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Santamaría, P.; Garde-Cerdán, T. Methyl jasmonate treatment to increase grape and wine phenolic content in Tempranillo and Graciano varieties during two growing seasons. Sci. Hortic. 2018, 240, 378–386. [Google Scholar] [CrossRef]
- Román, S.M.S.; Garde-Cerdán, T.; Baroja, E.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Foliar application of phenylalanine plus methyl jasmonate as a tool to improve Grenache grape aromatic composition. Sci. Hortic. 2020, 272, 109515. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Gutiérrez-Gamboa, G.; Baroja, E.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Influence of methyl jasmonate foliar application to vineyard on grape volatile composition over three consecutive vintages. Food Res. Int. 2018, 112, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Portu, J.; Santamaría, P.; López, R.; Garde-Cerdán, T. Effects on grape amino acid concentration through foliar application of three different elicitors. Food Res. Int. 2017, 99, 688–692. [Google Scholar] [CrossRef]
- Nair, A.; Kolet, S.P.; Thulasiram, H.V.; Bhargava, S. Role of methyl jasmonate in the expression of mycorrhizal induced resistance against Fusarium oxysporum in tomato plants. Physiol. Mol. Plant Pathol. 2015, 92, 139–145. [Google Scholar] [CrossRef]
- Wang, K.; Liao, Y.; Kan, J.; Han, L.; Zheng, Y. Response of direct or priming defense against Botrytis cinerea to methyl jasmonate treatment at different concentrations in grape berries. Int. J. Food Microbiol. 2015, 194, 32–39. [Google Scholar] [CrossRef]
- Burdziej, A.; Bellée, A.; Bodin, E.; Valls Fonayet, J.; Magnin, N.; Szakiel, A.; Richard, T.; Cluzet, S.; Corio-Costet, M.F. Three Types of Elicitors Induce Grapevine Resistance against Downy Mildew via Common and Specific Immune Responses. J. Agric. Food Chem. 2021, 69, 1781–1795. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, X.; Wu, C.; Fan, G.; Li, T.; Dong, C. Retardation of postharvest softening of blueberry fruit by methyl jasmonate is correlated with altered cell wall modification and energy metabolism. Sci. Hortic. 2021, 276, 109752. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B.; Gil-Muñoz, R. Influence of methyl jasmonate and benzothiadiazole on the composition of grape skin cell walls and wines. Food Chem. 2019, 277, 691–697. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Gil-Muñoz, R. Application of Elicitors at Two Maturation Stages of Vitis vinifera L. cv Monastrell: Changes in Skin Cell Walls. Chemistry 2022, 4, 98–111. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Ruiz-García, Y.; Williams, P.; Gil-Muñoz, R.; Gómez-Plaza, E.; Doco, T. Preharvest Application of Elicitors to Monastrell Grapes: Impact on Wine Polysaccharide and Oligosaccharide Composition. J. Agric. Food Chem. 2018, 66, 11151–11157. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M. Effect of Methyl Jasmonate Doped Nanoparticles on Nitrogen Composition of Monastrell Grapes and Wines. Biomolecules 2021, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Gimenez-Bañon, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Gil-Munoz, R.; Delgado-López, J.M. Nanoelicitors with prolonged retention and sustained release to produce beneficial compounds in wines. Environ. Sci. Nano 2021, 8, 3524–3535. [Google Scholar] [CrossRef]
- Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Delgado-López, J.M.; Gil-Muñoz, R. Effects of Methyl Jasmonate and Nano-Methyl Jasmonate Treatments on Monastrell Wine Volatile Composition. Molecules 2022, 27, 2878. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Sáenz de Urturi, I.; Rubio-Bretón, P.; Marín-San Román, S.; Baroja, E.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Pérez-Álvarez, E.P. Foliar application of methyl jasmonate and methyl jasmonate supported on nanoparticles: Incidence on grape phenolic composition over two seasons. Food Chem. 2023, 402, 134244. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Rubio-Bretón, P.; Intrigliolo, D.S.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Garde-Cerdán, T. Year, watering regime and foliar methyl jasmonate doped nanoparticles treatments: Effects on must nitrogen compounds in Monastrell grapes. Sci. Hortic. 2022, 297, 110944. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Differences in morphology and composition of skin and pulp cell walls from grapes (Vitis vinifera L.): Technological implications. Eur. Food Res. Technol. 2008, 227, 223–231. [Google Scholar] [CrossRef]
- Clemente, H.S.; Kolkas, H.; Canut, H.; Jamet, E. Plant Cell Wall Proteomes: The Core of Conserved Protein Families and the Case of Non-Canonical Proteins. Int. J. Mol. Sci. 2022, 23, 4273. [Google Scholar] [CrossRef]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef]
- Hernández-Hierro, J.M.; Quijada-Morín, N.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Relationship between skin cell wall composition and anthocyanin extractability of Vitis vinifera L. cv. Tempranillo at different grape ripeness degree. Food Chem. 2014, 146, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.M.; Huang, H.B.; Wang, H.C. Cell walls of loosening skin in post-veraison grape berries lose structural polysaccharides and calcium while accumulate structural proteins. Sci. Hortic. 2005, 104, 249–263. [Google Scholar] [CrossRef]
- Nunan, K.J.; Sims, I.M.; Bacic, A.; Robinson, S.P.; Fincher, G.B. Changes in cell wall composition during ripening of grape berries. Plant Physiol. 1998, 118, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Matsumoto, S.; Yamada, K. Methyl jasmonate treatment promotes flower opening of cut Eustoma by inducing cell wall loosening proteins in petals. Postharvest Biol. Technol. 2013, 82, e0248962. [Google Scholar] [CrossRef]
- Mnich, E.; Bjarnholt, N.; Eudes, A.; Harholt, J.; Holland, C.; Jørgensen, B.; Larsen, F.H.; Liu, M.; Manat, R.; Meyer, A.S.; et al. Phenolic cross-links: Building and de-constructing the plant cell wall. Nat. Prod. Rep. 2020, 37, 919–961. [Google Scholar] [CrossRef] [PubMed]
- Napoleão, T.A.; Soares, G.; Vital, C.E.; Bastos, C.; Castro, R.; Loureiro, M.E.; Giordano, A. Methyl jasmonate and salicylic acid are able to modify cell wall but only salicylic acid alters biomass digestibility in the model grass Brachypodium distachyon. Plant Sci. 2017, 263, 46–54. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Rui, H.; Tang, S. Effect of methyl jasmonate on cell wall modification of loquat fruit in relation to chilling injury after harvest. Food Chem. 2010, 118, 641–647. [Google Scholar] [CrossRef]
- Jarvis, M.C. Structure and properties of pectin gels in plant cell walls. Plant, Cell Environ. 1984, 7, 153–164. [Google Scholar] [CrossRef]
- Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Gimenez-Bañon, M.J.; Cebrían-Pérez, A.; Fernández-Fernández, J.I.; Gómez-Martínez, J.C.; Bleda-Sánchez, J.A. Cell wall characterization of new Monastrell hybrid descendants and their phenolic wine composition. Eur. Food Res. Technol. 2022, 248, 1253–1265. [Google Scholar] [CrossRef]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol. Rep. 2018, 19, e00273. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Santamaría, P.; Garde-Cerdán, T. Elicitation with methyl jasmonate supported by precursor feeding with phenylalanine: Effect on Garnacha grape phenolic content. Food Chem. 2017, 237, 416–422. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Fernández-Fernández, J.I.; Moreno-Olivares, J.D.; Bleda-Sánchez, J.A.; Gómez-Martínez, J.C.; Martínez-Jiménez, J.A.; Gil-Muñoz, R. Application of Elicitors in Two Ripening Periods of Vitis vinifera L. cv Monastrell: Influence on Anthocyanin Concentration of Grapes and Wines. Molecules 2021, 26, 1689. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Goulao, L.F.; Fernandes, J.C.; Lopes, P.; Amâncio, S. The Biochemistry of the Grape Berry Chapter 9 Tackling the Cell Wall of the Grape Berry. In The Biochemistry of Grape Berry; Bentham Science Publisher: Sharjah, United Arab Emirates, 2012; pp. 172–193. ISBN 978-1-60805-360-5. [Google Scholar]
- Renard, C.M.G.C.; Watrelot, A.A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Scott, R.W. Colorimetric Determination of Hexuronic Acids in Plant Materials. Anal. Chem. 1979, 51, 936–941. [Google Scholar] [CrossRef]
- Glories, Y. La couleur des vins rouges. lre partie : Les équilibres des anthocyanes et des tanins. OENO One 1984, 18, 195. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Pontallier, P.; Glories, Y. Some interpretations of colour changes in young red wines during their conservation. J. Sci. Food Agric. 1983, 34, 505–516. [Google Scholar] [CrossRef]
- Ribereau-Gayon, J.; Ribereau-Gayon, P.; Peynaud, E.; Sudraud, P. Dosage des anthocyanes dans les vins rouges. Sci. Tech. Vin. 1972, 1, 497–499. [Google Scholar]
| Weight (g) (100 Berries) | Soluble Solids (°Brix) | TA (g of Tartaric Acid L−1) | ||||
|---|---|---|---|---|---|---|
| Harvest Date | pH | |||||
| 2019 | Control | 23 September 2019 | 154.98 ± 5.66 | 22.07 ± 0.58 | 4.17 ± 0.24 b | 3.74 ± 0.02 |
| MeJ | 23 September 2019 | 154.64 ± 10.99 | 22.27 ± 0.55 | 4.74 ± 0.17 a | 3.74 ± 0.05 | |
| Nano-MeJ | 23 September 2019 | 158.91 ± 5.24 | 22.72 ± 0.56 | 4.68 ± 0.01 a | 3.78 ± 0.02 | |
| p-value | ns | ns | * | ns | ||
| 2020 | Control | 29 September 2020 | 162.79 ± 3.69 | 25.75 ± 0.33 | 2.87 ± 0.00 b | 4.14 ± 0.06 |
| MeJ | 29 September 2020 | 165.29 ± 9.52 | 24.92 ± 0.38 | 3.08 ± 0.08 a | 4.13 ± 0.04 | |
| Nano-MeJ | 29 September 2020 | 170.87 ± 4.83 | 25.35 ± 0.35 | 2.87 ± 0.13 b | 4.16 ± 0.03 | |
| p-value | ns | ns | * | ns | ||
| 2021 | Control | 4 October 2021 | 143.70 ± 11.00 b | 23.27 ± 0.60 | 2.75 ± 0.20 b | 4.02 ± 0.03 |
| MeJ | 4 October 2021 | 165.84 ± 7.98 a | 23.83 ± 0.25 | 3.38 ± 0.16 a | 4.02 ± 0.04 | |
| Nano-MeJ | 4 October 2021 | 168.67 ± 0.59 a | 23.50 ± 0.17 | 2.72 ± 0.25 b | 4.04 ± 0.03 | |
| p-value | * | ns | * | ns |
| % Dry Skin | % CWM | ||
|---|---|---|---|
| In Grape | (In Dry Skin) | ||
| 2019 | Control | 3.02 b | 25.66 b |
| MeJ | 3.17 a | 28.55 a | |
| Nano-MeJ | 2.88 c | 28.30 a | |
| p-value | *** | *** | |
| 2020 | Control | 3.26 | 24.94 b |
| MeJ | 3.32 | 24.79 b | |
| Nano-MeJ | 2.98 | 27.76 a | |
| p-value | ns | *** | |
| 2021 | Control | 3.08 b | 22.62 c |
| MeJ | 3.33 a | 24.66 a | |
| Nano-MeJ | 3.15 b | 23.54 b | |
| p-value | * | *** |
| T (%) | S (%) | TxS (%) | Error (%) | |
|---|---|---|---|---|
| Uronic Acids | 24 *** | 37 *** | 31 *** | 7 |
| TPC | 15 ** | 40 *** | 13 * | 32 |
| Proteins | 7 *** | 69 *** | 17 *** | 8 |
| Cel-glu | 25 *** | 57 *** | 5 ns | 13 |
| Non-cel-glu | 4 *** | 88 *** | 2 * | 5 |
| WA | IPT | CI | ||
|---|---|---|---|---|
| (mg L−1) | ||||
| 2019 | Control | 529.00 ± 38.97 | 39.11 ± 1.05 b | 14.01 ± 1.03 |
| MeJ | 551.00 ± 24.00 | 41.85 ± 0.97 a | 14.01 ± 0.81 | |
| Nano-MeJ | 520.33 ± 44.00 | 41.32 ± 2.71 a | 12.94 ± 0.67 | |
| p-value | ns | * | ns | |
| 2020 | Control | 572.33 ± 29.78 b | 47.88 ± 1.76 b | 14.14 ± 0.85 |
| MeJ | 682.67 ± 49.51 a | 53.75 ± 0.96 a | 15.55 ± 3.52 | |
| Nano-MeJ | 527.67 ± 44.40 b | 44.89 ± 2.91 b | 12.43 ± 0.90 | |
| p-value | * | ** | ns | |
| 2021 | Control | 621.67 ± 56.72 b | 47.24 ± 2.45 | 16.37 ± 1.47 |
| MeJ | 739.00 ± 54.15 a | 45.52 ± 3.81 | 18.29 ± 0.88 | |
| Nano-MeJ | 635.33 ± 20.03 b | 47.81 ± 0.71 | 16.56 ± 0.48 | |
| p-value | * | ns | ns | |
| T (%) | 17 *** | 6 ** | 15 ** | |
| Multifactorial | S (%) | 63 *** | 61 *** | 55 *** |
| analysis | TxS (%) | 8 ** | 21 *** | 5 ns |
| Error (%) | 11 | 12 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giménez-Bañón, M.J.; Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Gil-Muñoz, R. Methyl Jasmonate and Nanoparticles Doped with Methyl Jasmonate affect the Cell Wall Composition of Monastrell Grape Skins. Molecules 2023, 28, 1478. https://doi.org/10.3390/molecules28031478
Giménez-Bañón MJ, Paladines-Quezada DF, Moreno-Olivares JD, Bleda-Sánchez JA, Fernández-Fernández JI, Parra-Torrejón B, Ramírez-Rodríguez GB, Delgado-López JM, Gil-Muñoz R. Methyl Jasmonate and Nanoparticles Doped with Methyl Jasmonate affect the Cell Wall Composition of Monastrell Grape Skins. Molecules. 2023; 28(3):1478. https://doi.org/10.3390/molecules28031478
Chicago/Turabian StyleGiménez-Bañón, María José, Diego Fernando Paladines-Quezada, Juan Daniel Moreno-Olivares, Juan Antonio Bleda-Sánchez, José Ignacio Fernández-Fernández, Belén Parra-Torrejón, Gloria Belén Ramírez-Rodríguez, José Manuel Delgado-López, and Rocío Gil-Muñoz. 2023. "Methyl Jasmonate and Nanoparticles Doped with Methyl Jasmonate affect the Cell Wall Composition of Monastrell Grape Skins" Molecules 28, no. 3: 1478. https://doi.org/10.3390/molecules28031478
APA StyleGiménez-Bañón, M. J., Paladines-Quezada, D. F., Moreno-Olivares, J. D., Bleda-Sánchez, J. A., Fernández-Fernández, J. I., Parra-Torrejón, B., Ramírez-Rodríguez, G. B., Delgado-López, J. M., & Gil-Muñoz, R. (2023). Methyl Jasmonate and Nanoparticles Doped with Methyl Jasmonate affect the Cell Wall Composition of Monastrell Grape Skins. Molecules, 28(3), 1478. https://doi.org/10.3390/molecules28031478











