Microbial Production of Human Milk Oligosaccharides
Abstract
:1. Introduction
2. HMO Production Methods
3. HMO Production by Microbial Cell Factories
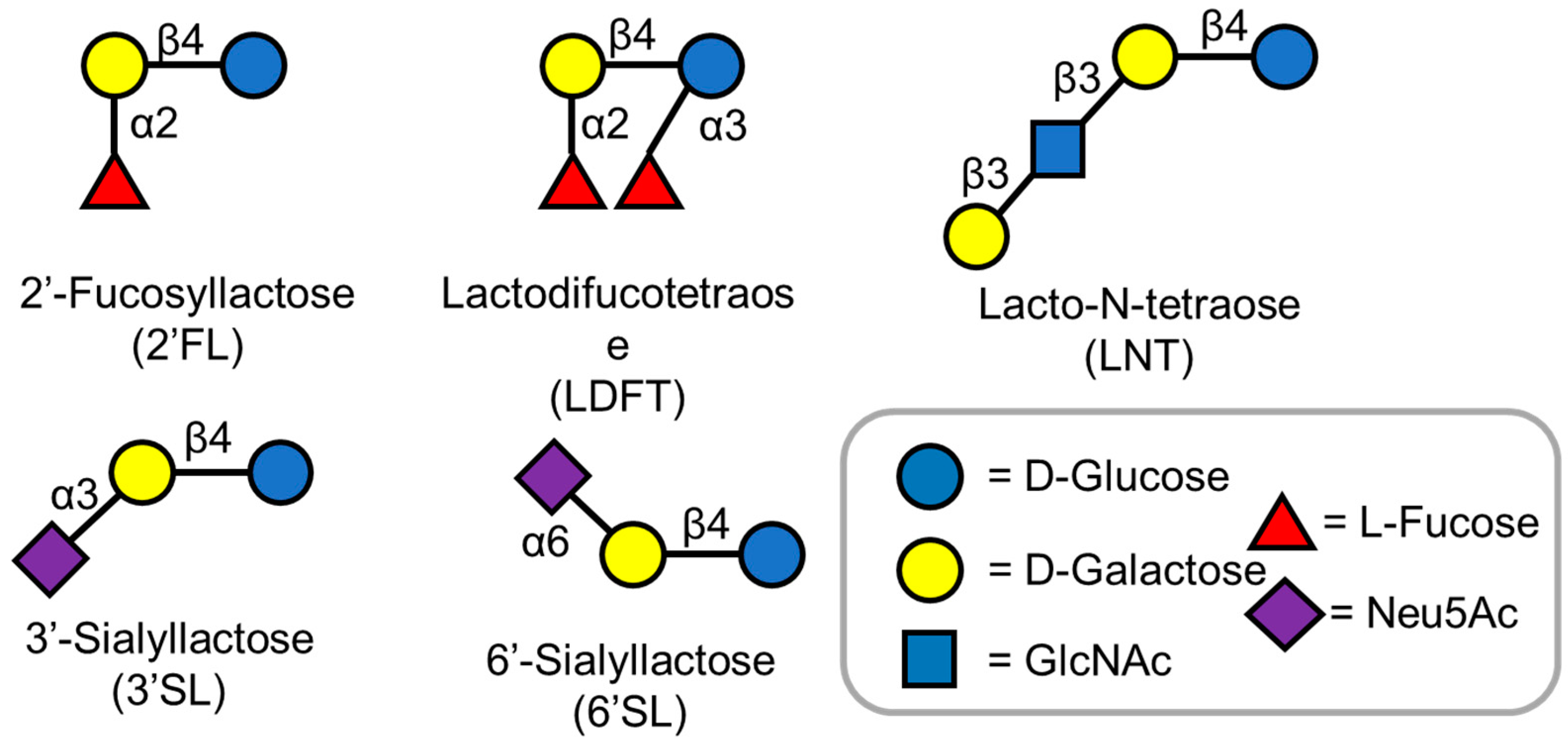
3.1. 2′-Fucosyllactose
3.2. LDFT (Lactodifucotetraose)
3.3. 3′ and 6′-Sialyllactose
3.4. LNT (Lacto-N-Tetraose)
4. Challenges
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jéquier, E. Carbohydrates as a Source of Energy. Am. J. Clin. Nutr. 1994, 59, 682S–685S. [Google Scholar] [CrossRef] [PubMed]
- Boquien, C.-Y. Human Milk: An Ideal Food for Nutrition of Preterm Newborn. Front. Pediatr. 2018, 6, 295. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Human Milk Oligosaccharides (HMOS): Structure, Function, and Enzyme-Catalyzed Synthesis. Adv. Carbohydr. Chem. Biochem. 2015, 72, 113–190. [Google Scholar] [CrossRef] [PubMed]
- Auer, F.; Jarvas, G.; Guttman, A. Recent Advances in the Analysis of Human Milk Oligosaccharides by Liquid Phase Separation Methods. J. Chromatogr. B 2021, 1162, 122497. [Google Scholar] [CrossRef] [PubMed]
- Soyyılmaz, B.; Mikš, M.H.; Röhrig, C.H.; Matwiejuk, M.; Meszaros-Matwiejuk, A.; Vigsnæs, L.K. The Mean of Milk: A Review of Human Milk Oligosaccharide Concentrations throughout Lactation. Nutrients 2021, 13, 2737. [Google Scholar] [CrossRef]
- Garwolińska, D.; Namieśnik, J.; Kot-Wasik, A.; Hewelt-Belka, W. Chemistry of Human Breast Milk—A Comprehensive Review of the Composition and Role of Milk Metabolites in Child Development. J. Agric. Food Chem. 2018, 66, 11881–11896. [Google Scholar] [CrossRef]
- Bode, L.; McGuire, M.; Rodriguez, J.M.; Geddes, D.T.; Hassiotou, F.; Hartmann, P.E.; McGuire, M.K. It’s Alive: Microbes and Cells in Human Milk and Their Potential Benefits to Mother and Infant. Adv. Nutr. 2014, 5, 571–573. [Google Scholar] [CrossRef]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef]
- Hill, D.R.; Chow, J.M.; Buck, R.H. Multifunctional Benefits of Prevalent HMOs: Implications for Infant Health. Nutrients 2021, 13, 3364. [Google Scholar] [CrossRef]
- Christian, P.; Smith, E.R.; Lee, S.E.; Vargas, A.J.; Bremer, A.A.; Raiten, D.J. The Need to Study Human Milk as a Biological System. Am. J. Clin. Nutr. 2021, 113, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Markets and Markets Website, Human Milk Oligosaccharides (HMO) Market. Available online: https://www.marketsandmarkets.com/Market-Reports/human-milk-oligosaccharides-hmo-market-220134357.html (accessed on 20 December 2022).
- Neves, P.A.; Barros, A.J.; Baker, P.; Piwoz, E.; Santos, T.M.; Gatica-Domínguez, G.; Vaz, J.S.; Rollins, N.; Victora, C.G. Consumption of Breast Milk, Formula and Other Non-Human Milk by Children Aged under 2 Years: Analysis of Eighty-Six Low- and Middle-Income Countries. Public Health Nutr. 2022, 25, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. From Lab Bench to Formulated Ingredient: Characterization, Production, and Commercialization of Human Milk Oligosaccharides. J. Funct. Foods 2020, 72, 104052. [Google Scholar] [CrossRef]
- Takamura, T.; Chiba, T.; Ishihara, H.; Tejima, S. Chemical Modification of Lactose. XIII. Synthesis of Lacto-N-Tetraose. Chem. Pharm. Bull. 1979, 27, 1497–1499. [Google Scholar] [CrossRef]
- Knerr, L.; Schmidt, R.R. Solid-Phase Synthesis of a Branched Hexasaccharide Related to Lacto-N-Hexaose. Eur. J. Org. Chem. 2000, 2000, 2803–2808. [Google Scholar] [CrossRef]
- Craft, K.M.; Townsend, S.D. Synthesis of Lacto-N-Tetraose. Carbohydr. Res. 2017, 440–441, 43–50. [Google Scholar] [CrossRef]
- Das, R.; Mukhopadhyay, B. Chemical O-Glycosylations: An Overview. ChemistryOpen 2016, 5, 401–433. [Google Scholar] [CrossRef]
- McKay, M.J.; Nguyen, H.M. Recent Advances in Transition Metal-Catalyzed Glycosylation. ACS Catal. 2012, 2, 1563–1595. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Romero, E.O.; Pyser, J.B.; Yazarians, J.A.; Narayan, A.R.H. Chemoenzymatic Total Synthesis of Natural Products. Acc. Chem. Res. 2021, 54, 1374–1384. [Google Scholar] [CrossRef]
- Muthana, S.; Cao, H.; Chen, X. Recent Progress in Chemical and Chemoenzymatic Synthesis of Carbohydrates. Curr. Opin. Chem. Biol. 2009, 13, 573–581. [Google Scholar] [CrossRef]
- Xiao, Z.; Guo, Y.; Liu, Y.; Li, L.; Zhang, Q.; Wen, L.; Wang, X.; Kondengaden, S.M.; Wu, Z.; Zhou, J.; et al. Chemoenzymatic Synthesis of a Library of Human Milk Oligosaccharides. J. Org. Chem. 2016, 81, 5851–5865. [Google Scholar] [CrossRef] [Green Version]
- McArthur, J.B.; Yu, H.; Chen, X. A Bacterial Β1–3-Galactosyltransferase Enables Multigram-Scale Synthesis of Human Milk Lacto- N -Tetraose (LNT) and Its Fucosides. ACS Catal. 2019, 9, 10721–10726. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xu, H.; Fang, J.; Zhang, X. Enzymatic and Chemoenzymatic Synthesis of Human Milk Oligosaccharides and Derivatives. Carbohydr. Polym. 2022, 291, 119564. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.L.; Townsend, S.D. Synthesis as an Expanding Resource in Human Milk Science. J. Am. Chem. Soc. 2021, 143, 11277–11290. [Google Scholar] [CrossRef]
- Zeuner, B.; Teze, D.; Muschiol, J.; Meyer, A.S. Synthesis of Human Milk Oligosaccharides: Protein Engineering Strategies for Improved Enzymatic Transglycosylation. Molecules 2019, 24, 2033. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.L.; Desai, S.H.; Atsumi, S. Microbial Production of Scent and Flavor Compounds. Curr. Opin. Biotechnol. 2016, 37, 8–15. [Google Scholar] [CrossRef]
- Chubukov, V.; Mukhopadhyay, A.; Petzold, C.J.; Keasling, J.D.; Martín, H.G. Synthetic and Systems Biology for Microbial Production of Commodity Chemicals. Npj Syst. Biol. Appl. 2016, 2, 16009. [Google Scholar] [CrossRef]
- Cho, J.S.; Kim, G.B.; Eun, H.; Moon, C.W.; Lee, S.Y. Designing Microbial Cell Factories for the Production of Chemicals. JACS Au 2022, 2, 1781–1799. [Google Scholar] [CrossRef]
- Tashiro, Y.; Rodriguez, G.M.; Atsumi, S. 2-Keto Acids Based Biosynthesis Pathways for Renewable Fuels and Chemicals. J. Ind. Microbiol. Biotechnol. 2015, 42, 361–373. [Google Scholar] [CrossRef]
- Bych, K.; Mikš, M.H.; Johanson, T.; Hederos, M.J.; Vigsnæs, L.K.; Becker, P. Production of HMOs Using Microbial Hosts—From Cell Engineering to Large Scale Production. Curr. Opin. Biotechnol. 2019, 56, 130–137. [Google Scholar] [CrossRef]
- Woo, J.E.; Seong, H.J.; Lee, S.Y.; Jang, Y.-S. Metabolic Engineering of Escherichia coli for the Production of Hyaluronic Acid From Glucose and Galactose. Front. Bioeng. Biotechnol. 2019, 7, 351. [Google Scholar] [CrossRef] [Green Version]
- Byun, S.-G.; Kim, M.-D.; Lee, W.-H.; Lee, K.-J.; Han, N.S.; Seo, J.-H. Production of GDP-L-Fucose, L-Fucose Donor for Fucosyloligosaccharide Synthesis, in Recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 2007, 74, 768–775. [Google Scholar] [CrossRef]
- Hegar, B.; Wibowo, Y.; Basrowi, R.W.; Ranuh, R.G.; Sudarmo, S.M.; Munasir, Z.; Atthiyah, A.F.; Widodo, A.D.; Supriatmo; Kadim, M.; et al. The Role of Two Human Milk Oligosaccharides, 2′-Fucosyllactose and Lacto-N-Neotetraose, in Infant Nutrition. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 330–340. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Duysburgh, C.; Vazquez, E.; Chow, J.; Buck, R.; Marzorati, M. 2′-Fucosyllactose Alters the Composition and Activity of Gut Microbiota from Formula-Fed Infants Receiving Complementary Feeding in a Validated Intestinal Model. J. Funct. Foods 2019, 61, 103484. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Ksiazyk, J.; Lagström, H.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.-M.; Picaud, J.-C.; Possner, M.; et al. Human Milk Oligosaccharides: 2′-Fucosyllactose (2′-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Agoston, K.; Hederos, M.J.; Bajza, I.; Dekany, G. Kilogram Scale Chemical Synthesis of 2′-Fucosyllactose. Carbohydr. Res. 2019, 476, 71–77. [Google Scholar] [CrossRef]
- Li, C.; Wu, M.; Gao, X.; Zhu, Z.; Li, Y.; Lu, F.; Qin, H.-M. Efficient Biosynthesis of 2′-Fucosyllactose Using an In Vitro Multienzyme Cascade. J. Agric. Food Chem. 2020, 68, 10763–10771. [Google Scholar] [CrossRef]
- Wan, L.; Zhu, Y.; Li, W.; Zhang, W.; Mu, W. Combinatorial Modular Pathway Engineering for Guanosine 5′-Diphosphate-l-Fucose Production in Recombinant Escherichia coli. J. Agric. Food Chem. 2020, 68, 5668–5675. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Han, D.; Pan, Y.; Wang, S.; Fang, J.; Wang, P.; Liu, X. Enhancing GDP-Fucose Production in Recombinant Escherichia coli by Metabolic Pathway Engineering. Enzyme Microb. Technol. 2015, 69, 38–45. [Google Scholar] [CrossRef]
- Seydametova, E.; Yu, J.; Shin, J.; Park, Y.; Kim, C.; Kim, H.; Yu, S.H.; Park, Y.; Kweon, D.-H. Search for Bacterial A1,2-Fucosyltransferases for Whole-Cell Biosynthesis of 2′-Fucosyllactose in Recombinant Escherichia coli. Microbiol. Res. 2019, 222, 35–42. [Google Scholar] [CrossRef]
- Parschat, K.; Schreiber, S.; Wartenberg, D.; Engels, B.; Jennewein, S. High-Titer De Novo Biosynthesis of the Predominant Human Milk Oligosaccharide 2′-Fucosyllactose from Sucrose in Escherichia coli. ACS Synth. Biol. 2020, 9, 2784–2796. [Google Scholar] [CrossRef]
- Engels, L.; Elling, L. WbgL: A Novel Bacterial A1,2-Fucosyltransferase for the Synthesis of 2′-Fucosyllactose. Glycobiology 2014, 24, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Wan, L.; Chen, R.; Zhang, W.; Mu, W. High-Level De Novo Biosynthesis of 2′-Fucosyllactose by Metabolically Engineered Escherichia coli. J. Agric. Food Chem. 2022, 70, 9017–9025. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Hu, M.; Zhang, T. Metabolic Engineering Strategies of de Novo Pathway for Enhancing 2′-Fucosyllactose Synthesis in Escherichia coli. Microb. Biotechnol. 2022, 15, 1561–1573. [Google Scholar] [CrossRef]
- Lee, J.W.; Kwak, S.; Liu, J.-J.; Yu, S.; Yun, E.J.; Kim, D.H.; Liu, C.; Kim, K.H.; Jin, Y.-S. Enhanced 2′-Fucosyllactose Production by Engineered Saccharomyces cerevisiae Using Xylose as a Co-Substrate. Metab. Eng. 2020, 62, 322–329. [Google Scholar] [CrossRef]
- Xu, M.; Meng, X.; Zhang, W.; Shen, Y.; Liu, W. Improved Production of 2′-Fucosyllactose in Engineered Saccharomyces cerevisiae Expressing a Putative α-1, 2-Fucosyltransferase from Bacillus Cereus. Microb. Cell Factories 2021, 20, 165. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Warren, C.D.; Altaye, M.; Morrow, A.L.; Ruiz-Palacios, G.; Pickering, L.K.; Newburg, D.S. Fucosylated Human Milk Oligosaccharides Vary between Individuals and over the Course of Lactation. Glycobiology 2001, 11, 365–372. [Google Scholar] [CrossRef]
- Yu, Z.-T.; Chen, C.; Newburg, D.S. Utilization of Major Fucosylated and Sialylated Human Milk Oligosaccharides by Isolated Human Gut Microbes. Glycobiology 2013, 23, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Wang, G.; Palcic, M.M.; Taylor, D.E. Cloning and Characterization of the α(1,3/4) Fucosyltransferase of Helicobacter Pylori. J. Biol. Chem. 2000, 275, 4988–4994. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, L.; Bai, Y.; Yu, H.; McArthur, J.B.; Chen, X.; Atsumi, S. Microbial Production of Human Milk Oligosaccharide Lactodifucotetraose. Metab. Eng. 2021, 66, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, Y.; Wu, Z.; Li, L.; Zeng, J.; Zhao, C.; Wu, Y.; Tasnima, N.; Wang, J.; Liu, H.; et al. Pylori A1–3/4-Fucosyltransferase (Hp3/4FT)-Catalyzed One-Pot Multienzyme (OPME) Synthesis of Lewis Antigens and Human Milk Fucosides. Chem. Commun. 2017, 53, 11012–11015. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Brand-Miller, J. The Role and Potential of Sialic Acid in Human Nutrition. Eur. J. Clin. Nutr. 2003, 57, 1351–1369. [Google Scholar] [CrossRef]
- Fierfort, N.; Samain, E. Genetic Engineering of Escherichia coli for the Economical Production of Sialylated Oligosaccharides. J. Biotechnol. 2008, 134, 261–265. [Google Scholar] [CrossRef]
- Drouillard, S.; Mine, T.; Kajiwara, H.; Yamamoto, T.; Samain, E. Efficient Synthesis of 6′-Sialyllactose, 6,6′-Disialyllactose, and 6′-KDO-Lactose by Metabolically Engineered E. coli Expressing a Multifunctional Sialyltransferase from the Photobacterium Sp. JT-ISH-224. Carbohydr. Res. 2010, 345, 1394–1399. [Google Scholar] [CrossRef]
- Hu, M.; Li, M.; Miao, M.; Zhang, T. Engineering Escherichia coli for the High-Titer Biosynthesis of Lacto-N-Tetraose. J. Agric. Food Chem. 2022, 70, 8704–8712. [Google Scholar] [CrossRef]
- Yao, W.; Yan, J.; Chen, X.; Wang, F.; Cao, H. Chemoenzymatic Synthesis of Lacto-N-Tetrasaccharide and Sialyl Lacto-N-Tetrasaccharides. Carbohydr. Res. 2015, 401, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Baumgärtner, F.; Conrad, J.; Sprenger, G.A.; Albermann, C. Synthesis of the Human Milk Oligosaccharide Lacto-N-Tetraose in Metabolically Engineered, Plasmid-Free E. Coli. Chembiochem Eur. J. Chem. Biol. 2014, 15, 1896–1900. [Google Scholar] [CrossRef] [PubMed]
- Baumgärtner, F.; Sprenger, G.A.; Albermann, C. Galactose-Limited Fed-Batch Cultivation of Escherichia coli for the Production of Lacto-N-Tetraose. Enzyme Microb. Technol. 2015, 75–76, 37–43. [Google Scholar] [CrossRef]
- Sugita, T.; Koketsu, K. Transporter Engineering Enables the Efficient Production of Lacto-N-Triose II and Lacto-N-Tetraose in Escherichia coli. J. Agric. Food Chem. 2022, 70, 5106–5114. [Google Scholar] [CrossRef] [PubMed]
- Blixt, O.; van Die, I.; Norberg, T.; van den Eijnden, D.H. High-Level Expression of the Neisseria Meningitidis LgtA Gene in Escherichia coli and Characterization of the Encoded N-Acetylglucosaminyltransferase as a Useful Catalyst in the Synthesis of GlcNAcβ1→3Gal and GalNAcβ1→3Gal Linkages. Glycobiology 1999, 9, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.P.; Hood, D.W.; Peak, I.R.; Virji, M.; Moxon, E.R. Molecular Analysis of a Locus for the Biosynthesis and Phase-Variable Expression of the Lacto-N-Neotetraose Terminal Lipopolysaccharide Structure in Neisseria Meningitidis. Mol. Microbiol. 1995, 18, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xia, C.; Li, L.; Guan, W.; Pettit, N.; Zhang, H.; Chen, M.; Wang, P.G. Characterization and Synthetic Application of a Novel Β1,3-Galactosyltransferase from Escherichia coli O55:H7. Bioorg. Med. Chem. 2009, 17, 4910–4915. [Google Scholar] [CrossRef] [PubMed]
- de Boer, H.A.; Comstock, L.J.; Vasser, M. The Tac Promoter: A Functional Hybrid Derived from the Trp and Lac Promoters. Proc. Natl. Acad. Sci. USA 1983, 80, 21–25. [Google Scholar] [CrossRef]
- Santillán, M.; Mackey, M.C. Quantitative Approaches to the Study of Bistability in the Lac Operon of Escherichia coli. J. R. Soc. Interface 2008, 5 (Suppl. S1), S29–S39. [Google Scholar] [CrossRef]
- Lee, S.J.; Trostel, A.; Adhya, S. Metabolite Changes Signal Genetic Regulatory Mechanisms for Robust Cell Behavior. MBio 2014, 5, e00972-13. [Google Scholar] [CrossRef] [PubMed]
- Koita, K.; Rao, C.V. Identification and analysis of the putative pentose sugar efflux transporters in Escherichia coli. Plos ONE 2012, 7, e43700. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; Zimmermann, K. Lipopolysaccharides in Food, Food Supplements, and Probiotics: Should We Be Worried? Eur. J. Microbiol. Immunol. 2018, 8, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ongkudon, C.M.; Chew, J.H.; Liu, B.; Danquah, M.K. Chromatographic Removal of Endotoxins: A Bioprocess Engineer’s Perspective. Int. Sch. Res. Not. 2012, 2012, e649746. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palur, D.S.K.; Pressley, S.R.; Atsumi, S. Microbial Production of Human Milk Oligosaccharides. Molecules 2023, 28, 1491. https://doi.org/10.3390/molecules28031491
Palur DSK, Pressley SR, Atsumi S. Microbial Production of Human Milk Oligosaccharides. Molecules. 2023; 28(3):1491. https://doi.org/10.3390/molecules28031491
Chicago/Turabian StylePalur, Dileep Sai Kumar, Shannon R. Pressley, and Shota Atsumi. 2023. "Microbial Production of Human Milk Oligosaccharides" Molecules 28, no. 3: 1491. https://doi.org/10.3390/molecules28031491
APA StylePalur, D. S. K., Pressley, S. R., & Atsumi, S. (2023). Microbial Production of Human Milk Oligosaccharides. Molecules, 28(3), 1491. https://doi.org/10.3390/molecules28031491





