Novel Indane Derivatives with Antioxidant Activity from the Roots of Anisodus tanguticus
Abstract
:1. Introduction
2. Results
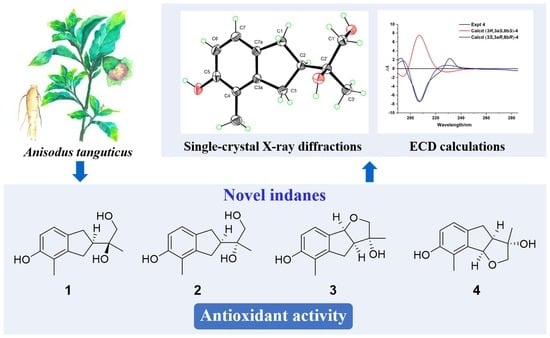
2.1. Structure Elucidation
2.2. Antioxidant Activities
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Physicochemical Properties and Spectroscopic Data of Compounds 1–4
4.5. X-ray Crystallographic Data of Compounds 1 and 3
4.6. ECD Calculation
4.7. Antioxidant Activity
4.7.1. ABTS•+ Assay
4.7.2. DPPH•+ Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zang, E.H.; Li, Q.Y.; Xu, J.F.; Zhang, Y.; Jiang, L.L.; Li, X.; Zhang, M.X.; Liu, Y.C.; Wu, Q.J.; Liu, Z.H.; et al. A preliminary pharmacophylogenetic study of Solanaceae medicinal plants containing tropane alkaloids. China J. Chin. Mater. Med. 2021, 46, 4344–4359. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Z.; Hu, G.; Li, G.; Niu, S.; Zhao, F. Separation, identification and pesticidal activity of alkaloids from Anisodus tanguticus (Maxim.) Pascher. Plant Prot. 2019, 45, 190–194. [Google Scholar] [CrossRef]
- Ma, L.; Gu, R.; Tang, L.; Chen, Z.E.; Di, R.; Long, C. Important poisonous plants in Tibetan ethnomedicine. Toxins 2015, 7, 138–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Wang, H. The variation of the contents of four tropane alkaloids in Anisodus tanguticus. Acta Bot. Boreali-Occident. Sin. 2002, 22, 630–634. [Google Scholar] [CrossRef]
- Huang, J.P.; Wang, Y.J.; Tian, T.; Wang, L.; Yan, Y.; Huang, S.X. Tropane alkaloid biosynthesis: A centennial review. Nat. Prod. Rep. 2021, 38, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, D.J.; Liu, C.; Su, D.F.; Shen, F.M. Beneficial effects of anisodamine in shock involved cholinergic anti-inflammatory pathway. Front. Pharmacol. 2011, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, Q.; Duan, X.; Li, L.; Zhang, S.; Chen, J.; Wang, Y. Evaluation of anisodamine-mediated amelioration of hypoxic injury in brain microvascular endothelial cells. Trop. J. Pharm. Res. 2022, 20, 1425–1432. [Google Scholar] [CrossRef]
- Xing, K.; Fu, X.; Jiang, L.; Wang, Y.; Li, W.; Gu, X.; Hao, G.; Miao, Q.; Ge, X.; Peng, Y.; et al. Cardioprotective effect of anisodamine against myocardial ischemia injury and its influence on cardiomyocytes apoptosis. Cell Biochem. Biophys. 2015, 73, 707–716. [Google Scholar] [CrossRef]
- Chen, L.; Lei, B.; Lou, Y.; Chen, L.; Jiang, J. Impact of intravenous administration of anisodamine on coronary microvascular dysfunction in patients with obstructive epicardial coronary artery disease after percutaneous coronary intervention. Trop. J. Pharm. Res. 2022, 21, 1045–1053. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Liu, J.; Zhu, H.; Liu, F.; Liu, Z.H.; Peng, C.; Xiong, L. New amides from the roots of Anisodus tanguticus. Biochem. Syst. Ecol. 2020, 91, 104082. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Zhou, Q.M.; Zhu, H.; Zhou, F.; Meng, C.W.; Shu, H.Z.; Liu, Z.H.; Peng, C.; Xiong, L. Anisotanols A–D, four norsesquiterpenoids with an unprecedented sesquiterpenoid skeleton from Anisodus anguinus. Chin. J. Chem. 2021, 39, 3375–3380. [Google Scholar] [CrossRef]
- Singh, A.; Fatima, K.; Singh, A.; Behl, A.; Mintoo, M.J.; Hasanain, M.; Ashraf, R.; Luqman, S.; Shanker, K.; Mondhe, D.M.; et al. Anticancer activity and toxicity profiles of 2-benzylidene indanone lead molecule. Eur. J. Pharm. Sci. 2015, 76, 57–67. [Google Scholar] [CrossRef]
- Almansour, A.I.; Ali, S.; Ali, M.A.; Ismail, R.; Choon, T.S.; Sellappan, V.; Elumalai, K.K.; Pandian, S. A regio- and stereoselective 1,3-dipolar cycloaddition for the synthesis of new-fangled dispiropyrrolothiazoles as antimycobacterial agents. Bioorg. Med. Chem. Lett. 2012, 22, 7418–7421. [Google Scholar] [CrossRef] [PubMed]
- Caputto, M.E.; Ciccarelli, A.; Frank, F.; Moglioni, A.G.; Moltrasio, G.Y.; Vega, D.; Lombardo, E.; Finkielsztein, L.M. Synthesis and biological evaluation of some novel 1-indanone thiazolylhydrazone derivatives as anti-Trypanosoma cruzi agents. Eur. J. Med. Chem. 2012, 55, 155–163. [Google Scholar] [CrossRef]
- Mor, S.; Pahal, P.; Narasimhan, B. Synthesis, characterization, antimicrobial activities and QSAR studies of some 10a-phenylbenzo[b]indeno[1,2-e][1,4]thiazin-11(10aH)-ones. Eur. J. Med. Chem. 2012, 53, 176–189. [Google Scholar] [CrossRef]
- Huang, H.C.; Chamberlain, T.S.; Selbert, K.; Koboldt, C.M.; Isakson, P.C.; Reitz, D.B. Diaryl indenes and benzofurans: Novel classes of potent and selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 1995, 5, 2377–2380. [Google Scholar] [CrossRef]
- Singh, P.; Prasher, P.; Dhillon, P.; Bhatti, R. Indole based peptidomimetics as anti-inflammatory and anti-hyperalgesic agents: Dual inhibition of 5-LOX and COX-2 enzymes. Eur. J. Med. Chem. 2015, 97, 104–123. [Google Scholar] [CrossRef]
- Cacabelos, R. Pharmacogenetic considerations when prescribing cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert Opin. Drug Metab. Toxicol. 2020, 16, 673–701. [Google Scholar] [CrossRef]
- Shin-Ya, K.; Furihata, K.; Teshima, Y.; Hayakawa, Y.; Seto, H. Structures of stealthins A and B, new free radical scavengers of microbial origin. Tetrahedron Lett. 1992, 33, 7025–7028. [Google Scholar] [CrossRef]
- Ueno, K.; Furumoto, T.; Umeda, S.; Mizutani, M.; Takikawa, H.; Batchvarova, R.; Sugimoto, Y. Heliolactone, a non-sesquiterpene lactone germination stimulant for root parasitic weeds from sunflower. Phytochemistry 2014, 108, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Prasher, P.; Sharma, M. Medicinal chemistry of indane and its analogues: A mini review. ChemistrySelect 2021, 6, 2658–2677. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Veltri, L. Recent advances in the synthesis of indanes and indenes. Chemistry 2016, 22, 5056–5094. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regener. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Ryan, L. Improving public health?: The role of antioxidant-rich fruit and vegetable beverages. Food Res. Int. 2011, 44, 3135–3148. [Google Scholar] [CrossRef]
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.N. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef]
- Helberg, J.; Pratt, D.A. Autoxidation vs. antioxidants—The fight for forever. Chem. Soc. Rev. 2021, 50, 7343–7358. [Google Scholar] [CrossRef]
- Evans, K.O.; Harry-O’kuru, R.E. Antioxidation behavior of milkweed oil 4-hydroxy-3-methoxycinnamate esters in phospholipid bilayers. J. Am. Oil Chem. Soc. 2013, 90, 1719–1727. [Google Scholar] [CrossRef]
- Agnihotri, V.K.; Elsohly, H.N.; Khan, S.I.; Smillie, T.J.; Khan, I.A.; Walker, L.A. Antioxidant constituents of Nymphaea caerulea flowers. Phytochemistry 2008, 69, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Yang, Z.D.; Ren, J.; Xue, P.H.; Yang, M.J. Screening of total alkaloids from Tibetan medicine for acetylcholinesterase inhibitory activity. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 194–196. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres Jr., V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Osawa, E. Corner flapping: A simple and fast algorithm for exhaustive generation of ring conformations. J. Am. Chem. Soc. 1989, 111, 8950–8951. [Google Scholar] [CrossRef]
- Goto, H.; Osawa, E. An efficient algorithm for searching low-energy conformers of cyclic and acyclic molecules. J. Chem. Soc. Perkin Trans. 1993, 2, 187–198. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Liu, Y.; Liu, F.; Qiao, M.M.; Guo, L.; Chen, M.H.; Peng, C.; Xiong, L. Curcumanes A and B, two bicyclic sesquiterpenoids with significant vasorelaxant activity from Curcuma longa. Org. Lett. 2019, 21, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. Spec Dis, version 1.7; University of Würzburg: Würzburg, Germany, 2017. [Google Scholar]




| No. | 1 a | 2 a | 3 b | 4 b | ||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | |
| 1 | 2.91 dd (15.0, 9.0) 2.76 dd (15.0, 9.0) | 33.5 | 2.82 dd (15.0, 9.0) 2.78 dd (15.0, 9.0) | 34.1 | ||||
| 2 | 2.65 m | 47.5 | 2.66 m | 47.5 | 3.58 dd (8.4, 0.7) 3.36 d (8.4) | 77.9 | 3.61 dd (9.1, 0.7) 3.37 d (9.1) | 78.1 |
| 3 | 2.82 m | 33.4 | 2.84 overlapped | 32.8 | 80.5 | 80.4 | ||
| 3a | 144.3 | 144.7 | 2.91 m | 55.5 | 2.91 overlapped | 55.4 | ||
| 4 | 120.4 | 120.5 | 2.95 dd (15.4, 9.1) 2.73 dd (15.4, 4.2) | 33.3 | 2.91 overlapped 2.76 dd (20.3, 9.8) | 33.8 | ||
| 4a | 145.1 | 134.7 | ||||||
| 5 | 154.4 | 154.4 | 120.1 | 6.81 d (7.7) | 122.5 | |||
| 6 | 6.59 d (8.4) | 113.6 | 6.59 d (8.4) | 113.6 | 156.5 | 6.74 d (7.7) | 116.6 | |
| 7 | 6.77 d (8.4) | 122.2 | 6.78 d (8.4) | 122.2 | 6.70 d (8.4) | 114.7 | 155.0 | |
| 7a | 134.7 | 134.4 | ||||||
| 8 | 6.97 d (8.4) | 123.9 | 122.3 | |||||
| 8a | 134.1 | 143.3 | ||||||
| 8b | 5.47 d (6.3) | 88.1 | 5.61 d (6.3) | 87.2 | ||||
| 1′ | 3.48 dd (10.8, 6.0) 3.45 dd (10.8, 6.0) | 69.8 | 3.49 dd (10.2, 6.0) 3.46 dd (10.2, 6.0) | 69.9 | 1.32 s | 20.9 | 1.30 s | 20.7 |
| 2′ | 73.7 | 73.7 | 2.09 s | 12.1 | 2.22 s | 12.0 | ||
| 3′ | 1.19 s | 22.9 | 1.17 s | 22.8 | ||||
| 4′ | 2.09 s | 12.4 | 2.09 s | 12.4 | ||||
| OH-3 | 3.83 s | 3.87 s | ||||||
| OH-5 | 7.77 s | 7.76 s | ||||||
| OH-6 | 8.10 s | |||||||
| OH-7 | 7.92 s | |||||||
| OH-1′ | 3.70 t (6.0) | 3.71 t (6.0) | ||||||
| OH-2′ | 3.28 s | 3.29 s | ||||||
| IC50 for ABTS•+ Scavenging Assay (μM) | IC50 for DPPH•+ Scavenging Assay (μM) | |
|---|---|---|
| 1 | 15.62 ± 1.85 | 68.46 ± 17.34 |
| 2 | 40.92 ± 7.02 | >100 |
| 3 | 43.93 ± 9.35 | >100 |
| 4 | 32.38 ± 6.29 | >100 |
| VC | 22.54 ± 5.18 | 10.19 ± 1.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, C.-W.; Zhao, H.-Y.; Zhu, H.; Peng, C.; Zhou, Q.-M.; Xiong, L. Novel Indane Derivatives with Antioxidant Activity from the Roots of Anisodus tanguticus. Molecules 2023, 28, 1493. https://doi.org/10.3390/molecules28031493
Meng C-W, Zhao H-Y, Zhu H, Peng C, Zhou Q-M, Xiong L. Novel Indane Derivatives with Antioxidant Activity from the Roots of Anisodus tanguticus. Molecules. 2023; 28(3):1493. https://doi.org/10.3390/molecules28031493
Chicago/Turabian StyleMeng, Chun-Wang, Hao-Yu Zhao, Huan Zhu, Cheng Peng, Qin-Mei Zhou, and Liang Xiong. 2023. "Novel Indane Derivatives with Antioxidant Activity from the Roots of Anisodus tanguticus" Molecules 28, no. 3: 1493. https://doi.org/10.3390/molecules28031493
APA StyleMeng, C.-W., Zhao, H.-Y., Zhu, H., Peng, C., Zhou, Q.-M., & Xiong, L. (2023). Novel Indane Derivatives with Antioxidant Activity from the Roots of Anisodus tanguticus. Molecules, 28(3), 1493. https://doi.org/10.3390/molecules28031493