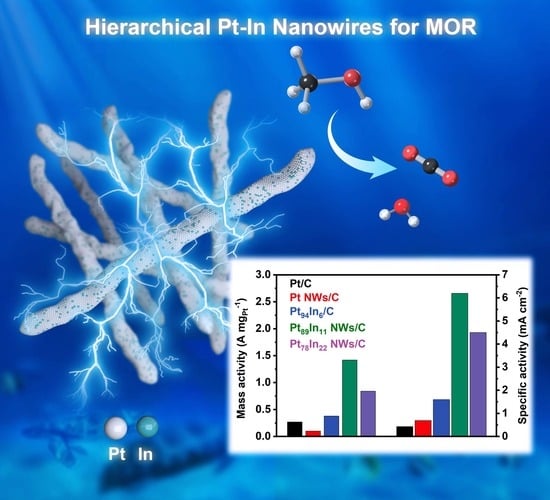
Hierarchical Pt-In Nanowires for Efficient Methanol Oxidation Electrocatalysis
Abstract
:1. Introduction
2. Result and Discussion
3. Experimental Section
3.1. Chemicals
3.2. Characterization
3.3. Preparation of Hierarchical Pt-In NWs
3.4. Loading the Obtained NWs onto Carbon
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liang, J.; Ma, F.; Hwang, S.; Wang, X.; Sokolowski, J.; Li, Q.; Wu, G.; Su, D. Atomic Arrangement Engineering of Metallic Nanocrystals for Energy-Conversion Electrocatalysis. Joule 2019, 3, 956–991. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic Nanocrystals: Syntheses, Properties, and Applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energy Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- Tian, X.L.; Wang, L.; Deng, P.; Chen, Y.; Xia, B.Y. Research advances in unsupported Pt-based catalysts for electrochemical methanol oxidation. J. Energy Chem. 2017, 26, 1067–1076. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Tong, Y.; Yan, X.; Liang, J.; Dou, S.X. Metal-Based Electrocatalysts for Methanol Electro-Oxidation: Progress, Opportunities, and Challenges. Small 2019, 17, 1904126. [Google Scholar] [CrossRef]
- Valdés-López, V.F.; Mason, T.; Shearing, P.R.; Brett, D.J.L. Carbon monoxide poisoning and mitigation strategies for polymer electrolyte membrane fuel cells—A review. Prog. Energy Combust. 2020, 79, 100842. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Hong, Y.; Kim, B.; Choi, S.-I.; Lee, K. Pt-Cu based nanocrystals as promising catalysts for various electrocatalytic reactions. J. Mater. Chem. A 2019, 7, 17183–17203. [Google Scholar] [CrossRef]
- Kong, F.; Liu, X.; Song, Y.; Qian, Z.; Li, J.; Zhang, L.; Yin, G.; Wang, J.; Su, D.; Sun, X. Selectively Coupling Ru Single Atoms to PtNi Concavities for High-Performance Methanol Oxidation via d-Band Center Regulation. Angew. Chem. Int. Ed. 2022, 61, e202207524. [Google Scholar] [CrossRef]
- Yang, J.; Hübner, R.; Zhang, J.; Wan, H.; Zheng, Y.; Wang, H.; Qi, H.; He, L.; Li, Y.; Dubale, A.A.; et al. A Robust PtNi Nanoframe/N-Doped Graphene Aerogel Electrocatalyst with Both High Activity and Stability. Angew. Chem. Int. Ed. 2021, 60, 9590–9597. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Zhang, L.; Yan, S.; Sharma, A.; Zhao, B.; Kumbhar, A.; Zhou, G.; Fang, J. Synthesis of Core@Shell Cu-Ni@Pt-Cu Nano-Octahedra and Their Improved MOR Activity. Angew. Chem. Int. Ed. 2021, 60, 7675–7680. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, D.; Zhang, Q.; Sun, Y.; Zhang, S.; Du, F.; Jin, X. Synthesis of PtCu-based nanocatalysts: Fundamentals and emerging challenges in energy conversion. J. Energy Chem. 2022, 64, 583–606. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Xu, S.; Xie, Y.; Ye, Y.; Zou, X.; Lin, S. Synthesis of Pt-Ni (trace)/GNs composite and its bi-functional electrocatalytic properties for MOR and ORR. J. Colloid Interface Sci. 2019, 554, 640–649. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lim, S.C.; Kuo, C.H.; Tuan, H.Y. Sub-1 nm PtSn ultrathin sheet as an extraordinary electrocatalyst for methanol and ethanol oxidation reactions. J. Colloid Interface Sci. 2019, 545, 54–62. [Google Scholar] [CrossRef]
- Chen, W.; Lei, Z.; Zeng, T.; Wang, L.; Cheng, N.; Tan, Y.; Mu, S. Structurally ordered PtSn intermetallic nanoparticles supported on ATO for efficient methanol oxidation reaction. Nanoscale 2019, 11, 19895–19902. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Deng, Q.; Bao, J.; Sheng, X.; Huang, Y.; Zhang, Y.; Zhou, Y. Controllable synthesis of efficient Ru-doped PtSn alloy nanoplate electrocatalysts for methanol oxidation reaction. Int. J. Hydrogen Energy 2022, 47, 32158–32166. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, T.; Yuan, M.; Li, Z.; Wang, W.; Bai, Y.; Liu, Z.; Li, S.; Zhang, G. Trimetallic synergy in dendritic intermetallic PtSnBi nanoalloys for promoting electrocatalytic alcohol oxidation. J. Colloid Interface Sci. 2021, 602, 504–512. [Google Scholar] [CrossRef]
- You, H.; Gao, F.; Wang, C.; Song, T.; Li, J.; Wang, X.; Zhang, Y.; Du, Y. Morphology Control Endows Palladium-Indium Nanocatalysts with High Catalytic Performance for Alcohol Oxidation. Chem Electro Chem 2021, 8, 3637–3642. [Google Scholar] [CrossRef]
- Zerdoumi, R.; Armbrüster, M. Insights into the Electronic Effects in Methanol Electro-Oxidation by Ternary In1–xSnxPd2 Intermetallic Compounds. ACS Appl. Energy Mater. 2021, 4, 11279–11289. [Google Scholar] [CrossRef]
- Wang, W.; Lv, F.; Lei, B.; Wan, S.; Luo, M.; Guo, S. Tuning Nanowires and Nanotubes for Efficient Fuel-Cell Electrocatalysis. Adv. Mater. 2016, 28, 10117–10141. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Zhang, W.; Luo, M.; Tao, L.; Sun, Y.; Xia, Z.; Chao, Y.; Yin, K.; Zhang, Q.; et al. Sub-Monolayer YO(x)/MoO(x) on Ultrathin Pt Nanowires Boosts Alcohol Oxidation Electrocatalysis. Adv. Mater. 2021, 33, e2103762. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Sun, L.; Zhao, X.; Huang, J.; Han, C.; Yang, X. One-pot synthesis of interconnected Pt95Co5 nanowires with enhanced electrocatalytic performance for methanol oxidation reaction. Nano Res. 2018, 11, 2562–2572. [Google Scholar] [CrossRef]
- Poerwoprajitno, A.R.; Gloag, L.; Cheong, S.; Gooding, J.J.; Tilley, R.D. Synthesis of low- and high-index faceted metal (Pt, Pd, Ru, Ir, Rh) nanoparticles for improved activity and stability in electrocatalysis. Nanoscale 2019, 11, 18995–19011. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Yu, Y.; Yu, R.; Lai, J.; Jung, J.C.-Y.; Zhang, S.; Zhao, Y.; Zhang, J.; Xia, Z. PtIrM (M = Ni, Co) jagged nanowires for efficient methanol oxidation electrocatalysis. J. Colloid Interface Sci. 2022, 625, 493–501. [Google Scholar] [CrossRef]
- Motin, A.M.; Haunold, T.; Bukhtiyarov, A.V.; Bera, A.; Rameshan, C.; Rupprechter, G. Surface science approach to Pt/carbon model catalysts: XPS, STM and microreactor studies. Appl. Surf. Sci. 2018, 440, 680–687. [Google Scholar] [CrossRef]
- Bergman, S.L.; Granestrand, J.; Tang, Y.; París, R.S.; Nilsson, M.; Tao, F.F.; Tang, C.; Pennycook, S.J.; Pettersson, L.J.; Bernasek, S.L. In-situ characterization by Near-Ambient Pressure XPS of the catalytically active phase of Pt/Al2O3 during NO and CO oxidation. Appl. Catal. B-Environ. 2018, 220, 506–511. [Google Scholar] [CrossRef]
- Scofield, M.E.; Koenigsmann, C.; Wang, L.; Liu, H.; Wong, S.S. Tailoring the composition of ultrathin, ternary alloy PtRuFe nanowires for the methanol oxidation reaction and formic acid oxidation reaction. Energy Environ. Sci. 2015, 8, 350–363. [Google Scholar] [CrossRef]
- Chhetri, M.; Rana, M.; Loukya, B.; Patil, P.K.; Datta, R.; Gautam, U.K. Mechanochemical Synthesis of Free-Standing Platinum Nanosheets and Their Electrocatalytic Properties. Adv. Mater. 2015, 27, 4430–4437. [Google Scholar] [CrossRef]
- Bu, L.; Zhang, N.; Guo, S.; Zhang, X.; Li, J.; Yao, J.; Wu, T.; Lu, G.; Ma, J.-Y.; Su, D.; et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Electrocatalysis 2016, 354, 1410–1414. [Google Scholar] [CrossRef]
- Zhang, N.; Bu, L.; Guo, S.; Guo, J.; Huang, X. Screw Thread-Like Platinum-Copper Nanowires Bounded with High-Index Facets for Efficient Electrocatalysis. Nano Lett. 2016, 16, 5037–5043. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Wang, Q.; Han, Y.; Fang, Y.; Dong, S. Shape-Control of Pt-Ru Nanocrystals: Tuning Surface Structure for Enhanced Electrocatalytic Methanol Oxidation. J. Am. Chem. Soc. 2018, 140, 1142–1147. [Google Scholar] [CrossRef]
- Lu, S.; Eid, K.; Ge, D.; Guo, J.; Wang, L.; Wang, H.; Gu, H. One-pot synthesis of PtRu nanodendrites as efficient catalysts for methanol oxidation reaction. Nanoscale 2017, 9, 1033–1039. [Google Scholar] [CrossRef]
- Kwon, T.; Jun, M.; Kim, H.Y.; Oh, A.; Park, J.; Baik, H.; Joo, S.H.; Lee, K. Vertex-Reinforced PtCuCo Ternary Nanoframes as Efficient and Stable Electrocatalysts for the Oxygen Reduction Reaction and the Methanol Oxidation Reaction. Adv. Funct. Mater. 2018, 28, 1706440. [Google Scholar] [CrossRef]
- Xue, S.; Deng, W.; Yang, F.; Yang, J.; Amiinu, I.S.; He, D.; Tang, H.; Mu, S. Hexapod PtRuCu Nanocrystalline Alloy for Highly Efficient and Stable Methanol Oxidation. ACS Catal. 2018, 8, 7578–7584. [Google Scholar] [CrossRef]
- Shang, C.; Guo, Y.; Wang, E. Facile fabrication of PdRuPt nanowire networks with tunable compositions as efficient methanol electrooxidation catalysts. Nano Res. 2018, 11, 4348–4355. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Zhang, X.; Ye, J.; Xue, F.; Zhen, C.; Liao, X.; Li, H.; Li, P.; Liu, M.; et al. Quatermetallic Pt-based ultrathin nanowires intensified by Rh enable highly active and robust electrocatalysts for methanol oxidation. Nano Energy 2020, 71, 104623. [Google Scholar] [CrossRef]
- Wang, K.; Huang, D.; Guan, Y.; Liu, F.; He, J.; Ding, Y. Fine-tuning the electronic structure of dealloyed PtCu nanowires for efficient methanol oxidation reaction. ACS Catal. 2021, 11, 14428–14438. [Google Scholar] [CrossRef]
- Chen, H.-S.; Benedetti, T.M.; Lian, J.; Cheong, S.; O’Mara, P.B.; Sulaiman, K.O.; Kelly, C.H.W.; Scott, R.W.J.; Gooding, J.J.; Tilley, R.D. Role of the Secondary Metal in Ordered and Disordered Pt-M Intermetallic Nanoparticles: An Example of Pt3Sn Nanocubes for the Electrocatalytic Methanol Oxidation. ACS Catal. 2021, 11, 2235–2243. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Zou, L.; Song, W. Hierarchical Pt-In Nanowires for Efficient Methanol Oxidation Electrocatalysis. Molecules 2023, 28, 1502. https://doi.org/10.3390/molecules28031502
Lu Z, Zou L, Song W. Hierarchical Pt-In Nanowires for Efficient Methanol Oxidation Electrocatalysis. Molecules. 2023; 28(3):1502. https://doi.org/10.3390/molecules28031502
Chicago/Turabian StyleLu, Zhao, Lu Zou, and Wulin Song. 2023. "Hierarchical Pt-In Nanowires for Efficient Methanol Oxidation Electrocatalysis" Molecules 28, no. 3: 1502. https://doi.org/10.3390/molecules28031502
APA StyleLu, Z., Zou, L., & Song, W. (2023). Hierarchical Pt-In Nanowires for Efficient Methanol Oxidation Electrocatalysis. Molecules, 28(3), 1502. https://doi.org/10.3390/molecules28031502