Synthesis and Properties of Norphthalocyanines Functionalized with a Tetrathiacrown Ether–Tetrathiafulvalene Substituent
Abstract
:1. Introduction
2. Results and Discussion
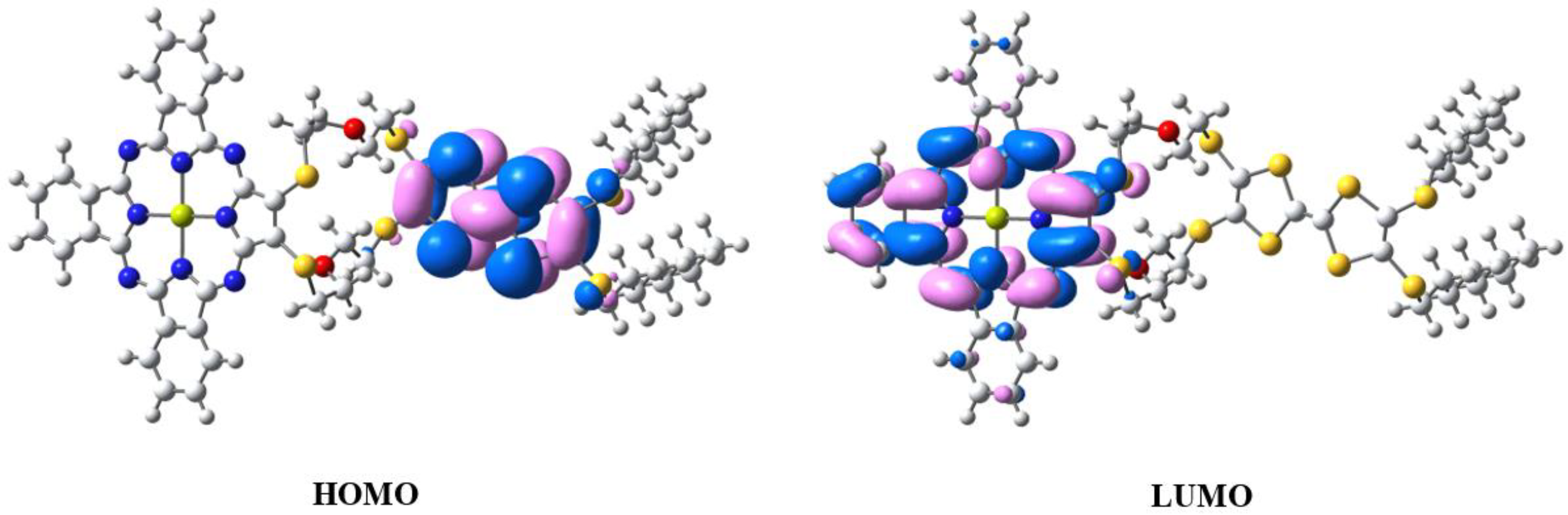
2.1. Synthesis and Quantum Chemical Calculations
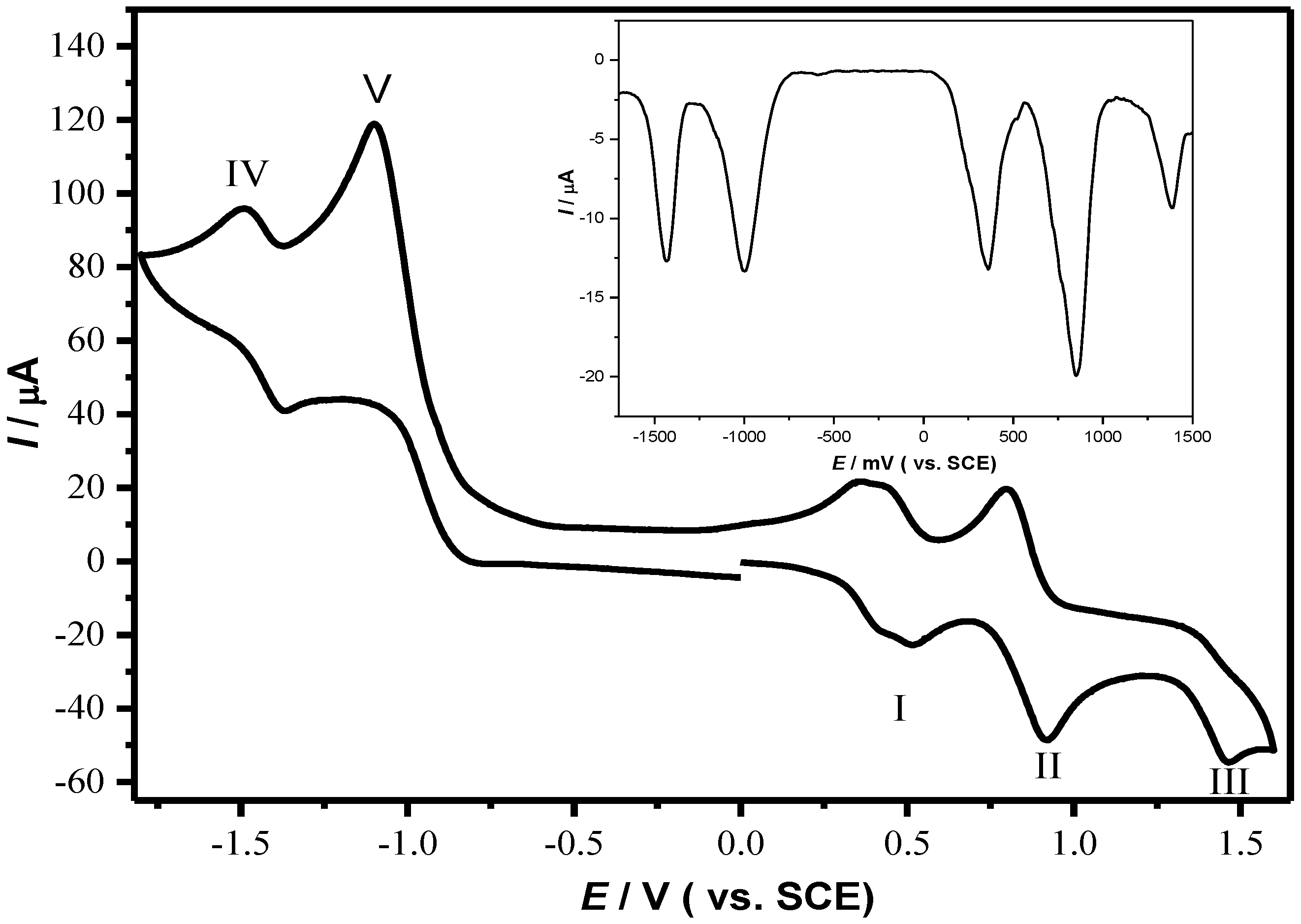
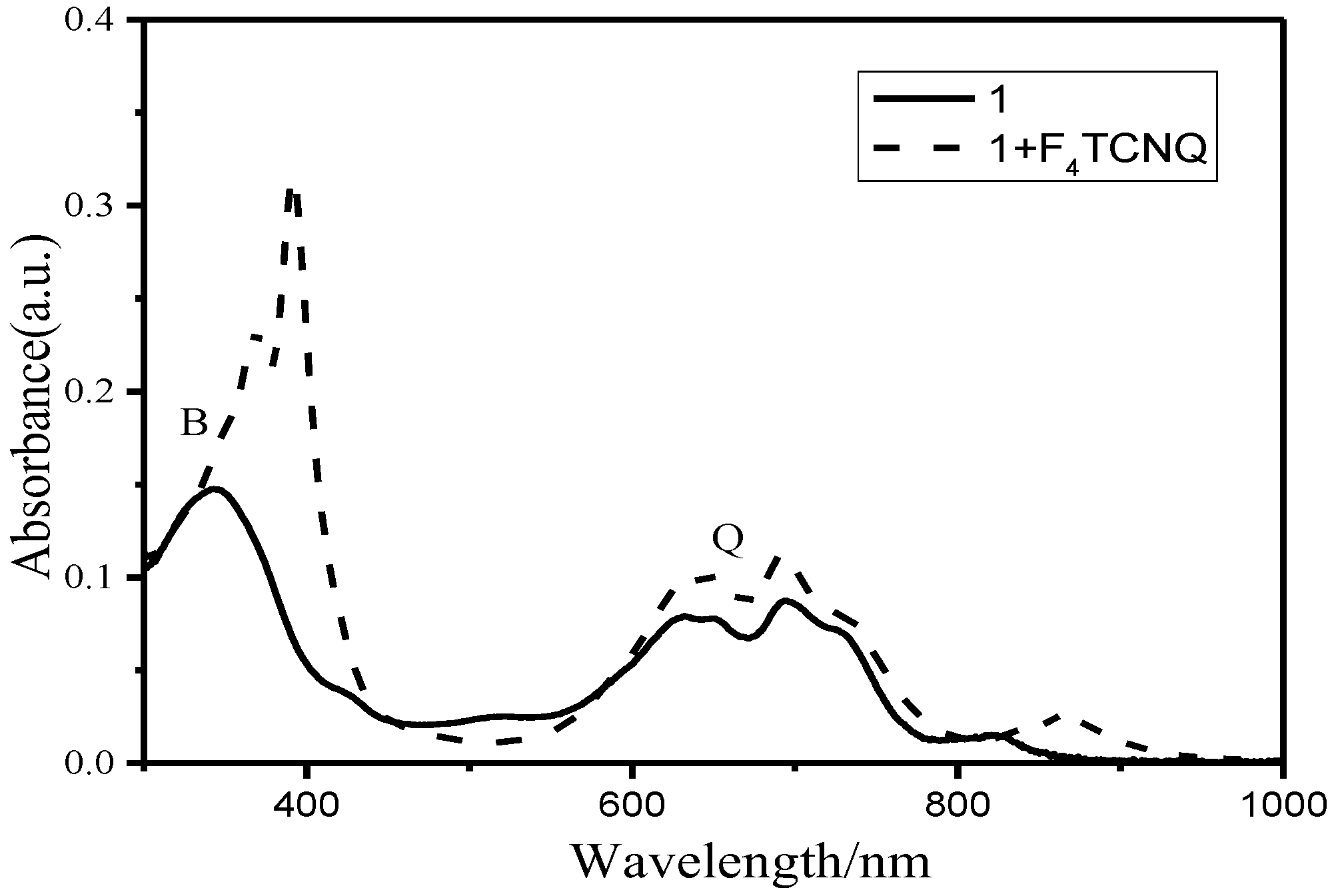
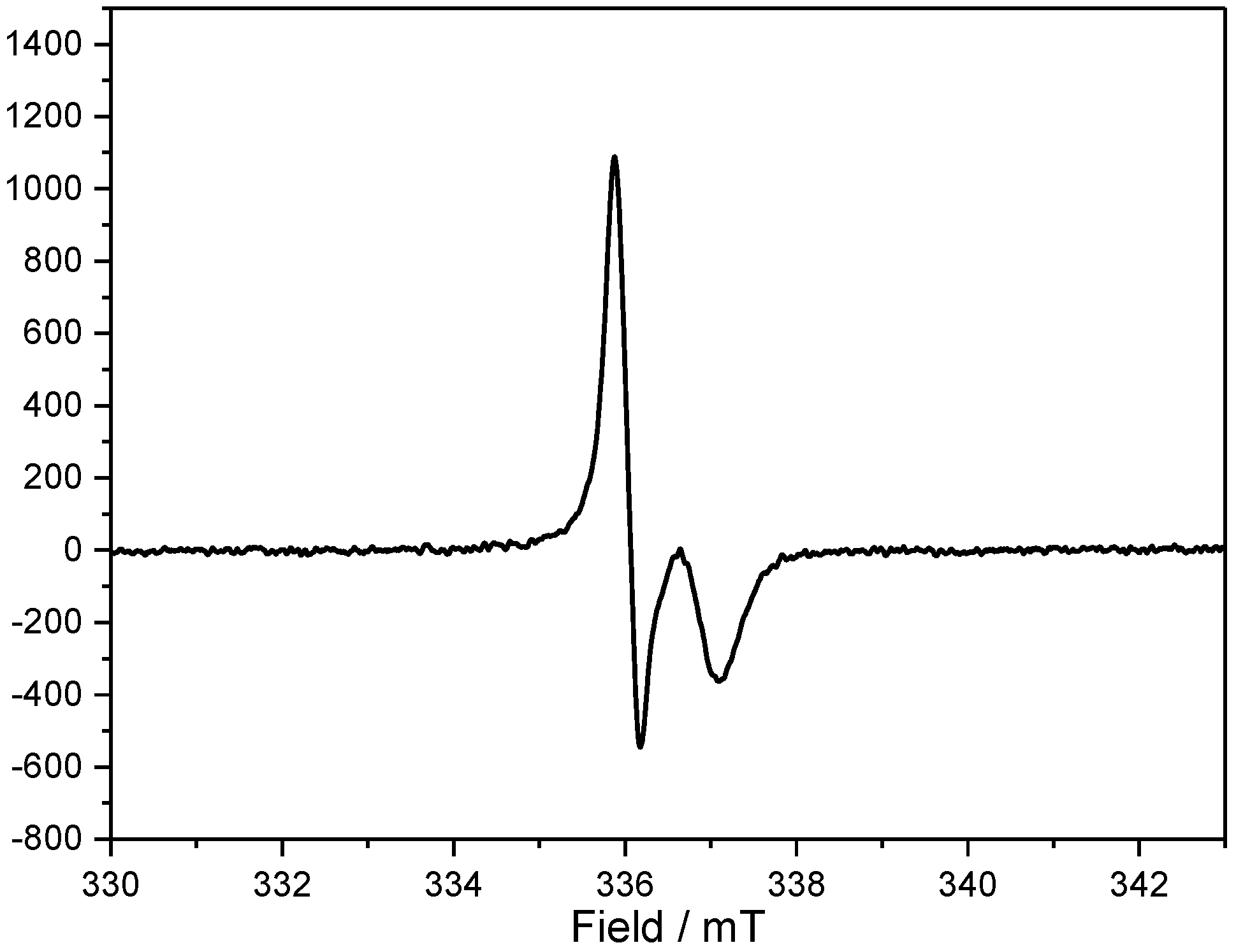
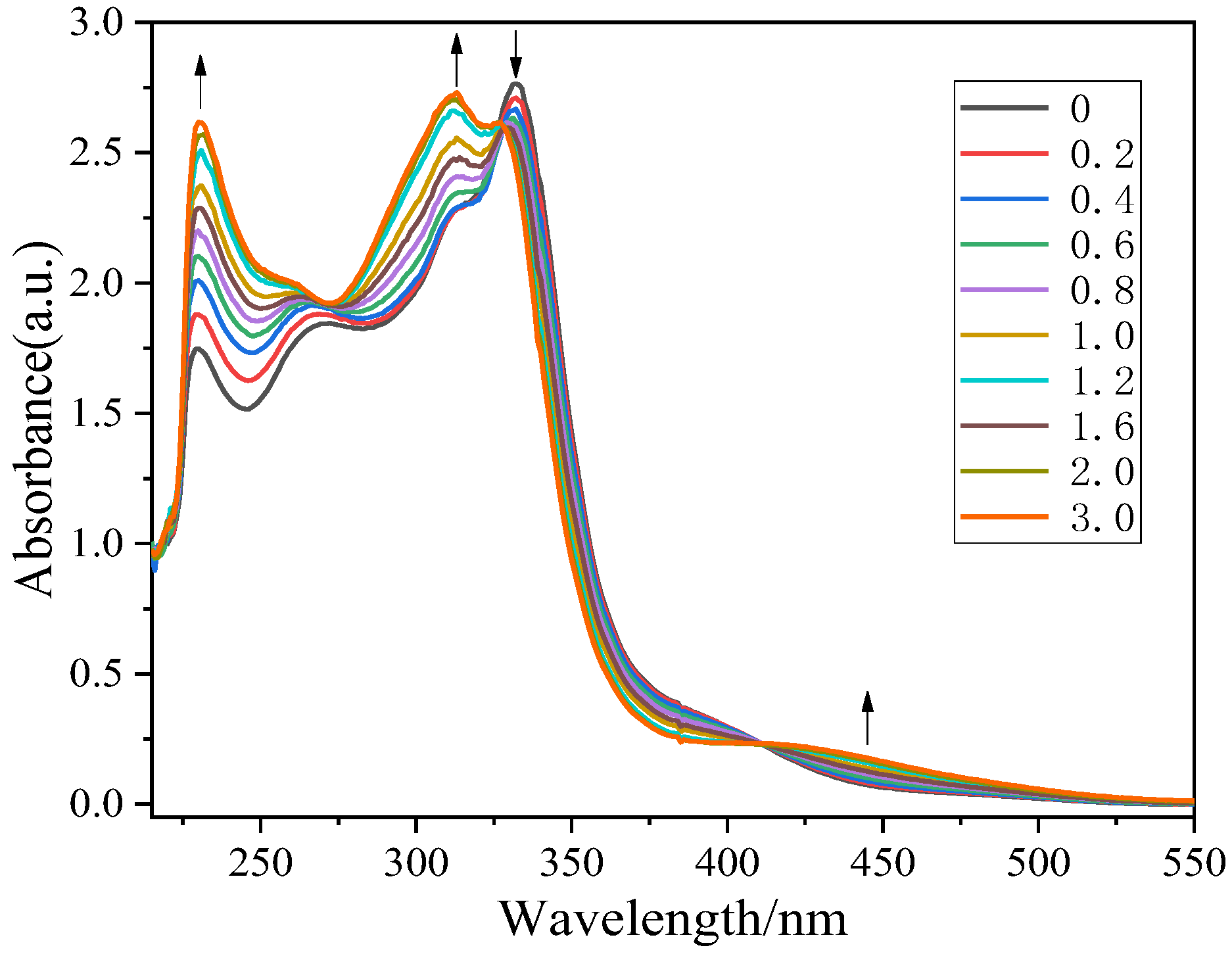
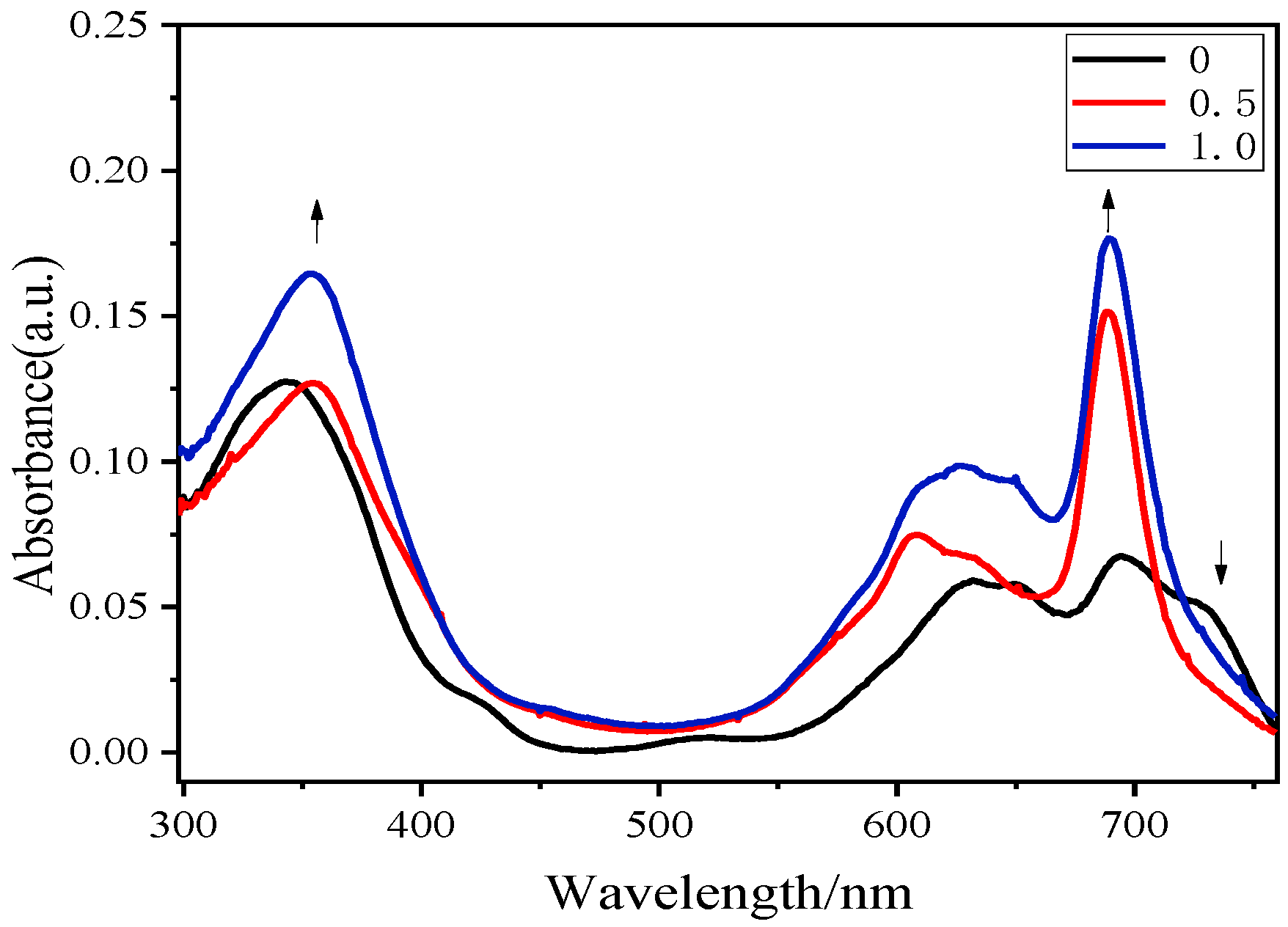
2.2. Electrochemical and Photophysical Properties
3. Experimental Section
Instruments and Chemicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Keller, K. Science and Technology of Photography; Wiley-VCH Publisher: New York, NY, USA, 1993. [Google Scholar]
- Wang, S.; Yan, X.; Cheng, Z.; Zhang, H.; Liu, Y.; Wang, Y. Highly Efficient Near-Infrared Delayed Fluorescence Organic Light Emitting Diodes Using a Phenanthrene-Based Charge-Transfer Compound. Angew. Chem. Int. Ed. 2015, 54, 13068–13072. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.Y.; Zhang, J.Q.; Bai, J.Y.; Zhang, L.G.; Wan, Z.Q.; Yao, X.J. Synthesis, physical properties and self-assembly of conjugated donoreacceptor system based on tetrathiafulvalene and functionalized with binding sites. Dyes Pigments 2012, 94, 403–409. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Liu, H.; Tian, M.Z.; Li, Y. Self-assembly of intramolecular charge-transfer compounds into functional molecular systems. Acc. Chem. Res. 2014, 47, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Huang, Q.; Zhong, Y.; Li, Z.; Li, H.; Lowry, M.; Escobedo, J.O.; Strongin, R.M. A dual emission fluorescent probe enables simultaneous detection of glutathione and cysteine/homocysteine. Chem. Sci. 2014, 5, 2177–2183. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent chemodosimeters for bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- Sly, J.; Kasak, P.; Gomar-Nadal, E.; Rovira, C.; Gorriz, L.; Thordarson, P.; Amabilino, D.B.; Rowan, A.E.; Nolte, R.J. Chiral molecular tapes from novel tetra(thiafulvalene-crown-ether)-substituted phthalocyanine building blocks. Chem. Commun. 2005, 10, 1255–1257. [Google Scholar] [CrossRef]
- Wang, R.; Liu, W.; Chen, Y.; Zuo, J.L.; You, X.Z. The synthesis and electrochemical properties of a new tetra-(crown-etherthiafulvalene)-annulated phthalocyanine derivative. Dyes Pigments 2009, 81, 40–44. [Google Scholar] [CrossRef]
- Wang, C.S.; Bryce, M.R.; Batsanov, A.S.; Howard, J.A.K. Synthesis of Pyrazinoporphyrazine Derivatives Functionalised with Tetrathiafulvalene (TTF) Units: X-Ray Crystal Structures of Two Related ttf Cyclophanes and Two Bis(1,3-Dithiole-2-Thione) Intermediates. Chem. Eur. J. 1997, 3, 1679–1690. [Google Scholar] [CrossRef]
- Loosli, C.; Jia, C.; Liu, S.X.; Haas, M.; Dias, M.; Levillain, E.; Neels, A.; Labat, G.; Hauser, A.; Decurtins, S. Synthesis and electrochemical and photophysical studies of tetrathiafulvalene-annulated phthalocyanines. J. Org. Chem. 2005, 70, 4988–4992. [Google Scholar] [CrossRef] [Green Version]
- Jana, A.; Ishida, M.; Park, J.S.; Bahring, S.; Jeppesen, J.O.; Sessler, J.L. Tetrathiafulvalene- (TTF-) Derived Oligopyrrolic Macrocycles. Chem. Rev. 2017, 117, 2641–2710. [Google Scholar] [CrossRef]
- Guo, J.; Xia, Y.; Li, D.; Hou, R. Synthesis and electron-donating properties of novel norphthalocyanines containing thiacrown ether-linked tetrathiafulvalene moieties. Tetrahedron Lett. 2016, 57, 570–573. [Google Scholar] [CrossRef] [Green Version]
- Hou, R.; Jiang, C.; Chen, T.; Jin, L.Y.; Yin, B. The Norphthalocyanines Bearing Two TTF Units: Synthesis, Photophysical and Electrochemical Properties. Heterocycles 2011, 83, 1859–1866. [Google Scholar]
- Hou, R.; Li, B.; Zhong, K.; Li, H.; Jin, L.Y.; Yin, B. Tetrakis(tetrathiafulvalene-tetrathiacrown ether)porphyrazine Triads: Synthesis, Photophysical, and Electrochemical Properties. Eur. J. Org. Chem. 2012, 2012, 1138–1146. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Liu, C.G.; Guan, W.; Song, P.; Yan, L.K.; Su, Z.M. Redox-switchable second-order nonlinear optical responses of push-pull monotetrathiafulvalene-metalloporphyrins. Inorg. Chem. 2009, 48, 6548–6554. [Google Scholar] [CrossRef]
- El-Khouly, M.E.; Jaggi, M.; Schmid, B.; Blum, C.; Liu, S.X.; Decurtins, S.; Ohkubo, K.; Fukuzumi, S. Annulation of Tetrathiafulvalene to the Bay Region of Perylenediimide: Fast Electron-Transfer Processes in Polar and Nonpolar Solvents. J. Phys. Chem. C 2011, 115, 8325–8334. [Google Scholar] [CrossRef]
- Leng, F.; Wang, X.; Jin, L.; Yin, B. The synthesis and properties of unsymmetrical porphyrazines annulated with a tetrathiafulvalene bearing two tetraethylene glycol units. Dyes Pigments 2010, 87, 89–94. [Google Scholar] [CrossRef]
- Farren, C.; Christensen, C.A.; FitzGerald, S.; Bryce, M.R.; Beeby, A. Synthesis of Novel Phthalocyanine-Tetrathiafulvalene Hybrids; Intramolecular Fluorescence Quenching Related to Molecular Geometry. J. Org. Chem. 2002, 67, 9130–9139. [Google Scholar] [CrossRef]
- Hou, R.; Wang, L.; Wei, F.; Xia, Y.; Li, D. Highly conjugated donor-acceptor dyad based on monotetrathiafulvalene covalently attached to a magnesium norphthalocyanine unit. J. Mol. Struct. 2020, 1208, 127890. [Google Scholar] [CrossRef]
- Yasuda, T.; Shimizu, T.; Liu, F.; Ungar, G.; Kato, T. Electro-functional octupolar pi-conjugated columnar liquid crystals. J. Am. Chem. Soc. 2011, 133, 13437–13444. [Google Scholar] [CrossRef]
- Wudl, F.; Smith, G.M.; Hufnagel, E.J. Bis-1,3-dithiolium chloride: An unusually stable organic radical cation. J. Chem. Soc. D 1970, 21, 1453–1454. [Google Scholar] [CrossRef]
- Akutagawa, T.; Kakiuchi, K.; Hasegawa, T.; Nakamura, T.; Christensen, C.A.; Becher, J. Langmuir-Blodgett Films of Amphiphilic Bis(tetrathiafulvalene) Macrocycles with Four Alkyl Chains. Langmuir 2004, 20, 4187–4195. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.J.; Sibert, J.W.; Barrett, A.G.M.; Hoffman, B.M. Synthesis and Coordination Chemistry of Unsymmetrical Tetraazaporphyrins Containing Single Oxathia- and Thiacrown Substituents. Tetrahedron 2000, 56, 7371–7377. [Google Scholar] [CrossRef]
- Wang, H.; Xue, L.; Jiang, H. Ratiometric Fluorescent Sensor for Silver Ion and Its Resultant Complex for Iodide Anion in Aqueous Solution. Org. Lett. 2011, 13, 3844–3847. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, R.; Liu, X.; Xia, Y.; Li, D. Synthesis and Properties of Norphthalocyanines Functionalized with a Tetrathiacrown Ether–Tetrathiafulvalene Substituent. Molecules 2023, 28, 916. https://doi.org/10.3390/molecules28030916
Hou R, Liu X, Xia Y, Li D. Synthesis and Properties of Norphthalocyanines Functionalized with a Tetrathiacrown Ether–Tetrathiafulvalene Substituent. Molecules. 2023; 28(3):916. https://doi.org/10.3390/molecules28030916
Chicago/Turabian StyleHou, Ruibin, Xiaoyu Liu, Yan Xia, and Dongfeng Li. 2023. "Synthesis and Properties of Norphthalocyanines Functionalized with a Tetrathiacrown Ether–Tetrathiafulvalene Substituent" Molecules 28, no. 3: 916. https://doi.org/10.3390/molecules28030916
APA StyleHou, R., Liu, X., Xia, Y., & Li, D. (2023). Synthesis and Properties of Norphthalocyanines Functionalized with a Tetrathiacrown Ether–Tetrathiafulvalene Substituent. Molecules, 28(3), 916. https://doi.org/10.3390/molecules28030916





