Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora
Abstract
1. Introduction
2. Results
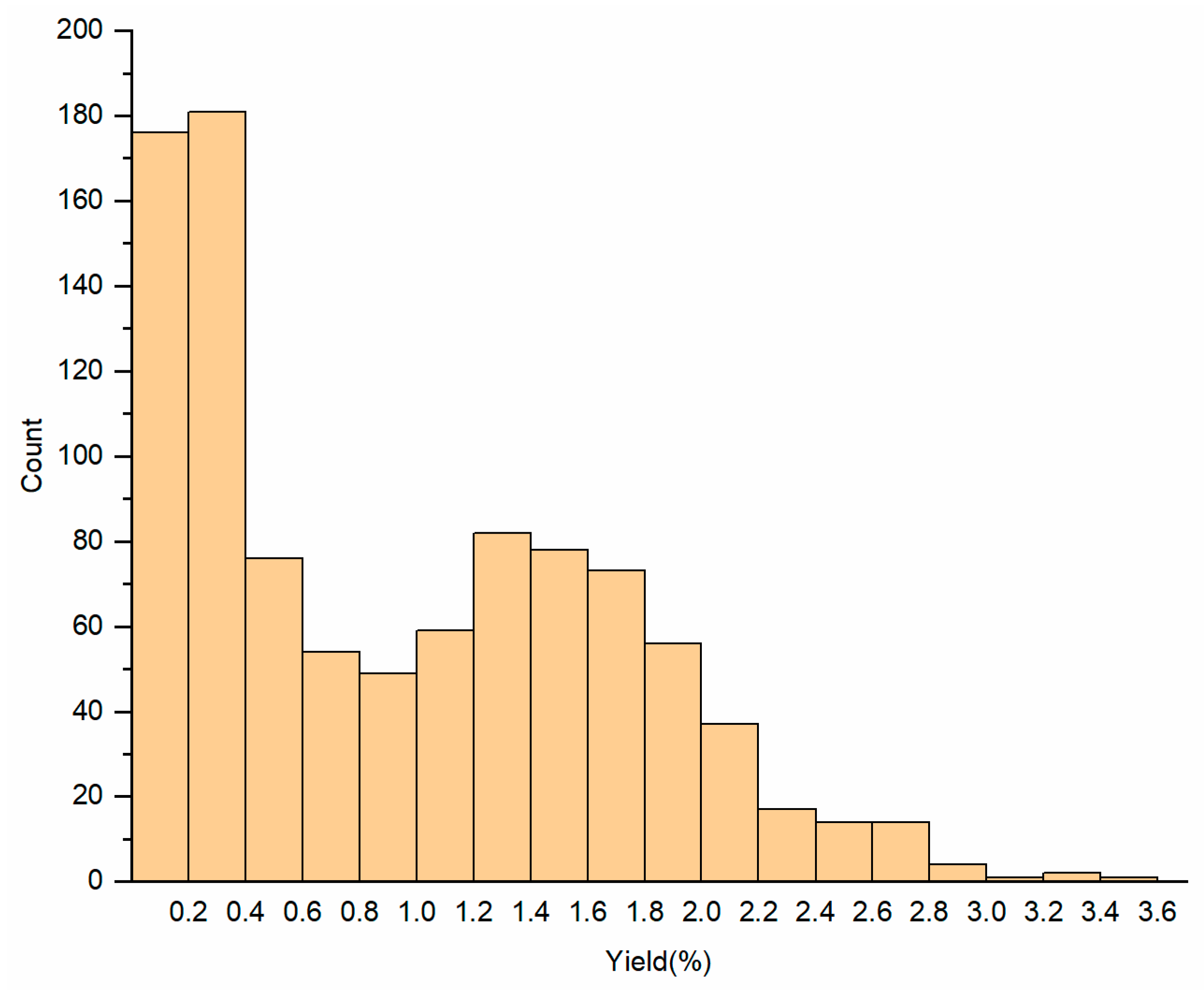
2.1. Variability in Essential Oil Yield
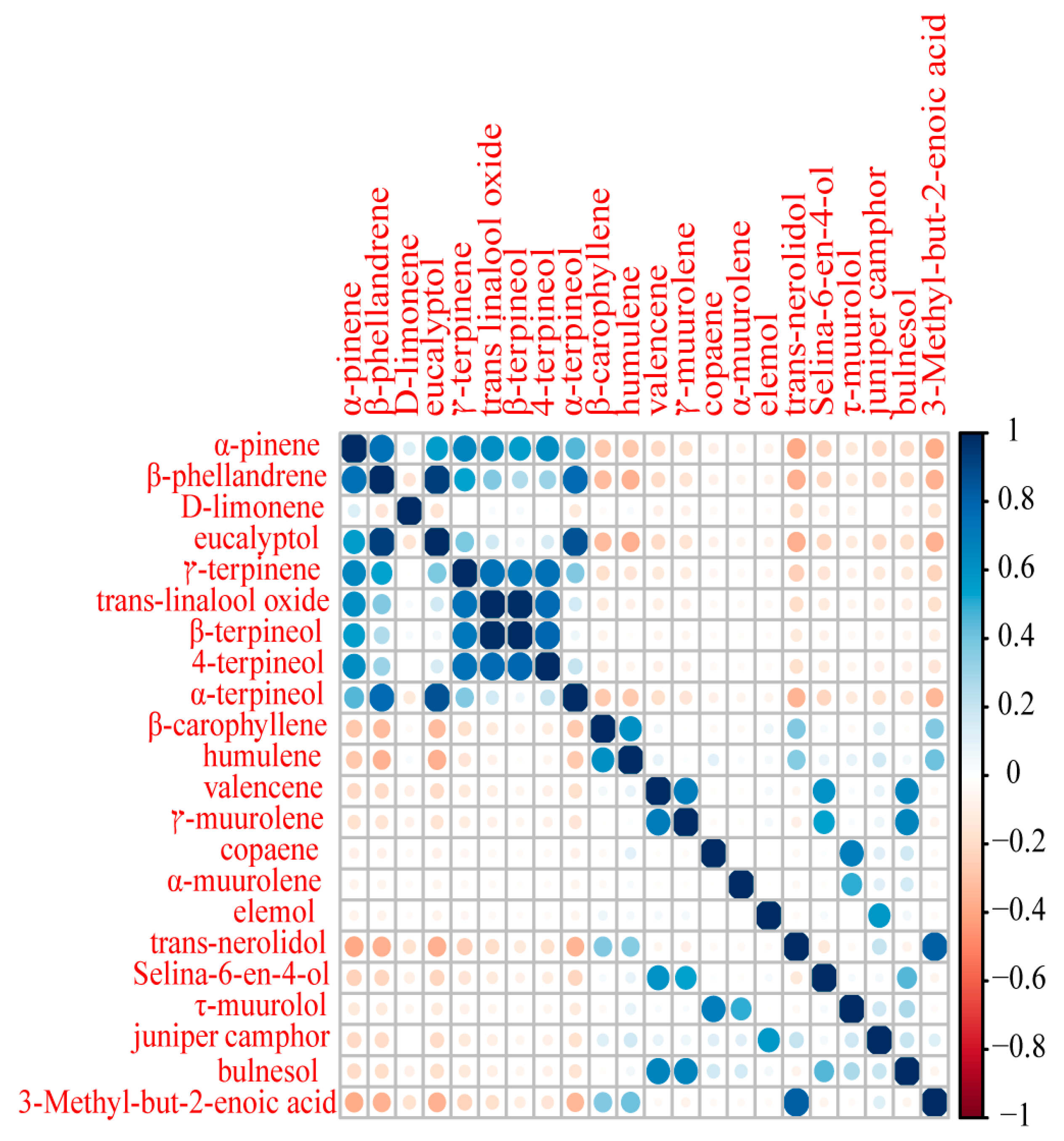
2.2. Essential Oil Profiles
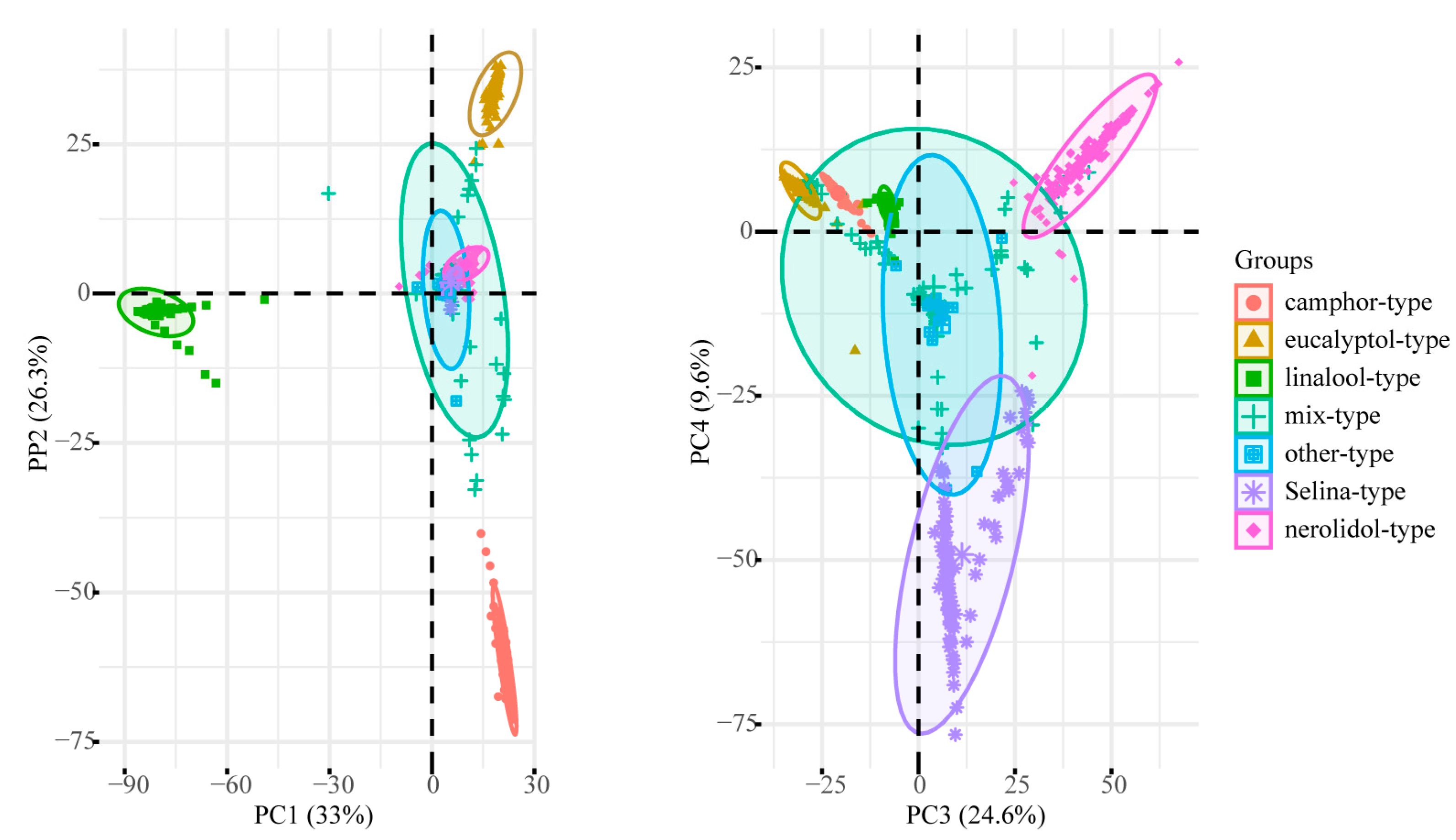
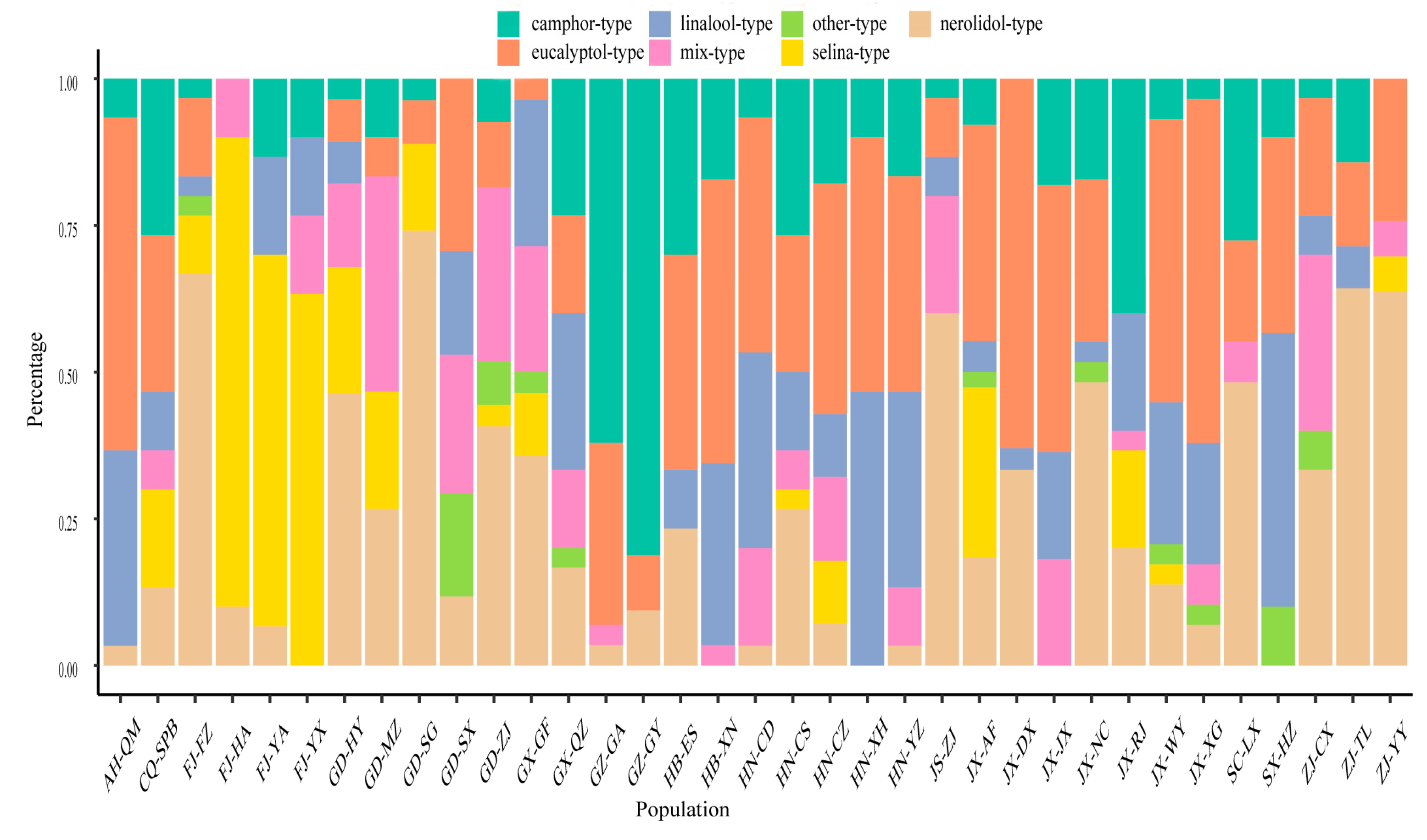
2.3. Chemotype Classification and Distribution
2.4. Exceptional Chemotypes
2.5. Impact of Environmental Factors on Essential Oil Variability
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction of the Essential Oils
4.3. Essential Oil Compound Identification
4.4. Compound Calculation
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ravindran, P.N.; Babu, K.N.; Shylaja, M. Cinnamon and Cassia: The Genus Cinnamomum; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Li, X.; Li, J.; Van der Werff, H. Cinnamomum. In Flora of China; Wu, Z., Raven, P.H., Hong, D., Eds.; Science Press: Beijing, China, 2008; pp. 166–187. [Google Scholar]
- Park, T.-J.; Park, Y.-S.; Lee, T.-G.; Ha, H.; Kim, K.-T. Inhibition of acetylcholine-mediated effects by borneol. Biochem. Pharmacol. 2003, 65, 83–90. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, L.; Lan, X.; Li, L.; Zhang, T.T.; Sun, J.H.; Du, G.H. Protection by borneol on cortical neurons against oxygen-glucose deprivation/reperfusion: Involvement of anti-oxidation and anti-inflammation through nuclear transcription factor κappaB signaling pathway. Neuroscience 2011, 176, 408–419. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, D.; Wu, J.; Ou, Y.; Mu, C.; Han, B.; Zhang, Q. Improved blood–brain barrier distribution: Effect of borneol on the brain pharmacokinetics of kaempferol in rats by in vivo microdialysis sampling. J. Ethnopharmacol. 2015, 162, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Geng, Z.; Zhang, W.; Liang, J.; Wang, C.; Deng, Z.; Du, S. The Chemical composition of essential oils from Cinnamomum camphora and their insecticidal activity against the stored product pests. Int. J. Mol. Sci. 2016, 17, 1836. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wu, Q.; Su, J.; Li, C.; Zhao, X.; Xie, J.; Gui, S.; Su, Z.; Zeng, H. Composition analysis of volatile oils from flowers, leaves and branches of Cinnamomum camphora chvar. Borneol in china. J. Essent. Oil Res. 2013, 25, 395–401. [Google Scholar] [CrossRef]
- Pragadheesh, V.S.; Saroj, A.; Yadav, A.; Chanotiya, C.S.; Alam, M.; Samad, A. Chemical characterization and antifungal activity of Cinnamomum camphora essential oil. Ind. Crops Prod. 2013, 49, 628–633. [Google Scholar] [CrossRef]
- He, X.; Wang, S.; Shi, J.; Sun, Z.; Lei, Z.; Yin, Z.; Qian, Z.; Tang, H.; Xie, H. Genotypic and Environmental Effects on the Volatile Chemotype of Valeriana jatamansi Jones. Front. Plant Sci. 2018, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Moniodis, J.; Renton, M.; Jones, C.G.; Barbour, L.; Byrne, M. Genetic and environmental parameters show associations with essential oil composition in West Australian sandalwood (Santalum spicatum). Aust. J. Bot. 2018, 66, 48–58. [Google Scholar] [CrossRef]
- Shi, W.; He, W.; Wen, G. Study on chemical constituents of the essential oil and classification of types from Cinnamomum camphora. J. Integr. Plant Biol. 1989, 31, 209–214. [Google Scholar]
- Zhang, G.F.; Chen, C.J.; Chen, Z.P.; Chen, R.Y.; Lin, X.S. Analysis of principle component and chemistry type of essential oil from Cinnamomum camphora leaf in Fujian Province. J. Plant Resour. Environ. 2008, 17, 24–27. [Google Scholar]
- Liu, H.; Shen, M.Y.; He, Z.Y. Five biochemical types of Cinnamomum camphora leaf oil in Guangxi. Guangxi For. Sci. Technol. 1992, 21, 181–186. [Google Scholar]
- Rahimmalek, M.; Heidari, E.F.; Ehtemam, M.H.; Mohammadi, S. Essential oil variation in Iranian Ajowan (Trachyspermum ammi (L.) Sprague) populations collected from different geographical regions in relation to climatic factors. Ind. Crops Prod. 2017, 95, 591–598. [Google Scholar] [CrossRef]
- Martínez-Natarén, D.A.; Parra-Tabla, V.; Dzib, G.; Acosta-Arriola, V.; Canul-Puc, K.A.; Calvo-Irabién, L.M. Essential oil yield variation within and among wild populations of Mexican oregano (Lippia graveolens H.B.K.-Verbenaceae), and its relation to climatic and edaphic conditions. J. Essent. Oil Bear. Plants 2012, 15, 589–601. [Google Scholar] [CrossRef]
- Kumar, R.; Kaundal, M.; Sharma, S.; Thakur, M.; Kumar, N.; Kaur, T.; Vyas, D.; Kumar, S. Effect of elevated [CO2] and temperature on growth, physiology and essential oil composition of Salvia sclarea L. in the western Himalayas. J. Appl. Res. Med. Aromat. Plants 2017, 6, 22–30. [Google Scholar]
- Curado, M.A.; Oliveira, C.B.A.; Jesus, J.G.; Santos, S.C.; Seraphin, J.C.; Ferri, P.H. Environmental factors influence on chemical polymorphism of the essential oils of Lychnophora ericoides. Phytochemistry 2006, 67, 2363–2369. [Google Scholar] [CrossRef] [PubMed]
- Mdoe, F.P.; Cheng, S.-S.; Msangi, S.; Nkwengulila, G.; Chang, S.-T.; Kweka, E.J. Activity of Cinnamomum osmophloeum leaf essential oil against Anopheles gambiae s.s. Parasites Vectors 2014, 7, 209. [Google Scholar] [CrossRef]
- Unlu, M.; Ergene, E.; Unlu, G.V.; Zeytinoglu, H.S.; Vural, N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food Chem. Toxicol. 2010, 48, 3274–3280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, C.; Xiao, Z.; Zhang, H.; Cao, M.; Liu, Y.; Jin, Z. Chemical constituents and chemotypes of fresh leaf essential oil of wild species belonging to Sect. Camphor (Trew.) Meissn. in southeastern China. J. Essent. Oil Bear. Plants 2019, 22, 1115–1122. [Google Scholar] [CrossRef]
- Chang, C.-T.; Chang, W.-L.; Hsu, J.-C.; Shih, Y.; Chou, S.-T. Chemical composition and tyrosinase inhibitory activity of Cinnamomum cassia essential oil. Bot. Stud. 2013, 54, 617–623. [Google Scholar] [CrossRef]
- Chang, H.-T.; Lin, C.-Y.; Hsu, L.-S.; Chang, S.-T. Thermal degradation of linalool-chemotype Cinnamomum osmophloeum leaf essential oil and its stabilization by microencapsulation with β-cyclodextrin. Molecules 2021, 26, 409. [Google Scholar] [CrossRef]
- Qiu, F.; Yang, H.; Zhang, T.; Wang, X.; Wen, S.; Su, X. Chemical Composition of Leaf Essential Oil of Cinnamomum porrectum (Roxb.) Kosterm. J. Essent. Oil Bear. Plants 2019, 22, 1313–1321. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, T.; Wang, X.; Wen, S.; Guo, Y.; Jiang, X. A study on the chemical components in essential oil from leaves of Cinnamomum kanehirae and chemotype divisions. Acta Agric. Univ. Jiangxiensis 2016, 38, 668–673. [Google Scholar]
- Frizzo, C.; Santos, A.; Paroul, N.; Serafini, L.; Dellacassa, E.; Lorenzo, D.; Moyna, P. Essential oils of camphor tree (Cinnamomum camphora Nees & Eberm) cultivated in southern Brazil. Braz. Arch. Biol. Technol. 2000, 43, 313–316. [Google Scholar]
- Rao, B.R.R.; Rajput, D.K.; Bhattacharya, A.K. Essential oil composition of petiole of Cinnamomum verum Bercht. & Presl. J. Spices Aromat. Crops 2007, 16, 38–41. [Google Scholar]
- Nath, S.C.; Sarma Baruah, A.K. Eugenol as the Major Component of the Leaf Oils of Cinnamomum impressinervium Meissn. J. Essent. Oil Res. 1994, 6, 211–212. [Google Scholar] [CrossRef]
- Patel, K.; Ali, S.; Sotheeswaran, S.; Dufour, J.-P. Composition of the leaf essential oil of Cinnamomum verum (Lauraceae) from Fiji islands. J. Essent. Oil Bear. Plants 2007, 10, 374–377. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Yeh, T.-F.; Hsu, F.-L.; Lin, C.-Y.; Chang, S.-T.; Chang, H.-T. Xanthine oxidase inhibitory activity and thermostability of cinnamaldehyde-chemotype leaf oil of Cinnamomum osmophloeum microencapsulated with β-cyclodextrin. Molecules 2018, 23, 1107. [Google Scholar] [CrossRef]
- Sriramavaratharajan, V.; Stephan, J.; Sudha, V.; Murugan, R. Leaf essential oil of Cinnamomum agasthyamalayanum from the Western Ghats, India—A new source of camphor. Ind. Crops Prod. 2016, 86, 259–261. [Google Scholar] [CrossRef]
- Liang, Z.; Li, G.; Qin, Z.; Liu, H. Study on the component of a new chemical type of leaves oil from Cinnamomum camphora. J. Fujian For. Sci. Technol. 2010, 37, 102–104. [Google Scholar]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Tian, Z.; Luo, Q.; Zhaojiang, Z. Seasonal emission of monoterpenes from four chemotypes of Cinnamomum camphora. Ind. Crops Prod. 2021, 163, 113327. [Google Scholar] [CrossRef]
- Yahyaa, M.; Berim, A.; Nawade, B.; Ibdah, M.; Dudareva, N.; Ibdah, M.J.P. Biosynthesis of methyleugenol and methylisoeugenol in Daucus carota leaves: Characterization of eugenol/isoeugenol synthase and O-Methyltransferase. Phytochemistry 2019, 159, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Kampranis, S.; Ioannidis, D.; Purvis, A.; Mahrez, W.; Ninga, E.; Katerelos, N.; Anssour, S.; Dunwell, J.; Degenhardt, J.; Makris, A.; et al. Rational conversion of substrate and product specificity in a Salvia monoterpene synthase: Structural insights into the evolution of terpene synthase function. Plant Cell 2007, 19, 1994–2005. [Google Scholar] [CrossRef]
- Croteau, R. Biosynthesis and catabolism of monoterpenoids. Chem. Rev. 1987, 87, 929–954. [Google Scholar] [CrossRef]
- Ma, R.; Su, P.; Jin, B.; Guo, J.; Tian, M.; Mao, L.; Tang, J.; Chen, T.; Lai, C.; Zeng, W.; et al. Molecular cloning and functional identification of a high-efficiency (+)-borneol dehydrogenase from Cinnamomum camphora (L.) Presl. Plant Physiol. Biochem. 2021, 158, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xie, C.; Zhan, T.; Li, L.; Liu, S.; Huang, Y.; An, W.; Zheng, X.; Huang, S. Genome-wide identification and functional characterization of the trans-Iisopentenyl diphosphate synthases gene family in Cinnamomum camphora. Front. Plant. Sci. 2021, 12, 708697. [Google Scholar] [CrossRef]
- Jochum, G.; Mudge, K.; Thomas, R. Elevated temperatures increase leaf senescence and root secondary metabolite concentrations in the understory herb Panax quinquefolius (Araliaceae). Am. J. Bot. 2007, 94, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Mirali, N.; Aziz, R.; Nabulsi, I. Genetic characterization of Rosa damascena species growing in different regions of Syria and its relationship to the quality of the essential oils. Int. J. Med. Aromat. Plants 2012, 2, 41–52. [Google Scholar]
- Zhang, T.; Yang, H.; Wen, S.; Qiu, F.; Liu, X. Effects of harvest season and storage time on the essential oil of the linalool chemotype of Cinnamomum camphora. J. Essent. Oil Bear. Plants 2019, 22, 1379–1385. [Google Scholar] [CrossRef]
- Herath, H.M.W.; Iruthayathas, E.E.; Ormrod, D.P. Temperature effects on essential oil composition of citronella selections. Econ. Bot. 1979, 33, 425–430. [Google Scholar] [CrossRef]
- Paula, J.A.M.; Ferri, P.H.; Bara, M.T.F.; Tresvenzol, L.M.F.; Sá, F.A.S.; Paula, J.R. Infraspecific chemical variability in the essential oils of Pimenta pseudocaryophyllus (Gomes) L.R. Landrum (Myrtaceae). Biochem. Syst. Ecol. 2011, 39, 643–650. [Google Scholar] [CrossRef]
- Suárez, A.I.; Vásquez, L.; Manzano, M.; Compagnone, R. Essential oil composition of Croton cuneatus and Croton malambo growing in Venezuela. Flavour Fragr. J. 2005, 20, 611–614. [Google Scholar] [CrossRef]
- Brum, R.; Honda, N.; Hess, S. Jatropha elliptica Muell. Arg. a source of δ-selinene. J. Essent. Oil Res. 1997, 9, 477–478. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Compontnts by Gas Chromatography/Mass Spectrometry; Allured Publishing: Carol Stream, IL, USA, 2017. [Google Scholar]
| Site | Province | Code | N 1 | E | MAAT | MAIT | MAP | Elevation | n | Oil Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Qimen | Anhui | AH-QM | 29.696716° | 117.506601° | 23 | 13 | 2396 | 90 | 30 | 2.01 ± 0.74 |
| Shapingba | Chongqing | CQ-SPB | 29.579210° | 106.417387° | 23 | 16 | 982 | 465 | 30 | 0.92 ± 0.16 |
| Fuzhou | Fujian | FJ-FZ | 26.157219° | 119.283930° | 27 | 18 | 1836 | 86 | 30 | 0.49 ± 0.50 |
| Huaan | Fujian | FJ-HA | 24.959486° | 117.534795° | 28 | 17 | 2989 | 205 | 10 | 0.48 ± 0.14 |
| Yongan | Fujian | FJ-YA | 26.011571° | 117.390753° | 27 | 16 | 2876 | 224 | 30 | 0.61 ± 0.44 |
| Youxi | Fujian | FJ-YX | 26.040294° | 118.128517° | 27 | 16 | 2769 | 176 | 30 | 0.55 ± 0.35 |
| Heyuan | Guangdong | GD-HY | 23.718087° | 115.209347° | 28 | 17 | 2648 | 343 | 28 | 0.66 ± 0.63 |
| Meizhou | Guangdong | GD-MZ | 24.146901° | 116.076344° | 28 | 18 | 2099 | 132 | 30 | 0.68 ± 0.59 |
| Shaoguan | Guangdong | GD-SG | 24.849486° | 113.562527° | 26 | 17 | 2814 | 82 | 27 | 0.28 ± 0.25 |
| Shixing | Guangdong | GD-SX | 24.855446° | 114.100072° | 27 | 16 | 2867 | 199 | 17 | 0.95 ± 0.92 |
| Zhanjiang | Guangdong | GD-ZJ | 21.126940° | 110.246939° | 28 | 21 | 1909 | 13 | 27 | 0.36 ± 0.47 |
| Gaofeng | Guangxi | GX-GF | 22.931939° | 108.357974° | 27 | 19 | 2014 | 118 | 28 | 1.10 ± 0.89 |
| Quanzhou | Guangxi | GX-QZ | 25.986144° | 110.921399° | 25 | 16 | 1979 | 184 | 30 | 0.91 ± 0.68 |
| Guian | Guizhou | GZ-GA | 26.412591° | 106.369501° | 20 | 13 | 1490 | 1226 | 29 | 1.47 ± 0.67 |
| Guiyang | GZ-GY | 26.496424° | 106.735431° | 19 | 12 | 1981 | 1124 | 32 | 1.00 ± 0.66 | |
| Enshi | Hubei | HB-ES | 30.281372° | 109.484778° | 23 | 14 | 1263 | 430 | 30 | 1.51 ± 0.86 |
| Xianning | Hubei | HB-XN | 29.569482° | 114.862824° | 23 | 13 | 1445 | 57 | 29 | 1.41 ± 0.46 |
| Changde | Hunan | HN-CD | 29.050278° | 111.592693° | 22 | 15 | 1327 | 91 | 30 | 1.35 ± 0.45 |
| Changsha | Hunan | HN-CS | 28.111381° | 113.054928° | 24 | 16 | 1591 | 90 | 30 | 0.69 ± 0.57 |
| Chenzhou | Hunan | HN-CZ | 25.789165° | 112.996943° | 23 | 15 | 2524 | 211 | 28 | 1.09 ± 0.70 |
| Xinhua | Hunan | HN-XH | 27.780197° | 111.180908° | 24 | 15 | 2204 | 181 | 30 | 1.70 ± 0.46 |
| Yongzhou | Hunan | HN-YZ | 26.391109° | 111.761389° | 24 | 16 | 822 | 176 | 30 | 1.02 ± 0.49 |
| Zhenjiang | Jiangsu | JS-ZJ | 32.175807° | 119.441861° | 21 | 12 | 1444 | 33 | 30 | 0.41 ± 0.47 |
| Anfu | Jiangxi | JX-AF | 27.406026° | 114.560550° | 25 | 15 | 2656 | 116 | 38 | 1.18 ± 1.00 |
| Dexing | Jiangxi | JX-DX | 28.844566° | 117.579117° | 25 | 15 | 2478 | 49 | 27 | 1.09 ± 0.84 |
| Jinxi | Jiangxi | JX-JX | 27.871332° | 116.584137° | 25 | 15 | 2821 | 47 | 11 | 0.89 ± 0.55 |
| Nanchang | Jiangxi | JX-NC | 28.779930° | 115.779720° | 24 | 16 | 2515 | 129 | 29 | 0.78 ± 0.69 |
| Ruijin | Jiangxi | JX-RJ | 25.864169° | 115.932219° | 26 | 16 | 2604 | 223 | 30 | 0.80 ± 0.54 |
| Wuyuan | Jiangxi | JX-WY | 29.252720° | 117.859167° | 25 | 14 | 2787 | 80 | 29 | 1.12 ± 0.73 |
| Xingguo | Jiangxi | JX-XG | 26.495143° | 115.771943° | 26 | 17 | 2341 | 265 | 29 | 1.42 ± 0.68 |
| Luxian | Sichuan | SC-LX | 29.138419° | 105.385134° | 23 | 15 | 1168 | 478 | 29 | 0.47 ± 0.35 |
| Hanzhong | Shaanxi | SX-HZ | 33.135874° | 107.301057° | 22 | 12 | 1764 | 443 | 30 | 1.54 ± 0.49 |
| Changxing | Zhejiang | ZJ-CX | 30.977313° | 119.609480° | 23 | 14 | 1614 | 115 | 30 | 0.60 ± 0.62 |
| Tonglu | Zhejiang | ZJ-TL | 29.818942° | 119.757321° | 23 | 13 | 2392 | 51 | 14 | 0.77 ± 0.65 |
| Yuyao | Zhejiang | ZJ-YY | 30.038909° | 120.992886° | 23 | 14 | 2972 | 19 | 33 | 0.44 ± 0.44 |
| Latitude | Longitude | |
|---|---|---|
| Yield | 0.162 ** | −0.174 ** |
| Eucalyptol | 0.175 ** | −0.014 |
| Linalool | 0.096 ** | −0.059 |
| Camphor | −0.014 | −0.282 ** |
| Selina-6-en-4-ol | −0.224 ** | 0.154 ** |
| trans-Nerolidol | 0.003 | 0.189 ** |
| Constituents | RI 1 | Mean Content 2 (%) | GD-SX -22 | FJ-YX -29 | HN-CS -14 | JX-AF -14 | GX-QZ -03 | GX-GF -22 | GX-GF -06 |
|---|---|---|---|---|---|---|---|---|---|
| α-Pinene | 939 | 1.20 | 2.91 | - | 0.63 | 0.06 | 1.56 | - | - |
| β-Phellandrene | 978 | 3.75 | 10.47 | - | - | 0.04 | 0.35 | 0.04 | - |
| α-Phellandrene | 1011 | 0.10 | 0.01 | - | - | 0.04 | 0.32 | 0.06 | - |
| D-limonene | 1035 | 0.40 | - | - | 1.15 | 0.17 | 1.90 | 0.05 | - |
| Eucalyptol | 1039 | 15.14 | 65.33 | 0.03 | 1.63 | 0.38 | 1.77 | 0.07 | - |
| γ-Terpinene | 1063 | 0.26 | - | - | - | - | - | 0.17 | - |
| trans-Linalool oxide | 1074 | 0.54 | 0.01 | 0.15 | 0.22 | 0.03 | 0.42 | 3.78 | - |
| Linalool | 1106 | 13.57 | 1.59 | 93.73 | - | 0.39 | 2.87 | 0.44 | 0.02 |
| β-Terpineol | 1109 | 0.28 | - | - | - | 0.04 | 0.16 | 3.58 | - |
| Camphor | 1155 | 12.77 | - | 0.23 | 84.35 | 0.75 | 23.37 | 0.01 | 0.02 |
| Borneol | 1181 | 0.47 | 0.13 | - | 0.22 | 0.09 | 52.22 | - | 0.15 |
| 4-Terpineol | 1188 | 0.95 | 2.99 | 0.04 | 0.52 | 0.03 | 0.46 | 4.49 | 0.02 |
| α-Terpineol | 1205 | 3.75 | 9.25 | 0.27 | 1.03 | 0.23 | 1.32 | 0.43 | 0.15 |
| trans-Geraniol | 1229 | 0.13 | 0.20 | - | - | 0.04 | - | 0.02 | - |
| Bornyl acetate | 1290 | 0.14 | - | - | - | 0.14 | - | 0.13 | 0.03 |
| δ-Elemene | 1342 | 0.43 | 0.05 | 0.08 | 0.30 | 0.17 | 0.58 | 0.36 | 0.53 |
| Methyl eugenol | 1401 | 0.21 | - | 0.01 | - | - | - | 7.03 | - |
| β-Caryophyllene | 1433 | 2.93 | 0.54 | 0.37 | 2.32 | 2.92 | 1.91 | 3.05 | 4.35 |
| γ-Elemene | 1439 | 0.10 | 0.01 | - | - | 0.09 | - | - | 0.01 |
| Humulene | 1469 | 1.53 | 0.01 | - | 1.14 | 0.89 | 1.28 | 2.36 | 2.46 |
| Valencene | 1485 | 0.23 | 0.01 | - | - | 0.03 | - | 0.07 | 0.62 |
| γ-Muurolene | 1483 | 0.20 | - | - | - | - | - | 0.02 | 1.00 |
| Germacrene D | 1489 | 1.04 | 0.17 | 0.31 | 1.40 | 0.93 | 0.41 | - | 0.34 |
| δ-Selinene | 1496 | 0.88 | - | - | 1.22 | - | 1.87 | - | 7.80 |
| α-Guaiene | 1503 | 0.89 | 0.06 | 0.07 | 1.27 | 0.04 | 1.33 | - | 0.29 |
| Methyl isoeugenol | 1509 | 1.73 | 0.01 | 0.43 | - | 0.32 | - | 71.99 | 0.30 |
| Copaene | 1542 | 0.36 | - | - | - | 0.03 | - | 0.01 | 0.07 |
| α-Muurolene | 1547 | 0.25 | - | - | - | - | - | 0.01 | - |
| Elemol | 1559 | 0.29 | - | 0.02 | 0.23 | 0.68 | - | 0.05 | 0.52 |
| trans-Nerolidol | 1564 | 13.93 | - | 0.02 | 0.23 | 60.30 | - | 0.04 | 0.43 |
| Germacrene B | 1574 | 0.50 | 0.05 | 0.07 | - | 0.01 | - | 0.02 | 1.13 |
| Spathulenol | 1589 | 0.85 | 0.01 | 0.10 | - | 0.46 | - | 0.02 | 1.90 |
| caryophyllene oxide | 1598 | 0.90 | 0.03 | 0.05 | - | 1.03 | - | 0.03 | 0.53 |
| Guaiol | 1609 | 0.24 | 0.04 | - | - | 2.71 | - | 0.12 | - |
| Selina-6-en-4-ol | 1637 | 5.58 | - | 0.02 | - | 0.11 | - | 0.01 | 71.20 |
| Viridiflorol | 1648 | 0.10 | - | - | - | 0.07 | - | 0.02 | 0.28 |
| τ-Muurolol | 1657 | 0.34 | 0.01 | - | - | 0.06 | - | - | 0.20 |
| α-Cadinol | 1667 | 0.18 | - | - | - | - | 0.17 | - | 0.81 |
| Juniper camphor | 1672 | 0.52 | 0.07 | 0.01 | - | 0.78 | - | 0.13 | - |
| Bulnesol | 1678 | 0.28 | 0.01 | 0.03 | - | 1.21 | - | 0.06 | 0.41 |
| 3-Methyl-but-2-enoic acid-1,7,7- trimethyl-bicyclo [2.2.1]hept-2-yl ester | 1731 | 4.39 | 0.46 | - | - | 20.88 | - | 0.03 | 0.36 |
| Monoterpene hydrocarbons | 19.28 | 13.39 | - | 1.78 | 0.31 | 4.13 | 0.32 | - | |
| Oxygenated Monoterpenes | 34.17 | 79.50 | 94.45 | 87.97 | 2.12 | 82.59 | 12.95 | 0.39 | |
| Sesquiterpenes hydrocarbons | 9.34 | 0.85 | 0.90 | 7.65 | 5.11 | 7.38 | 5.90 | 18.60 | |
| Oxygenated sesquiterpenes | 27.60 | 0.68 | 0.25 | 0.46 | 88.29 | 0.17 | 0.51 | 76.64 | |
| Phenylpropanoids | 1.94 | 0.01 | 0.44 | - | 0.32 | - | 79.02 | 0.30 |
| Chemotype | n | Mean Yield (%) | Min. Yield (%) | Max. Yield (%) |
|---|---|---|---|---|
| Eucalyptol | 249 | 1.64 ± 0.04a 1 | 0.09 | 3.28 |
| Camphor | 153 | 0.95 ± 0.04b | 0.04 | 3.46 |
| Linalool | 140 | 1.55 ± 0.04a | 0.27 | 2.83 |
| trans-Nerolidol | 234 | 0.22 ± 0.01e | 0.01 | 1.41 |
| Selina-6-en-4-ol | 97 | 0.45 ± 0.02d | 0.12 | 2.07 |
| Mix | 84 | 0.66 ± 0.07c | 0.05 | 2.76 |
| Other | 17 | 0.34 ± 0.08de | 0.01 | 0.99 |
| Compounds | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| α-Pinene | 0.36 | 0.18 | −0.54 | −0.14 |
| β-Phellandrene | 0.32 | 0.62 | −0.62 | −0.19 |
| α-Phellandrene | 0.034 | −0.06 | −0.04 | 0.01 |
| D-limonene | 0.13 | −0.45 | −0.15 | −0.04 |
| Eucalyptol | 0.33 | 0.65 | −0.64 | −0.21 |
| γ-Terpinene | 0.18 | 0.26 | −0.32 | −0.05 |
| trans-Linalool oxide | 0.08 | 0.13 | −0.19 | −0.00 |
| Linalool | −0.99 | −0.04 | −0.11 | −0.10 |
| β-Terpineol | 0.06 | 0.09 | −0.09 | 0.04 |
| Camphor | 0.28 | −0.89 | −0.31 | −0.14 |
| Borneol | 0.06 | −0.13 | −0.04 | −0.01 |
| 4-Terpineol | 0.10 | 0.14 | −0.16 | 0.02 |
| α-Terpineol | 0.30 | 0.56 | −0.58 | −0.18 |
| trans-Geraniol | 0.03 | 0.04 | 0.02 | 0.01 |
| Bornyl acetate | 0.11 | −0.27 | −0.05 | −0.05 |
| δ-Elemene | 0.14 | −0.10 | 0.36 | 0.11 |
| Methyl eugenol | 0.01 | 0.01 | 0.01 | 0.04 |
| β-caryophyllene | 0.15 | −0.10 | 0.56 | −0.00 |
| γ-Elemene | 0.04 | 0.02 | 0.18 | 0.08 |
| Humulene | 0.19 | −0.14 | 0.46 | 0.08 |
| Valencene | 0.05 | 0.02 | 0.15 | 0.61 |
| γ-Muurolene | 0.04 | 0.02 | 0.09 | 0.55 |
| Germacrene D | 0.18 | −0.20 | 0.46 | −0.20 |
| δ-Selinene | 0.05 | −0.06 | 0.06 | 0.40 |
| α-Guaiene | 0.21 | −0.42 | 0.10 | −0.03 |
| Methyl isoeugenol | 0.03 | 0.05 | 0.17 | 0.02 |
| Copaene | 0.02 | 0.01 | 0.04 | 0.10 |
| α-Muurolene | 0.01 | 0.01 | 0.02 | 0.06 |
| Elemol | 0.01 | −0.01 | 0.03 | 0.09 |
| trans-Nerolidol | 0.18 | 0.10 | 0.91 | −0.33 |
| Germacrene B | 0.04 | 0.04 | 0.15 | 0.60 |
| Spathulenol | 0.07 | 0.03 | 0.30 | 0.39 |
| caryophyllene oxide | 0.11 | 0.05 | 0.53 | 0.20 |
| Guaiol | 0.05 | 0.04 | 0.19 | 0.12 |
| Selina-6-en-4-ol | 0.06 | 0.02 | 0.14 | 0.95 |
| Viridiflorol | 0.04 | 0.02 | 0.18 | 0.11 |
| τ-Muurolol | 0.03 | 0.01 | 0.07 | 0.15 |
| α-Cadinol | 0.04 | 0.00 | 0.12 | 0.26 |
| Juniper camphor | 0.07 | 0.03 | 0.29 | 0.09 |
| Bulnesol | 0.05 | 0.02 | 0.12 | 0.52 |
| 3-Methyl-but-2-enoic acid-1,7,7- trimethyl-bicyclo [2.2.1]hept-2-yl ester | 0.16 | 0.10 | 0.81 | −0.21 |
| % of Variance | 33.01 | 26.37 | 24.72 | 9.59 |
| Characters | Standardized Canonical Coefficients | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Environmental factors | MAAT | 0.94 | −1.05 | −1.55 | −1.41 |
| MAIT | −0.26 | 0.34 | 1.95 | 0.68 | |
| MAP | 0.06 | −0.19 | 0.31 | 1.25 | |
| Elevation | −0.35 | −1.12 | 0.00 | −0.23 | |
| Oil characters | Oil yield | −0.19 | −0.60 | −0.59 | 0.16 |
| Eucalyptol | −0.46 | 0.75 | −0.58 | 1.21 | |
| Linalool | −0.25 | 0.58 | −0.74 | 0.30 | |
| Camphor | −0.99 | −0.25 | −0.21 | 0.77 | |
| trans-Nerolidol | −0.33 | 0.22 | −0.35 | 1.42 | |
| Selina-6-en-4-ol | 0.25 | −0.57 | −0.64 | 0.86 | |
| Eigen-value | 0.46 | 0.38 | 0.21 | 0.12 | |
| p value | 0.00 | 0.00 | 0.000 | 0.00 | |
| Cumulative variance | 55.00 | 88.20 | 97.20 | 100.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Zheng, Y.; Fu, C.; Yang, H.; Liu, X.; Qiu, F.; Wang, X.; Wang, Z. Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora. Molecules 2023, 28, 973. https://doi.org/10.3390/molecules28030973
Zhang T, Zheng Y, Fu C, Yang H, Liu X, Qiu F, Wang X, Wang Z. Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora. Molecules. 2023; 28(3):973. https://doi.org/10.3390/molecules28030973
Chicago/Turabian StyleZhang, Ting, Yongjie Zheng, Chao Fu, Haikuan Yang, Xinliang Liu, Fengying Qiu, Xindong Wang, and Zongde Wang. 2023. "Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora" Molecules 28, no. 3: 973. https://doi.org/10.3390/molecules28030973
APA StyleZhang, T., Zheng, Y., Fu, C., Yang, H., Liu, X., Qiu, F., Wang, X., & Wang, Z. (2023). Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora. Molecules, 28(3), 973. https://doi.org/10.3390/molecules28030973







