Differentiation between Isomeric 4,5-Functionalized 1,2,3-Thiadiazoles and 1,2,3-Triazoles by ESI-HRMS and IR Ion Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. (+)ESI-MS/MS Study of Compounds 1a

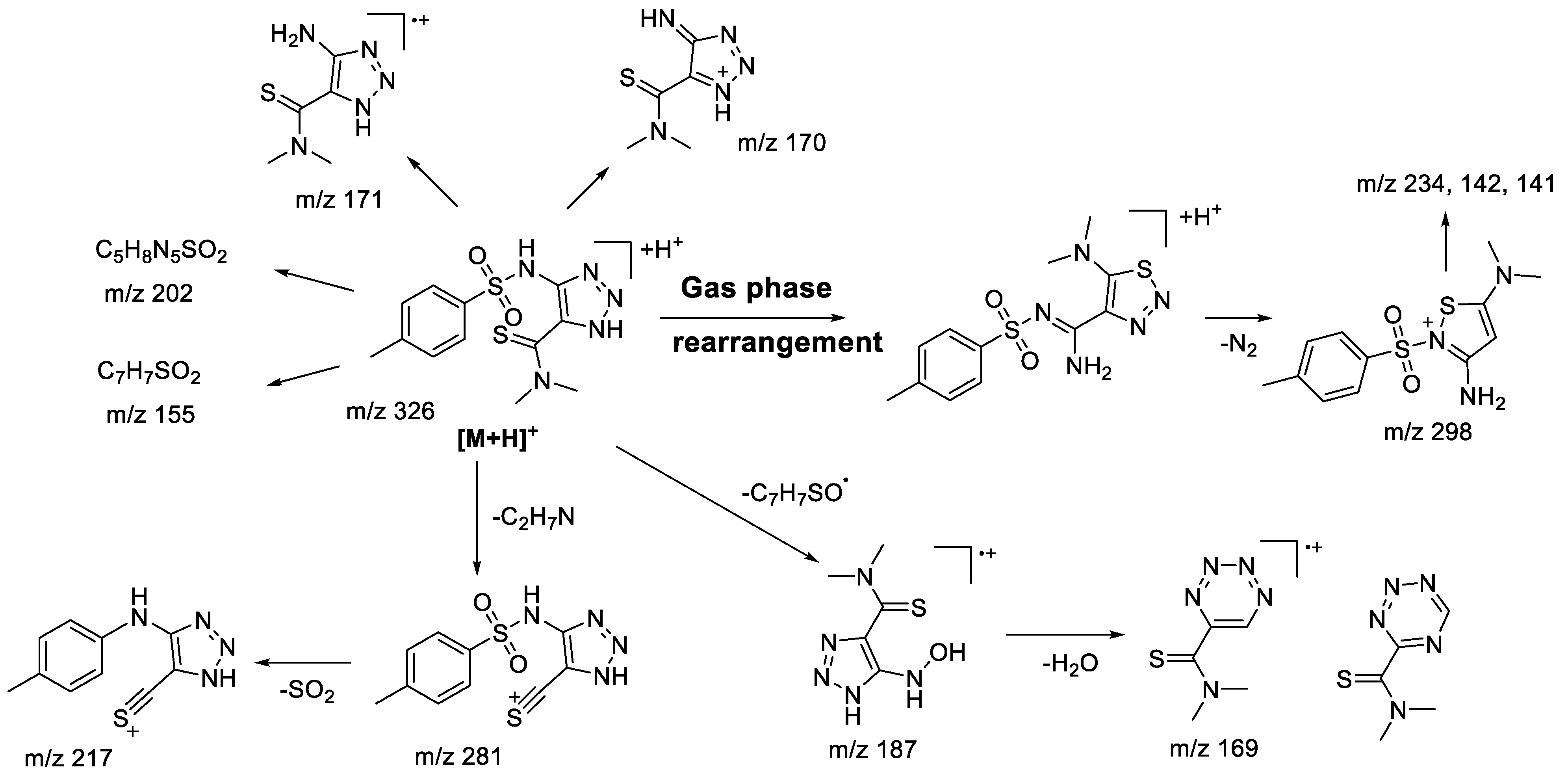
2.2. (+)ESI-MS/MS Study of Compounds of 1b-g


2.3. (+)ESI-MS/MS-IRMPD Spectroscopy Study of Isomeric Structures
2.4. (−)ESI-MS/MS Study of Compounds 1b-g


3. Materials and Methods
3.1. Synthesis of 4,5-Functionalized 1,2,3-Thiadiazoles and 1,2,3-Triazoles
3.2. Mass Spectrometry
3.3. Ion Spectroscopy
3.4. Computational Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- da Silva, F.G.; Do Carmo Cardoso, M.F.; Ferreira, G.P.; Ferreira, V.F. Biological properties of 1H-1,2,3- and 2H-1,2,3-Triazoles. In Chemistry of 1,2,3-Triazoles; Dehaen, W., Bakulev, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 40, pp. 117–165. [Google Scholar]
- Muthipeedika, N.J.; Bodke, Y.D.; Telkar, S.; Bakulev, V.A. Synthesis of Coumarins Linked With 1,2,3-Triazoles under Microwave Irradiation and Evaluation of Their Antimicrobial and Antioxidant Activity. J. Mex. Chem. Soc. 2020, 64, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Shafran, Y.; Glukhareva, T.; Dehaen, W.; Bakulev, V. Recent Developments in the Chemistry of 1,2,3-Thiadiazoles. Adv. Heterocycl. Chem. 2018, 126, 109–172. [Google Scholar] [CrossRef]
- Belskaya, N.P.; Demina, M.A.; Sapozhnikova, S.G.; Fan, Z.-J.; Zhang, H.; Dehaen, W.; Bakulev, V.A. 2-arylhydrazono-2-cyanoacetamidines to 5-amino-2-aryl-2H-[1,2,3]triazole-4-carbonitrile. Arkivoc 2008, 16, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Motornov, V.; Beier, P. Chemoselective Aza-[4+3]-annulation of N-Perfluoroalkyl-1,2,3-triazoles with 1,3-Dienes: Access to N-Perfluoroalkyl-Substituted Azepines. J. Org. Chem. 2018, 83, 15195–15201. [Google Scholar] [CrossRef]
- Markos, A.; Voltrova, S.; Motornov, V.; Tichy, D.; Klepetarova, B.; Beier, P. Stereoselective Synthesis of (Z)-β-Enamido Triflates and Fluorosulfonates from N-Fluoroalkylated Triazoles. Chem.–Eur. J. 2019, 25, 7640–7644. [Google Scholar] [CrossRef]
- Khaidarov, A.R.; Rostovskii, N.V.; Zolotarev, A.A.; Khlebnikov, A.F.; Novikov, M.S. Synthesis of 1-(2-Aminovinyl)indoles and 1,3′- Biindoles by Reaction of 2,2-Diaryl-Substituted 2H-Azirines with α- Imino Rh(II) Carbenoids. J. Org. Chem. 2019, 84, 3743–3753. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazolecontaining Hybrids as Leads in Medicinal Chemistry: A Recent Overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Watkinson, M. Click Triazoles as Chemosensors. In Click Triazoles; Košmrlj, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 28, pp. 109–136. [Google Scholar]
- Singh, G.; George, N.; Singh, R.; Singh, G.; Sushma; Kaur, G.; Singh, H.; Singh, J. Ion recognition by 1,2,3-triazole moieties synthesized via “click chemistry”. Appl. Organomet. Chem. 2023, 37, e6897. [Google Scholar] [CrossRef]
- Filimonov, V.O.; Dianova, L.N.; Galata, K.A.; Beryozkina, T.V.; Novikov, M.S.; Berseneva, V.S.; Eltsov, O.S.; Lebedev, A.T.; Slepukhin, P.A.; Bakulev, V.A. Switchable Synthesis of 4,5-Functionalized 1,2,3-Thiadiazoles and 1,2,3-Triazoles from 2-Cyanothioacetamides under Diazo Group Transfer Conditions. J. Org. Chem. 2017, 82, 4056–4071. [Google Scholar] [CrossRef]
- Filimonov, V.O.; Dianova, L.N.; Beryozkina, T.V.; Mazur, D.M.; Beliaev, N.A.; Volkova, N.N.; Ilkin, V.G.; Dehaen, W.; Lebedev, A.T.; Bakulev, V.A. Water/Alkali-Catalyzed Reactions of Azides with 2-Cyanothioacetamides. Eco-Friendly Synthesis of Monocyclic and Bicyclic 1,2,3-Thiadiazole-4-carbimidamides and 5-Amino-1,2,3-triazole-4-carbothioamides. J. Org. Chem. 2019, 84, 21–13430. [Google Scholar] [CrossRef]
- Lebedev, A.T. Mass spectrometry of diazocompounds. Mass Spectrom. Rev. 1991, 10, 91–132. [Google Scholar] [CrossRef]
- Eichinger, P.C.; Dua, S.; Bowie, J.H. A comparison of skeletal rearrangement reactions of even-electron anions in solution and in the gas phase. Int. J. Mass Spectrom. Ion Processes 1994, 133, 1–12. [Google Scholar] [CrossRef]
- Lobodin, V.V.; Lebedev, A.T. Analogies of monomolecular transformations of organic compounds in solution and mass spectrometry experiments. Mass-Spectrometria (Rus) 2005, 2, 91–128. [Google Scholar] [CrossRef]
- Eberlin, M.N. Electrospray ionization mass spectrometry: A major tool to investigate reaction mechanisms in both solution and the gas phase. Eur. J. Mass Spectrom. 2007, 13, 19–28. [Google Scholar] [CrossRef]
- Coelho, F.; Eberlin, M.N. The bridge connecting Gas-Phase and Solution Chemistries. Angew. Chem. Int. Ed. 2011, 50, 5261–5263. [Google Scholar] [CrossRef]
- Lebedev, A.T.; Alekseeva, T.N.; Kutateladze, T.G.; Mochalov, S.S.; Shabarov, Y.S.; Petrosyan, V.S. The electron impact-induced cyclization of o-carboxy- and o-carboxamidocyclopropylbenzenes. Org. Mass Spectrom. 1989, 24, 149–152. [Google Scholar] [CrossRef]
- Lebedev, A.T.; Bakulev, V.A.; Hayes, R.N.; Bowie, J.H. Anionic rearrangement in the gas phase. The collision-induced dissociations of deprotonated 2-diazo-2-cyanoacetamides. Rapid Commun. Mass Spectrom. 1991, 5, 234–237. [Google Scholar] [CrossRef]
- Dua, S.K.; Whait, R.B.; Alexander, M.J.; Hayes, R.N.; Lebedev, A.T.; Eichinger, P.C.N.; Bowie, J.H. The search for the gas-phase negative ion pinacol rearrangement. J. Am. Chem. Soc. 1993, 115, 5709–5715. [Google Scholar] [CrossRef]
- Zelenin, K.N.; Khrustalev, V.A.; Alekseev, V.V.; Sharbatyan, P.A.; Lebedev, A.T. 2-Phenyl-1,3,4-thiadiazolines-2. Khimiya Geterotsiklicheskikh Soedinenii. 1982, 7, 904–910. [Google Scholar]
- Mazur, D.M.; Zimens, M.E.; Bakulev, V.A.; Lebedev, A.T. Identification and Interconversion of Isomeric 4,5-Functionalized 1,2,3-Thiadiazoles and 1,2,3-Triazoles in Conditions of Electrospray Ionization. J. Pharm. Biomed. Anal. 2017, 145, 315–321. [Google Scholar] [CrossRef]
- Polfer, N.C.; Oomens, J. Reaction products in mass spectrometry elucidated with infrared spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 3804–3817. [Google Scholar] [CrossRef]
- Martens, J.; van Outersterp, R.E.; Vreeken, R.J.; Cuyckens, F.; Coene, K.L.M.; Engelke, U.F.; Kluijtmans, L.A.J.; Wevers, R.A.; Buydens, L.M.C.; Redlich, B.; et al. Infrared ion spectroscopy: New opportunities for small-molecule identification in mass spectrometry-A tutorial perspective. Anal. Chim. Acta 2020, 1093, 1–15. [Google Scholar] [CrossRef]
- Cismesia, A.P.; Bell, M.R.; Tesler, L.F.; Alves, M.; Polfer, N.C. Infrared ion spectroscopy: An analytical tool for the study of metabolites. Analyst 2018, 143, 1615–1623. [Google Scholar] [CrossRef]
- Davidson, J.T.; Piacentino, E.L.; Sasiene, Z.J.; Abiedalla, Y.; DeRuiter, J.; Clark, C.R.; Berden, G.; Oomens, J.; Ryzhov, V.; Jackson, G.P. Identification of novel fragmentation pathways and fragment ion structures in the tandem mass spectra of protonated synthetic cathinones. Forensic Chem. 2020, 19, 100245. [Google Scholar] [CrossRef]
- Martens, J.; Berden, G.; Bentlage, H.; Coene, K.L.M.; Engelke, U.F.; Wishart, D.; van Scherpenzeel, M.; Kluijtmans, L.A.J.; Wevers, R.A.; Oomens, J. Unraveling the unknown areas of the human metabolome: The role of infrared ion spectroscopy. J. Inherited Metab. Dis. 2018, 41, 367–377. [Google Scholar] [CrossRef] [Green Version]
- van Outersterp, R.E.; Houthuijs, K.J.; Berden, G.; Engelke, U.F.; Kluijtmans, L.A.J.; Wevers, R.A.; Coene, K.L.M.; Oomens, J.; Martens, J. Reference-standard free metabolite identification using infrared ion spectroscopy. Int. J. Mass Spectrom. 2019, 443, 77–85. [Google Scholar] [CrossRef]
- Bell, M.R.; Tesler, L.F.; Polfer, N.C. Cryogenic infrared ion spectroscopy for the structural elucidation of drug molecules: MDMA and its metabolites. Int. J. Mass Spectrom. 2019, 443, 101–108. [Google Scholar] [CrossRef]
- Dynesen, E.; Lawesson, S.-O.; Schroll, G.; Bowie, J.H.; Cooks, R.G. Electron-impact studies. Part XXI. The mass spectra of sulphonamides and sulphonyl chlorides: The formation of C–O and C–N bonds upon electron impact. J. Chem. Soc. B. 1968, 15–21. [Google Scholar] [CrossRef]
- Meyerson, S.; Drews, H.; Fields, E.K. Mass Spectra of Diaryl Sulfones. Anal. Chem. 1964, 36, 1294–1300. [Google Scholar] [CrossRef]
- Martens, J.; Berden, G.; Gebhardt, C.R.; Oomens, J. Infrared ion spectroscopy in a modified quadrupole ion trap mass spectrometer at the FELIX free electron laser laboratory. Rev. Sci. Instrum. 2016, 87, 103108. [Google Scholar] [CrossRef] [Green Version]
- Berden, G.; Derksen, M.; Houthuijs, K.J.; Martens, J.; Oomens, J. An automatic variable laser attenuator for IRMPD spectroscopy and analysis of power-dependence in fragmentation spectra. Int. J. Mass Spectrom. 2019, 443, 1–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, D.M.; Piacentino, E.L.; Berden, G.; Oomens, J.; Ryzhov, V.; Bakulev, V.A.; Lebedev, A.T. Differentiation between Isomeric 4,5-Functionalized 1,2,3-Thiadiazoles and 1,2,3-Triazoles by ESI-HRMS and IR Ion Spectroscopy. Molecules 2023, 28, 977. https://doi.org/10.3390/molecules28030977
Mazur DM, Piacentino EL, Berden G, Oomens J, Ryzhov V, Bakulev VA, Lebedev AT. Differentiation between Isomeric 4,5-Functionalized 1,2,3-Thiadiazoles and 1,2,3-Triazoles by ESI-HRMS and IR Ion Spectroscopy. Molecules. 2023; 28(3):977. https://doi.org/10.3390/molecules28030977
Chicago/Turabian StyleMazur, Dmitrii M., Elettra L. Piacentino, Giel Berden, Jos. Oomens, Victor Ryzhov, Vasiliy A. Bakulev, and Albert T. Lebedev. 2023. "Differentiation between Isomeric 4,5-Functionalized 1,2,3-Thiadiazoles and 1,2,3-Triazoles by ESI-HRMS and IR Ion Spectroscopy" Molecules 28, no. 3: 977. https://doi.org/10.3390/molecules28030977






