Potential Treatment of Dermatophyte Trichophyton rubrum in Rat Model Using Topical Green Biosynthesized Silver Nanoparticles with Achillea santolina Extract
Abstract
:1. Introduction
2. Results
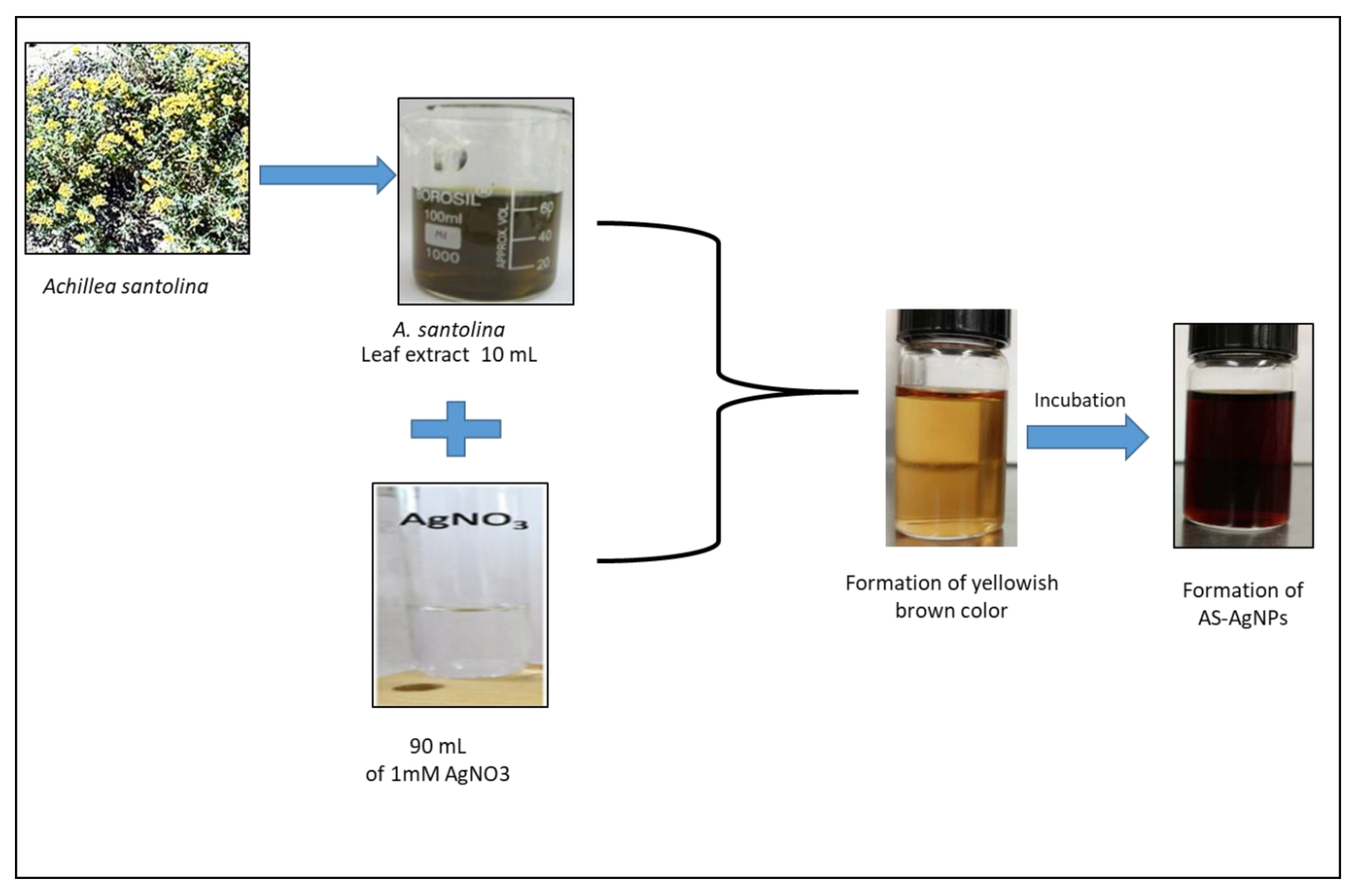
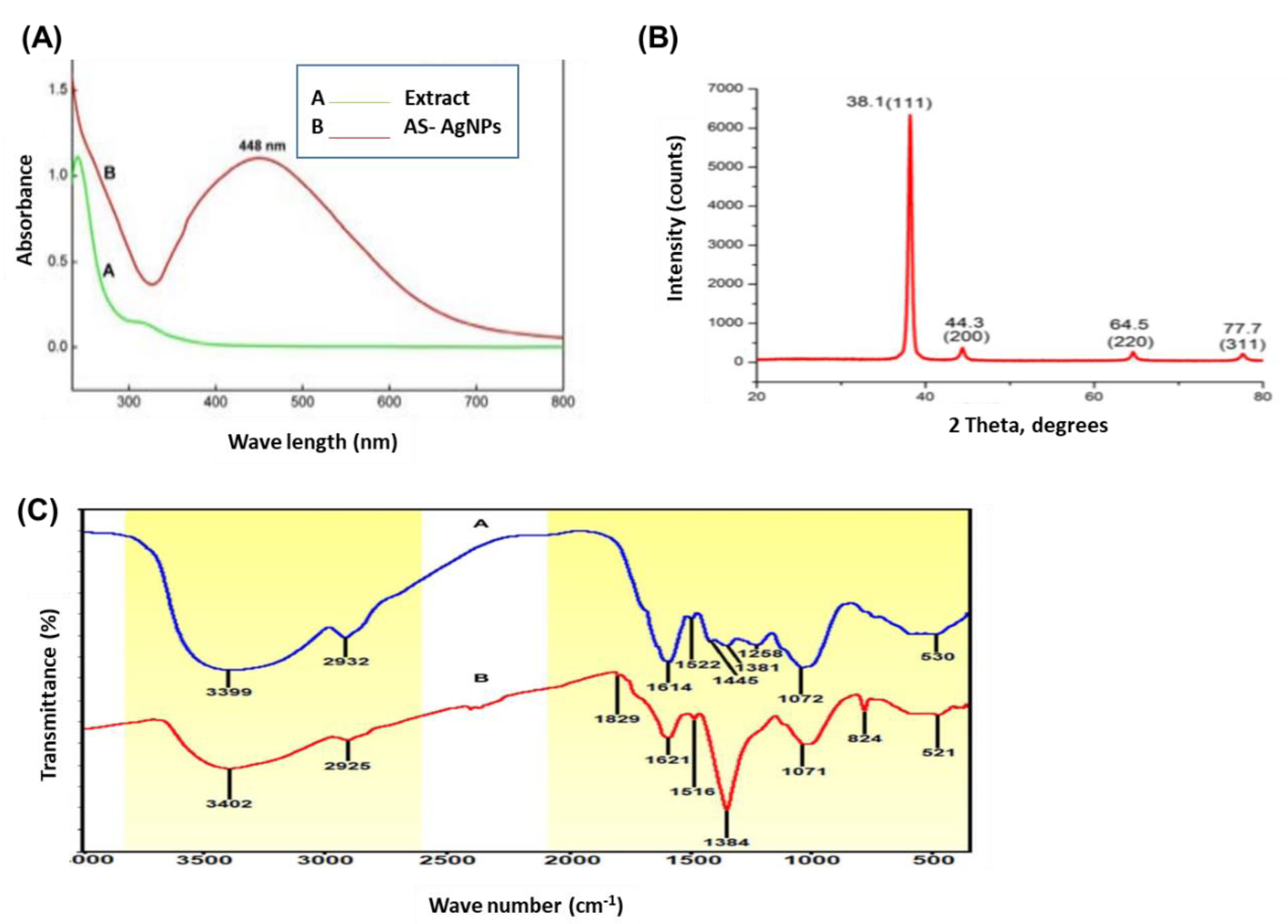
2.1. Biosynthesis and Characterization of AS-AgNPs
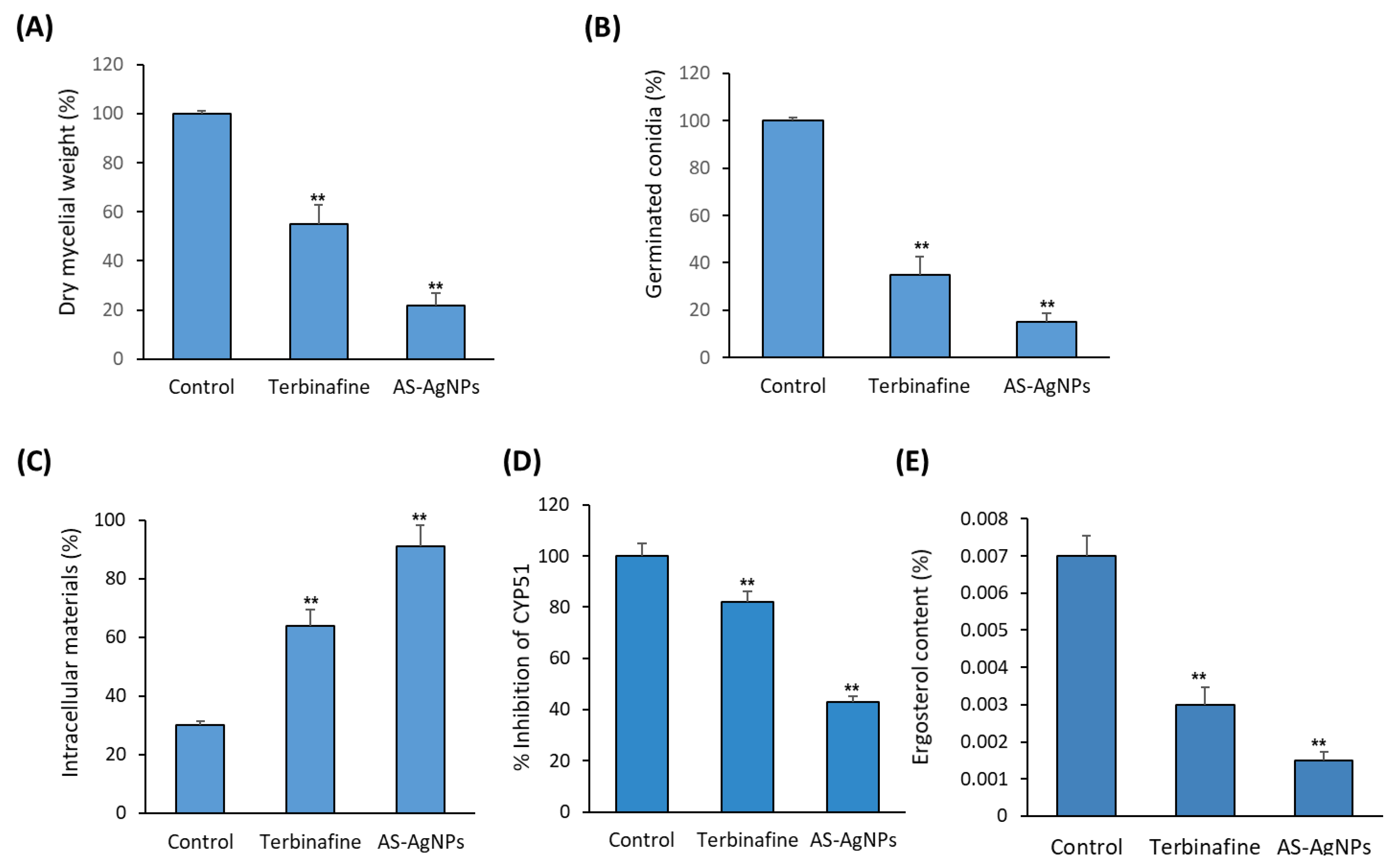
2.2. In Vitro Antifungal Activity of AS-AgNPs against T. rubrum
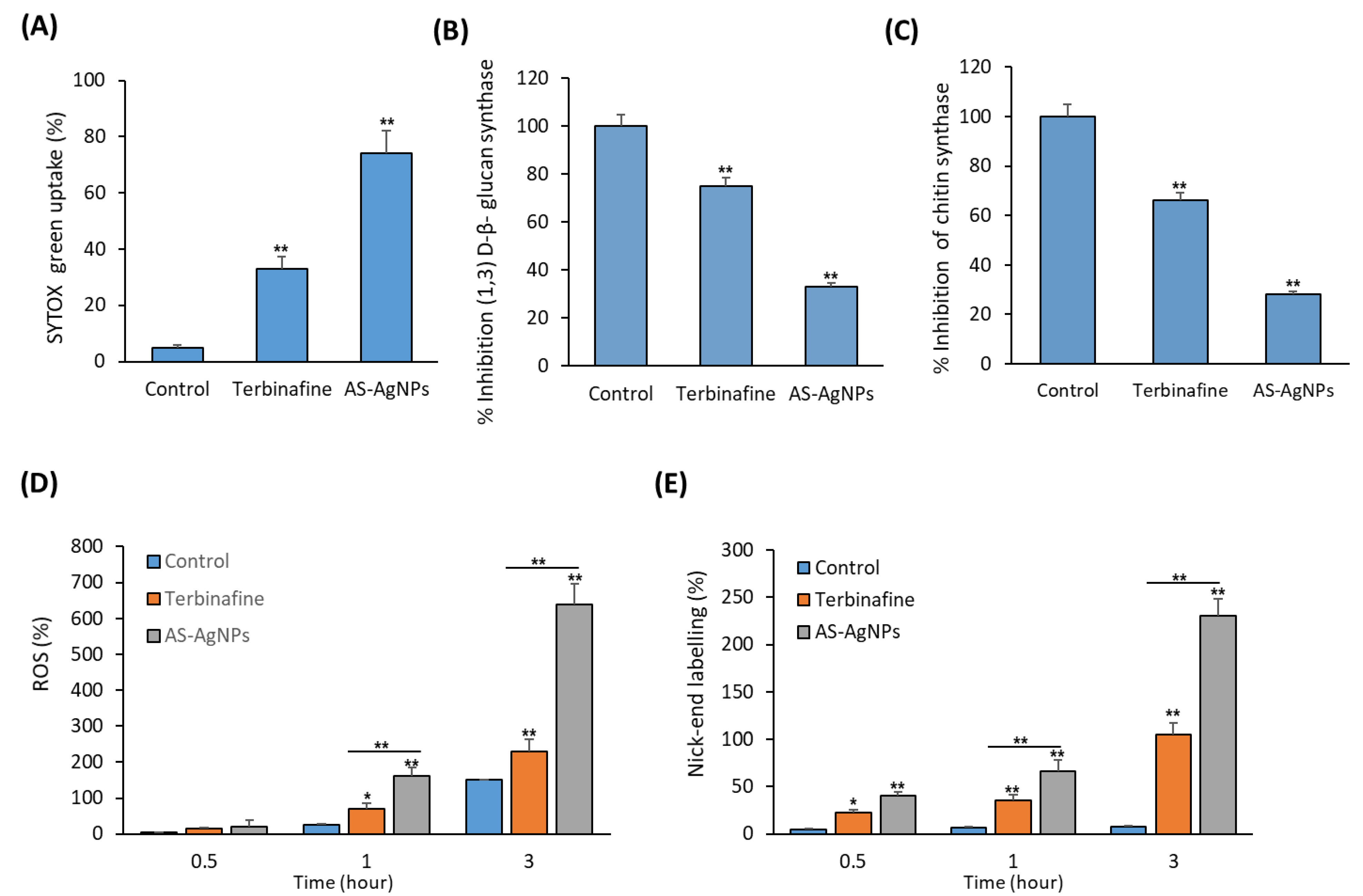
2.3. In Vitro Studies on the Antifungal Mechanism of AS-AgNPs
2.4. Effect of AS-AgNPs on Ultrastructural Changes in T. rubrum
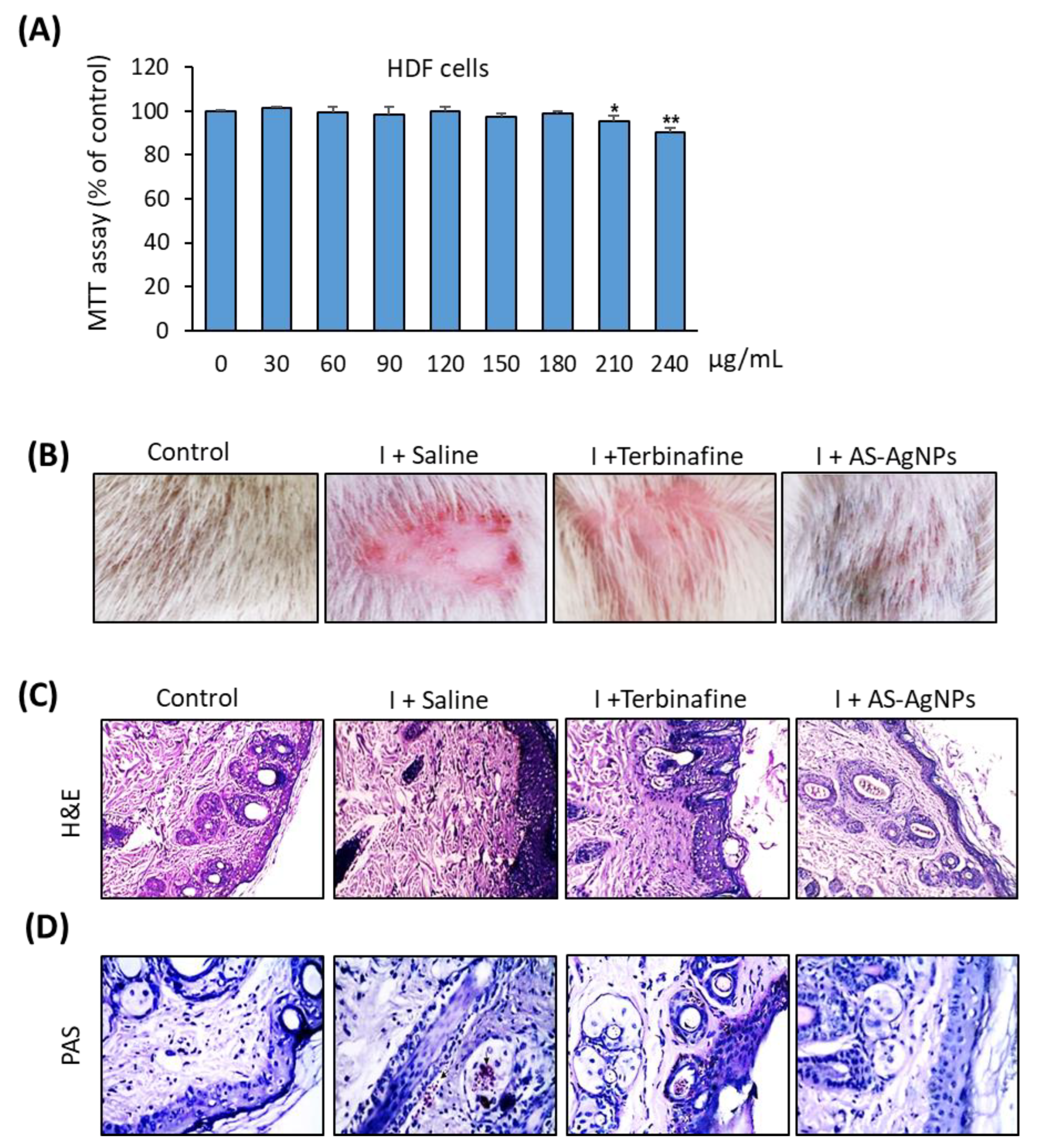
2.5. Effective In Vivo Topical Treatment of Dermal T. rubrum Infection Using AS-AgNPs
2.6. AS-AgNPs Significantly Reduce Fungal Burden and Tissue Inflammation in Rat Model of T. rubrum Deramatophytosis
3. Materials and Methods
3.1. Plant Material and Preparation of the Extract
3.2. Biosynthesis of AS-AgNPs
3.3. Characterization of AS-AgNPs
3.4. Fungal Strain
3.5. Minimum Inhibitory Concentration (MIC)
3.6. Hyphal Growth Inhibition
3.7. Effects on Conidial Germination
3.8. Membrane Permeability Assays
3.8.1. Release of Intracellular Material
3.8.2. Ergosterol Quantitation
3.8.3. Activity of CYP51 Enzyme
3.8.4. SYTOX® Green Uptake
3.9. Cell Wall Integrity Assays
Activity of β-(1,3)-d-Glucan Synthase and Chitin Synthase
3.10. Reactive Oxygen Species (ROS) Assay
3.11. DNA Fragmentation Assay
3.12. Effects on Morphology and Ultrastructure
3.12.1. Inverted Phase Contrast Microscopy
3.12.2. Scanning and Transmission Electron Microscopy
3.13. Cell culture and Cytotoxicity Assay
3.14. Rat Dermatophytosis Model
3.15. Fungal Burgen
3.16. Histopathology
3.17. Measurement of Cytokine Levels in Skin Tissue
3.18. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vermout, S.; Tabart, J.; Baldo, A.; Mathy, A.; Losson, B.; Mignon, B. Pathogenesis of dermatophytosis. Mycopathologia 2008, 166, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M. Boric Acid Inhibition of Trichophyton rubrum Growth and Conidia Formation. Biol. Trace Elem. Res. 2017, 180, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Blutfield, M.S.; Lohre, J.M.; Pawich, D.A.; Vlahovic, T.C. The Immunologic Response to Trichophyton Rubrum in Lower Extremity Fungal Infections. J. Fungi 2015, 1, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Baldo, A.; Monod, M.; Mathy, A.; Cambier, L.; Bagut, E.T.; Defaweux, V.; Symoens, F.; Antoine, N.; Mignon, B. Mechanisms of skin adherence and invasion by dermatophytes. Mycoses 2012, 55, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Ishizaki, H.; Matsumoto, T.; Hori, Y. In vitro release of granulocyte/macrophage colony-stimulating factor by peripheral blood mononuclear cells of dermatophytosis patients in response to stimulation with trichophytin. Clin. Exp. Dermatol. 1994, 19, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Matsumoto, Y.; Yamada, T.; Abe, S.; Sekimizu, K. An invertebrate infection model for evaluating anti-fungal agents against dermatophytosis. Sci. Rep. 2017, 7, 12289. [Google Scholar] [CrossRef]
- Yamada, T.; Maeda, M.; Alshahni, M.M.; Tanaka, R.; Yaguchi, T.; Bontems, O.; Salamin, K.; Fratti, M.; Monod, M. Terbinafine Resistance of Trichophyton Clinical Isolates Caused by Specific Point Mutations in the Squalene Epoxidase Gene. Antimicrob. Agents Chemother. 2017, 61, e00115-17. [Google Scholar] [CrossRef]
- Darier, S.; Tammam, A. Potentially phytotoxic effect of aqueous extract of Achillea santolina induced oxidative stress on Vicia faba and Hordeum vulgare. Rom. J. Biol. 2012, 57, 1–5. [Google Scholar]
- Yazdanparast, R.; Ardestani, A.; Jamshidi, S. Experimental diabetes treated with Achillea santolina: Effect on pancreatic oxidative parameters. J. Ethnopharmacol. 2007, 112, 13–18. [Google Scholar] [CrossRef]
- Zaringhalam, J.; Akbari, A.; Tekieh, E.; Manaheji, H.; Rezazadeh, S. Achillea santolina reduces serum interleukin-6 level and hyperalgesia during complete Freund’s adjuvant-induced inflammation in male Wistar rats. J. Chin. Integr. Med. 2010, 8, 1180–1189. [Google Scholar] [CrossRef]
- Barda, C.; Grafakou, M.-E.; Tomou, E.-M.; Skaltsa, H. Phytochemistry and Evidence-Based Traditional Uses of the Genus achillea L.: An Update (2011–2021). Sci. Pharm. 2021, 89, 50. [Google Scholar] [CrossRef]
- Faisal, M.; Inayat, A.; Nabi, M.; Hayat, W.; Khan, M.; Iqbal, W. Screening of achillea santolina for anti-diabetic activity and its comparison with caralluma tuberculata. original prof-0-4066. Prof. Med. J. 2020, 27, 1414–1419. [Google Scholar]
- Sabbagh, F.; Kiarostami, K.; Mahmoudi Khatir, N.; Rezania, S.; Muhamad, I.I. Green Synthesis of Mg(0.99) Zn(0.01)O Nanoparticles for the Fabrication of κ-Carrageenan/NaCMC Hydrogel in order to Deliver Catechin. Polymers 2020, 12, 861. [Google Scholar] [CrossRef] [PubMed]
- Some, S.; Mondal, R.; Dam, P.; Mandal, A. Synthesis of biogenic silver nanoparticles using medicinal plant extract: A new age in nanomedicine to combat multidrug-resistant pathogens. Green Synth. Silver Nanomater. 2021, 2022, 359–387. [Google Scholar]
- Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application. Nanomaterials 2022, 12, 673. [Google Scholar] [CrossRef]
- Essghaier, B.; Dridi, R.; Mottola, F.; Rocco, L.; Zid, M.F.; Hannachi, H. Biosynthesis and Characterization of Silver Nanoparticles from the Extremophile Plant Aeonium haworthii and Their Antioxidant, Antimicrobial and Anti-Diabetic Capacities. Nanomaterials 2022, 13, 100. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Ali, E.M. Therapeutic Effect of Green Synthesized Silver Nanoparticles Using Erodium glaucophyllum Extract against Oral Candidiasis: In Vitro and In Vivo Study. Molecules 2022, 27, 4221. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Ali, E.M. Green Synthesis of Silver Nanoparticles Using the Lotus lalambensis Aqueous Leaf Extract and Their Anti-Candidal Activity against Oral Candidiasis. ACS Omega 2021, 6, 8151–8162. [Google Scholar] [CrossRef]
- Ali, E.M.; Abdallah, B.M. Effective Inhibition of Candidiasis Using an Eco-Friendly Leaf Extract of Calotropis-gigantean-Mediated Silver Nanoparticles. Nanomaterials 2020, 10, 422. [Google Scholar] [CrossRef]
- Ali, E.M.; Abdallah, B.M. Effective Inhibition of Invasive Pulmonary Aspergillosis by Silver Nanoparticles Biosynthesized with Artemisia sieberi Leaf Extract. Nanomaterials 2021, 12, 51. [Google Scholar] [CrossRef]
- Jennings, K.R. Organic Mass Spectrometry. In Spectrometric Identification of Organic Compounds, 5th ed.; Silverstein, R.M., Bassler, G.C., Morrill, T.C., Eds.; Wiley: New York, NY, USA, 1991; Volume 26, p. 813. ISBN 0471 63404 2. [Google Scholar]
- Shameli, K.; Bin Ahmad, M.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Mahdavi, M.; Abdollahi, Y. Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method. Int. J. Mol. Sci. 2012, 13, 6639–6650. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Pulit-Prociak, J. Proecological method for the preparation of metal nanoparticles. J. Clean. Prod. 2017, 141, 1030–1039. [Google Scholar] [CrossRef]
- Malik, M.; Aamir Iqbal, M.; Iqbal, Y.; Malik, M.; Bakhsh, S.; Irfan, S.; Ahmad, R.; Pham, P.V. Biosynthesis of silver nanoparticles for biomedical applications: A mini review. Inorg. Chem. Commun. 2022, 145, 109980. [Google Scholar] [CrossRef]
- Rakib-Uz-Zaman, S.M.; Hoque Apu, E.; Muntasir, M.N.; Mowna, S.A.; Khanom, M.G.; Jahan, S.S.; Akter, N.; RKhan, M.A.; Shuborna, N.S.; Shams, S.M.; et al. Biosynthesis of Silver Nanoparticles from Cymbopogon citratus Leaf Extract and Evaluation of Their Antimicrobial Properties. Challenges 2022, 13, 18. [Google Scholar] [CrossRef]
- Naveed, M.; Bukhari, B.; Aziz, T.; Zaib, S.; Mansoor, M.A.; Khan, A.A.; Shahzad, M.; Dablool, A.S.; Alruways, M.W.; Almalki, A.A.; et al. Green Synthesis of Silver Nanoparticles Using the Plant Extract of Acer oblongifolium and Study of Its Antibacterial and Antiproliferative Activity via Mathematical Approaches. Molecules 2022, 27, 4226. [Google Scholar] [CrossRef]
- Shanmugam, J.; Dhayalan, M.; Savaas Umar, M.R.; Gopal, M.; Ali Khan, M.; Simal-Gandara, J.; Cid-Samamed, A. Green Synthesis of Silver Nanoparticles Using Allium cepa var. Aggregatum Natural Extract: Antibacterial and Cytotoxic Properties. Nanomaterials 2022, 12, 1725. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, J.M.; Ansari, M.A.; Khan, H.M.; Alzohairy, M.A.; Choi, I. Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Sci. Rep. 2016, 6, 20414. [Google Scholar] [CrossRef]
- Sastry, M.; Mayya, K.S.; Bandyopadhyay, K. pH Dependent changes in the optical properties of carboxylic acid derivatized silver colloidal particles. Colloids Surf. A Physicochem. Eng. Asp. 1997, 127, 221–228. [Google Scholar] [CrossRef]
- Hg, B.; Rane, N.; Sm, S.; Bhangale, H. Microbial Synthesis of Gold and Silver Nanoparticles and their Characterization. Int. J. Life Sci. 2022, 38, 2254–2260. [Google Scholar]
- Frattini, A.; Pellegri, N.; Nicastro, D.; de Sanctis, O. Effect of amine groups in the synthesis of Ag nanoparticles using aminosilanes. Mater. Chem. Phys. 2005, 94, 148–152. [Google Scholar] [CrossRef]
- Binsalah, M.; Devanesan, S.; AlSalhi, M.S.; Nooh, A.; Alghamdi, O.; Nooh, N. Biomimetic Synthesis of Silver Nanoparticles Using Ethyl Acetate Extract of Urtica diocia Leaves; Characterizations and Emerging Antimicrobial Activity. Microorganisms 2022, 10, 789. [Google Scholar] [CrossRef]
- Khan, M.; Karuppiah, P.; Alkhathlan, H.Z.; Kuniyil, M.; Khan, M.; Adil, S.F.; Shaik, M.R. Green Synthesis of Silver Nanoparticles Using Juniperus procera Extract: Their Characterization, and Biological Activity. Crystals 2022, 12, 420. [Google Scholar] [CrossRef]
- Skandalis, N.; Dimopoulou, A.; Georgopoulou, A.; Gallios, N.; Papadopoulos, D.; Tsipas, D.; Theologidis, I.; Michailidis, N.; Chatzinikolaidou, M. The Effect of Silver Nanoparticles Size, Produced Using Plant Extract from Arbutus unedo, on Their Antibacterial Efficacy. Nanomaterials 2017, 7, 178. [Google Scholar] [CrossRef]
- Ghandour, W.; Hubbard, J.A.M.; Deistung, J.; Hughes, M.N.; Poole, R.K. The uptake of silver ions by Escherichia coli K12: Toxic effects and interaction with copper ions. Appl. Microbiol. Biotechnol. 1988, 28, 559–565. [Google Scholar] [CrossRef]
- Ayatollahi Mousavi, S.A.; Salari, S.; Hadizadeh, S. Evaluation of Antifungal Effect of Silver Nanoparticles Against Microsporum canis, Trichophyton mentagrophytes and Microsporum gypseum. Iran. J. Biotechnol. 2015, 13, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Igaz, N.; Gopisetty, M.K.; Szerencsés, B.; Kovács, D.; Papp, C.; Vágvölgyi, C.; Boros, I.M.; Kónya, Z.; Kiricsi, M.; et al. Biosynthesized silver and gold nanoparticles are potent antimycotics against opportunistic pathogenic yeasts and dermatophytes. Int. J. Nanomed. 2018, 13, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Ouf, S.A.; El-Adly, A.A.; Mohamed, A.H. Inhibitory effect of silver nanoparticles mediated by atmospheric pressure air cold plasma jet against dermatophyte fungi. J. Med. Microbiol. 2015, 64, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, J.; Sowmya, B.; Venkat Kumar, S.; Rajeshkumar, S. Toxicology evaluation and antidermatophytic activity of silver nanoparticles synthesized using leaf extract of Passiflora caerulea. S. Afr. J. Chem. Eng. 2019, 29, 17–23. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, R.; Yin, T.; Wang, Q.; Guo, Z.; Qiwen, T.; Bilal, M.; He, S.; Zhu, X.; Shi, H.; et al. Novel bio-fabrication of silver nanoparticles using the cell-free extract of Lysinibacillus fusiformis sp. and their potent activity against pathogenic fungi. Mater. Res. Express 2019, 6, 1250f1252. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Ali, F.; Rahul Naz, F.; Jyoti, S.; Siddique, Y. Health Functionality of Apigenin: A Review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Bhatia, V.K.; Sharma, P.C. Epidemiological studies on Dermatophytosis in human patients in Himachal Pradesh, India. SpringerPlus 2014, 3, 134. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Sung, W.S.; Suh, B.K.; Moon, S.K.; Choi, J.S.; Kim, J.G.; Lee, D.G. Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2009, 22, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Dantas Ada, S.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative stress responses in the human fungal pathogen, Candida albicans. Biomolecules 2015, 5, 142–165. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, Y.; Takahata, S.; Abe, S. Morphological Effect of the New Antifungal Agent ME1111 on Hyphal Growth of Trichophyton mentagrophytes, Determined by Scanning and Transmission Electron Microscopy. Antimicrob. Agents Chemother. 2017, 61, e01195-16. [Google Scholar] [CrossRef]
- Hultenby, K.; Chryssanthou, E.; Klingspor, L.; Rensfeldt, K.; Strömbeck, L.; Faergemann, J. The effect of K101 Nail Solution on Trichophyton rubrum and Candida albicans growth and ultrastructure. Mycoses 2014, 57, 630–638. [Google Scholar] [CrossRef]
- Hata, M.; Ishii, Y.; Watanabe, E.; Uoto, K.; Kobayashi, S.; Yoshida, K.-I.; Otani, T.; Ando, A. Inhibition of ergosterol synthesis by novel antifungal compounds targeting C-14 reductase. Med. Mycol. 2010, 48, 613–621. [Google Scholar] [CrossRef]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58 Pt 11, 1454–1462. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Reddy Mudiam, M.K.; Kumar, M.; Dwivedi, S.P.; Singh, S.P.; Prasad, T. Silver nanoparticles induced alterations in multiple cellular targets, which are critical for drug susceptibilities and pathogenicity in fungal pathogen (Candida albicans). Int. J. Nanomed. 2018, 13, 2647–2663. [Google Scholar] [CrossRef]
- Jia, D.; Sun, W. Silver nanoparticles offer a synergistic effect with fluconazole against fluconazole-resistant Candida albicans by abrogating drug efflux pumps and increasing endogenous ROS. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2021, 93, 104937. [Google Scholar] [CrossRef]
- Lima, S.L.; Colombo, A.L.; de Almeida Junior, J.N. Fungal Cell Wall: Emerging Antifungals and Drug Resistance. Front. Microbiol. 2019, 10, 2573. [Google Scholar] [CrossRef]
- Kuang, R.; Wu, H.; Ting, P.C.; Aslanian, R.G.; Cao, J.; Kim, D.W.; Lee, J.F.; Schwerdt, J.; Zhou, G.; Herr, R.J.; et al. The optimization of pyridazinone series of glucan synthase inhibitors. Bioorganic Med. Chem. Lett. 2012, 22, 5268–5271. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.C.G.; Curto, M.; Carvalho, V.S.D.; Pérez, P.; Ribas, J.C. The fungal cell wall as a target for the development of new antifungal therapies. Biotechnol. Adv. 2019, 37, 107352. [Google Scholar] [CrossRef] [PubMed]
- Culakova, H.; Dzugasova, V.; Valencikova, R.; Gbelska, Y.; Subik, J. Stress response and expression of fluconazole resistance associated genes in the pathogenic yeast Candida glabrata deleted in the CgPDR16 gene. Microbiol. Res. 2015, 174, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, M.J.; Yun, S.J.; Kim, K.; Choi, I.H.; Park, S. Silver nanoparticles induce reactive oxygen species-mediated cell cycle delay and synergistic cytotoxicity with 3-bromopyruvate in Candida albicans, but not in Saccharomyces cerevisiae. Int. J. Nanomed. 2019, 14, 4801–4816. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.R.; Russo, M.; Gomes, E.; Almeida, S.R. Stimulation, inhibition and death of macrophages infected with Trichophyton rubrum. Microbes Infect. 2006, 8, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Calderon, R.A.; Hay, R.J. Fungicidal activity of human neutrophils and monocytes on dermatophyte fungi, Trichophyton quinckeanum and Trichophyton rubrum. Immunology 1987, 61, 289–295. [Google Scholar]
- Mignon, B.; Tabart, J.; Baldo, A.; Mathy, A.; Losson, B.; Vermout, S. Immunization and dermatophytes. Curr. Opin. Infect. Dis. 2008, 21, 134–140. [Google Scholar] [CrossRef]
- Gonzalez Segura, G.; Cantelli, B.A.; Peronni, K.; Rodrigo Sanches, P.; Komoto, T.T.; Rizzi, E.; Beleboni, R.O.; Junior, W.; Martinez-Rossi, N.M.; Marins, M.; et al. Cellular and Molecular Response of Macrophages THP-1 during Co-Culture with Inactive Trichophyton rubrum Conidia. J. Fungi 2020, 6, 363. [Google Scholar] [CrossRef]
- Mordorski, B.; Costa-Orlandi, C.B.; Baltazar, L.M.; Carreño, L.J.; Landriscina, A.; Rosen, J.; Navati, M.; Mendes-Giannini, M.J.S.; Friedman, J.M.; Nosanchuk, J.D.; et al. Topical nitric oxide releasing nanoparticles are effective in a murine model of dermal Trichophyton rubrum dermatophytosis. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2267–2270. [Google Scholar] [CrossRef]
- Cascione, M.; Rizzello, L.; Manno, D.; Serra, A.; De Matteis, V. Green Silver Nanoparticles Promote Inflammation Shutdown in Human Leukemic Monocytes. Materials 2022, 15, 775. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; Banerjee, P. Green nanotechnology—A new hope for medical biology. Environ. Toxicol. Pharmacol. 2013, 36, 997–1014. [Google Scholar] [CrossRef]
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwar, V. Eco-Friendly Greener Synthesis of Nanoparticles. Adv. Pharm. Bull. 2020, 10, 566–576. [Google Scholar] [CrossRef]
- Ouf, S.A.; Mohamed, A.-A.H.; El-Adly, A.A. Enhancement of the antidermatophytic activity of silver nanoparticles by Q-switched Nd:YAG laser and monoclonal antibody conjugation. Med. Mycol. 2016, 55, 495–506. [Google Scholar] [CrossRef]
- Von White, G.; Kerscher, P.; Brown, R.M.; Morella, J.D.; McAllister, W.; Dean, D.; Kitchens, C.L. Green Synthesis of Robust, Biocompatible Silver Nanoparticles Using Garlic Extract. J. Nanomater. 2012, 2012, 730746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jegadeeswaran, P.; Shivaraj, R.; Rajendran, V. Green synthesis of silver nanoparticles from extract of Padina tetrastromatica leaf. Dig. J. Nanomater. Biostructures 2012, 7, 991–998. [Google Scholar]
- Devadiga, A.; Shetty, K.V.; Saidutta, M.B. Timber industry waste-teak (Tectona grandis Linn.) leaf extract mediated synthesis of antibacterial silver nanoparticles. Int. Nano Lett. 2015, 5, 205–214. [Google Scholar] [CrossRef]
- Santos, D.A.; Hamdan, J.S. Evaluation of broth microdilution antifungal susceptibility testing conditions for Trichophyton rubrum. J. Clin. Microbiol. 2005, 43, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tripathi, A. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol. Res. 2008, 163, 337–344. [Google Scholar] [CrossRef]
- Pereira, F.; Moura Mendes Arrua, J.; Lima, E. Investigation on mechanism of antifungal activity of eugenol against Trichophyton rubrum. Med. Mycol. Off. Publ. Int. Soc. Hum. Anim. Mycol. 2012, 51, 507–513. [Google Scholar]
- Arthington-Skaggs, B.A.; Motley, M.; Warnock, D.W.; Morrison, C.J. Comparative evaluation of PASCO and national committee for clinical laboratory standards M27-A broth microdilution methods for antifungal drug susceptibility testing of yeasts. J. Clin. Microbiol. 2000, 38, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Jin, L.; Yang, C.; Zhu, Y.; Ye, X.; Li, X.; Zhang, B. Antifungal activity and potential mechanism of magnoflorine against Trichophyton rubrum. J. Antibiot. 2021, 74, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Hagen, S.; Marx, F.; Ram, A.; Meyer, V. The Antifungal Protein AFP from Aspergillus giganteus Inhibits Chitin Synthesis in Sensitive Fungi. Appl. Environ. Microbiol. 2007, 73, 2128–2134. [Google Scholar] [CrossRef]
- Kobayashi, D.; Kondo, K.; Uehara, N.; Otokozawa, S.; Tsuji, N.; Yagihashi, A.; Watanabe, N. Endogenous reactive oxygen species is an important mediator of miconazole antifungal effect. Antimicrob. Agents Chemother. 2002, 46, 3113–3117. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Krom, B.P.; Meijering, R.A.; Peters, B.M.; Zhu, J.; Scheper, M.A.; Harris, M.L.; Jabra-Rizk, M.A. Farnesol-induced apoptosis in Candida albicans. Antimicrob. Agents Chemother. 2009, 53, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Mady, O.Y.; Al-Madboly, L.A.; Donia, A.A. Preparation, and Assessment of Antidermatophyte Activity of Miconazole-Urea Water-Soluble Film. Front. Microbiol. 2020, 11, 385. [Google Scholar] [CrossRef] [PubMed]
- Athie-García, M.S.; Piñón-Castillo, H.A.; Muñoz-Castellanos, L.N.; Ulloa-Ogaz, A.L.; Martínez-Varela, P.I.; Quintero-Ramos, A.; Duran, R.; Murillo-Ramirez, J.G.; Orrantia-Borunda, E. Cell wall damage and oxidative stress in Candida albicans ATCC10231 and Aspergillus niger caused by palladium nanoparticles. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2018, 48, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Baltazar Lde, M.; Santos, P.C.; Paula, T.P.; Rachid, M.A.; Cisalpino, P.S.; Souza, D.G.; Santos, D.A. IFN-γ impairs Trichophyton rubrum proliferation in a murine model of dermatophytosis through the production of IL-1β and reactive oxygen species. Med. Mycol. 2014, 52, 293–302. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, B.M.; Rajendran, P.; Ali, E.M. Potential Treatment of Dermatophyte Trichophyton rubrum in Rat Model Using Topical Green Biosynthesized Silver Nanoparticles with Achillea santolina Extract. Molecules 2023, 28, 1536. https://doi.org/10.3390/molecules28041536
Abdallah BM, Rajendran P, Ali EM. Potential Treatment of Dermatophyte Trichophyton rubrum in Rat Model Using Topical Green Biosynthesized Silver Nanoparticles with Achillea santolina Extract. Molecules. 2023; 28(4):1536. https://doi.org/10.3390/molecules28041536
Chicago/Turabian StyleAbdallah, Basem M., Peramaiyan Rajendran, and Enas M. Ali. 2023. "Potential Treatment of Dermatophyte Trichophyton rubrum in Rat Model Using Topical Green Biosynthesized Silver Nanoparticles with Achillea santolina Extract" Molecules 28, no. 4: 1536. https://doi.org/10.3390/molecules28041536
APA StyleAbdallah, B. M., Rajendran, P., & Ali, E. M. (2023). Potential Treatment of Dermatophyte Trichophyton rubrum in Rat Model Using Topical Green Biosynthesized Silver Nanoparticles with Achillea santolina Extract. Molecules, 28(4), 1536. https://doi.org/10.3390/molecules28041536






