Preliminary Structural Characterization of Selenium Nanoparticle Composites Modified by Astragalus Polysaccharide and the Cytotoxicity Mechanism on Liver Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Synthesis of AASP−SeNPs
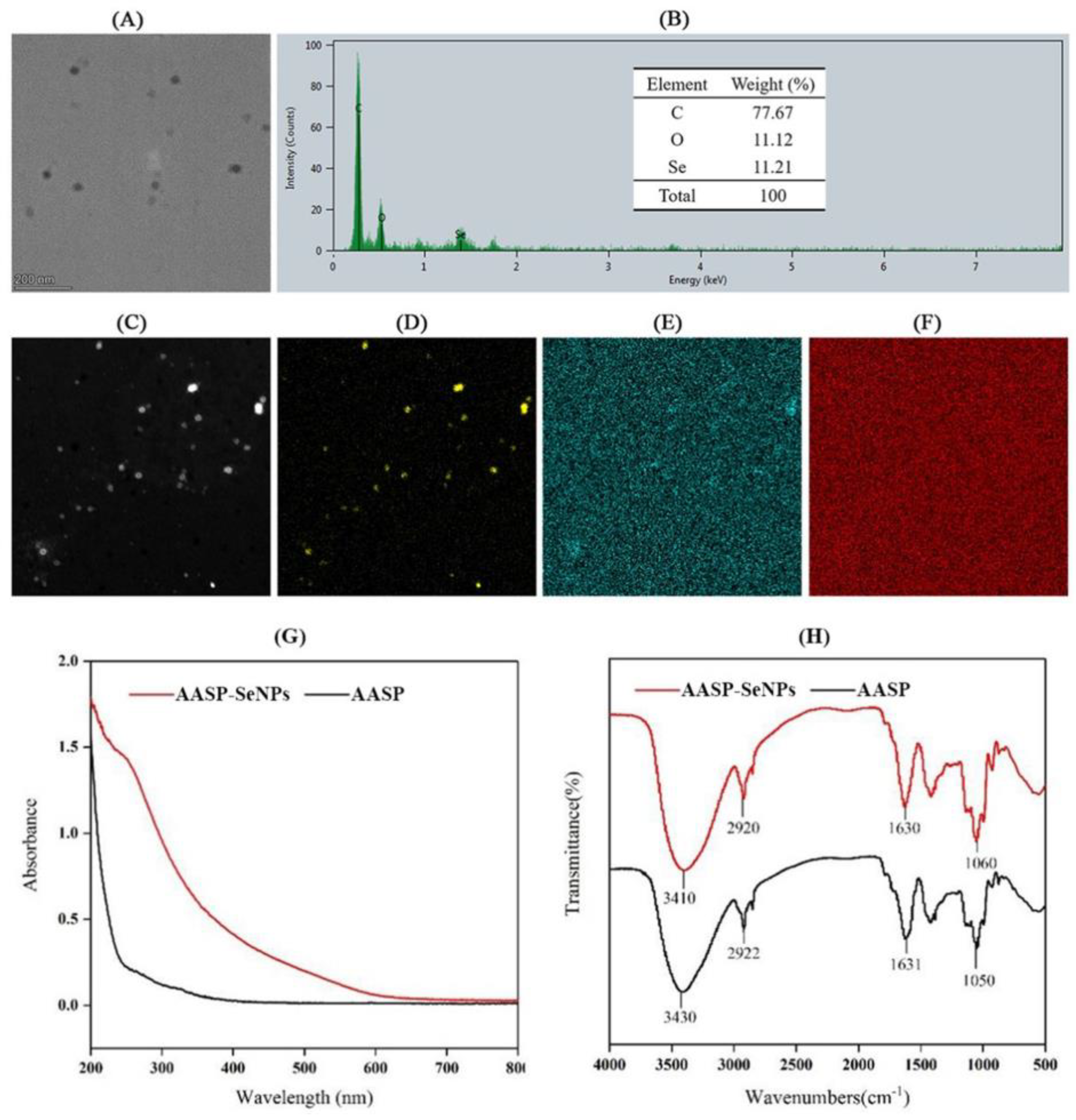
2.2. Characterization and Synthetic Mechanism of AASP−SeNPs
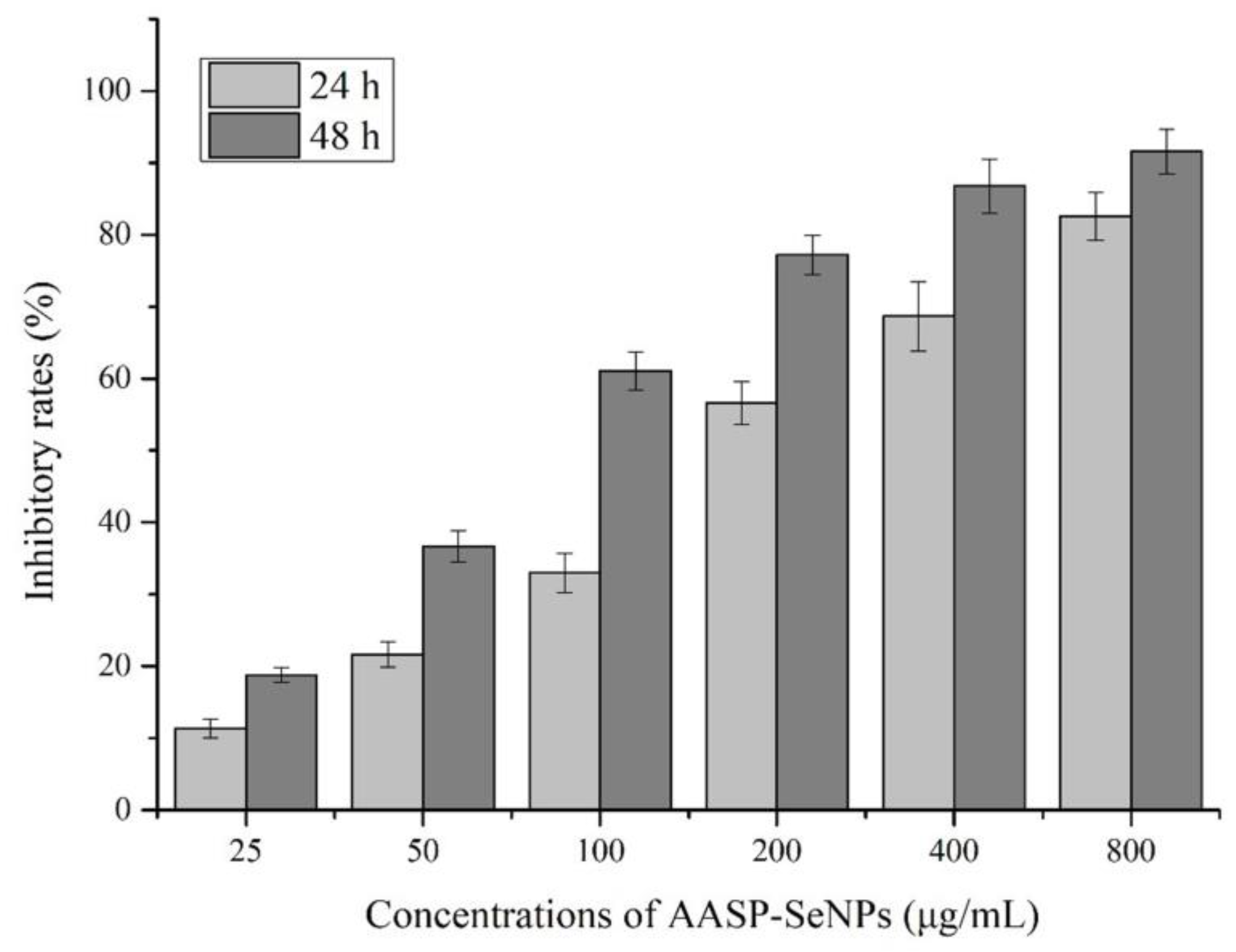
2.3. Inhibitory Effects of AASP−SeNPs on HepG2 Cells
2.4. Morphological Alterations and Apoptotic Rates of HepG2 Cells
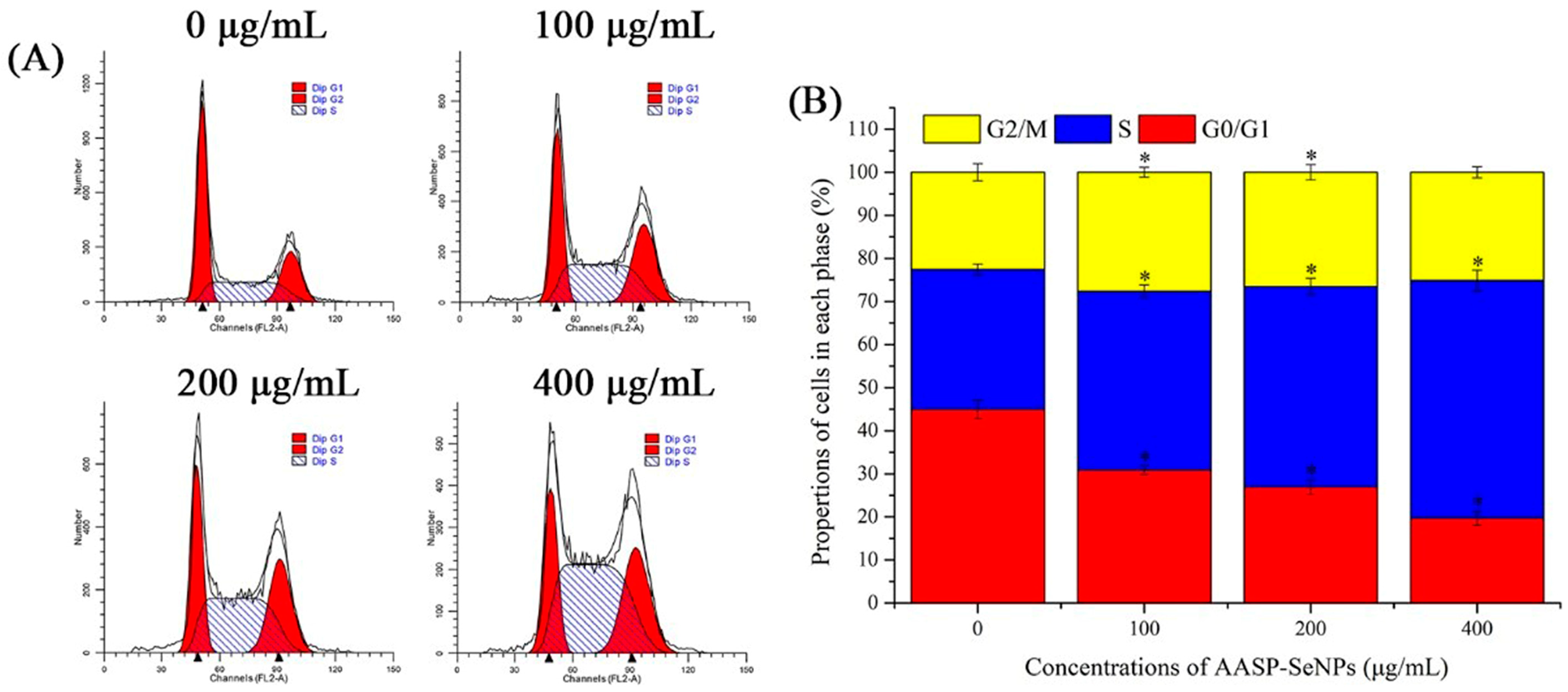
2.5. Effects of AASP−SeNPs on Cell Cycle Distributions of HepG2 Cells
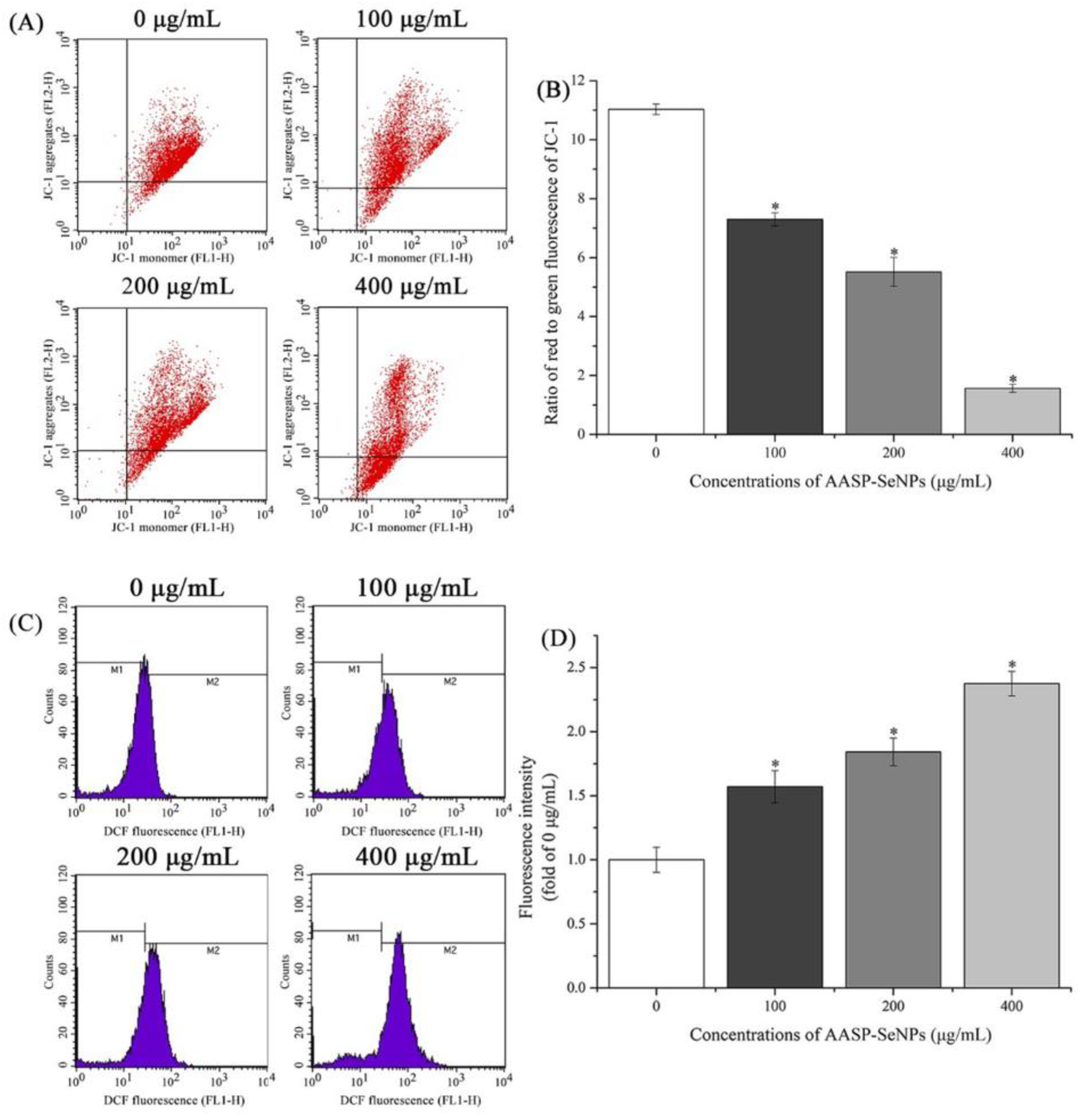
2.6. ROS and ΔΨm Levels in Mitochondria of HepG2 Cells
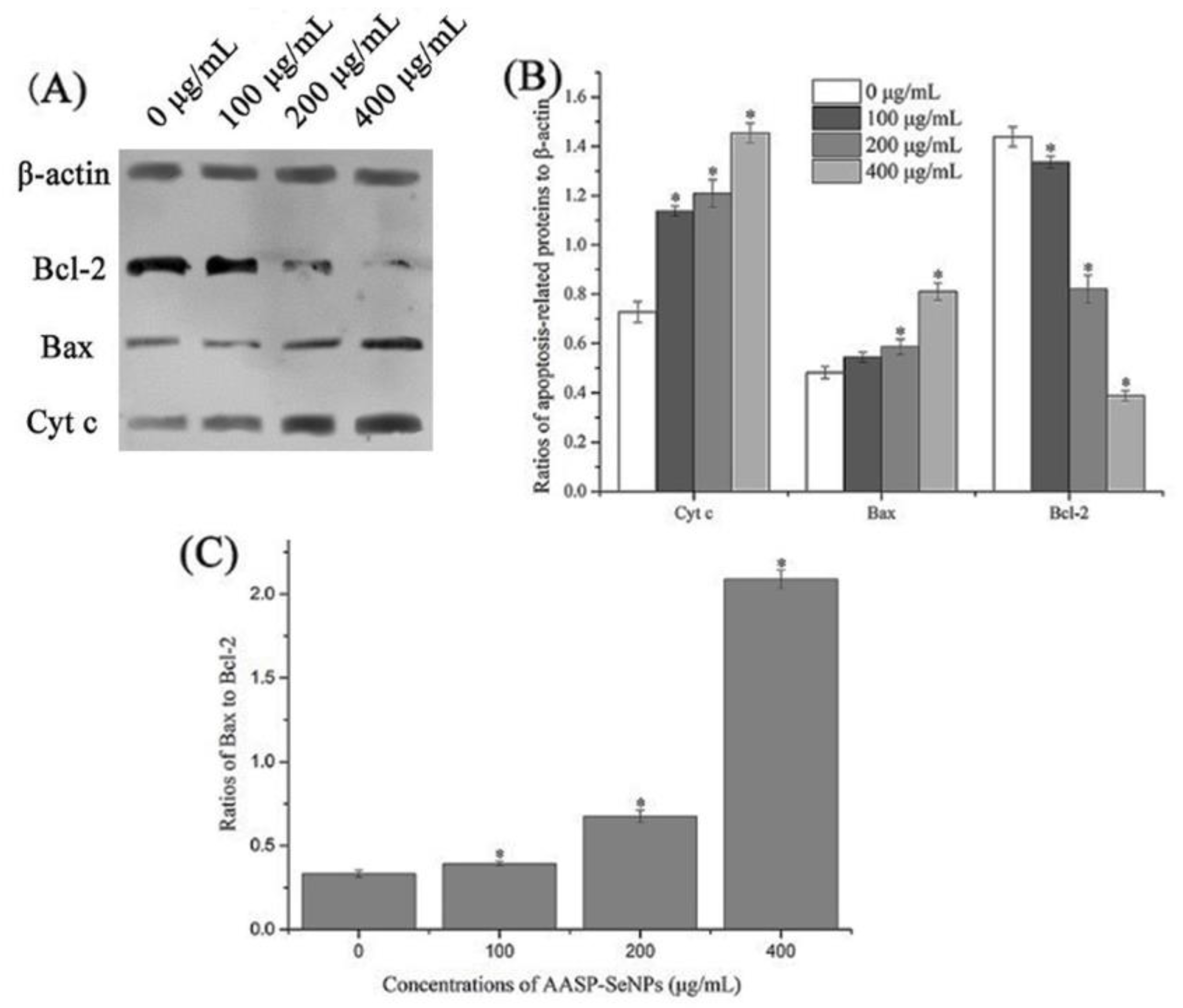
2.7. Apoptosis-Associated Protein Levels of HepG2 Cells
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of AASP−SeNPs
3.3. Physicochemical Properties of AASP−SeNPs
3.4. Anti-Hepatoma Activity of AASP−SeNPs In Vitro
3.4.1. MTT Assay
3.4.2. Cell Morphological Observation
3.4.3. Cell Apoptosis Assay
3.4.4. Cell Cycle Distribution
3.4.5. Mitochondrial Membrane Potential (ΔΨm) Determination
3.4.6. ROS Assay
3.4.7. Apoptosis-Related Protein Detection
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Peng, Z.W.; Zhang, Y.J.; Liang, H.H.; Lin, X.J.; Guo, R.P.; Chen, M.S. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: A prospective randomized trial. Radiology 2012, 262, 689–700. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, M.; Zhu, J.; Qu, J.; Qin, K.; Zhao, D.; Wang, L.; Dong, L.; Zhang, X. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed. Pharmacother. 2020, 132, 110797. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.P.; Zhang, X.F.; Zhang, G.L.; Huang, Y.F.; Gurunathan, S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int. J. Nanomed. 2017, 12, 6521–6535. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef]
- Dolgova, N.V.; Hackett, M.J.; MacDonald, T.C.; Nehzati, S.; James, A.K.; Krone, P.H.; George, G.N.; Pickering, I.J. Distribution of selenium in zebrafish larvae after exposure to organic and inorganic selenium forms. Metallomics 2016, 8, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Sanmartin, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef]
- Li, J.; Shen, B.; Nie, S.; Duan, Z.; Chen, K. A combination of selenium and polysaccharides: Promising therapeutic potential. Carbohydr. Polym. 2019, 206, 163–173. [Google Scholar] [CrossRef]
- Zhang, C.; Zhai, X.; Zhao, G.; Ren, F.; Leng, X. Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydr. Polym. 2015, 134, 158–166. [Google Scholar] [CrossRef]
- Menon, S.; Ks, S.D.; Santhiya, R.; Rajeshkumar, S.; Kumar, V. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf. B Biointerfaces 2018, 170, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, X.; Hunziker, P. Carbohydrate-based amphiphilic nano delivery systems for cancer therapy. Nanoscale 2016, 8, 16091–16156. [Google Scholar] [CrossRef] [PubMed]
- Tugarova, A.V.; Kamnev, A.A. Proteins in microbial synthesis of selenium nanoparticles. Talanta 2017, 174, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Ma, J.; Liang, T.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Selenium nanoparticles fabricated in laminarin polysaccharides solutions exert their cytotoxicities in HepG2 cells by inhibiting autophagy and promoting apoptosis. Int. J. Biol. Macromol. 2019, 137, 829–835. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Qiu, W.-Y.; Sun, L.; Ding, Z.-C.; Yan, J.-K. Preparation, characterization, and antioxidant capacities of selenium nanoparticles stabilized using polysaccharide–protein complexes from Corbicula fluminea. Food Biosci. 2018, 26, 177–184. [Google Scholar] [CrossRef]
- Cai, W.; Hu, T.; Bakry, A.M.; Zheng, Z.; Xiao, Y.; Huang, Q. Effect of ultrasound on size, morphology, stability and antioxidant activity of selenium nanoparticles dispersed by a hyperbranched polysaccharide from Lignosus rhinocerotis. Ultrason. Sonochem. 2018, 42, 823–831. [Google Scholar] [CrossRef]
- Yu, J.; Ji, H.-Y.; Liu, A.-J. Alcohol-soluble polysaccharide from Astragalus membranaceus: Preparation, characteristics and antitumor activity. Int. J. Biol. Macromol. 2018, 118, 2057–2064. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Q.; Zheng, Z.; Guan, H.; Liu, S. Construction of a Cordyceps sinensis exopolysaccharide-conjugated selenium nanoparticles and enhancement of their antioxidant activities. Int. J. Biol. Macromol. 2017, 99, 483–491. [Google Scholar] [CrossRef]
- Yan, J.-K.; Qiu, W.-Y.; Wang, Y.-Y.; Wang, W.-H.; Yang, Y.; Zhang, H.-N. Fabrication and stabilization of biocompatible selenium nanoparticles by carboxylic curdlans with various molecular properties. Carbohydr. Polym. 2018, 179, 19–27. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, P.; Zhao, G.; Sun, K.; Li, D.; Wan, X.; Zhang, J. Inverse relationship between elemental selenium nanoparticle size and inhibition of cancer cell growth in vitro and in vivo. Food Chem. Toxicol. 2015, 85, 71–77. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, Q.; Guo, J.; Guo, R.; Fan, X.; Bi, Y. Synthesis and evaluation of Grateloupia Livida polysaccharides-functionalized selenium nanoparticles. Int. J. Biol. Macromol. 2021, 191, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-Y.; Liu, C.; Dai, K.-Y.; Yu, J.; Liu, A.-J.; Chen, Y.-F. The extraction, structure, and immunomodulation activities in vivo of polysaccharides from Salvia miltiorrhiza. Ind. Crop. Prod. 2021, 173, 114085. [Google Scholar] [CrossRef]
- Liu, G.; Yang, X.; Zhang, J.; Liang, L.; Miao, F.; Ji, T.; Ye, Z.; Chu, M.; Ren, J.; Xu, X. Synthesis, stability and anti-fatigue activity of selenium nanoparticles stabilized by Lycium barbarum polysaccharides. Int. J. Biol. Macromol. 2021, 179, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Huang, Z.; Zheng, W.; Fan, C.; Chen, T. Enhancement of cell permeabilization apoptosis-inducing activity of selenium nanoparticles by ATP surface decoration. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 74–84. [Google Scholar] [CrossRef]
- Sinha, R.; El-Bayoumy, K. Apoptosis is a Critical Cellular Event in Cancer Chemoprevention and Chemotherapy by Selenium Compounds. Curr. Cancer Drug Targets 2004, 4, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, Y.; Zheng, W.; Fan, C.; Chen, T. Positive surface charge enhances selective cellular uptake and anticancer efficacy of selenium nanoparticles. Inorg. Chem. 2012, 51, 8956–8963. [Google Scholar] [CrossRef]
- Lemieszek, K.M.; Stepulak, A.; Sawa-Wejksza, K.; Czerwonka, A.; Ikonomidou, C.; Rzeski, W. Riluzole Inhibits Proliferation, Migration and Cell Cycle Progression and Induces Apoptosis in Tumor Cells of Various Origins. Anti-Cancer Agents Med. Chem. 2018, 18, 565–572. [Google Scholar] [CrossRef]
- Tabish, T.A.; Narayan, R.J. Mitochondria-targeted graphene for advanced cancer therapeutics. Acta Biomater. 2021, 129, 43–56. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Liu, M.; Wu, X.; Cui, Y.; Liu, P.; Xiao, B.; Zhang, X.; Zhang, J.; Sun, Z.; Song, M.; Shao, B.; et al. Mitophagy and apoptosis mediated by ROS participate in AlCl3-induced MC3T3-E1 cell dysfunction. Food Chem. Toxicol. 2021, 155, 112388. [Google Scholar] [CrossRef]
- Helaly, N.A.; Esheba, N.E.; Ammo, D.E.A.; Elwan, N.M.; Elkholy, R.A. High Bax/Bcl-2 ratio is associated with good prognosis and better survival in patients with B cell chronic lymphocytic leukemia. Leuk. Res. 2021, 107, 106604. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Yang, F.; Bai, Y.; Yu, W.; Qiao, N.; Han, Q.; Zhang, H.; Guo, J.; Hu, L.; Li, Y.; et al. Metabolomics analysis reveals the effects of copper on mitochondria-mediated apoptosis in kidney of broiler chicken (Gallus gallus). J. Inorg. Biochem. 2021, 224, 111581. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.-Y.; Huang, Q.; Wang, Y.-L.; Yang, X.-Q.; Su, D.-X.; He, S.; Tan, J.-C.; Zeng, Q.-Z.; Yuan, Y. Development, structure characterization and stability of food grade selenium nanoparticles stabilized by tilapia polypeptides. J. Food Eng. 2020, 275, 109878. [Google Scholar] [CrossRef]
- Ji, H.-Y.; Yu, J.; Jiao, J.-S.; Dong, X.-D.; Yu, S.-S.; Liu, A.-J. Ultrasonic-Assisted Extraction of Codonopsis pilosula Glucofructan: Optimization, Structure, and Immunoregulatory Activity. Nutrients 2022, 14, 927. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Wang, H.; Liu, R.; Xing, W.; Li, J.; Man, Y.B.; Wu, F. Size-dependent transformation, uptake, and transportation of SeNPs in a wheat–soil system. J. Hazard. Mater. 2022, 424, 127323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, J.; Ding, D.; Zhang, L.; Muehlmann, L.A.; Deng, S.E.; Wang, X.; Li, W.; Zhang, W. Synthesis and antioxidant properties of Lycium barbarum polysaccharides capped selenium nanoparticles using tea extract. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1463–1470. [Google Scholar] [CrossRef]
- Carou, M.C.; Cruzans, P.R.; Maruri, A.; Stockert, J.C.; Lombardo, D.M. Apoptosis in ovarian granulosa cells of cattle: Morphological features and clearance by homologous phagocytosis. Acta Histochem. 2015, 117, 92–103. [Google Scholar] [CrossRef]
- Shams, A.; Ahmed, A.; Khan, A.; Khawaja, S.; Rehman, N.U.; Qazi, A.S.; Khan, A.; Bawazeer, S.; Ali, S.A.; Al-Harrasi, A. Naturally Isolated Sesquiterpene Lactone and Hydroxyanthraquinone Induce Apoptosis in Oral Squamous Cell Carcinoma Cell Line. Cancers 2023, 15, 557. [Google Scholar] [CrossRef]
- Selvarathinam, K.; Subramani, P.; Thekkumalai, M.; Vilwanathan, R.; Selvarajan, R.; Abia, A.L.K. Wnt Signaling Pathway Collapse upon β-Catenin Destruction by a Novel Antimicrobial Peptide SKACP003: Unveiling the Molecular Mechanism and Genetic Activities Using Breast Cancer Cell Lines. Molecules 2023, 28, 930. [Google Scholar]
- Cao, T.-Q.; An, H.-X.; Ma, R.-J.; Dai, K.-Y.; Ji, H.-Y.; Liu, A.-J.; Zhou, J.-P. Structural characteristics of a low molecular weight velvet antler protein and the anti-tumor activity on S180 tumor-bearing mice. Bioorg. Chem. 2023, 131, 106304. [Google Scholar] [CrossRef]
- Wang, X.; Ni, H.; Xu, W.; Wu, B.; Xie, T.; Zhang, C.; Cheng, J.; Li, Z.; Tao, L.; Zhang, Y. Difenoconazole induces oxidative DNA damage and mitochondria mediated apoptosis in SH-SY5Y cells. Chemosphere 2021, 283, 131160. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Chen, Y.; Wang, H.; Zhang, R.; Zhang, Q.; Wei, Y.; Shi, S.; Li, X. Calreticulin regulated intrinsic apoptosis through mitochondria-dependent and independent pathways mediated by ER stress in arsenite exposed HT-22 cells. Chemosphere 2020, 251, 126466. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Liao, J.; Li, Q.; Shi, J.; Zhang, H.; Guo, J.; Han, Q.; Hu, L.; Li, Y.; Pan, J.; et al. Copper induces mitochondria-mediated apoptosis via AMPK-mTOR pathway in hypothalamus of Pigs. Ecotoxicol. Environ. Saf. 2021, 220, 112395. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Dong, X.-D.; Jiao, J.-S.; Ji, H.-Y.; Liu, A.-J. Antitumor and immunoregulatory activities of a novel polysaccharide from Astragalus membranaceus on S180 tumor-bearing mice. Int. J. Biol. Macromol. 2021, 189, 930–938. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Lou, X.; Jiao, J.; Li, Y.; Dai, K.; Jia, X. Preliminary Structural Characterization of Selenium Nanoparticle Composites Modified by Astragalus Polysaccharide and the Cytotoxicity Mechanism on Liver Cancer Cells. Molecules 2023, 28, 1561. https://doi.org/10.3390/molecules28041561
Ji H, Lou X, Jiao J, Li Y, Dai K, Jia X. Preliminary Structural Characterization of Selenium Nanoparticle Composites Modified by Astragalus Polysaccharide and the Cytotoxicity Mechanism on Liver Cancer Cells. Molecules. 2023; 28(4):1561. https://doi.org/10.3390/molecules28041561
Chicago/Turabian StyleJi, Haiyu, Xiaowei Lou, Jianshuang Jiao, Yang Li, Keyao Dai, and Xiaoyu Jia. 2023. "Preliminary Structural Characterization of Selenium Nanoparticle Composites Modified by Astragalus Polysaccharide and the Cytotoxicity Mechanism on Liver Cancer Cells" Molecules 28, no. 4: 1561. https://doi.org/10.3390/molecules28041561
APA StyleJi, H., Lou, X., Jiao, J., Li, Y., Dai, K., & Jia, X. (2023). Preliminary Structural Characterization of Selenium Nanoparticle Composites Modified by Astragalus Polysaccharide and the Cytotoxicity Mechanism on Liver Cancer Cells. Molecules, 28(4), 1561. https://doi.org/10.3390/molecules28041561






