Redox Biomarkers Assessment after Oral Administration of Wine Extract and Grape Stem Extract in Rats and Mice
Abstract
:1. Introduction
2. Results
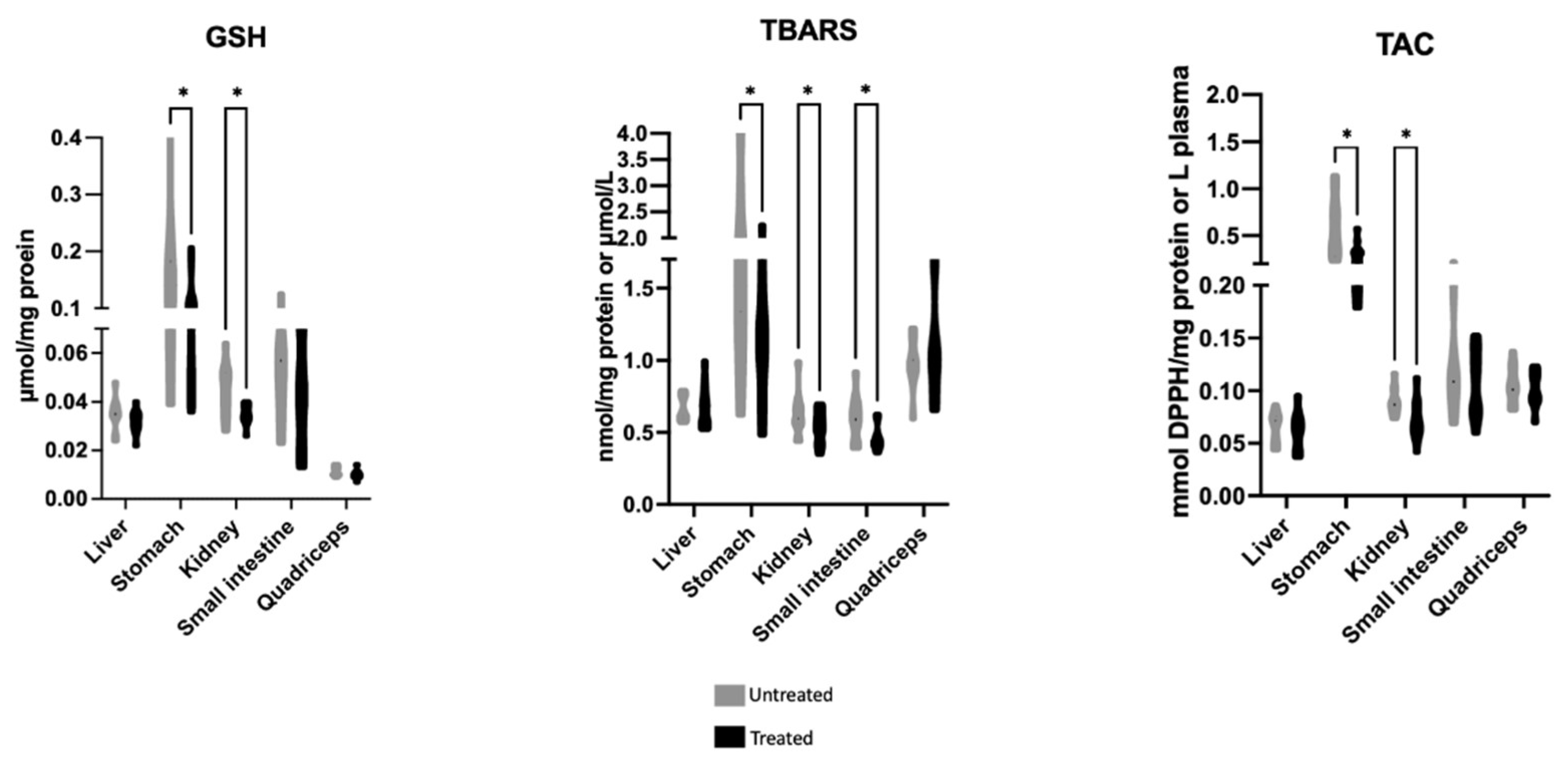
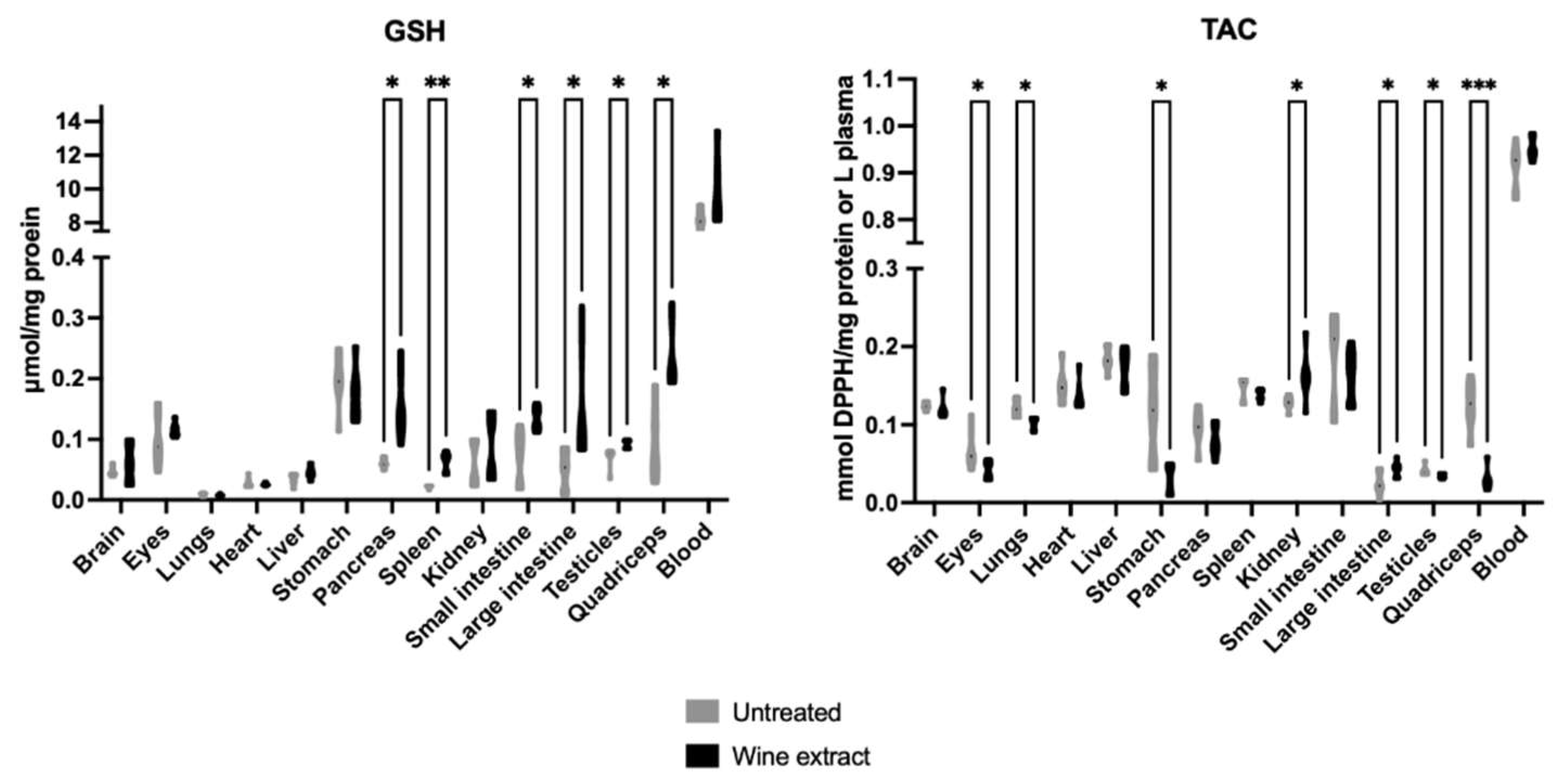
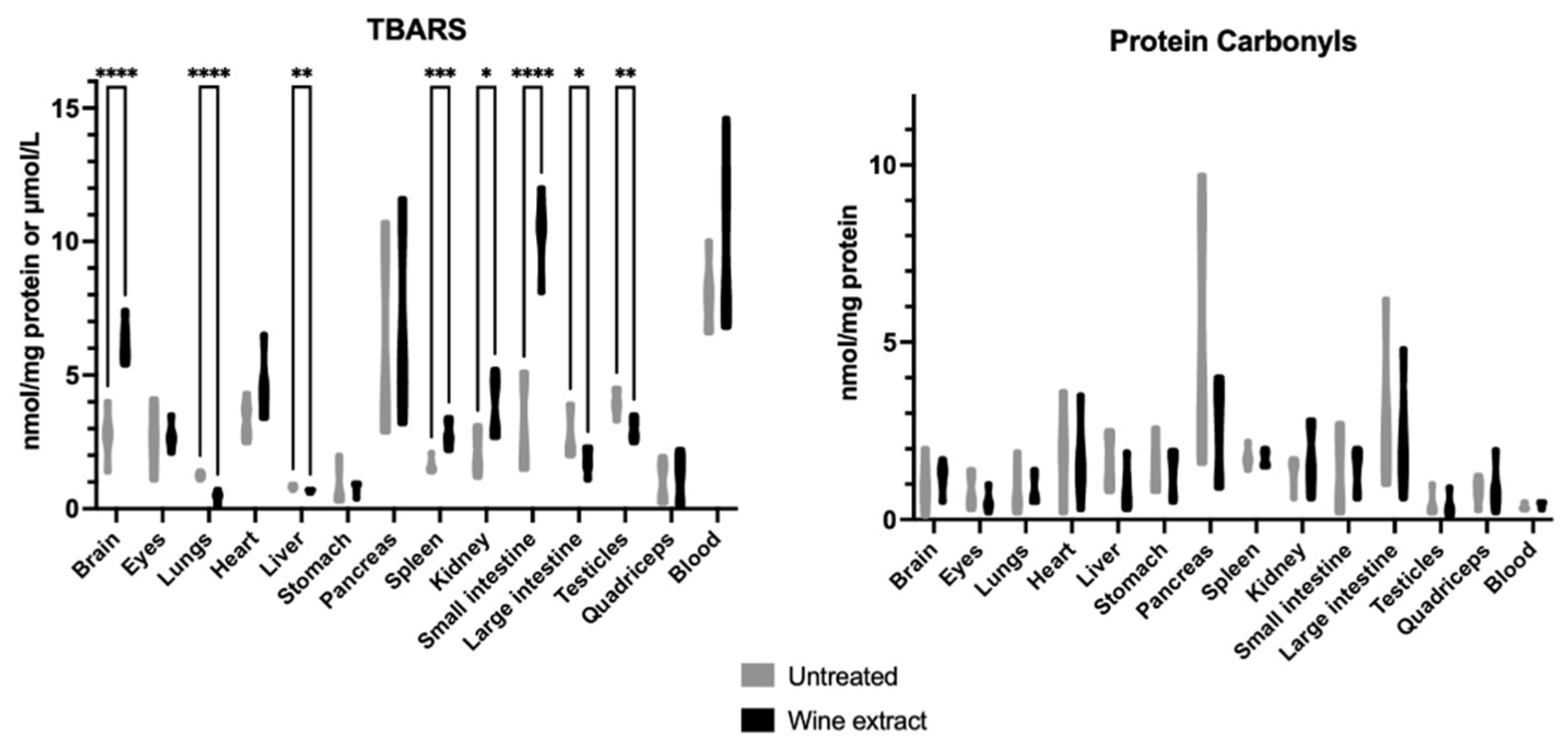
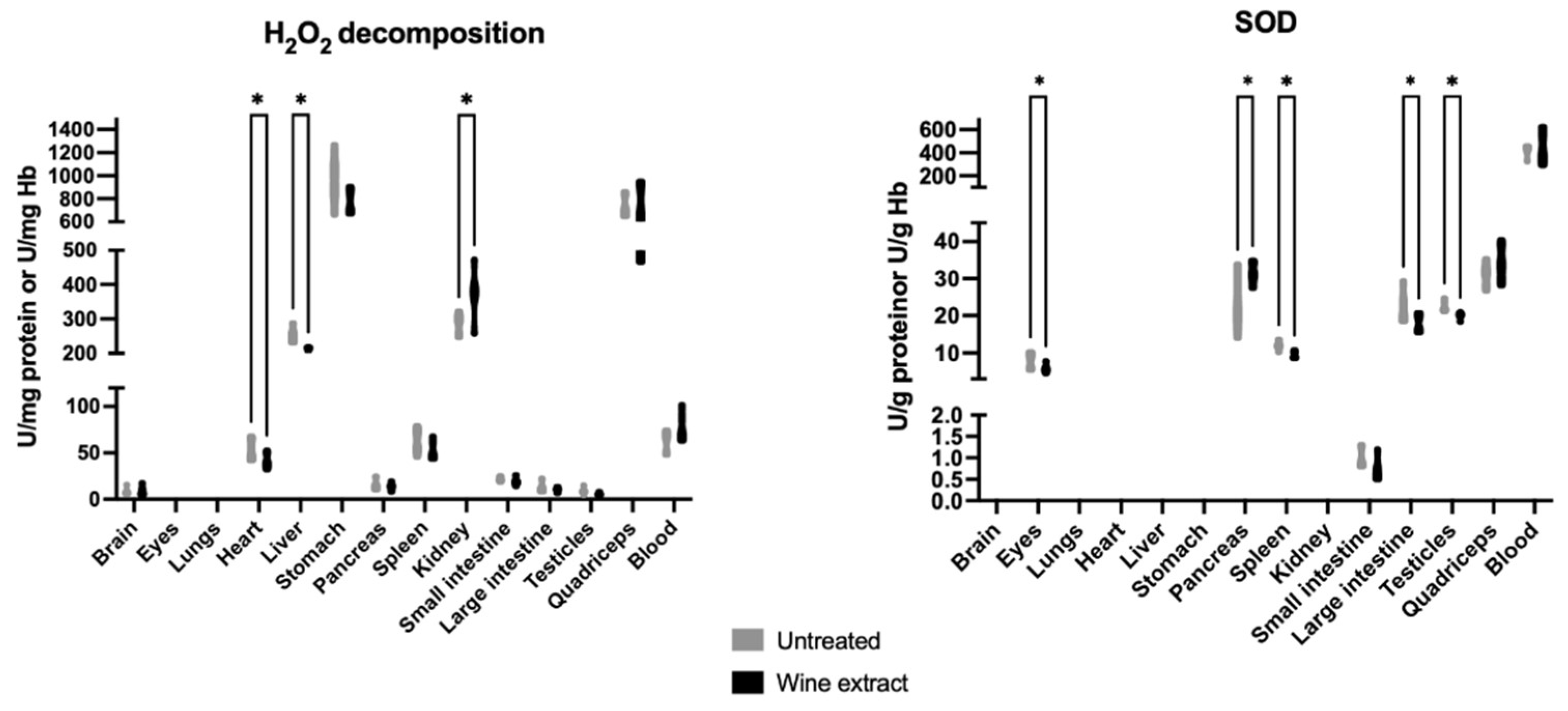
2.1. Effects of Mavrodaphne Grape Stem Extract on Mice Redox Biomarkers
2.2. Effects of Xinomavro Wine Extract on Rat Redox Biomarkers
2.3. Effects of Xinomavro Wine Extract on Rat Body Weight and Growth Rate
2.4. Clinical Examination
2.5. Effects of Xinomavro Wine Extract on Rat Hematological and Clinical Chemistry Parameters
2.6. Gross Necropsy
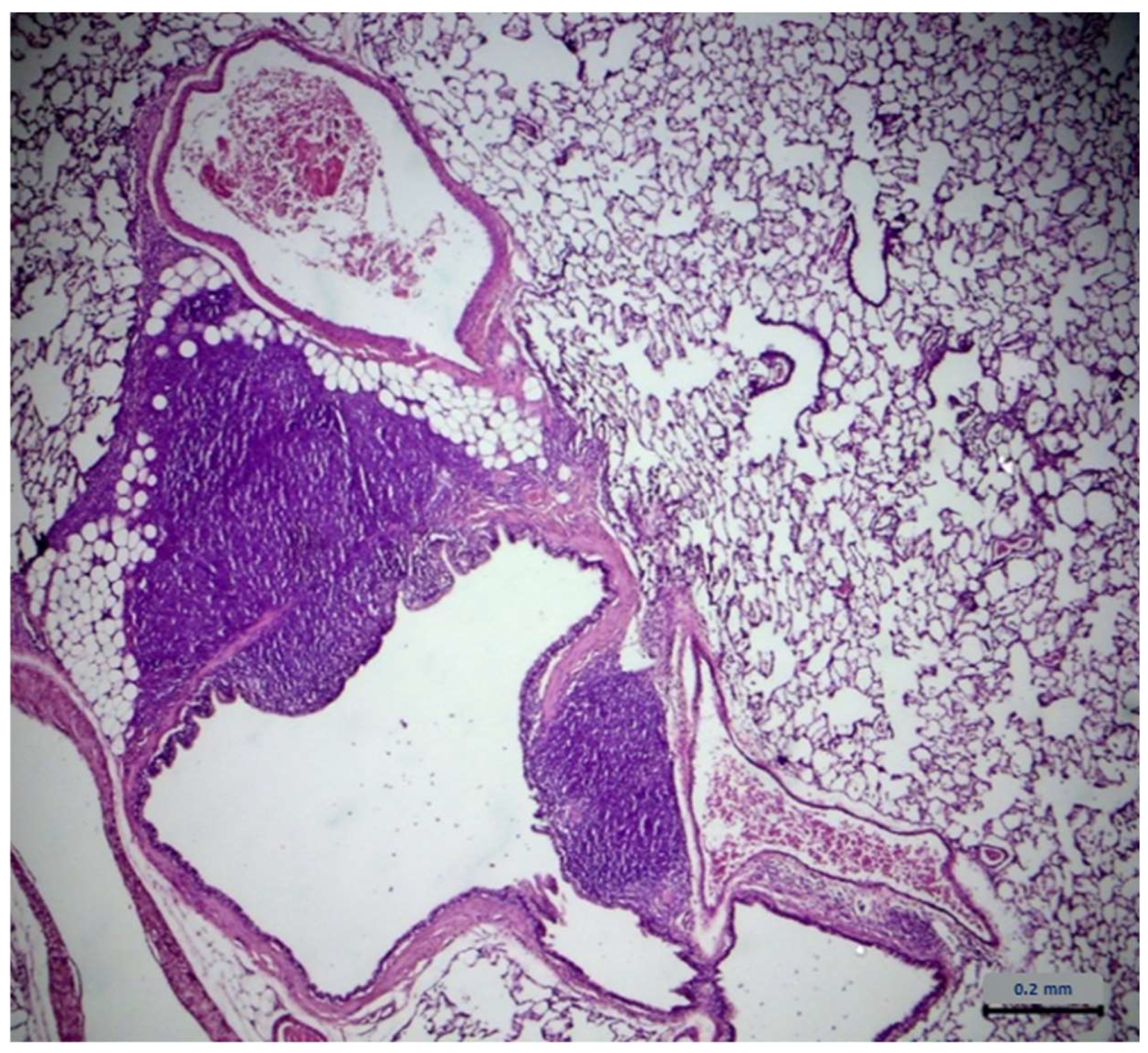
2.7. Histopathological Assessment
3. Discussion
4. Materials and Methods
4.1. Chemical Composition and Dose Preparation
4.2. Animals and Housing Conditions
4.3. Redox Biomarkers
4.3.1. Tissue Homogenization
4.3.2. Determination of GSH
4.3.3. Determination of H2O2 Decomposition Rate
4.3.4. Determination of TAC
4.3.5. Determination of TBARS
4.3.6. Determination of Protein Carbonyls
4.3.7. Determination of SOD Activity
4.3.8. Determination of GPx Activity
4.3.9. Determination of GR Activity
4.4. Body Weight and Growth Rate
4.5. Hematological and Clinical Chemistry Parameters
4.6. Gross Necropsy
4.7. Histopathology
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Giovannelli, L.; Testa, G.; De Filippo, C.; Cheynier, V.; Clifford, M.; Dolara, P. Effect of complex polyphenols and tannins from red wine on DNA oxidative damage of rat colon mucosa in vivo. Eur. J. Nutr. 2000, 39, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Facinó, R.; Carini, M.; Aldini, G.; Berti, F.; Rossoni, G.; Bombardelli, E.; Morazzoni, P. Procyanidines from Vitis vinifera Seeds Protect Rabbit Heart from Ischemia/Reperfusion Injury: Antioxidant Intervention and/or Iron and Copper Sequestering Ability. Planta Med. 1996, 62, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Tran, M.X.; Stohs, S.J. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997, 95, 179–189. [Google Scholar] [PubMed]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Bagchi, D.J.; Balmoori, J.; Stohs, S.J. Protective Effects of Grape Seed Proanthocyanidins and Selected Antioxidants against TPA-Induced Hepatic and Brain Lipid Peroxidation and DNA Fragmentation, and Peritoneal Macrophage Activation in Mice. Gen. Pharmacol. 1998, 30, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Zafirov, D.; Bredy-Dobreva, G.; Litchev, V.; Papasova, M. Antiexudative and capillaritonic effects of procyanidines isolated from grape seeds (V. vinifera). Acta Physiol. Pharmacol. Bulg. 1990, 16, 50–54. [Google Scholar]
- Frankel, E.N.; Waterhouse, A.L.; Teissedre, P.L. Principal Phenolic Phytochemicals in Selected California Wines and Their Antioxidant Activity in Inhibiting Oxidation of Human Low-Density Lipoproteins. J. Agric. Food Chem. 1995, 43, 890–894. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef]
- Salucci, S.; Burattini, S.; Giordano, F.M.; Lucarini, S.; Diamantini, G.; Falcieri, E. Further Highlighting on the Prevention of Oxidative Damage by Polyphenol-Rich Wine Extracts. J. Med. Food 2017, 20, 410–419. [Google Scholar] [CrossRef]
- Fauconneau, B.; Waffo-Teguo, P.; Huguet, F.; Barrier, L.; Decendit, A.; Merillon, J.-M. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci. 1997, 61, 2103–2110. [Google Scholar] [CrossRef]
- Biasi, F.; Deiana, M.; Guina, T.; Gamba, P.; Leonarduzzi, G.; Poli, G. Wine consumption and intestinal redox homeostasis. Redox Biol. 2014, 2, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolara, P.; Luceri, C.; De Filippo, C.; Femia, A.P.; Giovannelli, L.; Caderni, G.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat. Res. Mol. Mech. Mutagen. 2005, 591, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Miranda, A.; Vergara, L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin. Chim. Acta 2011, 412, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Ferreira, E.; Freitas, V.; Almeida, L.; Barbosa, R.M.; Laranjinha, J. Intestinal anti-inflammatory activity of red wine extract: Unveiling the mechanisms in colonic epithelial cells. Food Funct. 2013, 4, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Pace-Asciak, C.R. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen. Pharmacol. Vasc. Syst. 1996, 27, 363–366. [Google Scholar] [CrossRef]
- Basli, A.; Soulet, S.; Chaher, N.; Mérillon, J.-M.; Chibane, M.; Monti, J.-P.; Richard, T. Wine Polyphenols: Potential Agents in Neuroprotection. Oxidative Med. Cell. Longev. 2012, 2012, 805762. [Google Scholar] [CrossRef]
- Caimi, G.; Carollo, C.; Presti, R.L. Wine and endothelial function. Drugs Under Exp. Clin. Res. 2003, 29, 235–242. [Google Scholar]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef]
- Rasines-Perea, Z.; Teissedre, P.-L. Grape Polyphenols’ Effects in Human Cardiovascular Diseases and Diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef]
- Gresele, P.; Cerletti, C.; Guglielmini, G.; Pignatelli, P.; de Gaetano, G.; Violi, F. Effects of resveratrol and other wine polyphenols on vascular function: An update. J. Nutr. Biochem. 2011, 22, 201–211. [Google Scholar] [CrossRef]
- Riccioni, G.; Gammone, M.A.; Tettamanti, G.; Bergante, S.; Pluchinotta, F.R.; D’Orazio, N. Resveratrol and anti-atherogenic effects. Int. J. Food Sci. Nutr. 2015, 66, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Babál, P.; Kristová, V.; Černá, A.; Janega, P.; Pecháňová, O.; Danihel, L.; Andriantsitohaina, R. Red wine polyphenols prevent endothelial damage induced by CCl4 administration. Physiol. Res. 2006, 55, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Morrissey, R.; Usborne, A.; Kapetanovic, I.; Crowell, J.; Muzzio, M.; McCormick, D. Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food Chem. Toxicol. 2011, 49, 3319–3327. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Knight, T.J.; Beitz, D.C.; Lewis, D.S.; Engen, R.L. Resveratrol promotes atherosclerosis in hypercholesterolemic rabbits. Life Sci. 1996, 59, PL15–PL21. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.A.; Giovannini, L.; Giannessi, D.; Migliori, M.; Bernini, W.; Fregoni, M. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int. J. Tissue React. 1995, 17, 1–3. [Google Scholar]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.T.; Evans, G.L.; Zhang, M.; Maran, A.; Sibonga, J.D. Is Resveratrol an Estrogen Agonist in Growing Rats? Endocrinology 1999, 140, 50–54. [Google Scholar] [CrossRef]
- Carbó, N.; Costelli, P.; Baccino, F.M.; López-Soriano, F.J.; Argilés, J.M. Resveratrol, a Natural Product Present in Wine, Decreases Tumour Growth in a Rat Tumour Model. Biochem. Biophys. Res. Commun. 1999, 254, 739–743. [Google Scholar] [CrossRef]
- Turrens, J.F.; Lariccia, J.; Nair, M.G. Resveratrol Has No Effect on Lipoprotein Profile and Does Not Prevent Peroxidation of Serum Lipids in Normal Rats. Free. Radic. Res. 1997, 27, 557–562. [Google Scholar] [CrossRef]
- Halpern, M.J.; Dahlgren, A.-L.; Laakso, I.; Seppänen-Laakso, T.; Dahlgren, J.; McAnulty, P.A. Red-wine Polyphenols and Inhibition of Platelet Aggregation: Possible Mechanisms, and Potential Use in Health Promotion and Disease Prevention. J. Int. Med. Res. 1998, 26, 171–180. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Chen, Y.; Agarwal, R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation–promotion protocol and identification of procyanidin B5-3′-gallate as the most effective antioxidant constituent. Carcinog. 1999, 20, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.; Singh, R.P.; Kaul, N.; Agarwal, R.; Agarwal, C. Dietary Feeding of Grape Seed Extract Prevents Intestinal Tumorigenesis in APCmin/+ Mice. Neoplasia 2010, 12, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Wine Phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Saric, S.; Sivamani, R.K. Polyphenols and Sunburn. Int. J. Mol. Sci. 2016, 17, 1521. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Afaq, F.; Azizuddin, K.; Mukhtar, H. Inhibition of UVB-Induced Oxidative Stress-Mediated Phosphorylation of Mitogen-Activated Protein Kinase Signaling Pathways in Cultured Human Epidermal Keratinocytes by Green Tea Polyphenol (−)-Epigallocatechin-3-gallate. Toxicol. Appl. Pharmacol. 2001, 176, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Filip, A.; Daicoviciu, D.; Clichici, S.; Bolfa, P.; Catoi, C.; Baldea, I.; Bolojan, L.; Olteanu, D.; Muresan, A.; Postescu, I. The effects of grape seeds polyphenols on SKH-1 mice skin irradiated with multiple doses of UV-B. J. Photochem. Photobiol. B Biol. 2011, 105, 133–142. [Google Scholar] [CrossRef]
- Bouhamidi, R.; Prévost, V.; Nouvelot, A. High protection by grape seed proanthocyanidins (GSPC) of polyunsaturated fatty acids against UV-C induced peroxidation. C. R. Acad. Sci. III 1998, 321, 31–38. [Google Scholar] [CrossRef]
- Choi, J.; Ryu, S.-J.; Kim, K.-J.; Kim, H.-M.; Chung, H.-C.; Lee, B.-Y. Single, 14-Day, and 13-Week Repeated Dose Toxicity Studies of Daily Oral Gelidium elegans Extract Administration to Rats. Molecules 2018, 23, 217. [Google Scholar] [CrossRef]
- Vijayasteltar, L.; Nair, G.G.; Maliakel, B.; Kuttan, R.; Krishnakumar, I.M. Safety assessment of a standardized polyphenolic extract of clove buds: Subchronic toxicity and mutagenicity studies. Toxicol. Rep. 2016, 3, 439–449. [Google Scholar] [CrossRef]
- Cladis, D.P.; Li, S.; Reddivari, L.; Cox, A.; Ferruzzi, M.G.; Weaver, C.M. A 90 day oral toxicity study of blueberry polyphenols in ovariectomized sprague-dawley rats. Food Chem. Toxicol. 2020, 139, 111254. [Google Scholar] [CrossRef]
- Tanaka, W.; Yokoyama, D.; Matsuura, Y.; Nozaki, M.; Hirozawa, N.; Kunitake, H.; Sakono, M.; Sakakibara, H. Subchronic toxicity evaluation of leaves from rabbiteye blueberry (Vaccinium virgatum Aiton) in rats. Toxicol. Rep. 2019, 6, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Takami, S.; Imai, T.; Hasumura, M.; Cho, Y.-M.; Onose, J.; Hirose, M. Evaluation of toxicity of green tea catechins with 90-day dietary administration to F344 rats. Food Chem. Toxicol. 2008, 46, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Lluís, L.; Muñoz, M.; Nogués, M.R.; Sánchez-Martos, V.; Romeu, M.; Giralt, M.; Valls, J.; Solà, R. Toxicology evaluation of a procyanidin-rich extract from grape skins and seeds. Food Chem. Toxicol. 2011, 49, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Morikawa, T.; Takahashi, M.; Yoshida, M.; Ogawa, K. A 13-week subchronic toxicity study of grape skin extract in F344 rats. J. Toxicol. Sci. 2013, 38, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef]
- Bentivegna, S.; Whitney, K. Subchronic 3-month oral toxicity study of grape seed and grape skin extracts. Food Chem. Toxicol. 2002, 40, 1731–1743. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, P.; Rueda-Robles, A.; Borrás-Linares, I.; Quirantes-Piné, R.M.; Emanuelli, T.; Segura-Carretero, A.; Lozano-Sánchez, J. Grape and Grape-Based Product Polyphenols: A Systematic Review of Health Properties, Bioavailability, and Gut Microbiota Interactions. Horticulturae 2022, 8, 583. [Google Scholar] [CrossRef]
- Giovinazzo, G.; Carluccio, M.A.; Grieco, F. Wine Polyphenols and Health. In Bioactive Molecules in Food; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2019; pp. 1135–1155. [Google Scholar] [CrossRef]
- Tekos, F.; Makri, S.; Skaperda, Z.-V.; Patouna, A.; Terizi, K.; Kyriazis, I.; Kotseridis, Y.; Mikropoulou, E.; Papaefstathiou, G.; Halabalaki, M.; et al. Assessment of Antioxidant and Antimutagenic Properties of Red and White Wine Extracts In Vitro. Metabolites 2021, 11, 436. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Vassi, E.; Poulas, K.; Kokkinakis, E.; Asprodini, E.; Haroutounian, S.; Kouretas, D. Grape Stem Extracts From Three Native Greek Vine Varieties Exhibit Strong Antioxidant and Antimutagenic Properties. Anticancer. Res. 2020, 40, 2025–2032. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef]
- Troncozo, M.I.; Lješević, M.; Beškoski, V.P.; Anđelković, B.; Balatti, P.A.; Saparrat, M.C. Fungal transformation and reduction of phytotoxicity of grape pomace waste. Chemosphere 2019, 237, 124458. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Vassi, E.; Veskoukis, A.S.; Tekos, F.; Skaperda, Z.; Poulas, K.; Haroutounian, S.; Kouretas, D. Biological effects of grape stem extracts on human cancer cell lines. Int. J. Funct. Nutr. 2022, 3, 1–8. [Google Scholar] [CrossRef]
- Priftis, A.; Soursou, V.; Makiou, A.-S.; Tekos, F.; Veskoukis, A.S.; Tsantarliotou, M.P.; Taitzoglou, I.A.; Kouretas, D. A lightly roasted coffee extract improves blood and tissue redox status in rats through enhancement of GSH biosynthesis. Food Chem. Toxicol. 2019, 125, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.E.; Vinardell, M.P.; Planas, J.M. The Daily Oral Administration of High Doses of trans-Resveratrol to Rats for 28 Days Is Not Harmful. J. Nutr. 2002, 132, 257–260. [Google Scholar] [CrossRef]
- Makri, S.; Kafantaris, I.; Stagos, D.; Chamokeridou, T.; Petrotos, K.; Gerasopoulos, K.; Mpesios, A.; Goutzourelas, N.; Kokkas, S.; Goulas, P.; et al. Novel feed including bioactive compounds from winery wastes improved broilers’ redox status in blood and tissues of vital organs. Food Chem. Toxicol. 2017, 102, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kim, I.-S.; More, S.V.; Kim, B.-W.; Choi, D.-K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat. Prod. Rep. 2014, 31, 109–139. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Skaperda, Z.; Argyriadou, A.; Nechalioti, P.; Alvanou, M.; Makri, S.; Bouroutzika, E.; Kyriazis, I.; Tekos, F.; Veskoukis, A.; Kallitsis, T.; et al. Redox Biomarker Baseline Levels in Cattle Tissues and Their Relationships with Meat Quality. Antioxidants 2021, 10, 958. [Google Scholar] [CrossRef]
- Kusano, C.; Ferrari, B. Total Antioxidant Capacity: A Biomarker in Biomedical and Nutritional Studies. J. Cell Mol. Biol. 2008, 7, 1–15. [Google Scholar]
- Kafantaris, I.; Stagos, D.; Kotsampasi, B.; Hatzis, A.; Kypriotakis, A.; Gerasopoulos, K.; Makri, S.; Goutzourelas, N.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Animal 2018, 12, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Kerasioti, E.; Terzopoulou, Z.; Komini, O.; Kafantaris, I.; Makri, S.; Stagos, D.; Gerasopoulos, K.; Anisimov, N.Y.; Tsatsakis, A.M.; Kouretas, D. Tissue specific effects of feeds supplemented with grape pomace or olive oil mill wastewater on detoxification enzymes in sheep. Toxicol. Rep. 2017, 4, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Rivera, G.; Orellana, M.; Araya, J.; Bosco, C. Rat kidney antioxidant response to long-term exposure to flavonol rich red wine. Life Sci. 2002, 71, 2881–2895. [Google Scholar] [CrossRef]
- Lee, S.-J.; Choi, S.-K.; Seo, J.-S. Grape skin improves antioxidant capacity in rats fed a high fat diet. Nutr. Res. Pract. 2009, 3, 279–285. [Google Scholar] [CrossRef]
- Yu, Q.-M.; Lim, E.-J.; Choi, S.-K.; Seo, J.-S. Antioxidant effect of grapevine leaf extract on the oxidative stress induced by a high-fat diet in rats. Food Sci. Biotechnol. 2014, 23, 849–857. [Google Scholar] [CrossRef]
- Ray, S.; Bagchi, D.; Lim, P.M.; Bagchi, M.; Gross, S.M.; Kothari, S.C.; Preuss, H.G.; Stohs, S.J. Acute and long-term safety evaluation of a novel IH636 grape seed proanthocyanidin extract. Res. Commun. Mol. Pathol. Pharmacol. 2001, 109, 165–197. [Google Scholar]
- Wren, A.F.; Cleary, M.; Frantz, C.; Melton, S.; Norris, L. 90-Day Oral Toxicity Study of a Grape Seed Extract (IH636) in Rats. J. Agric. Food Chem. 2002, 50, 2180–2192. [Google Scholar] [CrossRef]
- Filho, W.J.; Lima, C.C.; Paunksnis, M.R.R.; Silva, A.A.; Perilhão, M.S.; Caldeira, M.; Bocalini, D.; De Souza, R.R. Reference database of hematological parameters for growing and aging rats. Aging Male 2018, 21, 145–148. [Google Scholar] [CrossRef]
- Albers, T.M.; Simon, M.A.; Clifford, C.B. Histopathology of Naturally Transmitted “Rat Respiratory Virus”: Progression of Lesions and Proposed Diagnostic Criteria. Vet. Pathol. 2009, 46, 992–999. [Google Scholar] [CrossRef]
- Baker, D.G. Natural Pathogens of Laboratory Mice, Rats, and Rabbits and Their Effects on Research. Clin. Microbiol. Rev. 1998, 11, 231–266. [Google Scholar] [CrossRef]
- Slaoui, M.; Dreef, H.C.; Van Esch, E. Inflammatory Lesions in the Lungs of Wistar Rats. Toxicol. Pathol. 1998, 26, 712–713, discussion 714. [Google Scholar] [CrossRef] [PubMed]
- Elwell, M.R.; Mahler, J.F.; Rao, G. “Have You Seen This?” Inflammatory Lesions in the Lungs of Rats. Toxicol. Pathol. 1997, 25, 529–531. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.; Murthy, S.V.; Dr, K.; Prabhakar, M.C. Role of Free Radicals and Antioxidants in Tuberculosis Patients. Indian J. Tuberc. 2004, 51, 213–218. [Google Scholar]
- Veskoukis, A.S.; Kyparos, A.; Paschalis, V.; Nikolaidis, M.G. Spectrophotometric assays for measuring redox biomarkers in blood. Biomarkers 2016, 21, 208–217. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar] [CrossRef]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef]
- Keles, M.; Taysi, S.; Sen, N.; Aksoy, H.; Akçay, F. Effect of Corticosteroid Therapy on Serum and CSF Malondialdehyde and Antioxidant Proteins in Multiple Sclerosis. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2001, 28, 141–143. [Google Scholar] [CrossRef]
- Spanidis, Y.; Goutzourelas, N.; Stagos, D.; Mpesios, A.; Priftis, A.; Bar-Or, D.; Spandidos, D.A.; Tsatsakis, A.M.; Leon, G.; Kouretas, D. Variations in oxidative stress markers in elite basketball players at the beginning and end of a season. Exp. Ther. Med. 2016, 11, 147–153. [Google Scholar] [CrossRef]
- Patsoukis, N.; Zervoudakis, G.; Panagopoulos, N.T.; Georgiou, C.D.; Angelatou, F.; Matsokis, N.A. Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neurosci. Lett. 2004, 357, 83–86. [Google Scholar] [CrossRef]
- Oberley, L.W.; Spitz, D.R. Assay of superoxide dismutase activity in tumor tissue. Methods Enzymol. 1984, 105, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef] [PubMed]
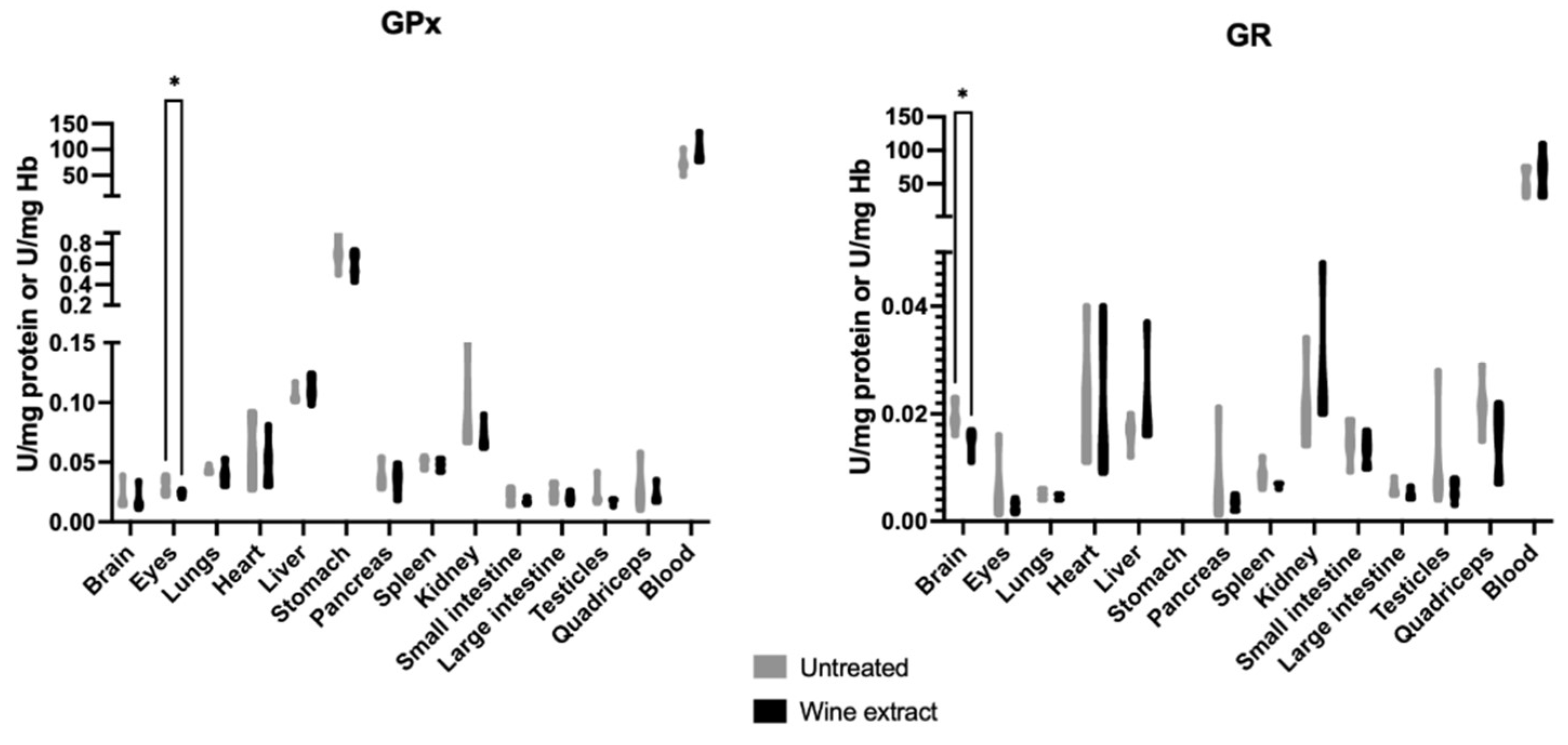

| Blood Parameters (Unit) | Control Group n = 6 | Extract Group n = 6 |
|---|---|---|
| Red blood cells (RBC)(M/μL) | 8.63 ± 0.45 | 8.42 ± 0.21 |
| Hemoglobin (HGB) (g/dL) | 15.97 ± 0.75 | 15.46 ± 0.58 |
| Μean corpuscular volume volume (MCV) (fl) | 50.63 ± 0.79 | 50.64 ± 1.51 |
| Mean corpuscular hemoglobin (MCH) (pg) | 16.58 ± 0.15 | 16.42 ± 0.83 |
| Mean corpuscular hemoglobin concentration (MCHC) (g/dL) | 32.78 ± 0.60 | 32.40 ± 0.72 |
| Hematocrit (HCT) (%) | 44.78 ± 2.94 | 45.68 ± 1.19 |
| White blood cells (WBC)(K/μL) | 5.78 ± 0.92 | 5.34 ± 2.34 |
| Neutrophils (K/μL %) | 19.77 ± 2.74 | 26.24 ± 9.82 |
| Lymphocytes (K/μL % | 75.67 ± 2.52 | 70.10 ± 9.99 |
| Monocytes (K/μL %) | 2.37 ± 0.23 | 2.08 ± 0.34 |
| Eosinophils (K/μL %) | 1.37 ± 0.60 | 0.86 ± 0.38 |
| Basophils (K/μL %) | 0.70 ± 0.15 | 0.62 ± 0.08 |
| Platelets (PLT) (K/μL) | 656.33 ± 65.48 | 645.80 ± 102.47 |
| Μean platelets volume (MPV) (fl) | 7.62 ± 0.56 | 8.34 ± 0.56 |
| Blood Serum Results | Control Group n = 6 | Extract Group n = 6 |
|---|---|---|
| Total proteins (g/dL) | 6.7 ± 0.21 | 6.68 ± 0.23 |
| Albumin (g/mL) | 4.32 ± 0.12 | 4.3 ± 0.10 |
| Urea (mg/mL) | 15.17 ± 4.96 | 14.33 ± 5.86 |
| Creatinine (mg/mL) | 0.6 ± 0 | 0.47 ± 0.06 |
| Alanine-aminotransferase (ALT) (U/lit) | 200.33 ± 11.5 | 250.67 ± 53.45 |
| Alkaline phosphatase (ALP) (U/lit) | 53.17 ± 5.27 | 51 ± 1.41 |
| Gamma-glutamyl transferase (GGT) (U/lit) | 0 | 0 |
| Lung Type of Lesions | Control Group n = 6 | Extract Group n = 6 |
|---|---|---|
| Architecture | - 1 | - |
| Cell degeneration/necrosis | - | - |
| Inflammatory filtration | 4 2 | 4 |
| Hemodynamic damage | - | - |
| Cell growth disorders | - | - |
| Lung Severity | Control Group n = 4 | Extract Group n = 4 |
|---|---|---|
| Minimum | - | - |
| Mild | 3 1 | 2 |
| Medium | 1 2 | 2 |
| Serious | - | - |
| A/A | Ingredients | Quantity | |
|---|---|---|---|
| 1 | Whey protein 80% (sheep goat) | 4000 | kg |
| 2 | Debatted cocoa powder | 3500 | kg |
| 3 | Coconut sugar | 3000 | kg |
| 4 | Pasteurized egg white | 2600 | kg |
| 5 | Olive oil | 2400 | lt |
| 6 | Coconut oil | 2300 | lt |
| 7 | Carob flour | 2000 | kg |
| 8 | Carob honey | 1500 | kg |
| 9 | Grape stem extract | 0.155 | kg |
| 10 | Sodium bicarbonate | 0.150 | kg |
| Total | 21,605 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekos, F.; Skaperda, Z.; Vardakas, P.; Kyriazi, D.; Maravelis, G.C.; Poulas, K.; Taitzoglou, I.A.; Nepka, C.; Kouretas, D. Redox Biomarkers Assessment after Oral Administration of Wine Extract and Grape Stem Extract in Rats and Mice. Molecules 2023, 28, 1574. https://doi.org/10.3390/molecules28041574
Tekos F, Skaperda Z, Vardakas P, Kyriazi D, Maravelis GC, Poulas K, Taitzoglou IA, Nepka C, Kouretas D. Redox Biomarkers Assessment after Oral Administration of Wine Extract and Grape Stem Extract in Rats and Mice. Molecules. 2023; 28(4):1574. https://doi.org/10.3390/molecules28041574
Chicago/Turabian StyleTekos, Fotios, Zoi Skaperda, Periklis Vardakas, Despina Kyriazi, Georgios C. Maravelis, Konstantinos Poulas, Ioannis A. Taitzoglou, Charitini Nepka, and Demetrios Kouretas. 2023. "Redox Biomarkers Assessment after Oral Administration of Wine Extract and Grape Stem Extract in Rats and Mice" Molecules 28, no. 4: 1574. https://doi.org/10.3390/molecules28041574








