Glucosinolates and Cytotoxic Activity of Collard Volatiles Obtained Using Microwave-Assisted Extraction
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Reagents
3.2. Isolation and Chemical Analysis
3.2.1. Isolation of Desulfoglucosinolates
3.2.2. UHPLC-DAD-MS/MS Analysis
3.2.3. Isolation of Volatiles
3.2.4. GC-MS Analysis
3.3. Cell Viability Assay (MTT Assay)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latté, K.P.; Appel, K.-E.; Lampen, A. Health benefits and possible risks of broccoli—An overview. Food Chem. Toxicol. 2011, 49, 3287–3309. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, D.G. The World Was My Garden: Travels of a Plant Explorer; C. Scribner’s Sons: New York, NY, USA, 1938; p. 173. [Google Scholar]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2019, 59, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Howard, N.P.; Albach, D.C. Different Shades of Kale—Approaches to Analyze Kale Variety Interrelations. Genes 2022, 13, 232. [Google Scholar] [CrossRef]
- Cartea, M.E.; Picoaga, A.; Soengas, P.; Ordas, A. Morphological characterization of kale populations from northwestern Spain. Euphytica 2002, 129, 25–32. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Ramos, I.N.; Brandao, T.R.S.; Silva, C.L.M. Effect of air-drying temperature on the quality and bioactive caracterisation of dried galega kale (Brassica oleracea L. var. acephala). J. Food Process. Preserv. 2015, 39, 2485–2496. [Google Scholar] [CrossRef]
- Pavicic, L.; Pirker-Mosher, G. The Best of Croatian Cooking, Expanded ed.; Hippocrene Books, Inc.: New York, NY, USA, 2004; p. 137. [Google Scholar]
- Radošević, K.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Rimac Brnčić, S.; Takacs, K.; Radojčić Redovniković, I. Assessment of glucosinolates, antioxidative and antiproliferative activity of broccoli and collard extracts. J. Food Compos. Anal. 2017, 61, 59–66. [Google Scholar] [CrossRef]
- Delonga, K.; Radojčić Redovniković, I.; Dragović-Uzelac, V.; Mrkić, V.; Vorkapić-Furač, J. Distribution of glucosinolates in some raw and processed brassica vegetables grown in Croatia. Acta Aliment. 2007, 36, 207–216. [Google Scholar] [CrossRef]
- Vadivambal, R.; Jayas, D.S. Changes in quality of microwave-treated agricultural products—A review. Biosyst. Eng. 2007, 98, 1–16. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Platz, S.; Mewis, I.; Schreiner, M.; Rohn, S.; Kroh, L.W. Thermally induced degradation of sulfur-containing aliphatic glucosinolates in broccoli sprouts (Brassica oleracea var. italica) and model systems. J. Agric. Food Chem. 2012, 60, 2231–2241. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Velasco, P.; Cartea, M.E.; Gonzalez, C.; Vilar, M.; Ooras, A. Factors affecting the glucosinolate content of kale (Brassica oleracea acephala Group). J. Agric. Food Chem. 2007, 55, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Sarıkamış, G.; Balkaya, A.; Yanmaz, R. Glucosinolates in kale genotypes from the Blacksea region of Turkey. Biotechnol. Biotechnol. Equip. 2008, 22, 942–946. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P.; Obregón, S.; Padilla, G.; de Haro, A. Seasonal variation in glucosinolate content in Brassica oleracea crops grown in northwestern Spain. Phytochemistry 2008, 69, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kapusta-Duch, J.; Kusznirewicz, B.; Leszczynska, T.; Borczak, B. Effect of conventional cooking on changes in the contents of basic composition and glucosinolates in kale. Ecol. Chem. Eng. A 2016, 23, 465–480. [Google Scholar] [CrossRef]
- Korus, A.; Słupski, J.; Gebczynski, P.; Bana, A. Effect of preliminary processing and method of preservation on the content of glucosinolates in kale (Brassica oleracea L. var. acephala) leaves. LWT Food Sci. Technol. 2014, 59, 1003–1008. [Google Scholar] [CrossRef]
- Charron, C.S.; Saxton, A.M.; Sams, C.E. Relationship of climate and genotype to seasonal variation in the glucosinolate–myrosinase system. I. Glucosinolate content in ten cultivars of Brassica oleracea grown in fall and spring seasons. J. Sci. Food Agric. 2005, 85, 671–681. [Google Scholar] [CrossRef]
- Hennig, K.; Verkerk, R.; Bonnema, G.; Dekker, M. Rapid estimation of glucosinolate thermal degradation rate constants in leaves of Chinese kale and broccoli (Brassica oleracea) in two seasons. J. Agric. Food Chem. 2012, 60, 7859–7865. [Google Scholar] [CrossRef]
- Hansen, B.G.; Kerwin, R.E.; Ober, J.A.; Lambrix, V.M.; Mitchell-Olds, T.; Gershenzon, J.; Halkier, B.A.; Kliebenstein, D.J. A novel 2-oxoacid-dependent dioxygenase involved in the formation of the goiterogenic 2-hydroxybut-3-enyl glucosinolate and generalist insect resistance in Arabidopsis. Plant Physiol. 2008, 148, 2096–2108. [Google Scholar] [CrossRef]
- Kliebenstein, D.J.; Cacho, N.I. Nonlinear selection and a blend of convergent, divergent and parallel evolution shapes natural variation in glucosinolates. Adv. Bot. Res. 2016, 80, 31–55. [Google Scholar] [CrossRef]
- Agerbirk, N.; Hansen, C.C.; Kiefer, C.; Hauser, T.P.; Ørgaard, M.; Lange, C.B.A.; Cipollini, D.; Koch, M.A. Comparison of glucosinolate diversity in the crucifer tribe Cardamineae and the remaining order Brassicales highlights repetitive evolutionary loss and gain of biosynthetic steps. Phytochemistry 2021, 185, 112668. [Google Scholar] [CrossRef]
- Robin, A.H.K.; Yi, G.-E.; Laila, R.; Yang, K.; Park, J.-I.; Kim, H.R.; Nou, I.-S. Expression profiling of glucosinolate biosynthetic genes in Brassica oleracea L. var. capitata inbred lines reveals their association with glucosinolate content. Molecules 2016, 21, 787. [Google Scholar] [CrossRef] [PubMed]
- Pfalz, M.; Vogel, H.; Kroymann, J. The gene controlling the indole glucosinolate modifier1 quantitative trait locus alters indole glucosinolate structures and aphid resistance in Arabidopsis. Plant Cell 2009, 21, 985–999. [Google Scholar] [CrossRef]
- Pfalz, M.; Mikkelsen, M.D.; Bednarek, P.; Olsen, C.E.; Halkier, B.A.; Kroymann, J. Metabolic engineering in Nicotiana benthamiana reveals key enzyme functions in Arabidopsis indole glucosinolate modification. Plant Cell 2011, 23, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Pfalz, M.; Mukhaimar, M.; Perreau, F.; Kirk, J.; Hansen, C.I.; Olsen, C.E.; Agerbirk, N.; Kroymann, J. Methyl transfer in glucosinolate biosynthesis mediated by indole glucosinolate O-methyltransferase 5. Plant Physiol. 2016, 172, 2190–2203. [Google Scholar] [CrossRef] [PubMed]
- Acheson, R.M. 1-Hydroxypyrroles, 1-hydroxyindoles and 9-hydroxycarbazoles. Adv. Heterocycl. Chem. 1990, 51, 105–175. [Google Scholar] [CrossRef]
- Acheson, R.M.; Littlewood, D.M.; Rosenberg, H.E. Synthesis of 1-methoxyindoles. J. Chem. Soc. Chem. Commun. 1974, 16, 671. [Google Scholar] [CrossRef]
- Somei, M. 1-Hydroxyindoles. Heterocycles 1999, 50, 1157–1211. [Google Scholar] [CrossRef]
- Đulović, A.; Popović, M.; Burčul, F.; Čikeš Čulić, V.; Marijan, S.; Ruščić, M.; Anđelković, N.; Blažević, I. Glucosinolates of Sisymbrium officinale and S. orientale. Molecules 2022, 27, 8431. [Google Scholar] [CrossRef]
- Blažević, I.; Đulović, A.; Čikeš Čulić, V.; Popović, M.; Guillot, X.; Burčul, F.; Rollin, P. Microwave-assisted versus conventional isolation of glucosinolate degradation products from Lunaria annua L. and their cytotoxic activity. Biomolecules 2020, 10, 215. [Google Scholar] [CrossRef]
- Al-Gendy, A.A.; Nematallah, K.A.; Zaghloul, S.S.; Ayoub, N.A. Glucosinolates profile, volatile constituents, antimicrobial, and cytotoxic activities of Lobularia libyca. Pharm. Biol. 2016, 54, 3257–3263. [Google Scholar] [CrossRef] [Green Version]
- Vrca, I.; Ramić, D.; Fredotović, Ž.; Smole Možina, S.; Blažević, I.; Bilušić, T. Chemical composition and biological activity of essential oil and extract from the seeds of Tropaeolum majus L. var. altum. Food Technol. Biotechnol. 2022, 60, 533–542. [Google Scholar] [CrossRef]
- Popović, M.; Maravić, A.; Čikeš Čulić, V.; Đulović, A.; Burčul, F.; Blažević, I. Biological effects of glucosinolate degradation products from horseradish: A horse that wins the race. Biomolecules 2020, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Blažević, I.; Đulović, A.; Maravić, A.; Čikeš Čulić, V.; Montaut, S.; Rollin, P. Antimicrobial and cytotoxic activities of Lepidium latifolium L. Hydrodistillate, extract and its major sulfur volatile allyl isothiocyanate. Chem. Biodivers. 2019, 16, e1800661. [Google Scholar] [CrossRef]
- Đulović, A.; Burčul, F.; Čulić, V.Č.; Ruščić, M.; Brzović, P.; Montaut, S.; Rollin, P.; Blažević, I. Lepidium graminifolium L.: Glucosinolate profile and antiproliferative potential of volatile isolates. Molecules 2021, 26, 5183. [Google Scholar] [CrossRef] [PubMed]
- Blažević, I.; Montaut, S.; Burčul, F.; Rollin, P. Glucosinolates: Novel sources and biological potential. In Glucosinolates, Reference Series in Phytochemistry; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2017; pp. 3–60. [Google Scholar]
- Mužek, M.N.; Omanović, D.; Đulović, A.; Burčul, F.; Svilović, S.; Blažević, I. The garden candytuft (Iberis umbellata L.): At the crossroad of copper accumulation and glucosinolates. Processes 2020, 8, 1116. [Google Scholar] [CrossRef]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Agerbirk, N.; Hansen, C.C.; Olsen, C.E.; Kiefer, C.; Hauser, T.P.; Christensen, S.; Jensen, K.R.; Ørgaard, M.; Pattison, D.I.; Lange, C.B.A.; et al. Glucosinolate profiles and phylogeny in Barbarea compared to other tribe Cardamineae (Brassicaceae) and Reseda (Resedaceae), based on a library of ion trap HPLC-MS/MS data of reference desulfoglucosinolates. Phytochemistry 2021, 185, 112658. [Google Scholar] [CrossRef]
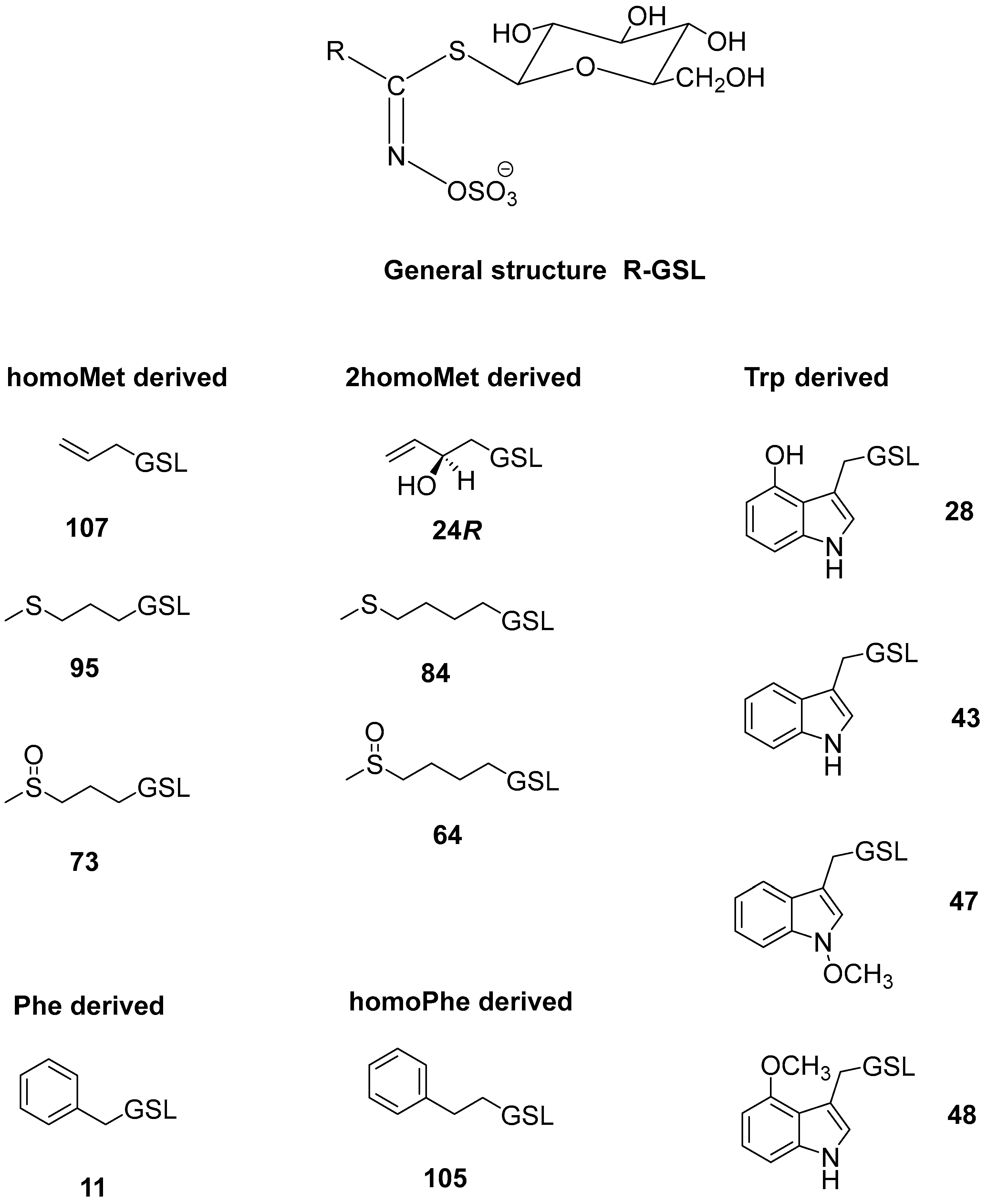
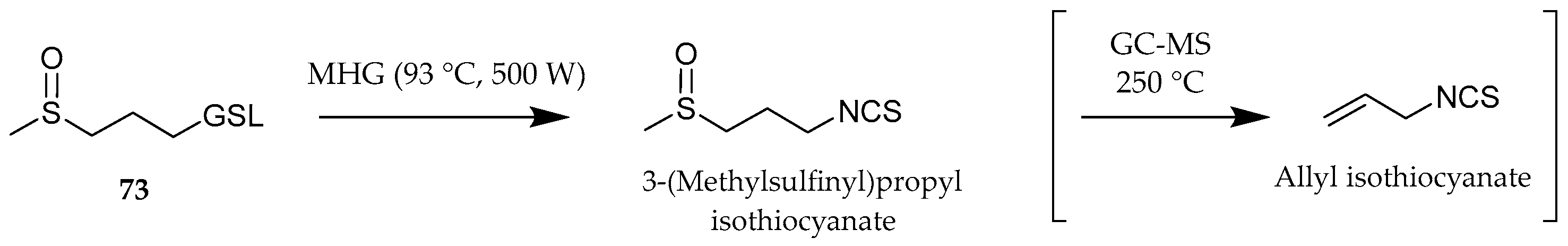

| No. * | Identified Glucosinolate | tR (min) | [M+Na]+ | Plant Tissue (μmol/g DW) | |||
|---|---|---|---|---|---|---|---|
| Flower | Leaf | Stem | Root | ||||
| Met-derived | |||||||
| 73 | (RS)-3-(Methylsulfinyl)propyl GSL (glucoiberin) | 1.25 | 366 | 3.36 ± 0.28 | 0.07 ± 0.00 | tr | tr |
| 24R | (2R)-2-Hydroxybut-3-enyl GSL (progoitrin) | 1.66 | 332 | n.d. | n.d. | 0.49 ± 0.00 | n.d. |
| 64 | (RS)-4-(Methylsulfinyl)butyl GSL (glucoraphanin) | 2.32 | 380 | 0.50 ± 0.00 | n.d. | n.d. | n.d. |
| 107 | Allyl GSL (sinigrin) | 2.57 | 302 | n.d. | n.d. | 0.40 ± 0.00 | 7.65 ± 1.78 |
| 95 | 3-(Methylsulfanyl)propyl GSL (glucoibervirin) | 5.73 | 350 | n.d. | n.d. | n.d. | 12.43 ± 1.12 |
| 84 | 4-(Methylsulfanyl)butyl GSL (glucoerucin) | 6.81 | 364 | n.d. | n.d. | n.d. | 1.88 ± 0.33 |
| Total aliphatic | 3.86 ± 0.28 | 0.07 ± 0.00 | 0.89 ± 0.00 | 21.96 ± 3.23 | |||
| Phe-derived | |||||||
| 11 | Benzyl GSL (glucotropaeolin) | 6.82 | 352 | n.d. | n.d. | 0.13 ± 0.00 | tr |
| 105 | 2-Phenylethyl GSL (gluconasturtiin) | 8.22 | 366 | n.d. | n.d. | 0.09 ± 0.00 | 34.02 ± 1.23 |
| Total arylaliphatic | n.d. | n.d. | 0.22 ± 0.00 | 34.02 ± 1.23 | |||
| Trp-derived | |||||||
| 28 | 4-Hydroxyindol-3-ylmethyl GSL (4-hydroxyglucobrassicin) | 5.89 | 407 | 1.99 ± 0.07 | 2.45 ± 0.08 | 0.69 ± 0.04 | n.d. |
| 43 | Indol-3-ylmethyl GSL (glucobrassicin) | 7.61 | 391 | 0.42 ± 0.08 | 0.12 ± 0.03 | 0.04 ± 0.00 | 0.81 ± 0.03 |
| 48 | 4-Methoxyindol-3-ylmethyl GSL (4-methoxyglucobrassicin) | 8.36 | 421 | n.d. | n.d. | 0.09 ± 0.00 | 1.93 ± 0.63 |
| 47 | N-Methoxyindol-3-ylmethyl GSL (neoglucobrassicin) | 9.66 | 421 | n.d. | n.d. | 0.24 ± 0.00 | 4.68 ± 0.44 |
| Total indole | 2.41 ± 0.15 | 2.57 ± 0.11 | 0.73 ± 0.04 | 7.42 ± 1.10 | |||
| Total GSLs | 6.27 ± 0.43 | 2.64 ± 0.11 | 1.84 ± 0.04 | 63.40 ± 5.56 | |||
| Compound | RI | Leaf | Stem | Root |
|---|---|---|---|---|
| Isothiocyanates | ||||
| Allyl isothiocyanate a | 884 | 40.16 | 11.57 | tr |
| 3-(Methylsulfanyl)propyl isothiocyanate a | 1308 | n.d. | n.d. | 3.93 |
| 4-(Methylsulfanyl)butyl isothiocyanate (erucin) b | 1431 | n.d. | n.d. | 0.61 |
| 2-Phenylethyl isothiocyanate a | 1464 | n.d. | 12.96 | 46.18 |
| Nitriles | ||||
| 4-(Methylsulfanyl)butanenitrile b | 1084 | n.d. | n.d. | 11.35 |
| 5-(Methylsulfanyl)pentanenitrile b | 1199 | n.d. | n.d. | 1.01 |
| 3-Phenylpropanenitrile a | 1241 | n.d | 63.48 | 34.31 |
| Others | ||||
| Dimethyl trisulfide a | 971 | n.d. | 2.60 | n.d. |
| Hex-(4E)-en-1-yl acetate b | 1007 | 21.10 | n.d. | n.d. |
| Benzyl alcohol a | 1034 | n.d. | n.d. | tr |
| Phenylacetaldehyde a | 1045 | 13.07 | 4.59 | tr |
| Nonanal b | 1103 | 23.83 | 2.14 | n.d. |
| 2-Phenylethyl alcohol a | 1112 | n.d. | n.d. | 0.50 |
| Total sum (%) | 98.16 | 97.34 | 97.89 | |
| Yield (µg/g FW) | 3.8 | 2.0 | 4.3 | |
| Isothiocyanates (%) | 40.16 | 24.53 | 50.72 | |
| Nitriles (%) | n.d. | 63.48 | 46.67 | |
| Others (%) | 58.00 | 9.33 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đulović, A.; Burčul, F.; Čikeš Čulić, V.; Rollin, P.; Blažević, I. Glucosinolates and Cytotoxic Activity of Collard Volatiles Obtained Using Microwave-Assisted Extraction. Molecules 2023, 28, 1657. https://doi.org/10.3390/molecules28041657
Đulović A, Burčul F, Čikeš Čulić V, Rollin P, Blažević I. Glucosinolates and Cytotoxic Activity of Collard Volatiles Obtained Using Microwave-Assisted Extraction. Molecules. 2023; 28(4):1657. https://doi.org/10.3390/molecules28041657
Chicago/Turabian StyleĐulović, Azra, Franko Burčul, Vedrana Čikeš Čulić, Patrick Rollin, and Ivica Blažević. 2023. "Glucosinolates and Cytotoxic Activity of Collard Volatiles Obtained Using Microwave-Assisted Extraction" Molecules 28, no. 4: 1657. https://doi.org/10.3390/molecules28041657
APA StyleĐulović, A., Burčul, F., Čikeš Čulić, V., Rollin, P., & Blažević, I. (2023). Glucosinolates and Cytotoxic Activity of Collard Volatiles Obtained Using Microwave-Assisted Extraction. Molecules, 28(4), 1657. https://doi.org/10.3390/molecules28041657











