Application of Olefin Metathesis in the Synthesis of Carbo- and Heteroaromatic Compounds—Recent Advances
Abstract
:1. Introduction
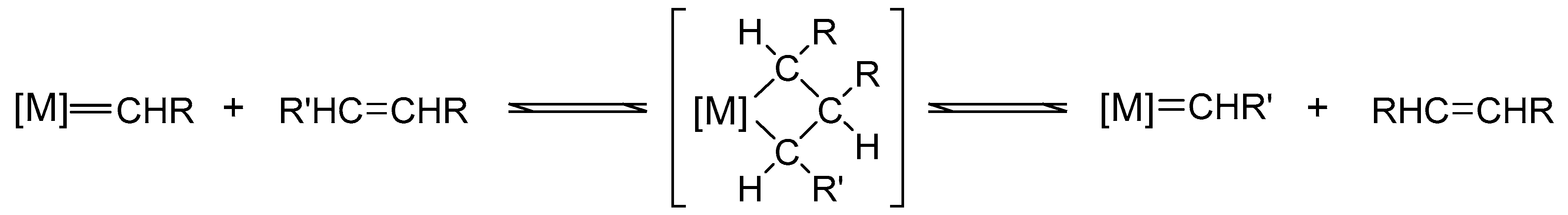
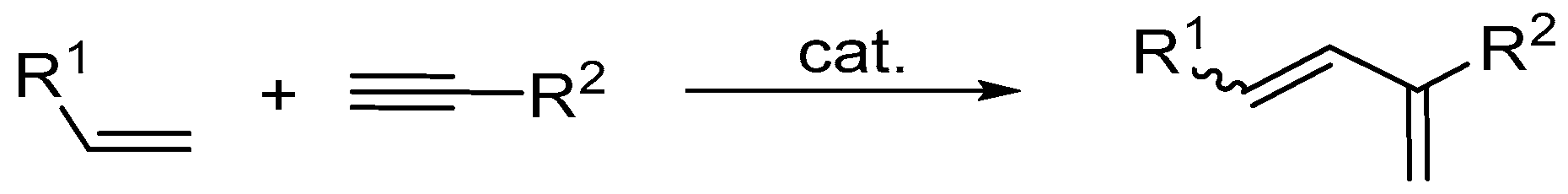
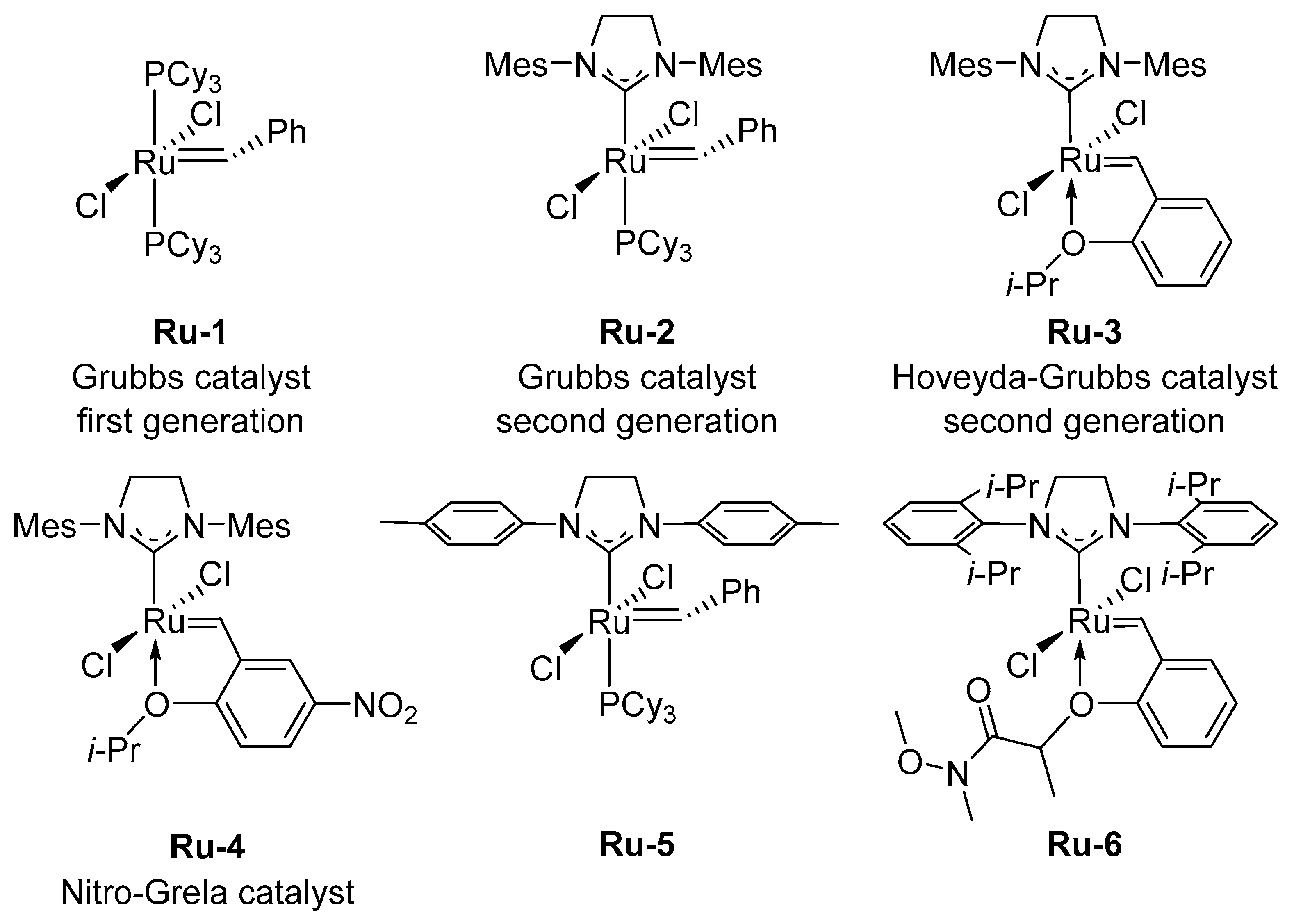
2. Olefin Metathesis
3. Synthesis of Aromatic Heterocycles
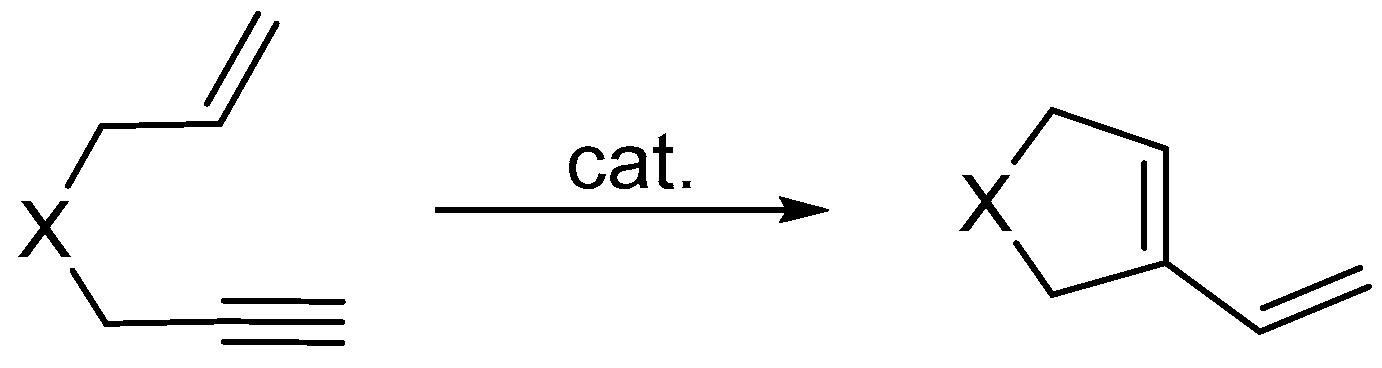
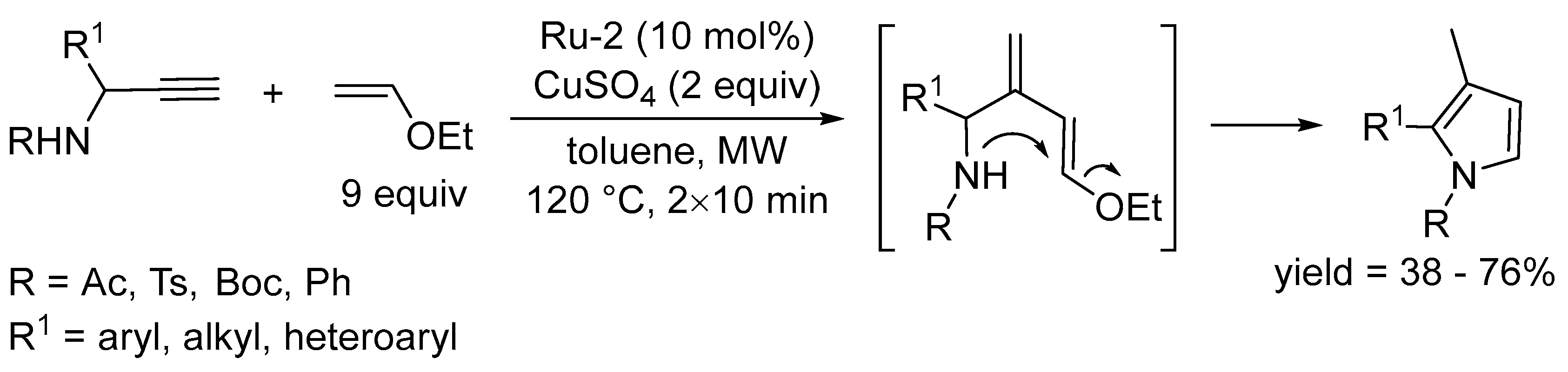
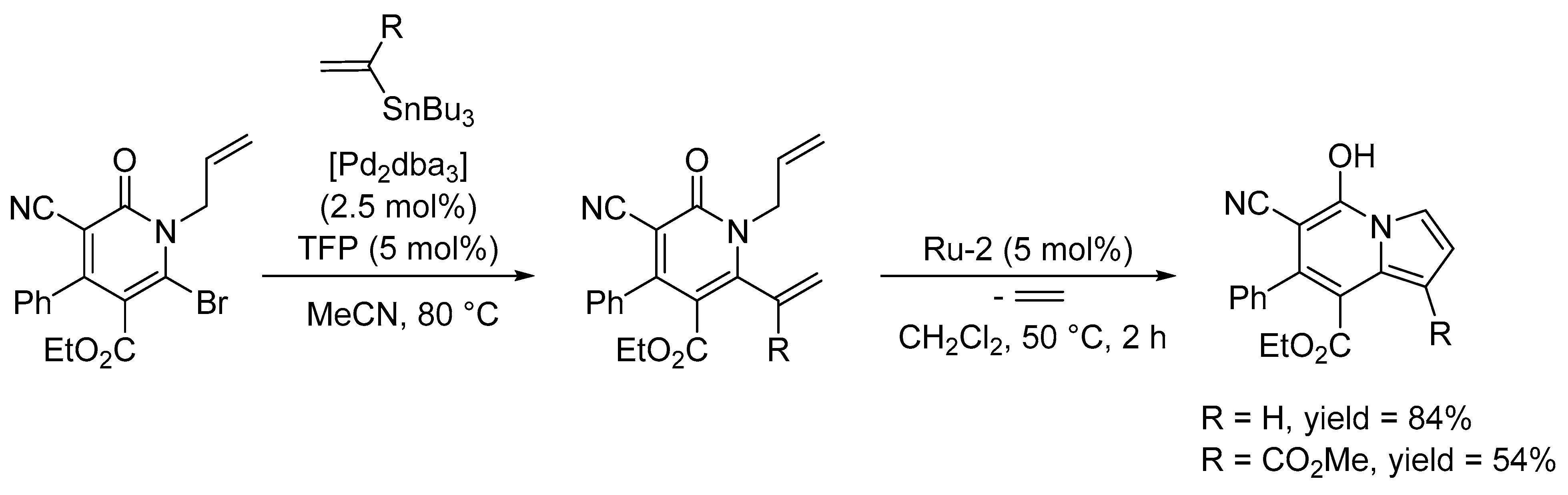
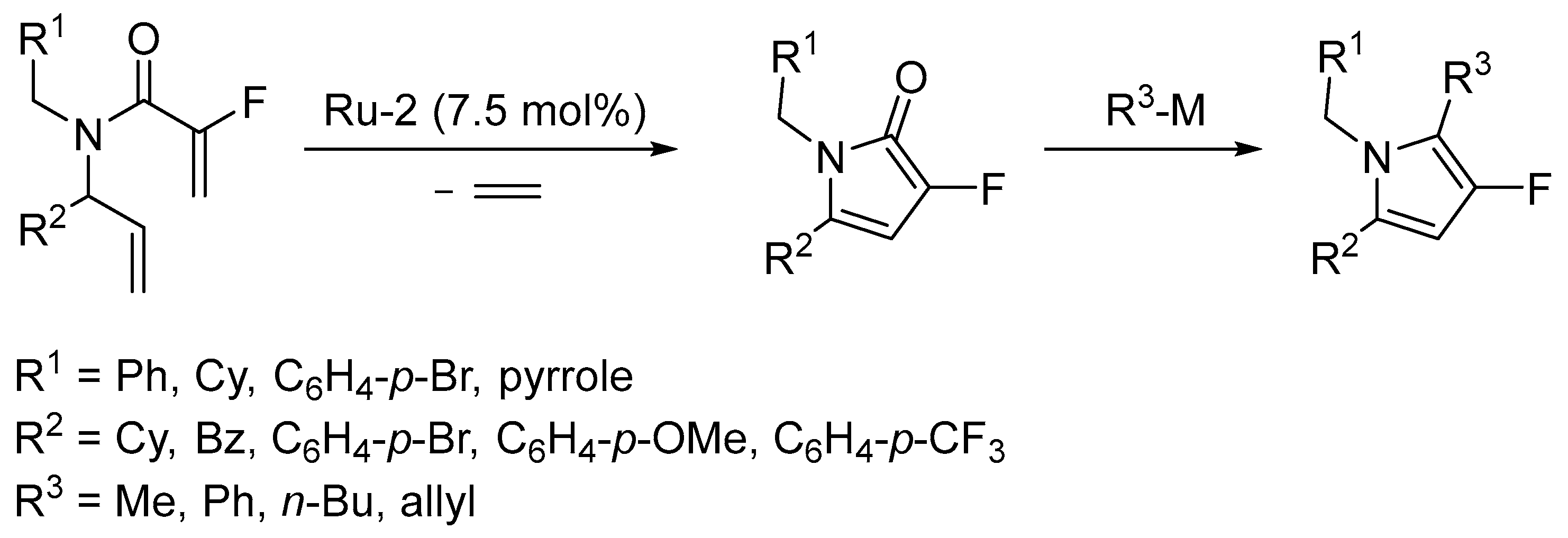
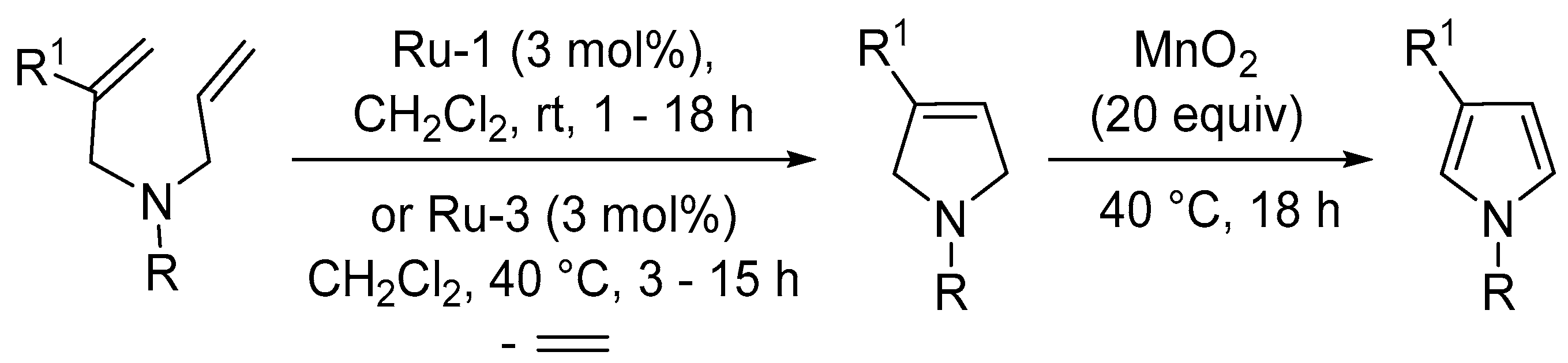
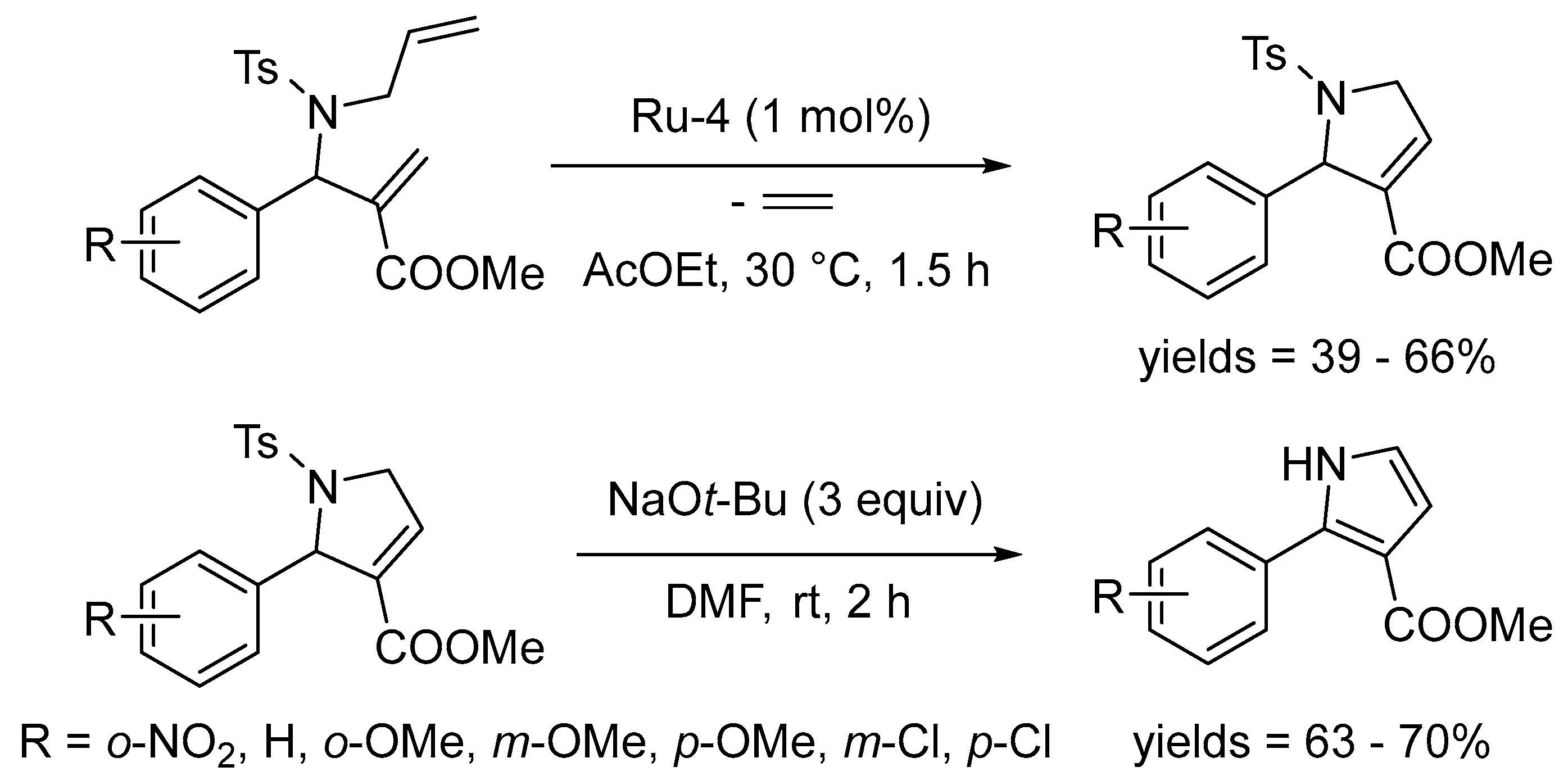
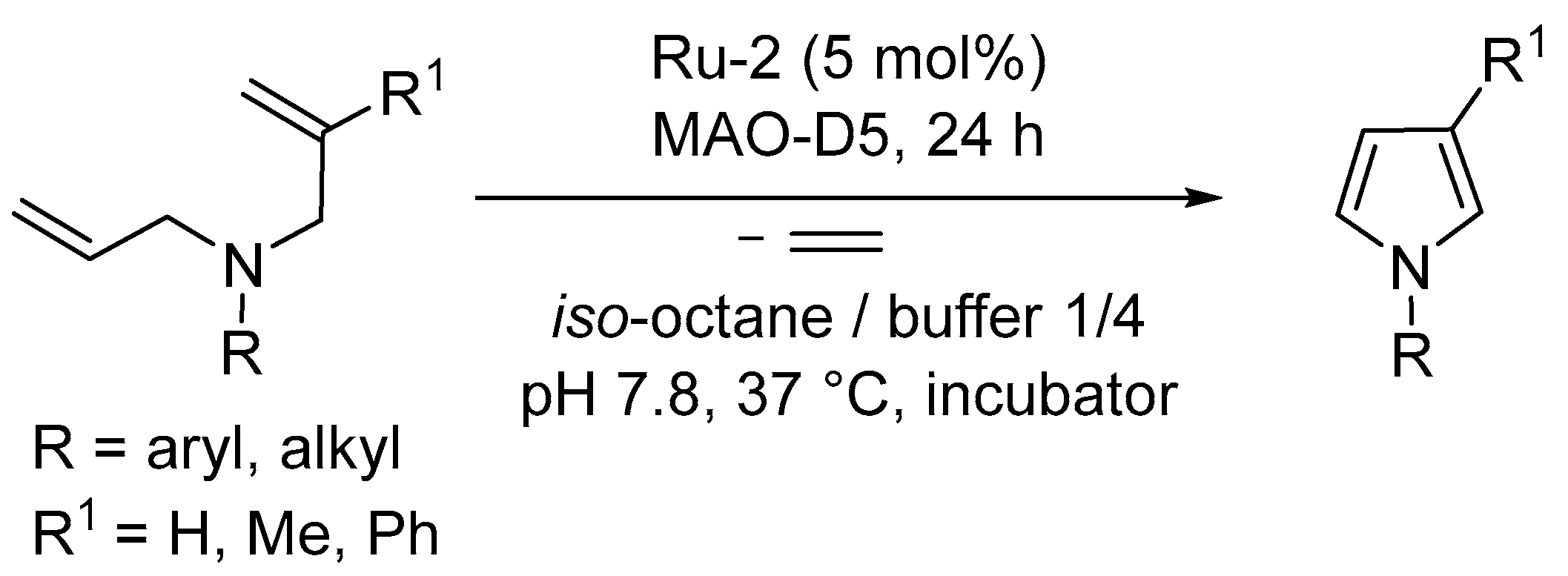
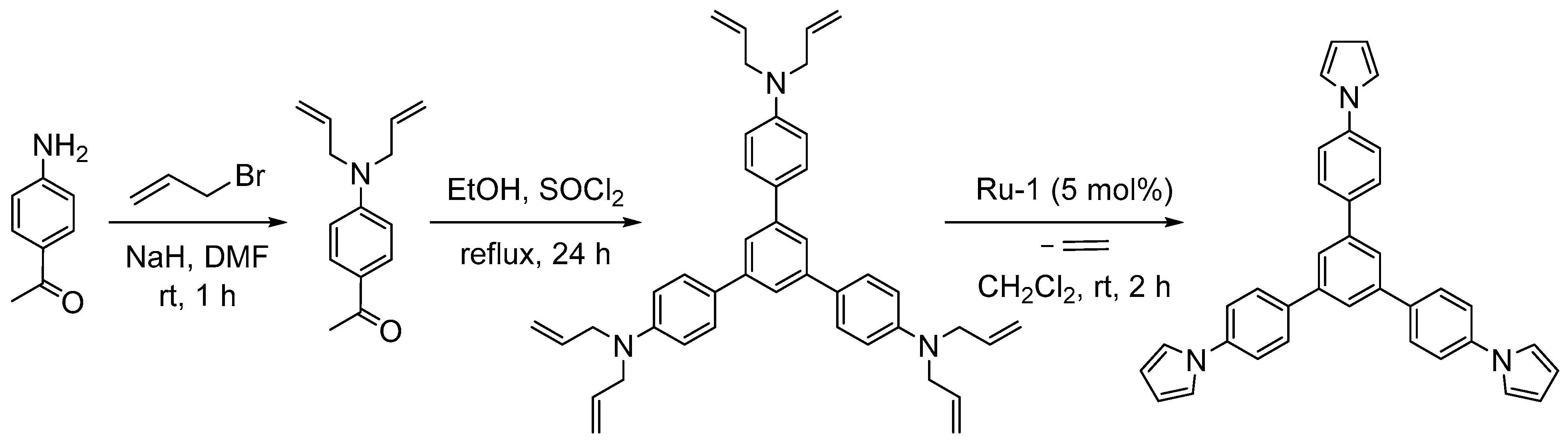
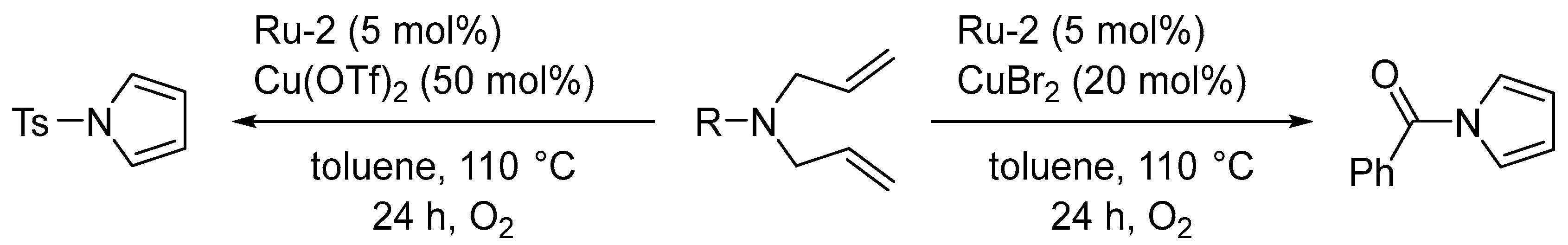
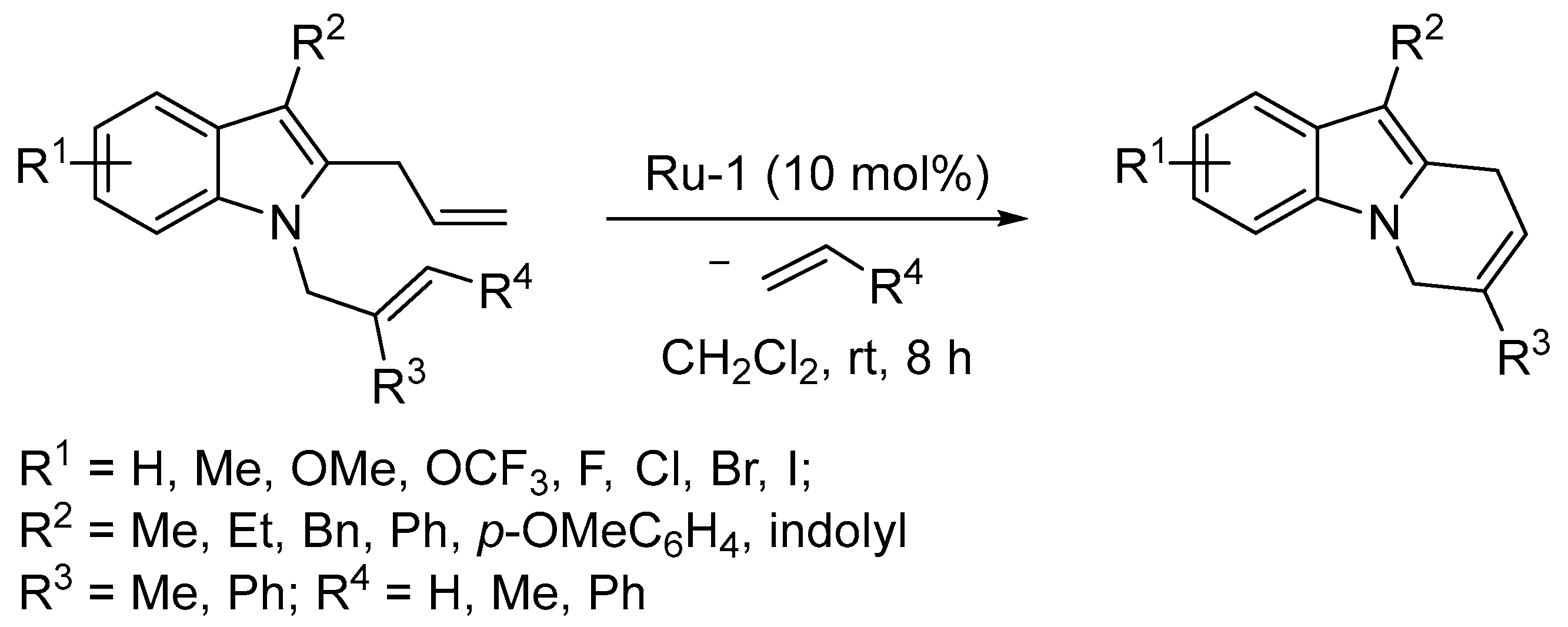
3.1. Synthesis of Five-Membered Rings
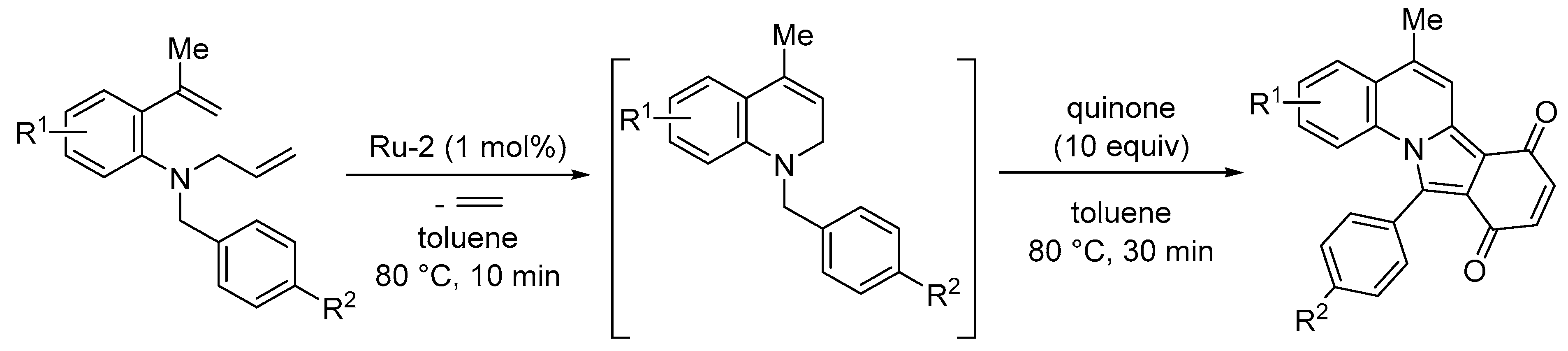
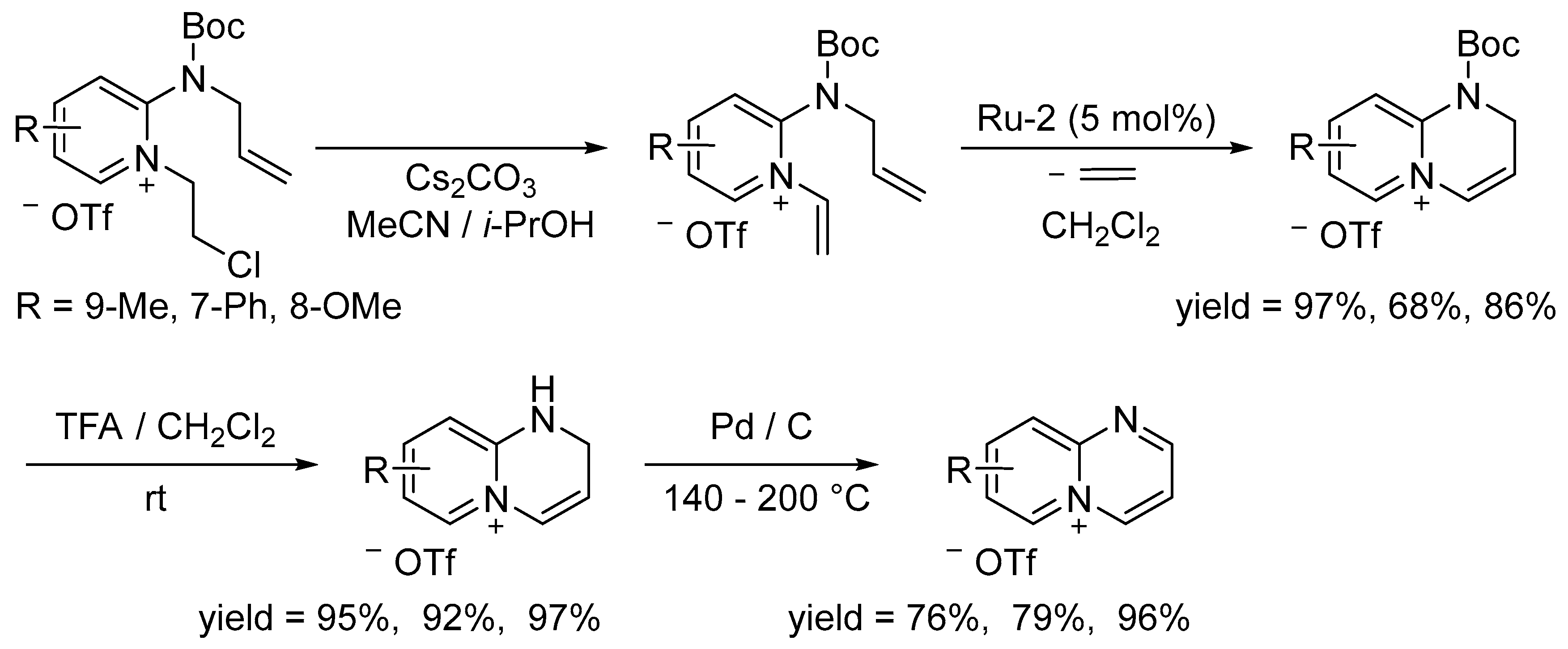
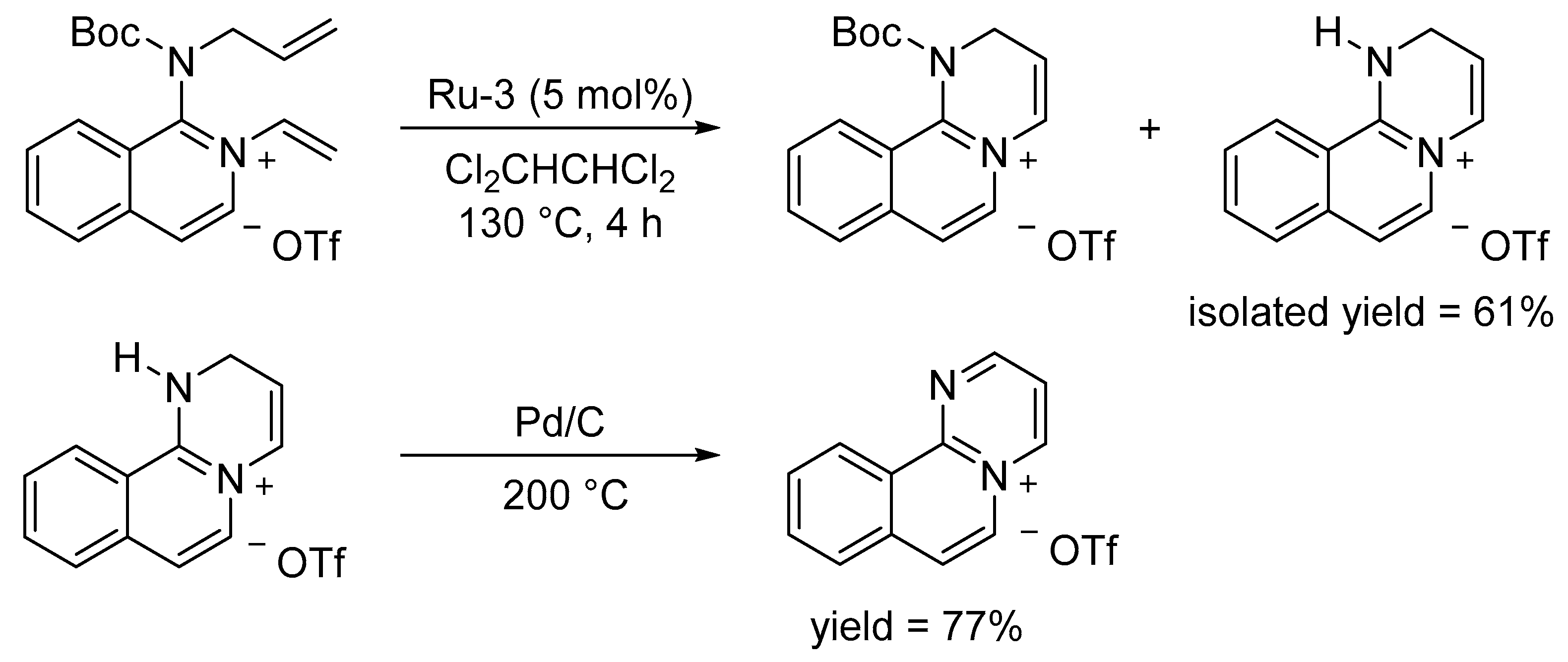
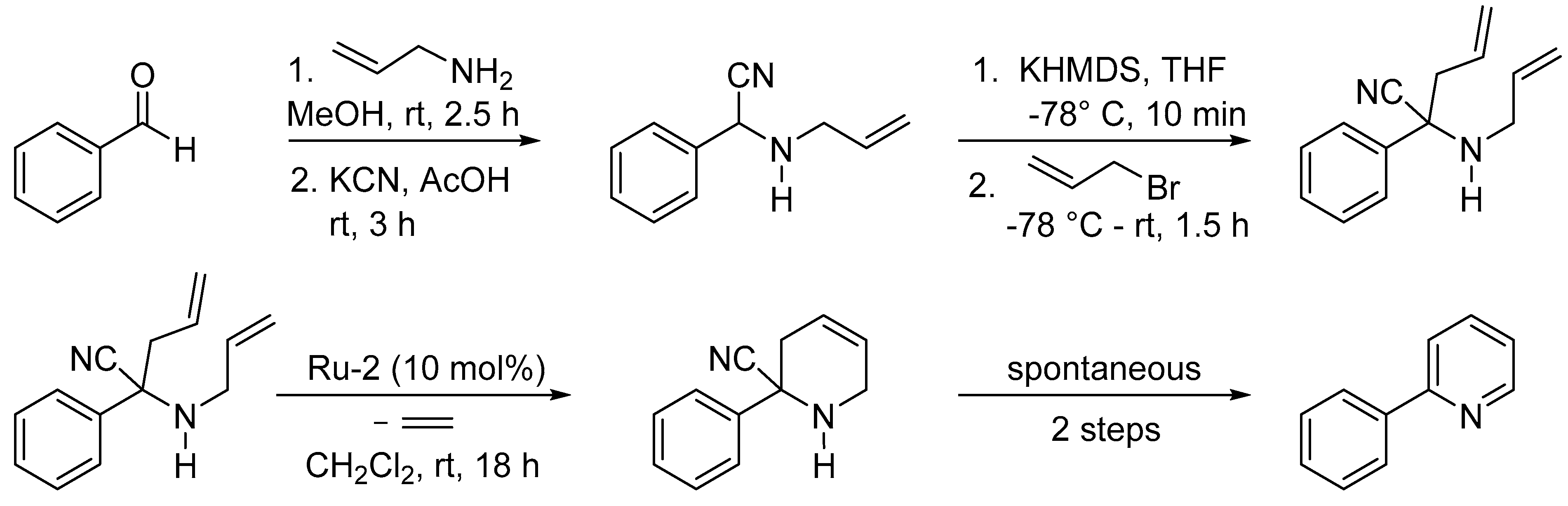
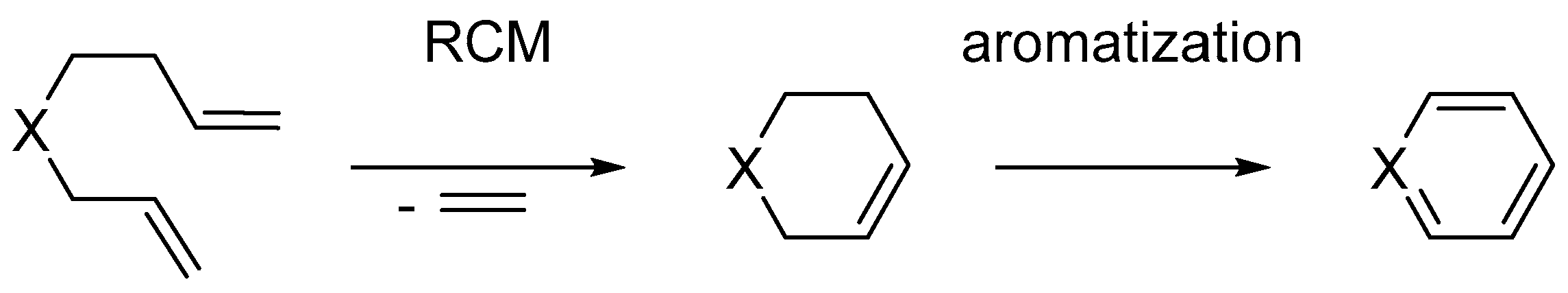
3.2. Synthesis of Six-Membered Rings
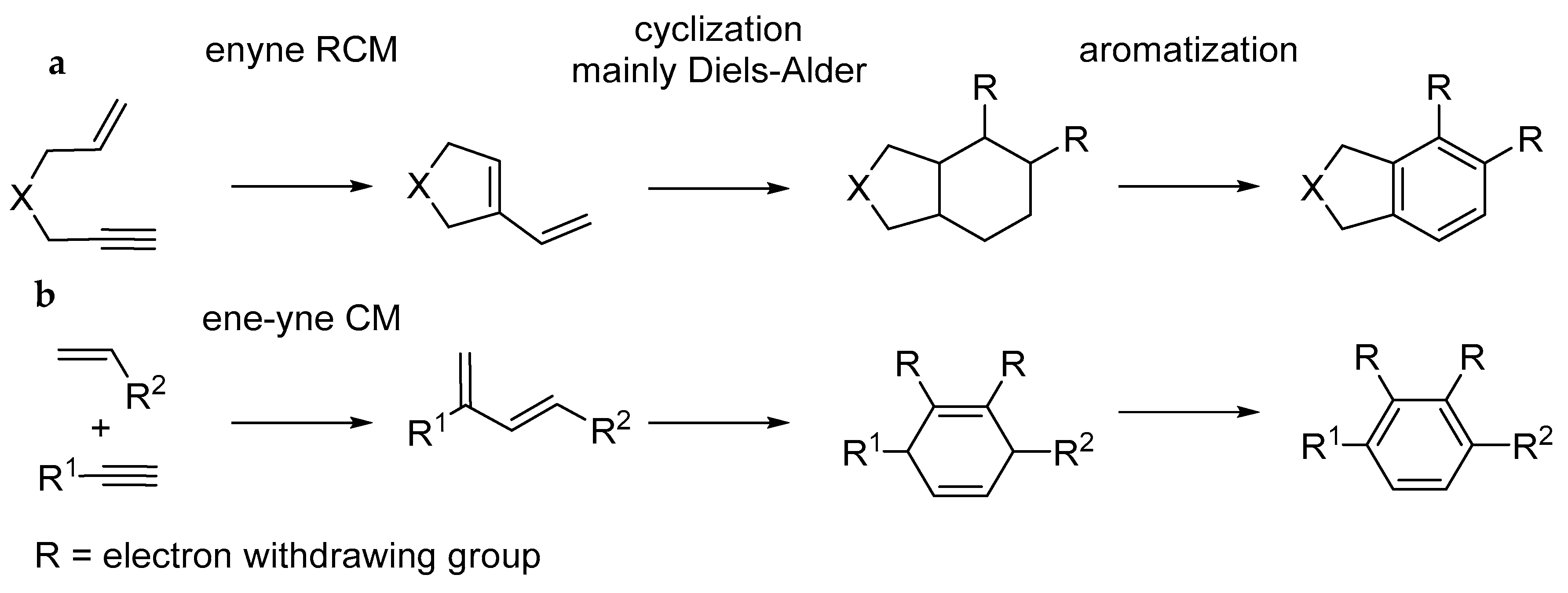
4. Synthesis of Aromatic Carbocycles
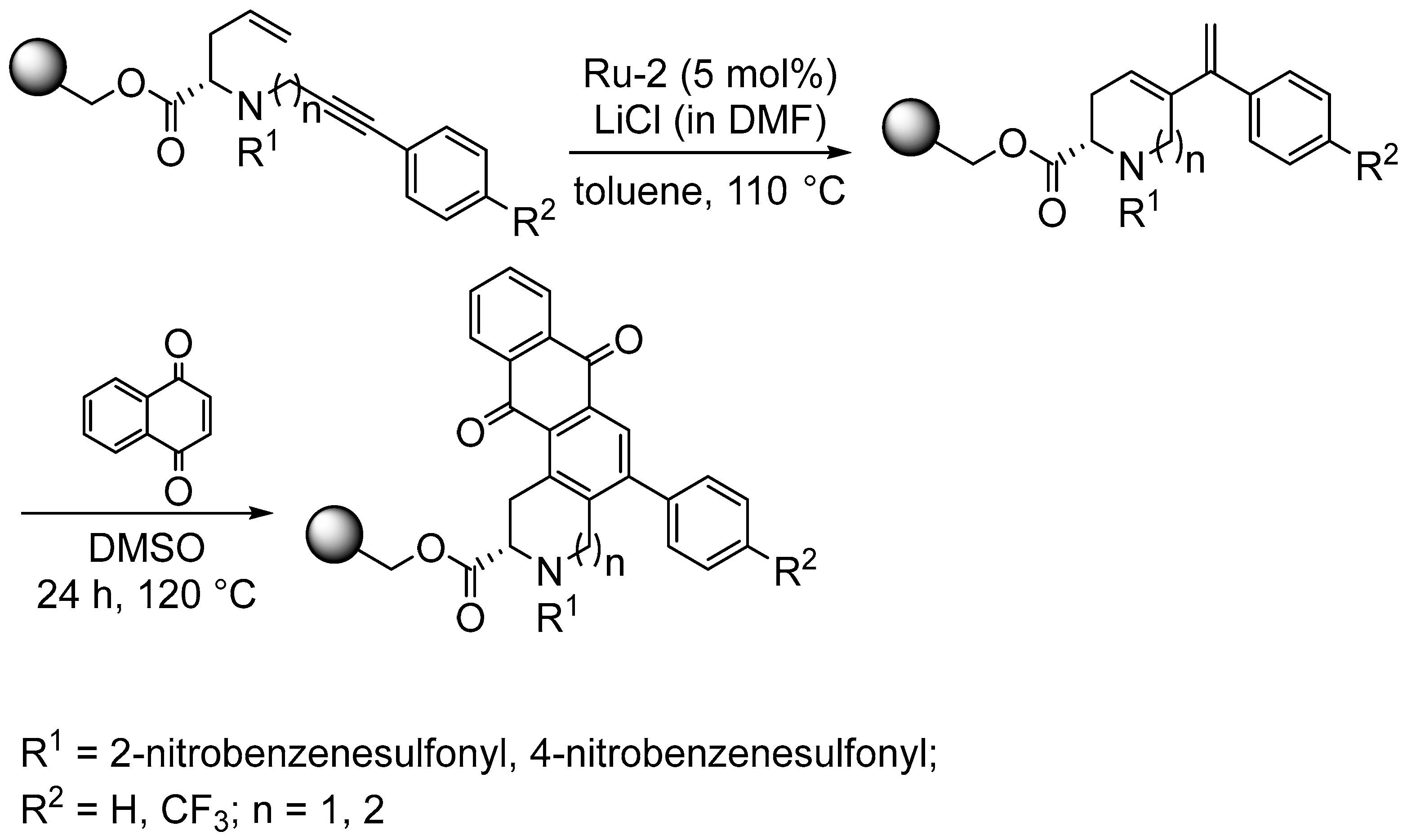
4.1. Synthesis of Aromatic Carbocycles Fused to Heterocyclic Rings
4.2. Synthesis of Benzene Derivatives
4.3. Synthesis of Aromatic Ring in Complex Molecules
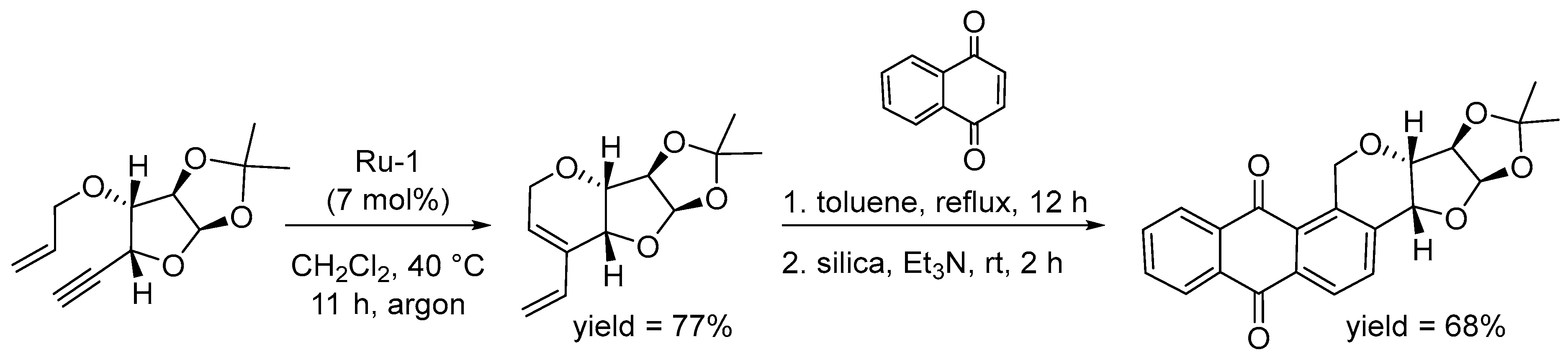
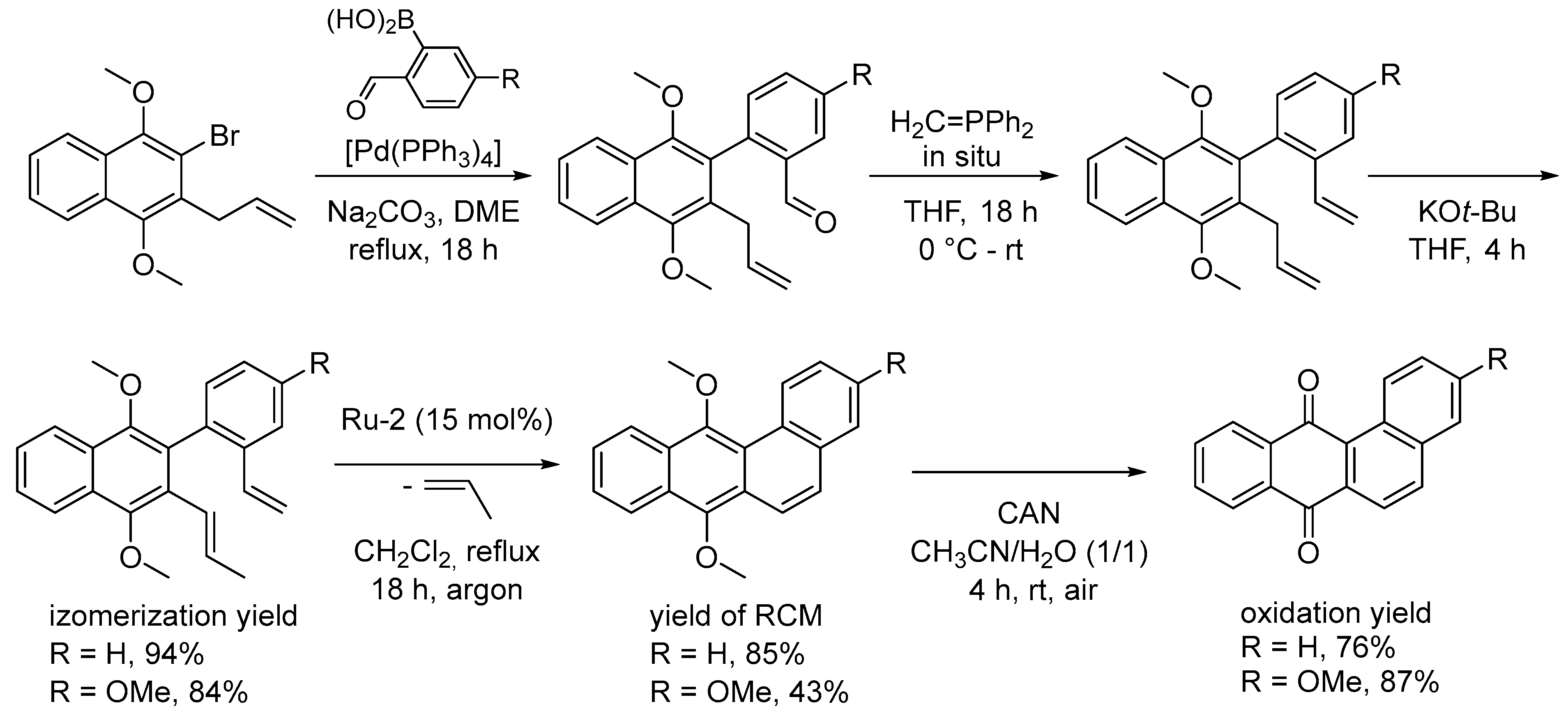
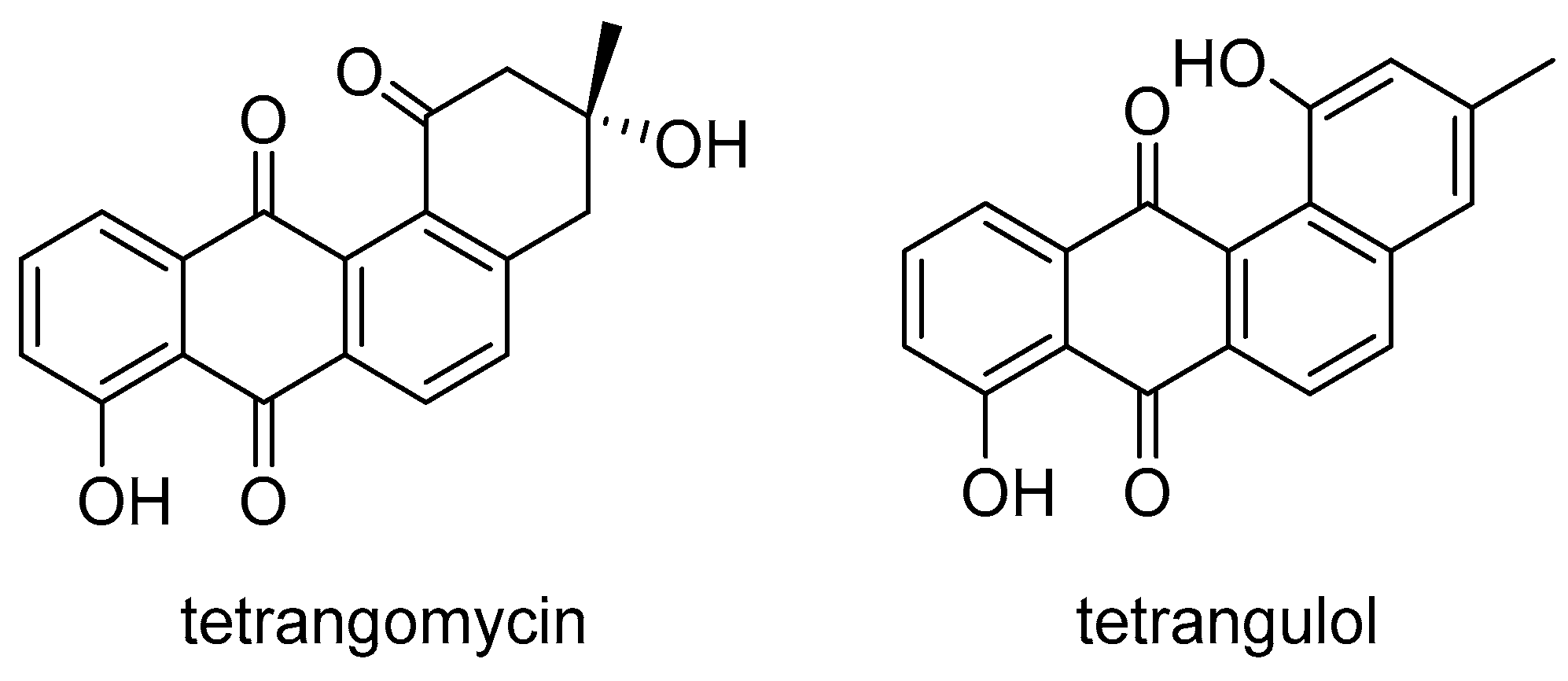
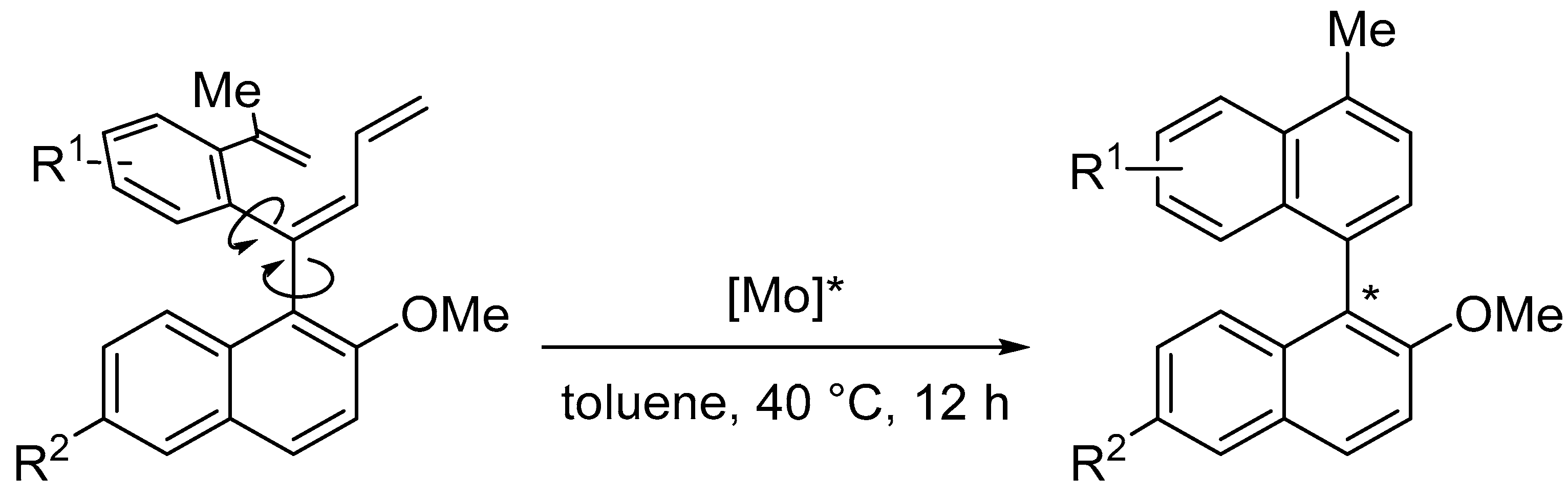
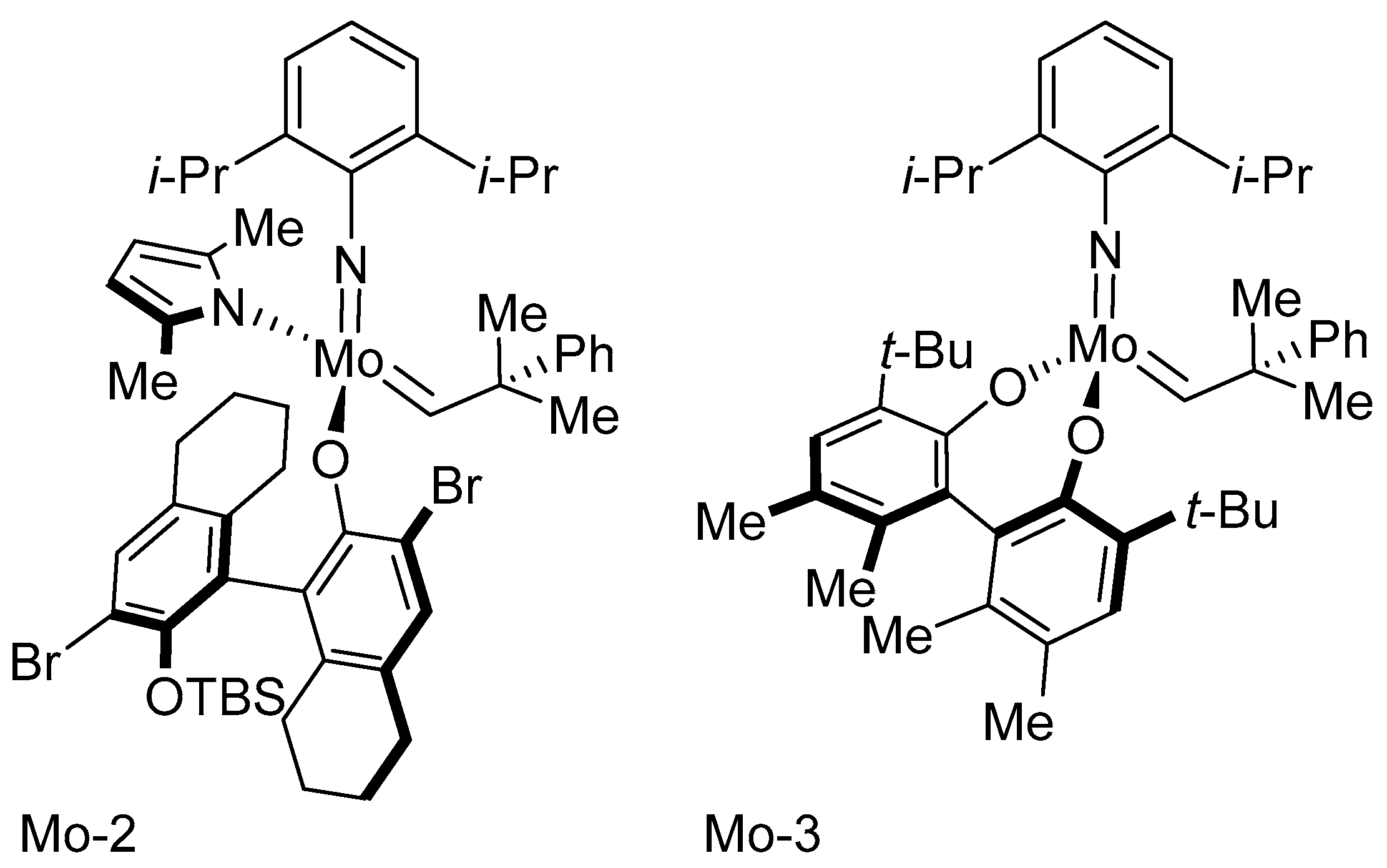
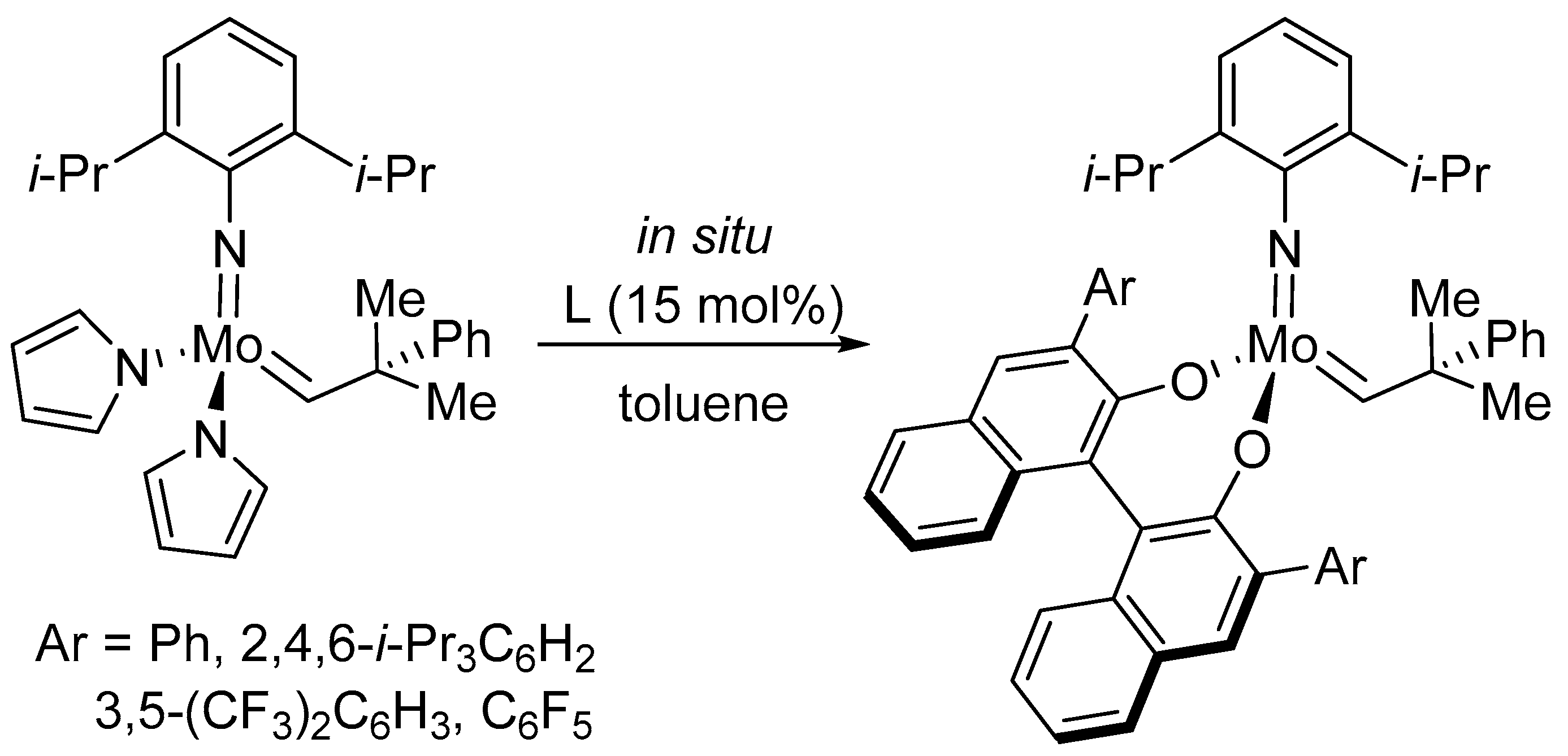
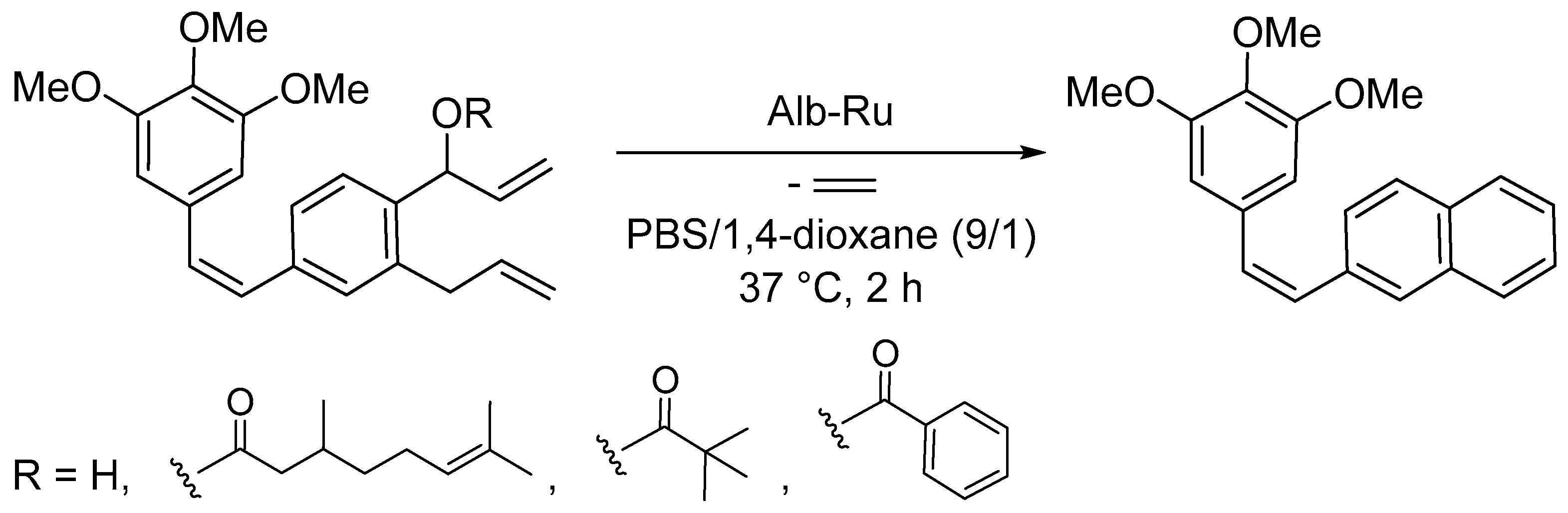
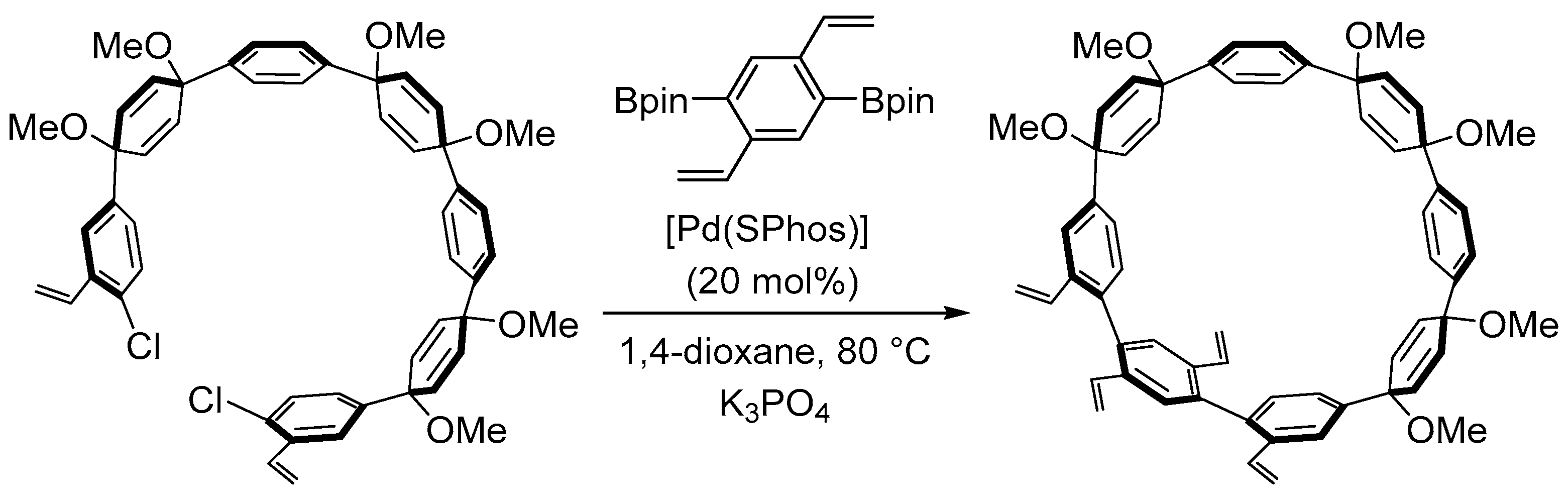
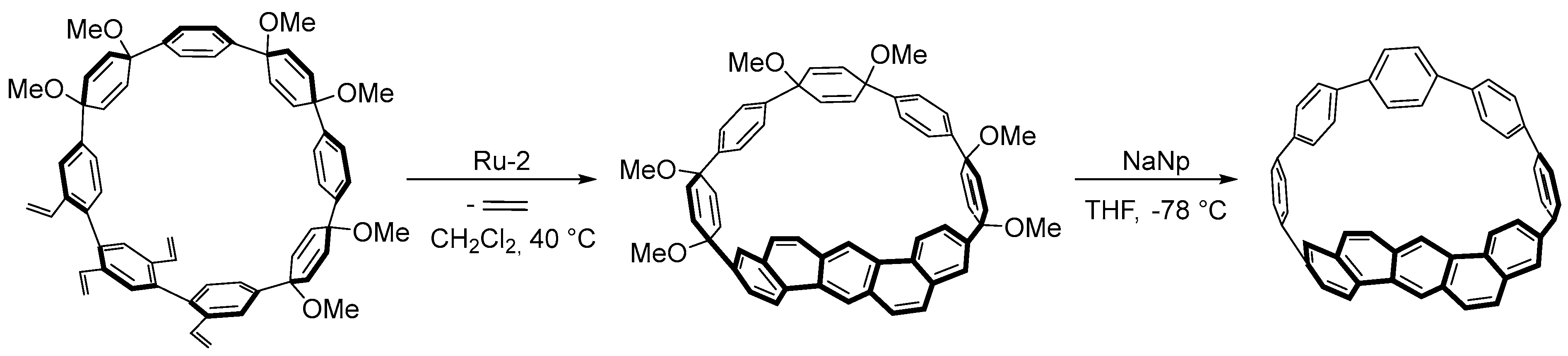
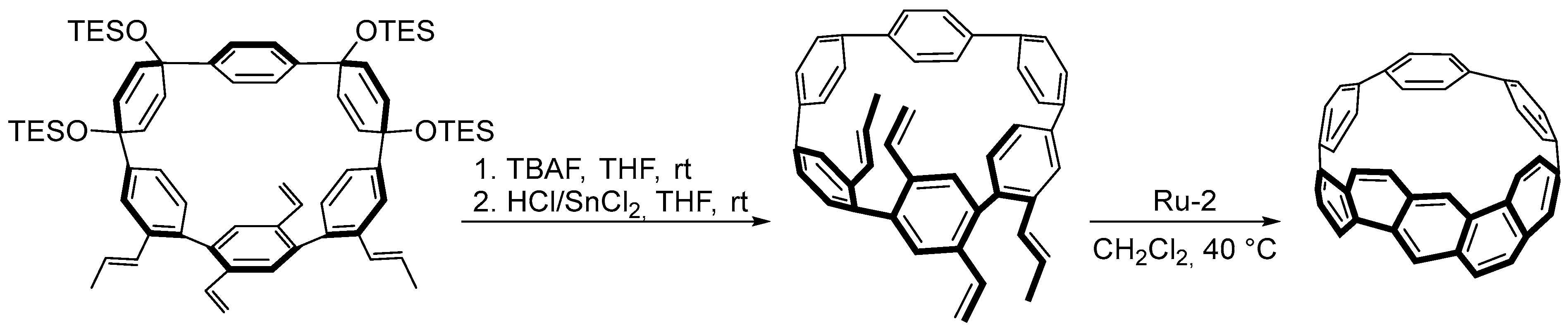
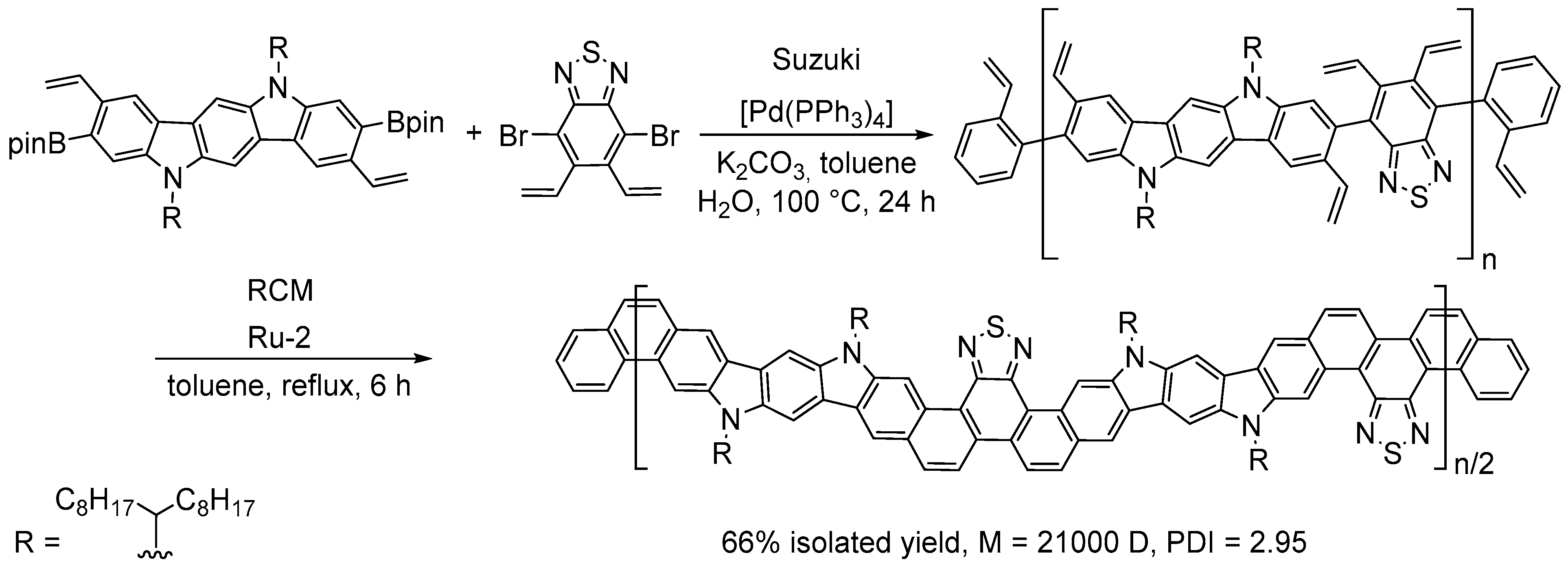
4.4. Synthesis of Polyarenes
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Handbook of Metathesis; Grubbs, R.H. (Ed.) Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Handbook of Metathesis; Grubbs, R.H.; Wenzel, A.G.; O’Leary, D.J.; Khosravi, E. (Eds.) Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Olefin Metathesis. Theory and Practice; Grela, K., Ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Metathesis in Natural Product Synthesis; Cossy, J.; Arseniyadis, S.; Meyer, C. (Eds.) Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Franc, H.-G.; Stadelhofer, J.W. Industrial Aromatic Chemistry; Springer: Berlin, Germany, 2011. [Google Scholar]
- Quin, L.D.; Tyrell, J.A. Fundamentals of Heterocyclic Chemistry. Importance in Nature and in the Synthesis of Pharmaceuticals; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Van Otterlo, W.A.L.; de Koning, C.B. Metathesis in the synthesis of aromatic compounds. Chem. Rev. 2009, 109, 3743–3782. [Google Scholar] [CrossRef] [PubMed]
- De Koning, C.B.; van Otterlo, W.A.L. Ring‐closing metathesis: Synthetic routes to carbocyclic aromatic compounds using ring-closing alkene and enyne metathesis. In Arene Chemistry. Reaction Mechanisms and Methods for Aromatic Compounds; Mortier, J., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 485–510. [Google Scholar]
- Yoshida, K. Metathesis reactions. In Transition-Metal-Mediated Aromatic Ring Construction; Tanaka, K., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 721–742. [Google Scholar]
- Donohoe, T.J.; Orr, A.J.; Bingham, M. Ring-Closing Metathesis as a basis for the construction of aromatic compounds. Angew. Chem. Int. Ed. 2006, 45, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Race, N.J.; Bower, J.F. Synthesis of heteroaromatic compounds by alkene and enyne metathesis. Top. Heterocycl. Chem. 2017, 47, 1–32. [Google Scholar]
- Donohoe, T.J.; Fishlock, L.P.; Procopiou, P.A. Ring-Closing Metathesis: Novel routes to aromatic heterocycles. Chem. Eur. J. 2008, 14, 5716–5726. [Google Scholar] [CrossRef]
- Potukuchi, H.K.; Colomer, I.; Donohoe, T.J. Synthesis of aromatic heterocycles using ring-closing metathesis. Adv. Heterocycl. Chem. 2016, 120, 43–65. [Google Scholar]
- Herisson, J.-L.; Chauvin, Y. Catalyse de transformation des olefines par les complexes du tungsten. II. Telomerisation des olefines cycliques en presence d’olefines acycliques. Macromol. Chem. 1971, 141, 161–176. [Google Scholar] [CrossRef]
- Hansen, E.C.; Lee, D. Search for solutions to the reactivity and selectivity problems in enyne metathesis. Acc. Chem. Res. 2006, 39, 509–519. [Google Scholar] [CrossRef]
- Hansen, E.C.; Lee, D. Ring-closing enyne metathesis: Control over mode selectivity and stereoselectivity. J. Am. Chem. Soc. 2004, 126, 15074–15080. [Google Scholar] [CrossRef]
- Trost, B.M.; Krische, M.J. Transition metal catalyzed cycloisomerizations. Synlett 1998, 1998, 1–16. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, G.; Tong, X.; Wang, F.; Xie, X.; Wang, J.; Jiang, L. Transition metal-catalyzed intramolecular enyne cyclization reaction. Curr. Org. Chem. 2006, 10, 1457–1478. [Google Scholar] [CrossRef]
- Diver, S.T.; Giessert, A.J. Enyne metathesis (enyne bond reorganization). Chem. Rev. 2004, 104, 1317–1382. [Google Scholar] [CrossRef] [PubMed]
- Villar, H.; Fringsa, M.; Bolm, C. Ring-closing enyne metathesis: A powerful tool for the synthesis of heterocycles. Chem. Soc. Rev. 2007, 36, 55–66. [Google Scholar] [CrossRef]
- Li, J.; Lee, D. Enyne metathesis. In Handbook of Metathesis; Grubbs, R.H., O’Leary, D.J., Eds.; Wiley-VCH: Weinheim, Germany, 2015; Volume 2, pp. 381–444. [Google Scholar]
- Trnka, T.N.; Grubbs, R.H. The development of L2X2Ru=CHR olefin metathesis catalysts: An organometallic success story. Acc. Chem. Res. 2001, 34, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Schrock, R.R. Olefin metathesis by molybdenum imido alkylidene catalysts. Tetrahedron 1999, 55, 8141–8153. [Google Scholar] [CrossRef]
- Zielinski, G.K.; Grela, K. Tandem catalysis utilizing olefin metathesis reactions. Chem. Eur. J. 2016, 22, 9440–9454. [Google Scholar] [CrossRef] [PubMed]
- Raviola, C.; Protti, S.; Ravelli, D.; Fagnoni, M. (Hetero)aromatics from dienynes, enediynes and enyne–allenes. Chem. Soc. Rev. 2016, 45, 4364–4390. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, T.J.; Bower, J.F.; Chan, L.K.M. Olefin cross-metathesis for the synthesis of heteroaromatic compounds. Org. Biomol. Chem. 2012, 10, 1322–1328. [Google Scholar] [CrossRef]
- Georgiades, S.N.; Nicolaou, P.G. Recent advances in carbazole syntheses. Adv. Heterocycl. Chem. 2019, 129, 1–88. [Google Scholar]
- Chachignon, H.; Scalacci, N.; Petricci, E.; Castagnolo, D. Synthesis of 1,2,3-substituted pyrroles from propargylamines via a one-pot tandem enyne cross metathesis–cyclization reaction. J. Org. Chem. 2015, 80, 5287–5295. [Google Scholar] [CrossRef]
- Frei, M.S.; Bilyard, M.K.; Alanine, T.A.; Galloway, W.R.J.D.; Stokes, J.E.; Spring, D.R. Studies towards the synthesis of indolizin-5(3H)-one derivatives and related 6,5-azabicyclic scaffolds by ring-closing metathesis. Bioorg. Med. Chem. 2015, 23, 2666–2679. [Google Scholar] [CrossRef]
- Bunrit, A.; Sawadjoon, S.; Tsupova, S.; Sjöberg, P.J.R.; Samec, J.S.M. A general route to β-substituted pyrroles by transition-metal catalysis. J. Org. Chem. 2016, 81, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, T.J.; Donald, C.S.; Marquez, R. Flexible synthesis of polyfunctionalised 3-fluoropyrroles. Org. Biomol. Chem. 2016, 14, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Keeley, A.; McCauley, S.; Evans, P. A ring-closing metathesis-manganese dioxide oxidation sequence for the synthesis of substituted pyrroles. Tetrahedron 2016, 72, 2552–2559. [Google Scholar] [CrossRef]
- Grychowska, K.; Kubica, B.; Drop, M.; Colacino, E.; Bantreil, X.; Paw1owski, M.; Martinez, J.; Subra, G.; Zajdel, P.; Lamaty, F. Application of the ring-closing metathesis to the formation of 2-aryl-1H-pyrrole-3-carboxylates as building blocks for biologically active compounds. Tetrahedron 2016, 72, 7462–7469. [Google Scholar] [CrossRef]
- Scalacci, N.; Black, G.W.; Mattedi, G.; Brown, N.L.; Turner, N.J.; Castagnolo, D. Unveiling the biocatalytic aromatizing activity of monoamine oxidases MAO-N and 6-HDNO: Development of chemoenzymatic cascades for the synthesis of pyrroles. ACS Catal. 2017, 7, 1295–1300. [Google Scholar] [CrossRef]
- Kotha, S.; Todeti, S.; Das, T.; Datta, A. Synthesis of star-shaped pyrrole-based C3-symmetric molecules via ring-closing metathesis, Buchwald–Hartwig cross-coupling and Clauson–Kaas pyrrole synthesis as key steps. Tetrahedron Lett. 2018, 59, 1023–1027. [Google Scholar] [CrossRef]
- Chena, W.; Zhanga, Y.-L.; Li, H.-J.; Nana, X.; Liua, Y.; Wu, Y.-C. Synthesis of N-sulfonyl- and N-acylpyrroles via a ring-closing metathesis/dehydrogenation tandem reaction. Synthesis 2019, 51, 3651–3666. [Google Scholar] [CrossRef]
- Fujii, Y.; Suwa, Y.; Wada, Y.; Takehara, T.; Suzuki, T.; Kawashima, Y.; Kawashita, N.; Takagi, T.; Fujioka, H.; Arisawa, M. Metal-free nitrogen-containing polyheterocyclic near-infrared (NIR) absorption dyes: Synthesis, absorption properties, and theoretical calculation of substituted 5-methylisoindolo[2,1-a]quinolones. ACS Omega 2019, 4, 5064–5075. [Google Scholar] [CrossRef]
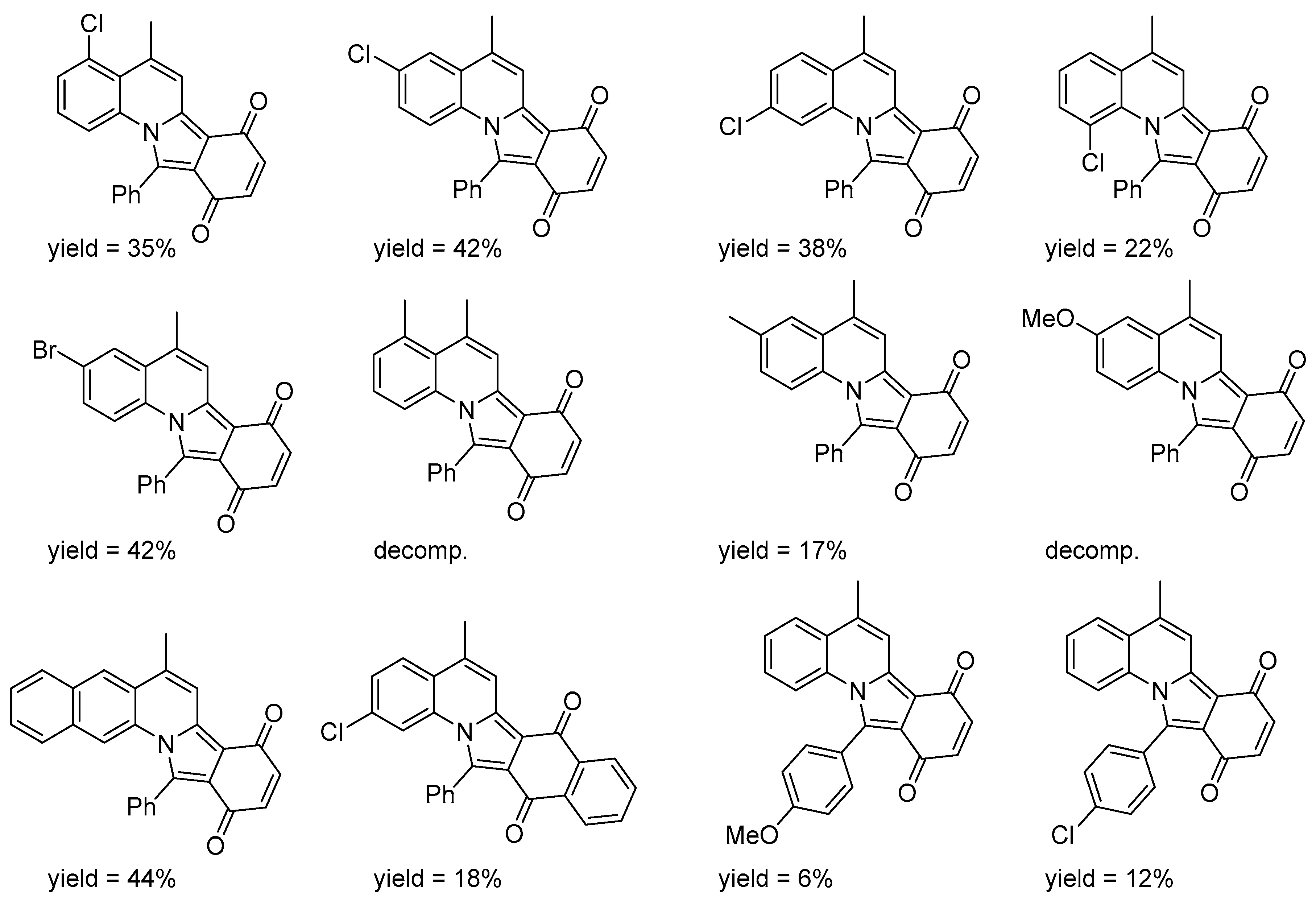
- Francois, B.; Eberlin, L.; Berrée, F.; Whiting, A.; Carboni, B. Access to fused pyrroles from cyclic 1,3-dienyl boronic esters and arylnitroso compounds. J. Org. Chem. 2020, 85, 5173–5182. [Google Scholar] [CrossRef]
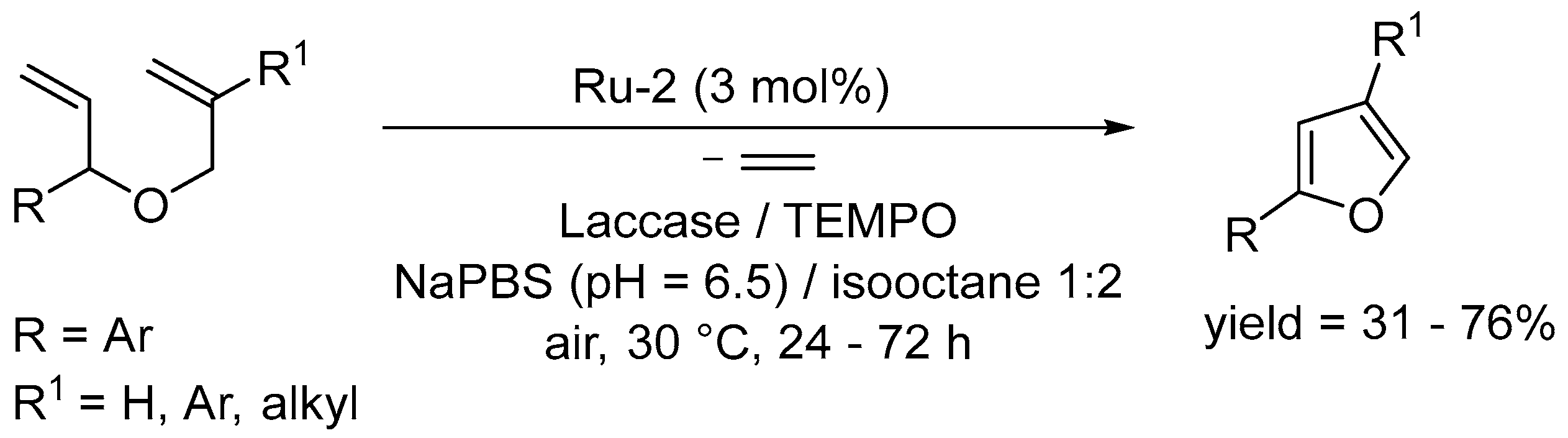
- Risi, C.; Zhao, F.; Castagnolo, D. Chemo-enzymatic metathesis/aromatization cascades for the synthesis of furans: Disclosing the aromatizing activity of Laccase/TEMPO in oxygen-containing heterocycles. ACS Catal. 2019, 9, 7264–7269. [Google Scholar] [CrossRef]
- Kotha, S.; Solanke, B.U.; Gupta, N.K. Design and synthesis of C3-symmetric molecules containing oxepine and benzofuran moieties via metathesis. J. Mol. Struct. 2021, 1244, 130907. [Google Scholar] [CrossRef]
- Kotha, S.; Solanke, B.U. Modular approach to benzofurans, 2H-chromenes and benzoxepines via Claisen rearrangement and ring-closing metathesis: Access to phenylpropanoids. Chem. Asian J. 2022, 17, e202200084. [Google Scholar] [CrossRef]
- Lood, K.; Tikk, T.; Krüger, M.; Schmidt, B. Methylene capping facilitates cross-metathesis reactions of enals: A short synthesis of 7-methoxywutaifuranal from the xylochemical isoeugenol. J. Org. Chem. 2022, 87, 3079–3088. [Google Scholar] [CrossRef]
- Sucunza, D.; Cuadro, A.M.; Alvarez-Builla, J.; Vaquero, J.J. Recent advances in the synthesis of azonia aromatic heterocycles. J. Org. Chem. 2016, 81, 10126–10135. [Google Scholar] [CrossRef]
- Abengózar, A.; Abarca, B.; Cuadro, A.M.; Sucunza, D.; Álvarez-Builla, J.; Vaquero, J.J. Azonia aromatic cations by ring-closing metathesis: Synthesis of azaquinolizinium cations. Eur. J. Org. Chem. 2015, 2015, 4214–4223. [Google Scholar] [CrossRef]
- Weber, C.; Nebe, M.M.; Kaluza, L.P.V.; Opatz, T. Short synthesis of pyridines from deprotonated α-aminonitriles by an alkylation/RCM sequence. Z. Naturforsch. 2016, 71b, 633–641. [Google Scholar] [CrossRef]
- Remya, P.R.; Suresh, C.H. Grubbs and Hoveyda-Grubbs catalysts for pyridine derivative synthesis: Probing the mechanistic pathways using DFT. Mol. Catal. 2018, 450, 29–38. [Google Scholar] [CrossRef]
- Mandal, T.; Chakraborti, G.; Karmakar, S.; Dash, J. Divergent and orthogonal approach to carbazoles and pyridoindoles from oxindoles via indole intermediates. Org. Lett. 2018, 20, 4759–4763. [Google Scholar] [CrossRef]
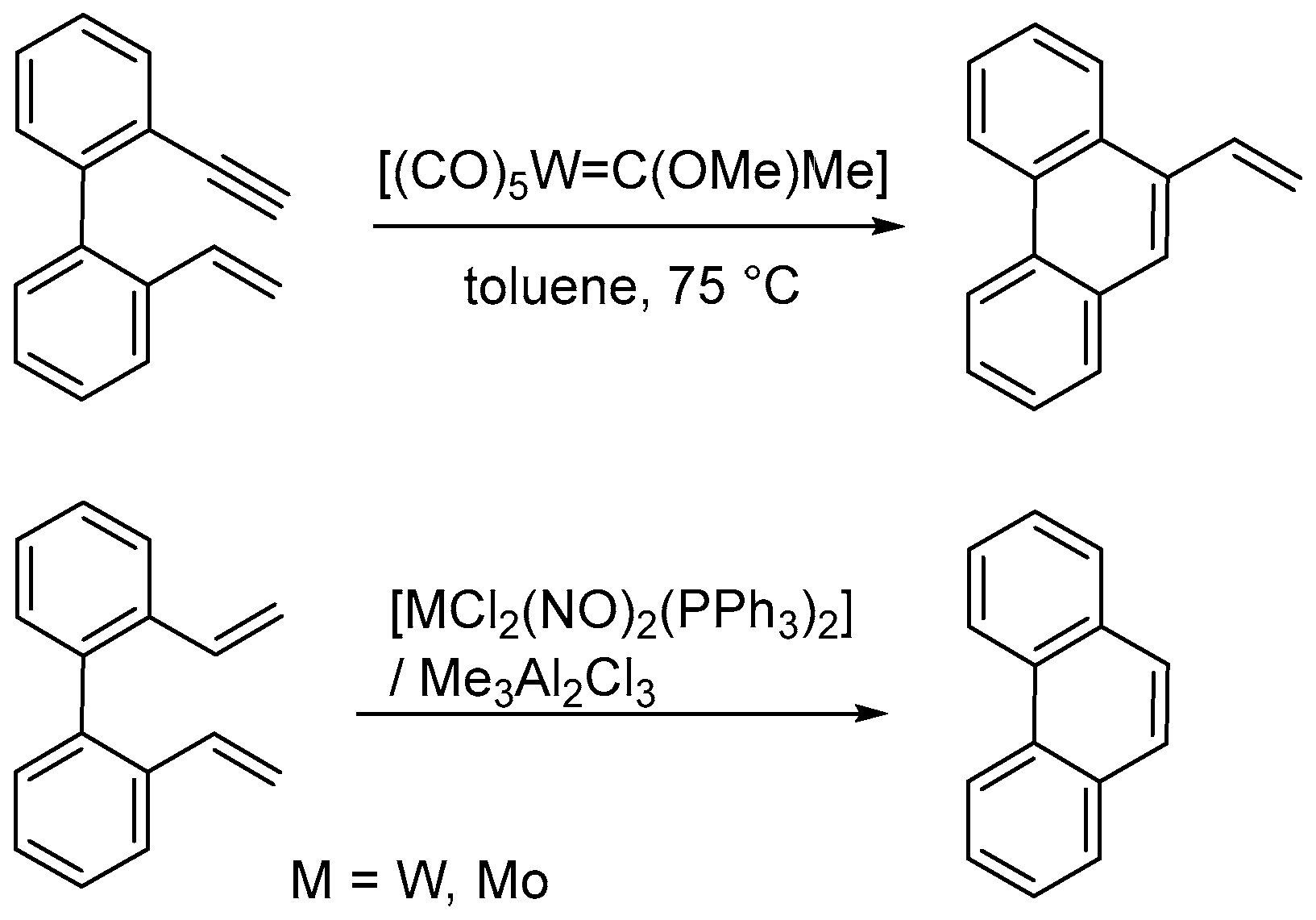
- Katz, T.J.; Sivavec, T.M. Metal-catalyzed rearrangement of alkene-alkynes and the stereochemistry of metallacyclobutene ring opening. J. Am. Chem. Soc. 1985, 107, 737–738. [Google Scholar] [CrossRef]
- Katz, T.J.; Rothchild, R. Mechanism of the olefin metathesis of 2,2′-divinylbiphenyl. J. Am. Chem. Soc. 1976, 98, 2519–2526. [Google Scholar] [CrossRef]
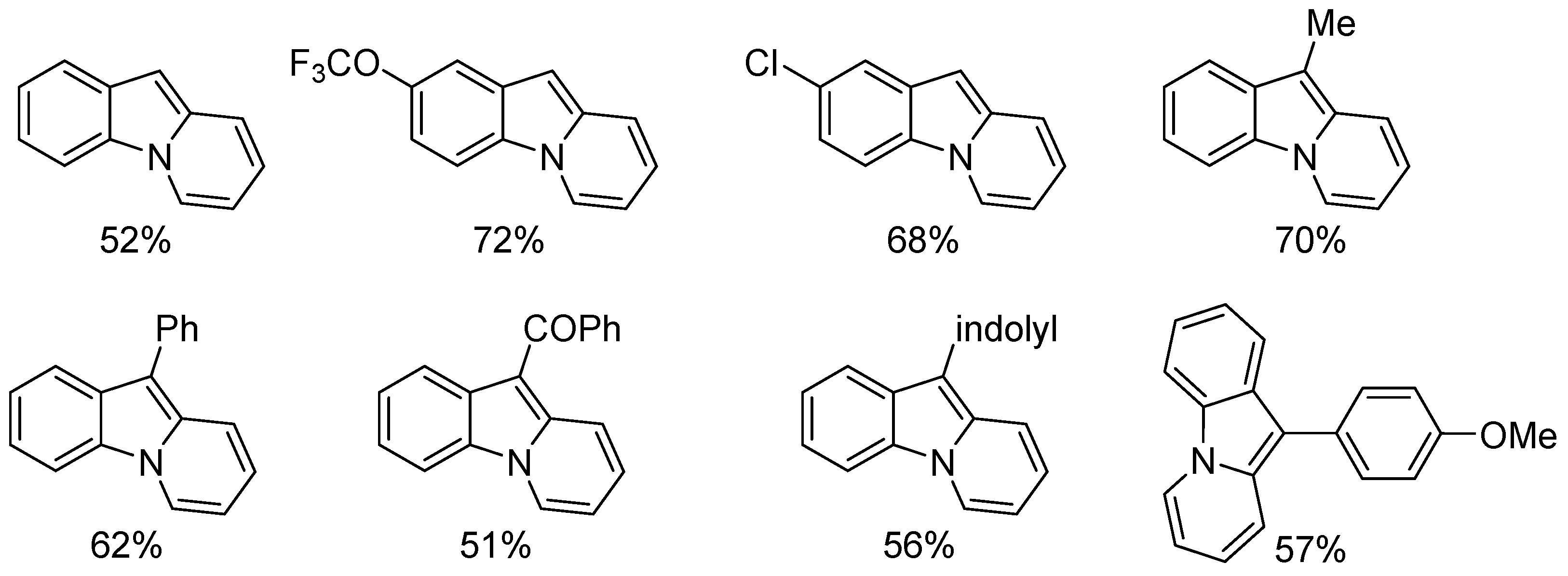
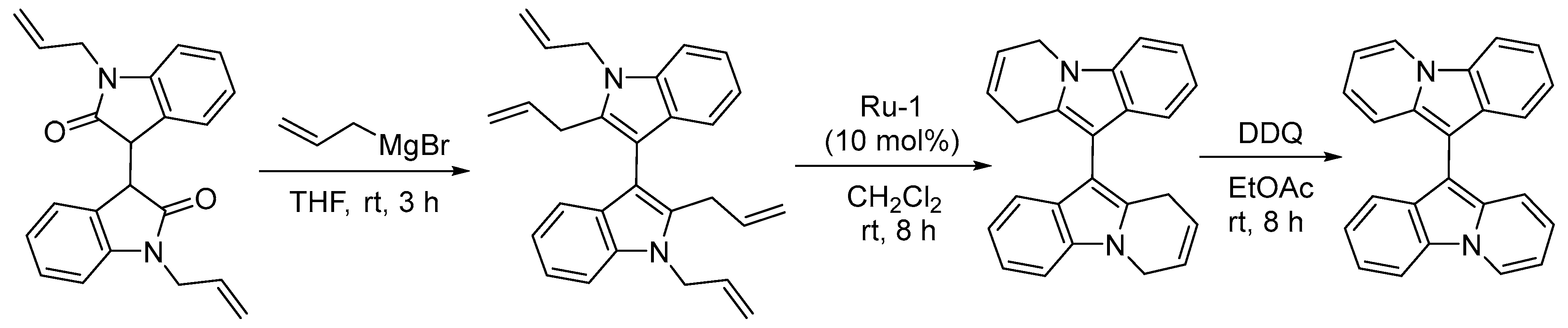
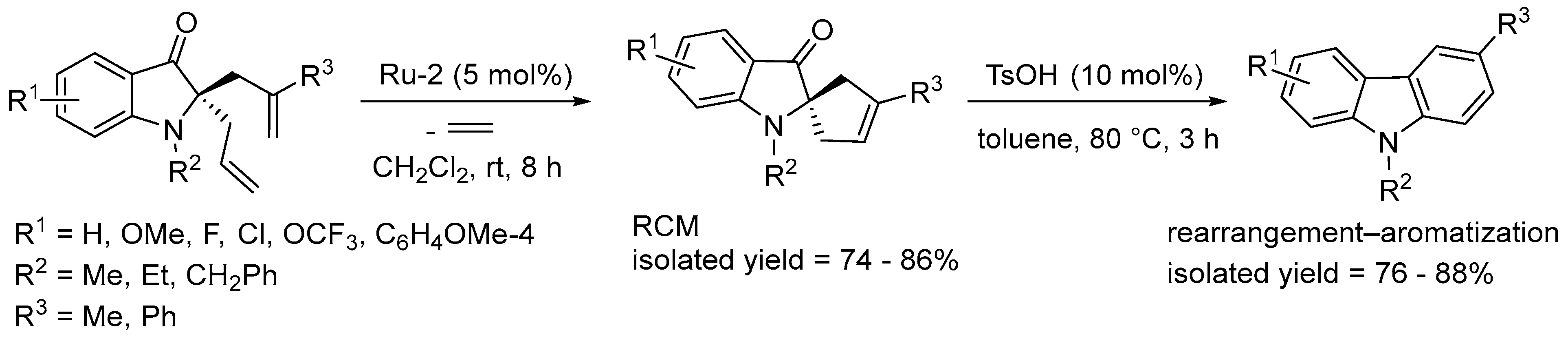
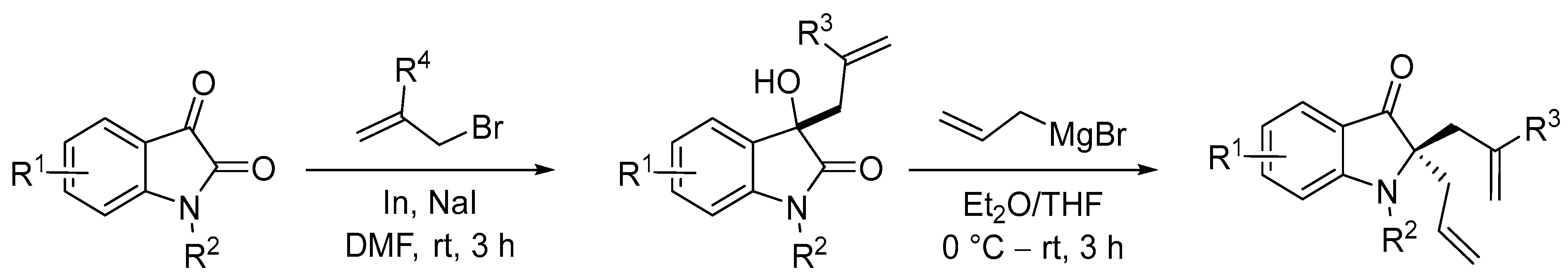
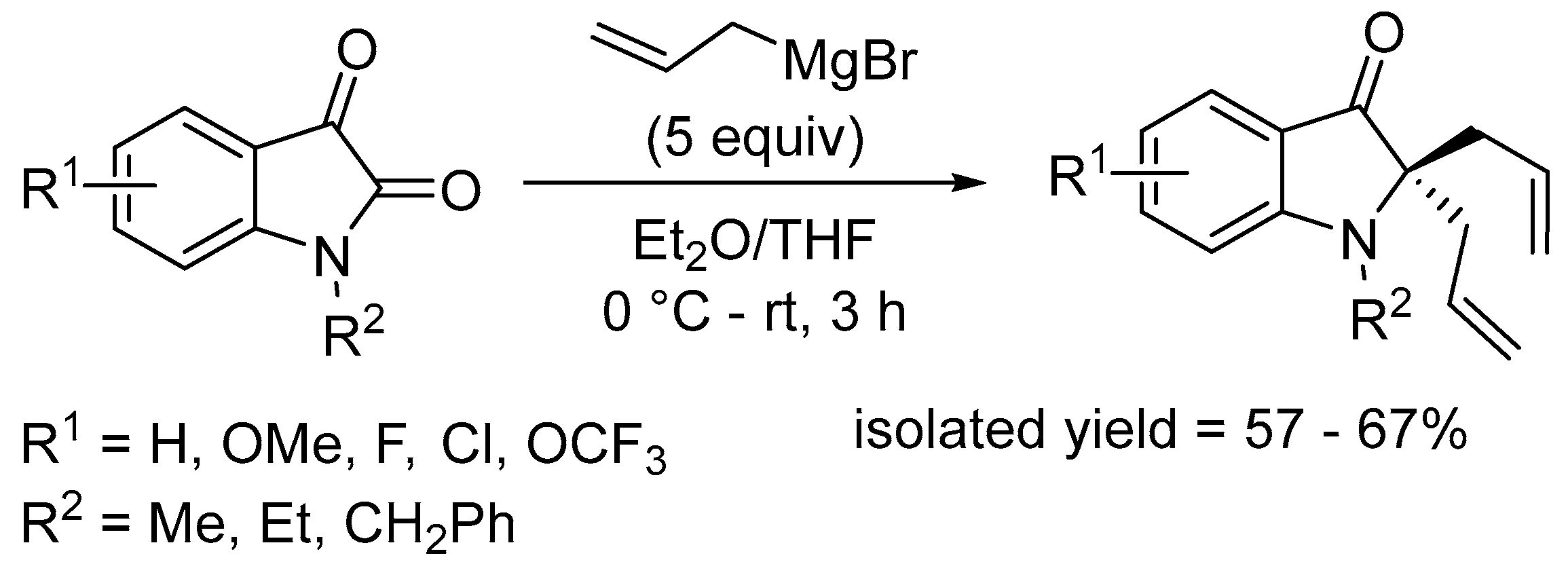
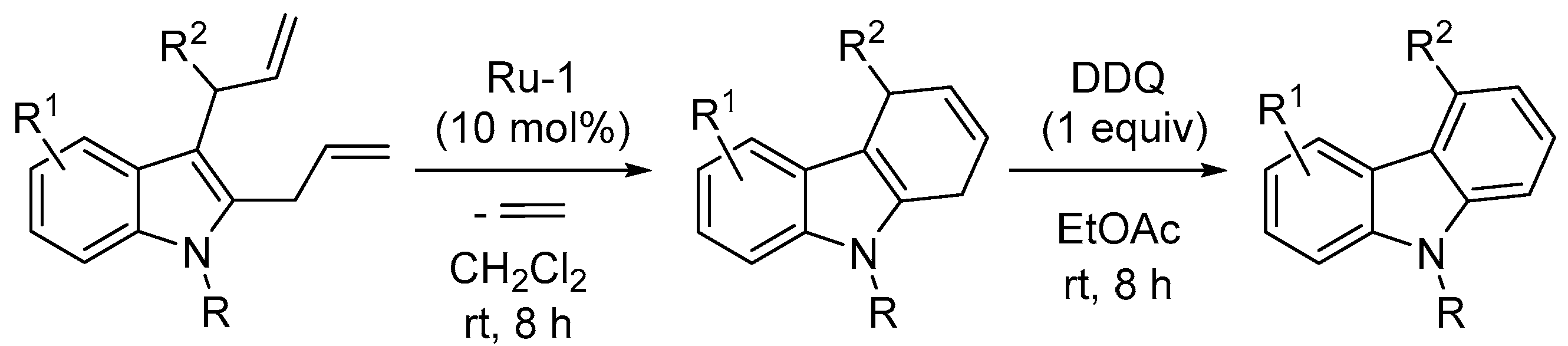
- Mandal, T.; Dash, J. Ring-closing metathesis for the construction of carbazole and indole-fused natural products. Org. Biomol. Chem. 2021, 19, 9797–9808. [Google Scholar] [CrossRef] [PubMed]
- Dhara, K.; Mandal, T.; Das, J.; Dash, J. Synthesis of carbazole alkaloids by ring-closing metathesis and ring rearrangement–aromatization. Angew. Chem. Int. Ed. 2015, 54, 15831–15835. [Google Scholar] [CrossRef] [PubMed]
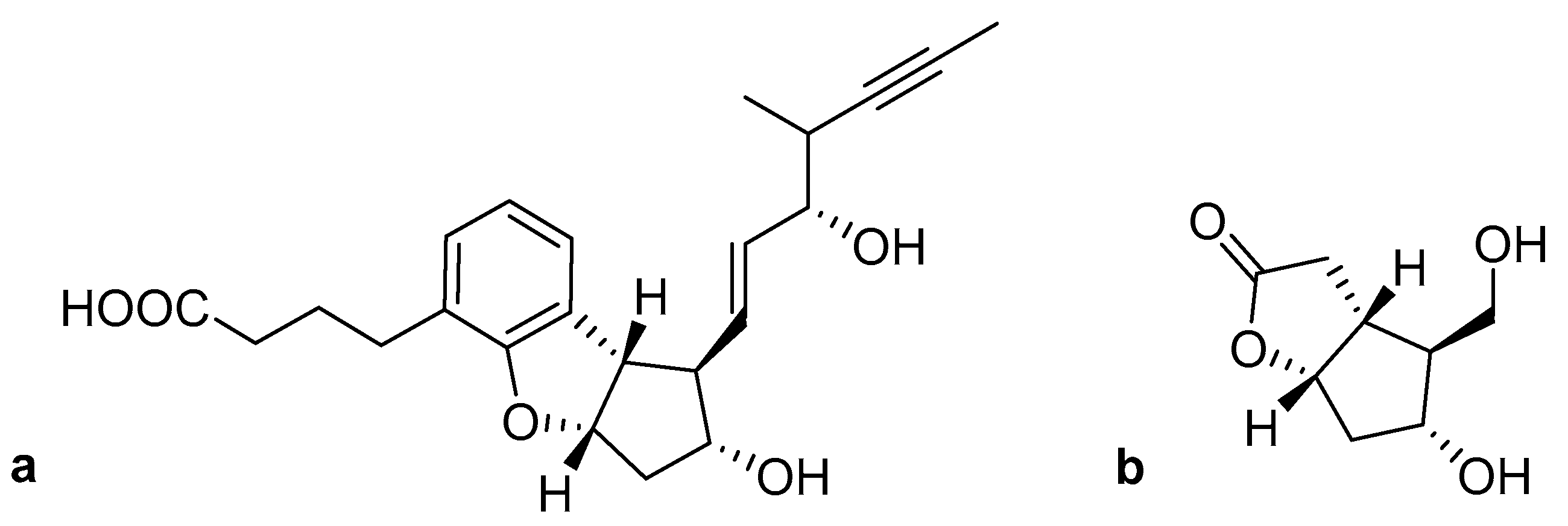
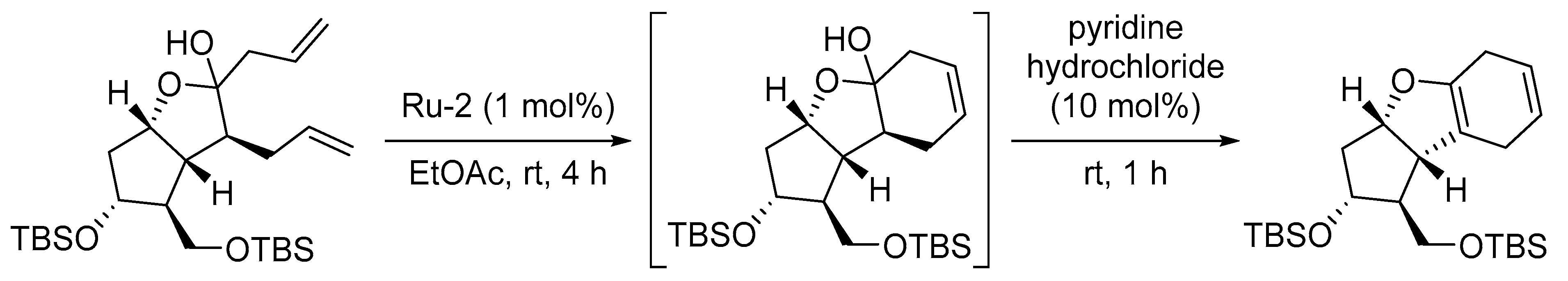
- Liu, X.; Tian, C.; Jiao, X.; Li, X.; Yang, H.; Yaoa, Y.; Xie, P. Practical synthesis of chiral tricyclic cyclopenta[b]benzofuran, a key intermediate of Beraprost. Org. Biomol. Chem. 2016, 14, 7715–7721. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, D.; Noda, K.; Komatsu, Y.; Yoshida, K.; Ueda, H.; Tokuyama, H. Aerobic dehydrogenation of N-heterocycles with Grubbs catalyst: Its application to assisted-tandem catalysis to construct N-containing fused heteroarenes. Chem. Eur. J. 2020, 26, 15793–15798. [Google Scholar] [CrossRef] [PubMed]
- Saraci, E.; Wang, L.; Theopold, K.H.; Lobo, R.F. Bioderived muconates by cross-metathesis and their conversion into terephthalates. ChemSusChem 2018, 11, 773–780. [Google Scholar] [CrossRef]
- Lozhkin, B.; Ward, T.R. A Close-to-aromatize approach for the late-stage functionalization through ring-closing metathesis. Helv. Chim. Acta 2021, 104, e2100024. [Google Scholar] [CrossRef]
- Kotha, S.; Seema, V.; Banerjee, S.; Dipak, M.K. Diversity oriented approach to polycyclics via cross-enyne metathesis and Diels–Alder reaction as key steps. J. Chem. Sci. (India) 2015, 127, 155–162. [Google Scholar] [CrossRef]
- Karmakar, S.; Mandal, T.; Dash, J. Ring-closing metathesis approach for the synthesis of o-terphenyl derivatives. Eur. J. Org. Chem. 2019, 2019, 5916–5924. [Google Scholar] [CrossRef]
- Sayyad, A.; Kaliappan, K.P. Sequential enyne-metathesis/Diels–Alder strategy: Rapid access to sugar–oxasteroid–quinone hybrids. Eur. J. Org. Chem. 2017, 2017, 5055–5065. [Google Scholar] [CrossRef]
- Johnson, M.M.; Ngwira, K.J.; Rousseau, A.L.; Lemmerer, A.; de Koning, C.B. Novel methodology for the synthesis of the benz[a]anthracene skeleton of the angucyclines using a Suzuki-Miyaura/isomerization/ring-closing metathesis strategy. Tetrahedron 2018, 74, 12–18. [Google Scholar] [CrossRef]
- Králová, P.; Soural, M. Synthesis of polycyclic tetrahydroisoquinolines and tetrahydrobenzo[d]azepines from polymer-supported allylglycine. J. Org. Chem. 2022, 87, 5242–5256. [Google Scholar] [CrossRef]
- Jončev, Z.; Sparr, C. Atroposelective arene-forming alkene metathesis. Angew. Chem. Int. Ed. 2022, 61, e202211168. [Google Scholar] [CrossRef]
- Nasibullin, I.; Smirnov, I.; Ahmadi, P.; Vong, K.; Kurbangalieva, A.; Tanaka, K. Synthetic prodrug design enables biocatalytic activation in mice to elicit tumor growth suppression. Nat. Commun. 2022, 13, 39. [Google Scholar] [CrossRef]
- Wu, D.; Cheng, W.; Ban, X.; Xia, J. Cycloparaphenylenes (CPPs): An overview of synthesis, properties, and potential applications. Asian J. Org. Chem. 2018, 7, 2161–2181. [Google Scholar] [CrossRef]
- Lewis, S.E. Cycloparaphenylenes and related nanohoops. Chem. Soc. Rev. 2015, 44, 2221–2304. [Google Scholar] [CrossRef]
- Mitra, N.K.; Meudom, R.; Gorden, J.D.; Merner, B.L. A Non-Cross-Coupling approach to arene-bridged macrocycles: Synthesis, structure, and direct, regioselective functionalization of a cycloparaphenylene fragment. Org. Lett. 2015, 17, 2700–2703. [Google Scholar] [CrossRef] [PubMed]
- Mitra, N.K.; Corzo, H.H.; Merner, B.L. A macrocyclic 1,4-diketone enables the synthesis of a p-phenylene ring that is more strained than a monomer unit of [4]cycloparaphenylene. Org. Lett. 2016, 18, 3278–3281. [Google Scholar] [CrossRef] [PubMed]
- Mitra, N.K.; Meudom, R.; Corzo, H.H.; Gorden, J.D.; Merner, B.L. Overcoming strain-induced rearrangement reactions: A mild dehydrative aromatization protocol for synthesis of highly distorted p-phenylenes. J. Am. Chem. Soc. 2016, 138, 3235–3240. [Google Scholar] [CrossRef] [PubMed]
- Mitra, N.K.; Merryman, C.P.; Merner, L.B. Highly Strained para-phenylene-bridged macrocycles from unstrained 1,4-diketo macrocycles. Synlett 2017, 28, 2205–2211. [Google Scholar]
- Golder, M.R.; Colwell, C.E.; Wong, B.M.; Zakharov, L.N.; Zhen, J.; Jasti, R. Iterative reductive aromatization/ring-closing metathesis. Strategy toward the synthesis of strained aromatic belts. J. Am. Chem. Soc. 2016, 138, 6577–6582. [Google Scholar] [CrossRef]
- Lee, J.; Kalin, A.J.; Wang, C.; Early, J.T.; Al-Hashimi, M.; Fang, L. Donor–acceptor conjugated ladder polymer via aromatization-driven thermodynamic annulation. Polym. Chem. 2018, 9, 1603–1609. [Google Scholar] [CrossRef]
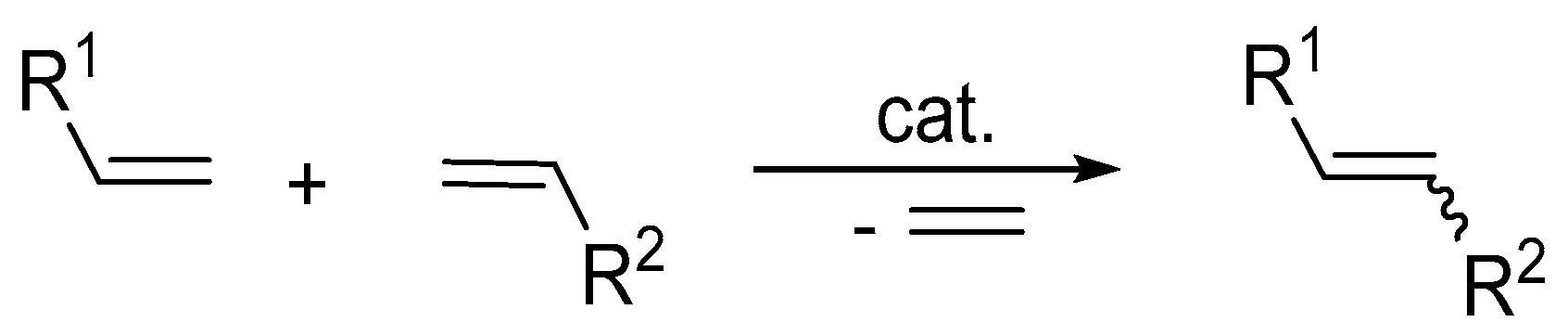
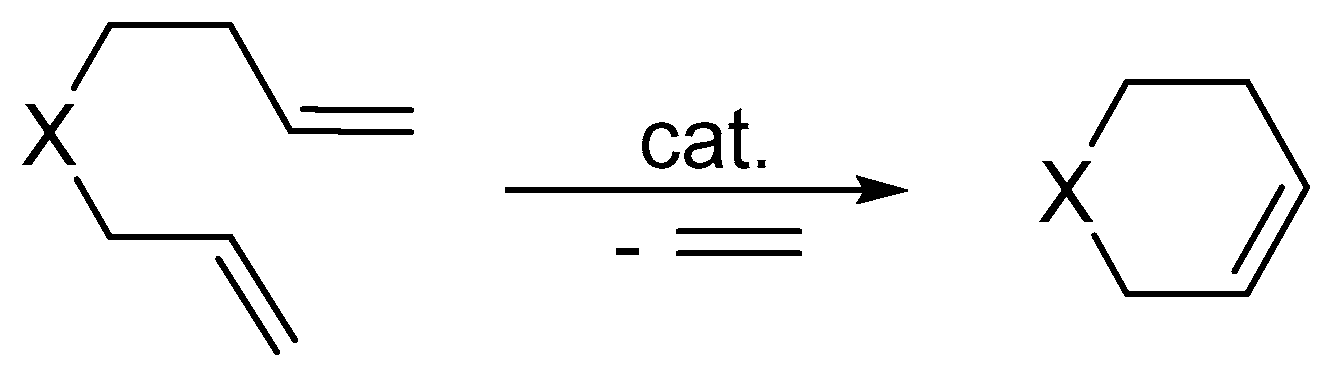
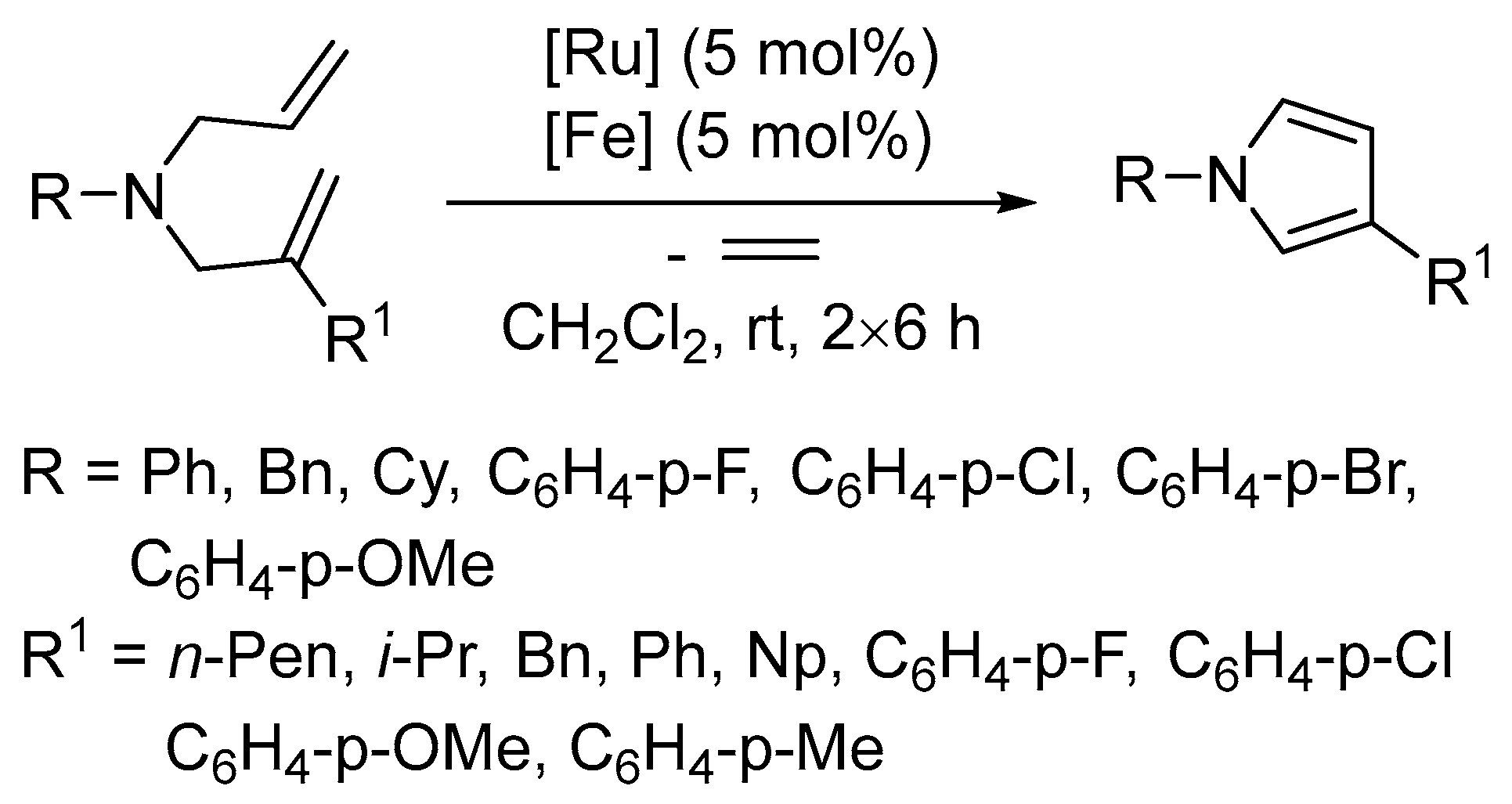
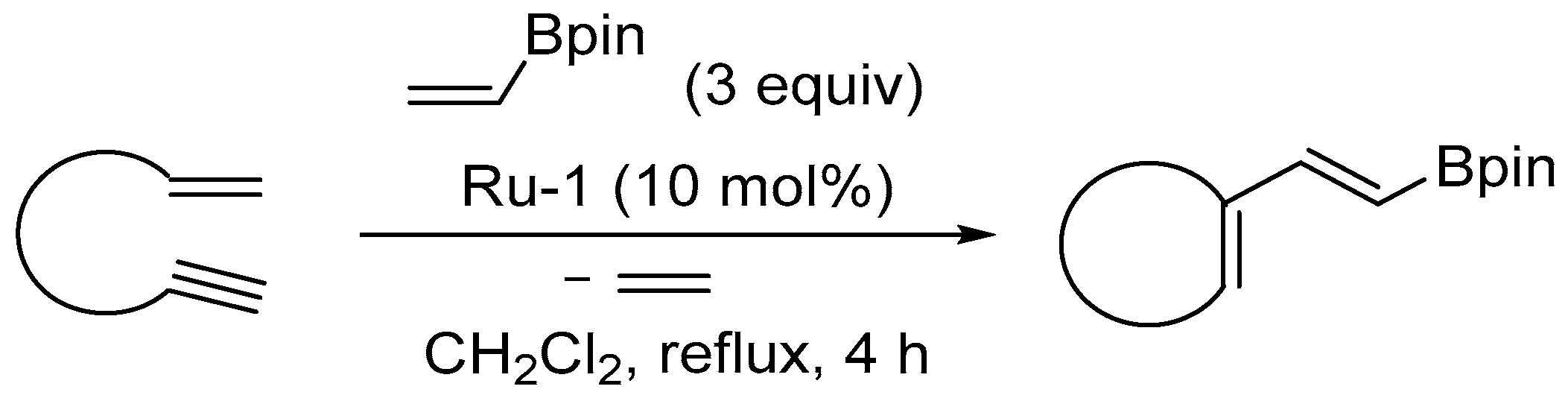
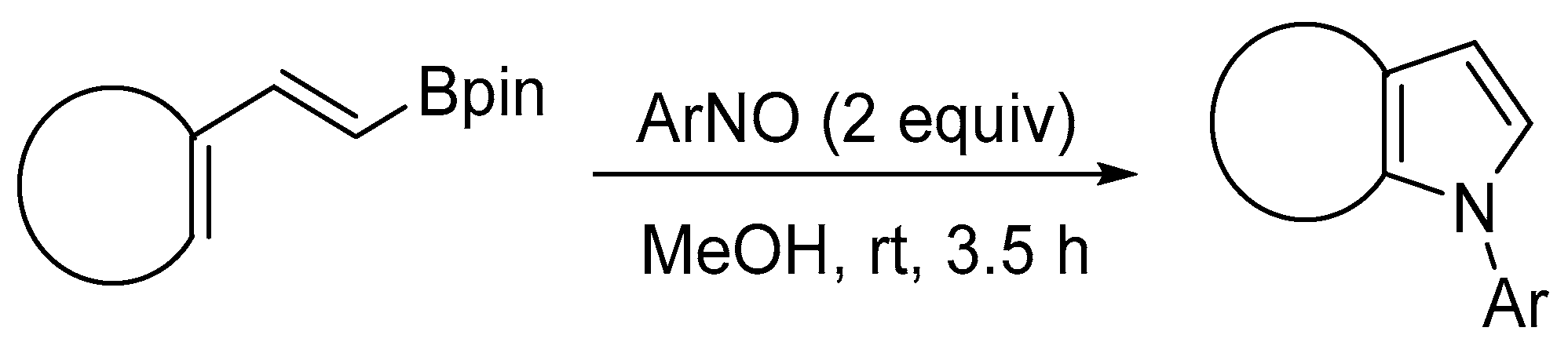
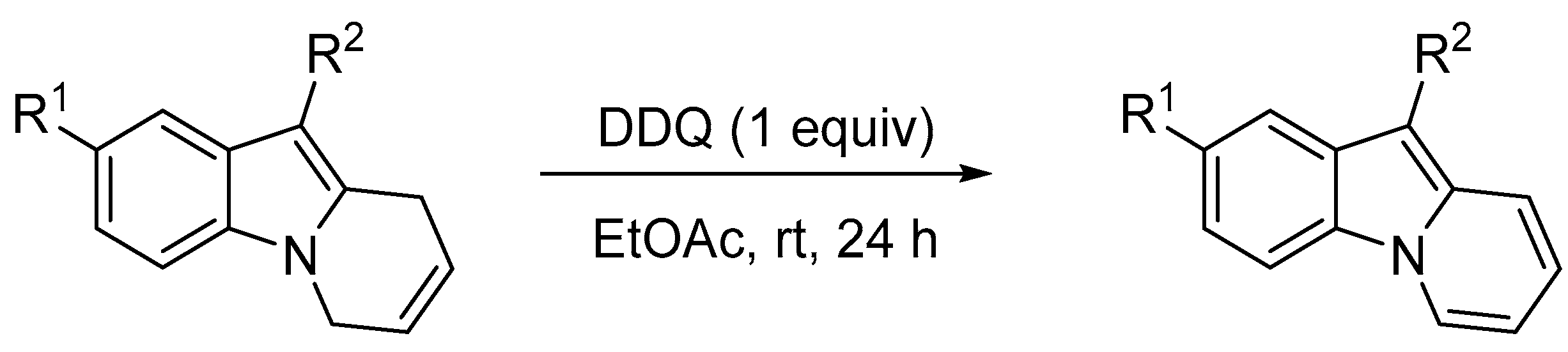
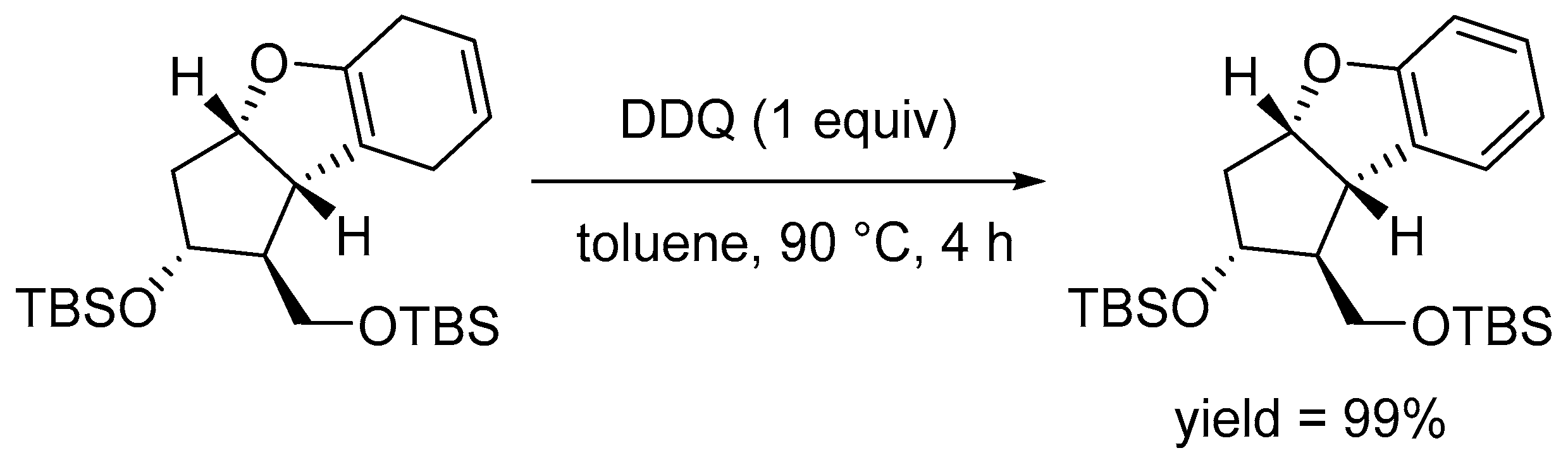
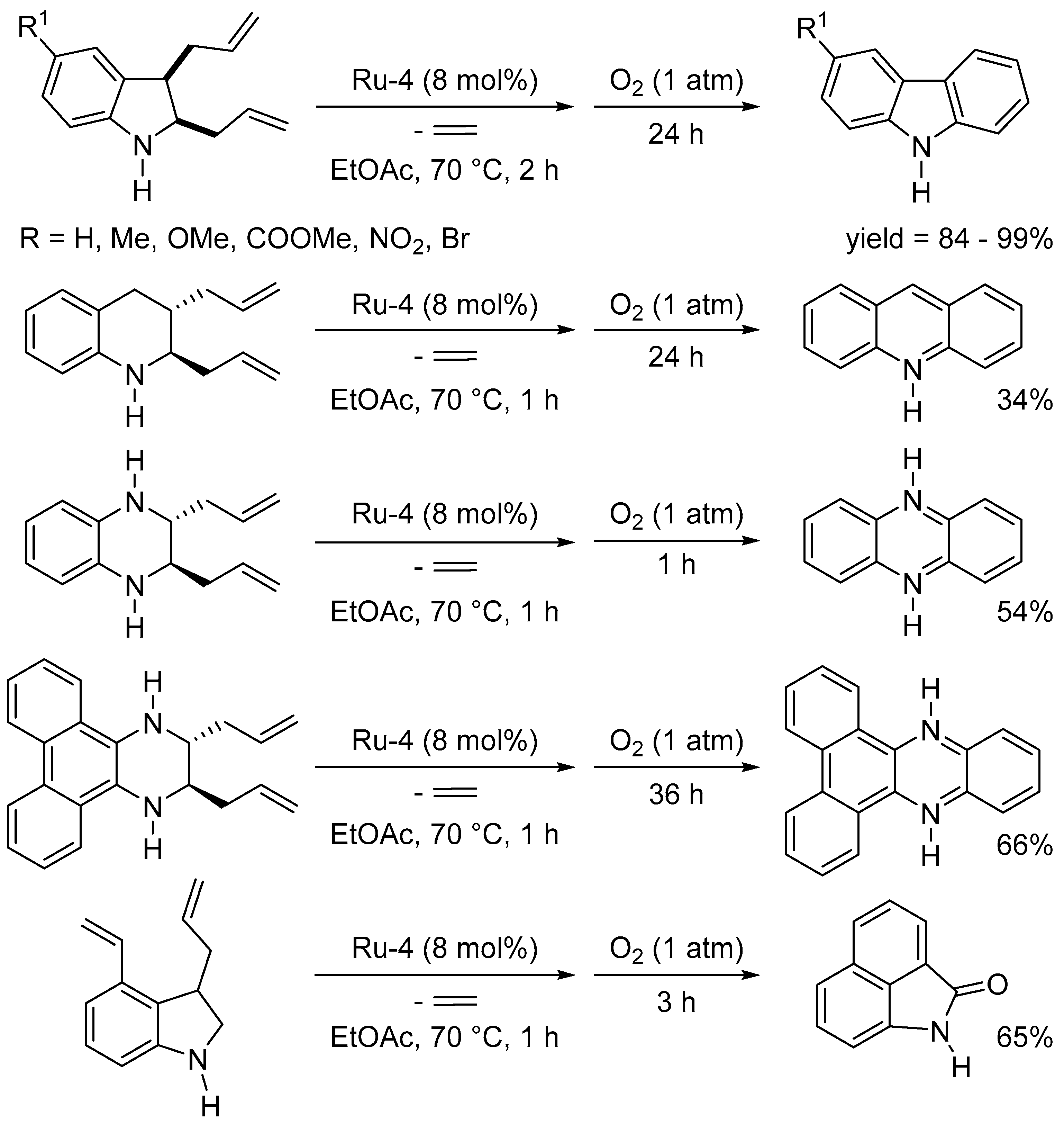
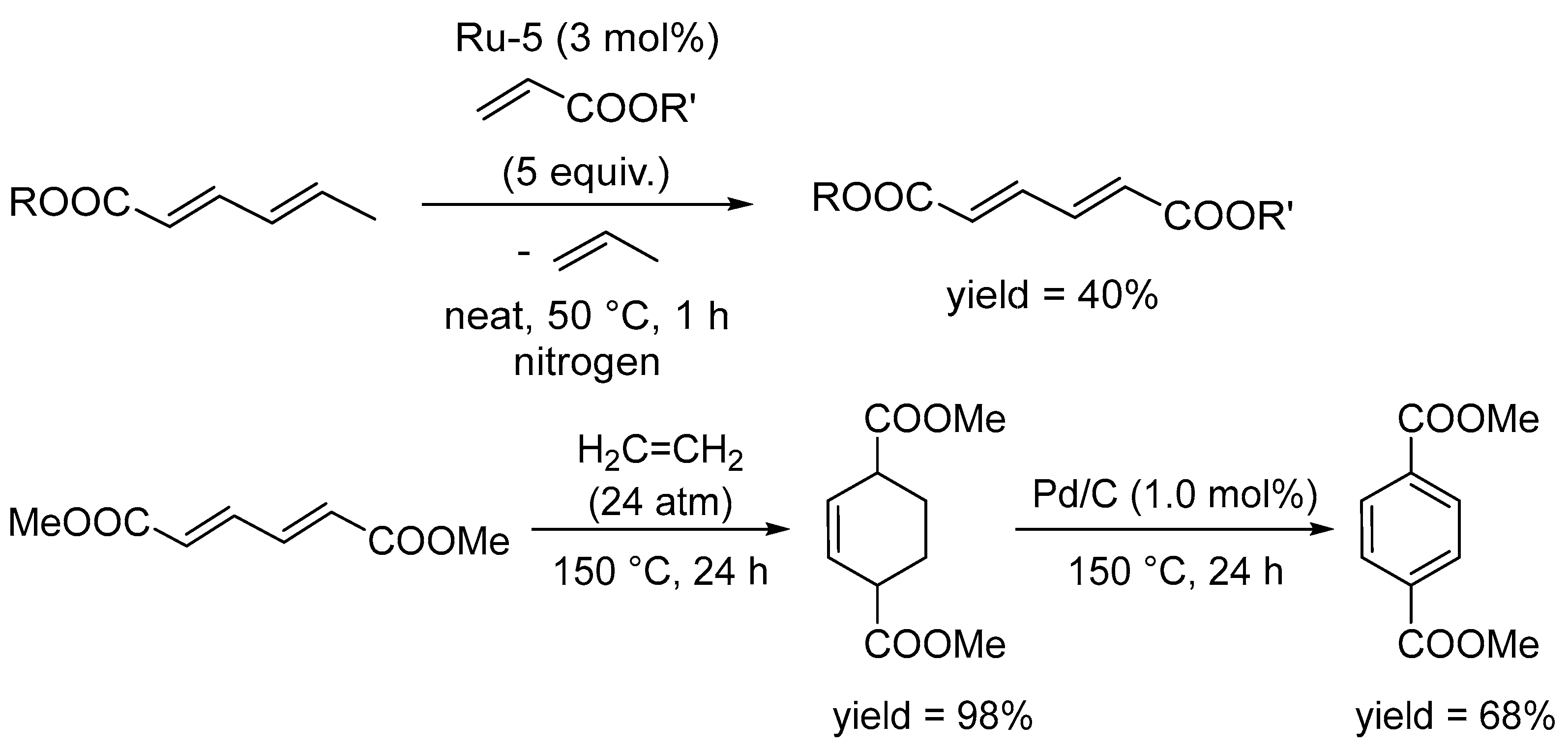
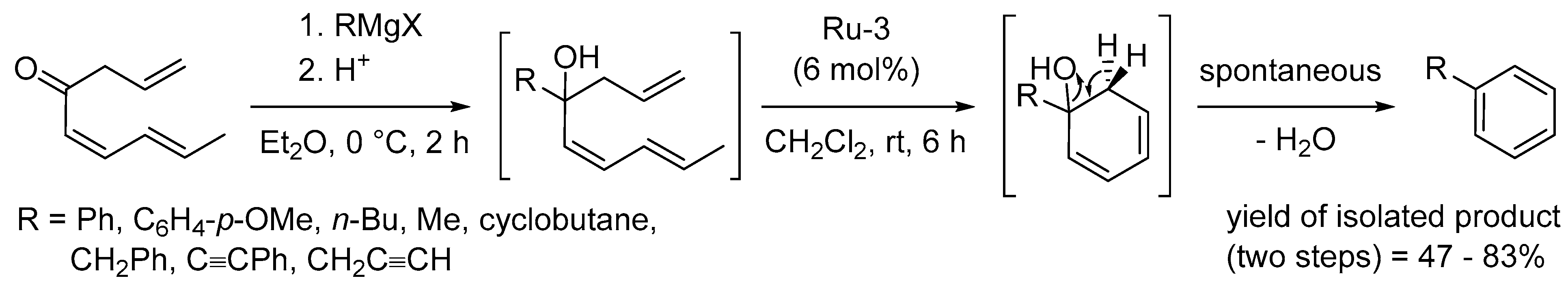
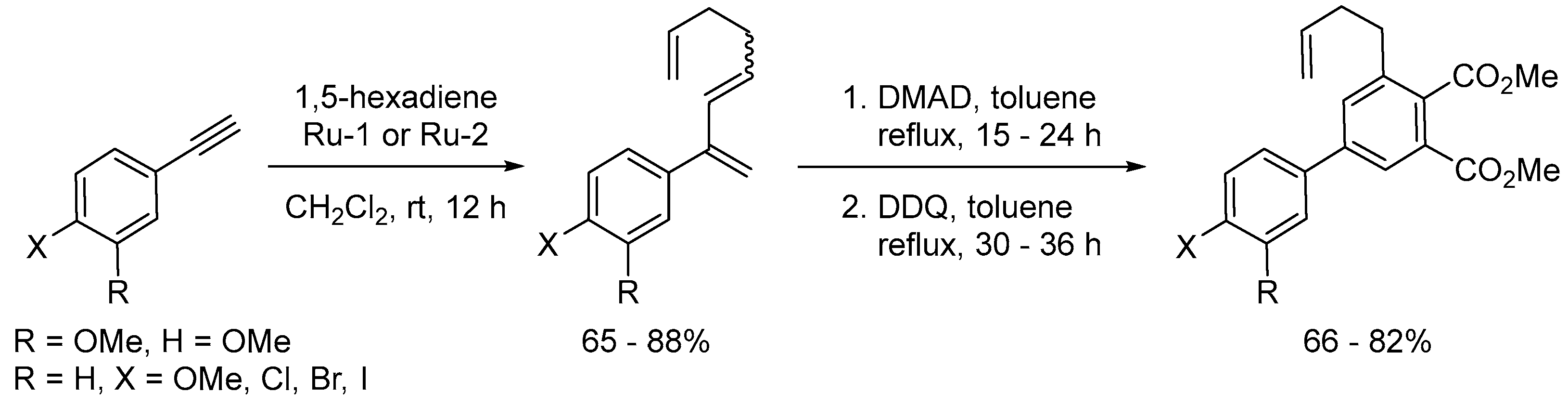
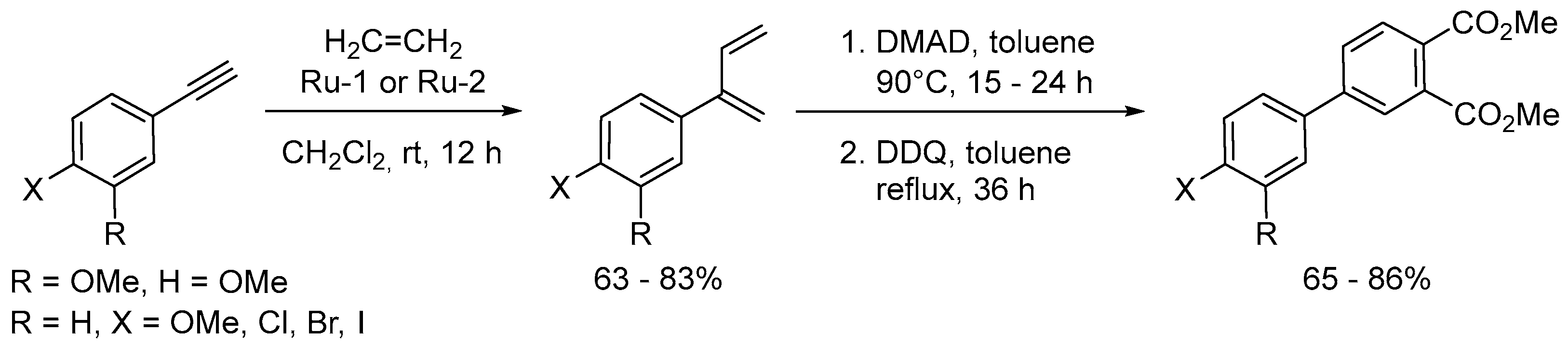
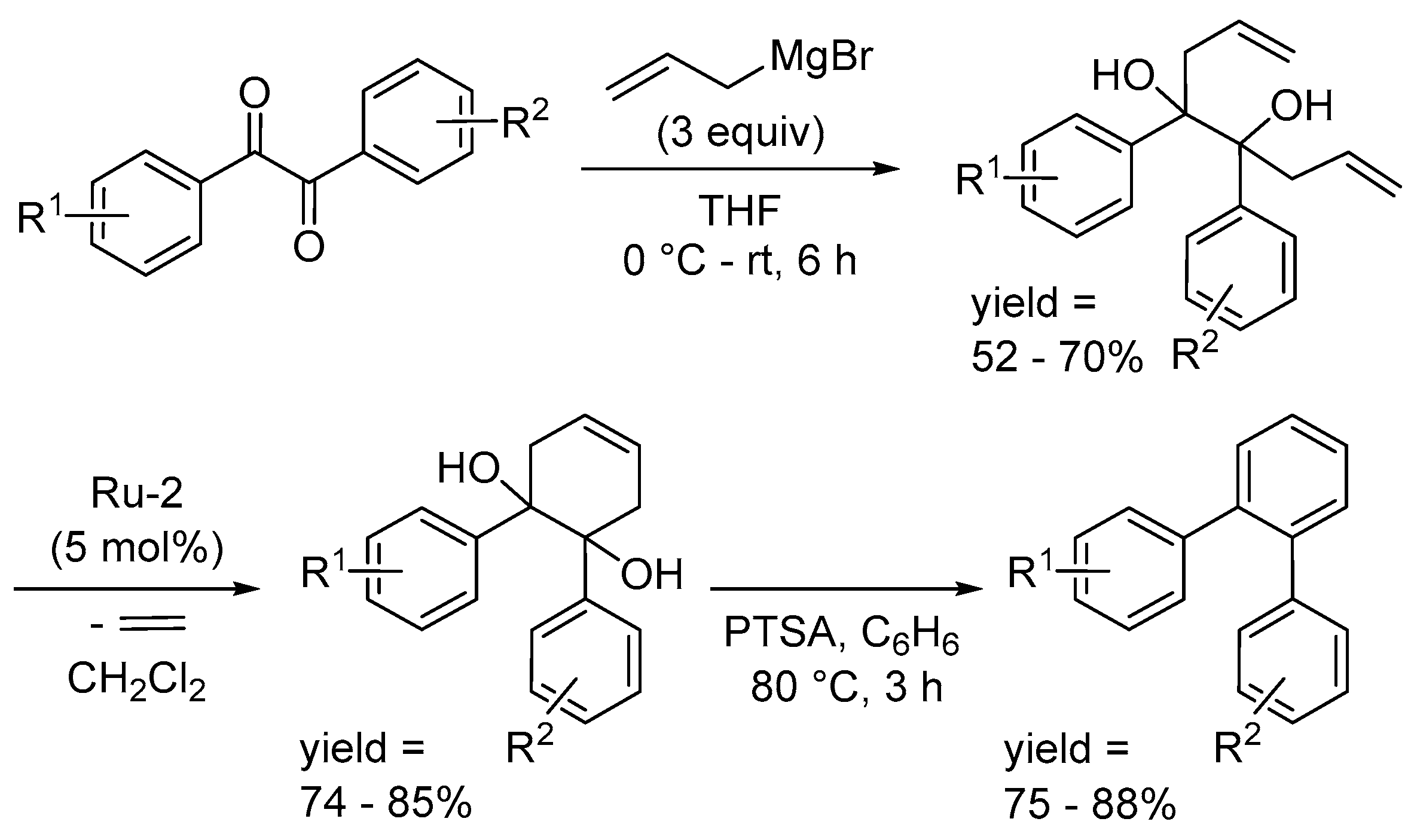
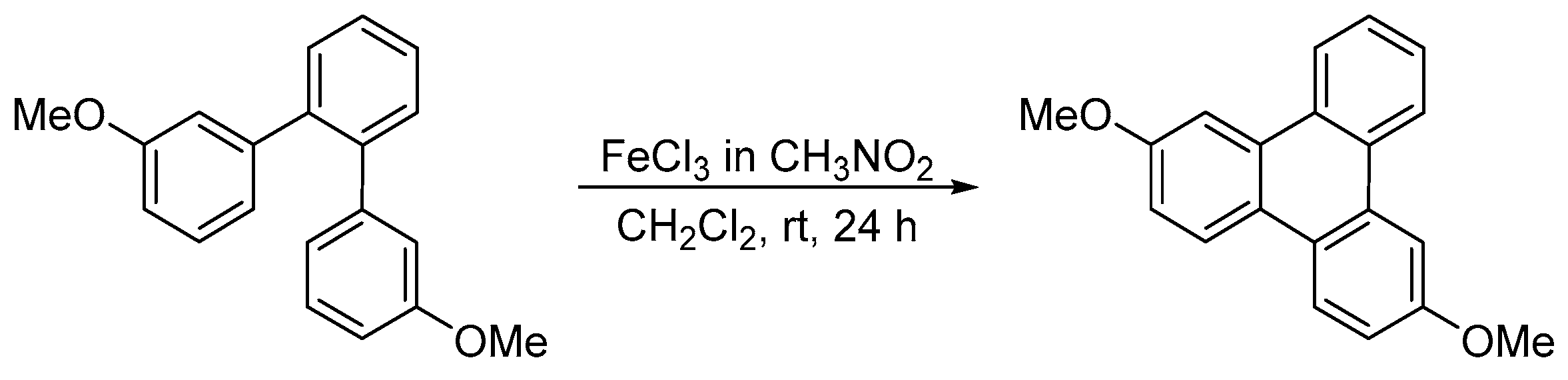

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogalski, S.; Pietraszuk, C. Application of Olefin Metathesis in the Synthesis of Carbo- and Heteroaromatic Compounds—Recent Advances. Molecules 2023, 28, 1680. https://doi.org/10.3390/molecules28041680
Rogalski S, Pietraszuk C. Application of Olefin Metathesis in the Synthesis of Carbo- and Heteroaromatic Compounds—Recent Advances. Molecules. 2023; 28(4):1680. https://doi.org/10.3390/molecules28041680
Chicago/Turabian StyleRogalski, Szymon, and Cezary Pietraszuk. 2023. "Application of Olefin Metathesis in the Synthesis of Carbo- and Heteroaromatic Compounds—Recent Advances" Molecules 28, no. 4: 1680. https://doi.org/10.3390/molecules28041680
APA StyleRogalski, S., & Pietraszuk, C. (2023). Application of Olefin Metathesis in the Synthesis of Carbo- and Heteroaromatic Compounds—Recent Advances. Molecules, 28(4), 1680. https://doi.org/10.3390/molecules28041680





