GC-MS Determination of Undeclared Phthalate Esters in Commercial Fragrances: Occurrence, Profiles and Assessment of Carcinogenic and Non-Carcinogenic Risk Associated with Their Consumption among Adult Consumers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Instrumentation
2.3. Sampling and Preparation
2.4. Statistical Analysis
2.5. Health Risk Assessment
3. Results and Discussion
3.1. Method Validation
3.2. Occurrence and Distribution of Phthalate Esters in the Analyzed Perfume Samples
3.3. Correlations among the Phthalate Esters Concentrations in the Analyzed Perfume Samples
3.4. Health Risk Assessment
3.4.1. Systemic Exposure Dosage (SED) of Phthalate Esters in Perfumes
| Systemic Exposure Dose (SED) | |||||
|---|---|---|---|---|---|
| NOAEL * | Male | Female | |||
| Mean | Maximum | Mean | Maximum | ||
| DMP | 3.75 | 1.15× 10−3 | 3.34× 10−3 | 1.26× 10−3 | 3.63× 10−3 |
| DEP | 0.15 | 4.29× 10−2 | 3.21× 10−1 | 4.68× 10−2 | 3.49× 10−1 |
| DBP | 0.66 | 8.45× 10−4 | 3.67× 10−1 | 9.20× 10−4 | 4.00× 10−3 |
| BBP | 50 | 4.59× 10−4 | 2.50× 10−3 | 5.00× 10−4 | 2.73× 10−3 |
| DEHP | 4.8 | 3.11× 10−3 | 2.10× 10−2 | 3.39× 10−3 | 2.29× 10−2 |
3.4.2. Non-Carcinogenic Risk Assessment
3.4.3. Carcinogenic Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, B.; Prasad, K.S.; Dave, H.; Das, A.; Asodariya, G.; Talati, N.; Swain, S.; Kapse, S. Cumulative human exposure and environmental occurrence of phthalate esters: A global perspective. Environ. Res. 2022, 210, 112987. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.E.; David, R.M.; Guinn, R.; Kramarz, K.W.; Lampi, M.A.; Staples, C.A. Modeling human exposure to phthalate esters: A comparison of indirect and biomonitoring estimation methods. Hum. Ecol. Risk. Assess. 2011, 17, 923–965. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.C.; Liao, K.W.; Chang, J.W.; Chan, S.H.; Lee, C.C. Characterization of phthalates exposure and risk for cosmetics and perfume sales clerks. Environ. Pollut. 2018, 233, 577–587. [Google Scholar] [CrossRef]
- Karim, A.V.; Krishnan, S.; Sethulekshmi, S.; Shriwastav, A. Phthalate Esters in the Environment: An Overview on the Occurrence, Toxicity, Detection, and Treatment Options. In New Trends in Emerging Environmental Contaminants; Springer: Singapore, 2022; pp. 131–160. Available online: https://link.springer.com/chapter/10.1007/978-981-16-8367-1_7 (accessed on 21 January 2023).
- Shaaban, H. High speed hydrophilic interaction liquid chromatographic method for simultaneous determination of selected pharmaceuticals in wastewater using a cyano-bonded silica column. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 180–187. [Google Scholar] [CrossRef]
- Mostafa, A.; Shaaban, H. Quantitative analysis and resolution of pharmaceuticals in the environment using multivariate curve resolution-alternating least squares (MCR-ALS). Acta Pharm. 2019, 69, 217–231. [Google Scholar] [CrossRef]
- Pagoni, A.; Arvaniti, O.S.; Kalantzi, O.I. Exposure to phthalates from personal care products: Urinary levels and predictors of exposure. Environ. Res. 2022, 212, 113194. [Google Scholar] [CrossRef]
- Fruh, V.; Preston, E.V.; Quinn, M.R.; Hacker, M.R.; Wylie, B.J.; O’Brien, K.; Hauser, R.; James-Todd, T.; Mahalingaiah, S. Urinary phthalate metabolite concentrations and personal care product use during pregnancy–Results of a pilot study. Sci. Total Environ. 2022, 835, 155439. [Google Scholar] [CrossRef]
- Fišerová, P.S.; Melymuk, L.; Komprdová, K.; Domínguez-Romero, E.; Scheringer, M.; Kohoutek, J.; Přibylová, P.; Andrýsková, L.; Piler, P.; Koch, H.M.; et al. Personal care product use and lifestyle affect phthalate and DINCH metabolite levels in teenagers and young adults. Environ. Res. 2022, 213, 113675. [Google Scholar] [CrossRef]
- Shih, Y.L.; Hsieh, C.J.; Lee, T.Y.; Liao, P.H.; Wu, H.T.; Liu, C.Y. Sex differences between urinary phthalate metabolites and metabolic syndrome in adults: A cross-sectional Taiwan Biobank Study. Int. J. Environ. Res. Public Health 2022, 19, 10458. [Google Scholar] [CrossRef]
- Ryva, B.A.; Haggerty, D.K.; Pacyga, D.C.; James-Todd, T.; Li, Z.; Flaws, J.A.; Strakovsky, R.S. Determinants of urinary phthalate biomarker concentrations in pre-and perimenopausal women with consideration of race. Environ. Res. 2022, 214, 114056. [Google Scholar] [CrossRef]
- Kim, S.; Min, H.S.; Lee, W.J.; Choe, S.A. Occupational differences in personal care product use and urinary concentration of endocrine disrupting chemicals by gender. J. Exp. Sci. Environ. Epidemiol. 2022, 1–7. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council. Off. J. Eur. Union L 2009, 342, 59. Available online: https://www.cirs-ck.com/Uploads/file/20171207/1512632679_83244.pdf. (accessed on 20 January 2023).
- Shaaban, H.; Mostafa, A.; Alhajri, W.; Almubarak, L.; AlKhalifah, K. Development and validation of an eco-friendly SPE-HPLC-MS method for simultaneous determination of selected parabens and bisphenol A in personal care products: Evaluation of the greenness profile of the developed method. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 621–628. [Google Scholar] [CrossRef]
- Mostafa, A.; Shaaban, H. Development and validation of a dispersive liquid–liquid microextraction method for the determination of phthalate esters in perfumes using gas chromatography-mass spectrometry. RSC Adv. 2018, 8, 26897–26905. [Google Scholar] [CrossRef]
- Senta, I.; Rodríguez-Mozaz, S.; Corominas, L.; Covaci, A.; Petrovic, M. Applicability of an on-line solid-phase extraction liquid chromatography–tandem mass spectrometry for the wastewater-based assessment of human exposure to chemicals from personal care and household products. Sci. Total Environ. 2022, 845, 157309. [Google Scholar] [CrossRef]
- Shaaban, H.; Issa, S.Y.; Ahmad, R.; Mostafa, A.; Refai, S.; Alkharraa, N.; Albaqshi, B.T.; Hussien, D.; Alqarni, A.M. Investigation on the elemental profiles of lip cosmetic products: Concentrations, distribution and assessment of potential carcinogenic and non-carcinogenic human health risk for consumer safety. Saudi Pharm. J. 2022, 30, 779–792. [Google Scholar] [CrossRef]
- Saudi Arabia Perfume Market. Available online: https://www.imarcgroup.com/saudi-arabia-perfume-market (accessed on 21 January 2023).
- Shaaban, H.; Alhajri, W. Usage Patterns of Cosmetic and Personal Care Products among Female Population in Saudi Arabia: Important Factors for Exposure and Risk Assessment. J. Environ. Public Health 2020, 2020, 8434508. [Google Scholar] [CrossRef]
- Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers. Available online: https://ec.europa.eu/health/ph_risk/committees/sccp/documents/out168_en.pdf. (accessed on 10 January 2023).
- Alexander, J.; Husøy, T.; Naterstad, K.; Paulsen, J.E.; Sanner, T.; Steffensen, I.L.; Dahl, K.H.; Binderup, M.L. Risk Assessment of Diethyl Phthalate (DEP) in Cosmetics. Opinion on the Panel on Food Additives, Flavourings, Procesing Aids, Materials in Contact with Food and Cosmetics in the Norwegian Scientific Committee for Food Safety; VKM Report; Norwegian Scientific Committee for Food Safety: Oslo, Norway, 2005; Available online: https://fhi.brage.unit.no/fhi-xmlui/bitstream/handle/11250/2471439/Alexander_2005_Ris.pdf?sequence=1 (accessed on 21 January 2023).
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; et al. Update of the risk assessment of di-butylphthalate (DBP), butyl-benzyl-phthalate (BBP), bis (2-ethylhexyl) phthalate (DEHP), di-isononylphthalate (DINP) and di-isodecylphthalate (DIDP) for use in food contact materials. EFSA J. 2019, 17, e05838. [Google Scholar]
- Tyl, R.W.; Myers, C.B.; Marr, M.C.; Fail, P.A.; Seely, J.C.; Brine, D.R.; Barter, R.A.; Butala, J.H. Reproductive toxicity evaluation of dietary butyl benzyl phthalate (BBP) in rats. Reprod. Toxicol. 2004, 18, 241–264. [Google Scholar] [CrossRef]
- Lee, K.Y.; Shibutani, M.; Takagi, H.; Kato, N.; Takigami, S.; Uneyama, C.; Hirose, M. Diverse developmental toxicity of di-n-butyl phthalate in both sexes of rat offspring after maternal exposure during the period from late gestation through lactation. Toxicology 2004, 203, 221–238. [Google Scholar] [CrossRef]
- Bernauer, U.; Bodin, L.; Chaudhry, Q.; Coenraads, P.J.; Dusinska, M.; Ezendam, J.; Gaffet, E.; Galli, C.L.; Granum, B.; Panteri, E.; et al. The SCCS Notes of Guidance for the testing of cosmetic ingredients and their safety evaluation, 11th revision, 30–31 March 2021, SCCS/1628/21. Regul. Toxicol. Pharmacol. 2021, 127, 105052. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, K.B.; Yoon, S.; Kim, H.S.; Lee, B.M. Risk assessment of unintentional phthalates contaminants in cosmetics. Regul. Toxicol. Pharmacol. 2020, 115, 104687. [Google Scholar] [CrossRef]
- Al Othaimeen, A.I.; Al Nozha, M.; Osman, A.K. Obesity: An emerging problem in Saudi Arabia. Analysis of data from the National Nutrition Survey. E. Mediterr. Health J. 2007, 13, 441–448. Available online: https://apps.who.int/iris/handle/10665/117265 (accessed on 19 January 2023).
- Dybing, E.; Sanner, T.; Roelfzema, H.; Kroese, D.; Tennant, R.W. T25: A simplified carcinogenic potency index: Description of the system and study of correlations between carcinogenic potency and species/site specificity and mutagenicity. Pharmacol. Toxicol. 1997, 80, 272–279. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Carcinogenesis Bioassay of di (2-ethylhexyl) phthalate (CAS No. 117-81-7) in F344 Rats and B6C3F1 Mice (Feed Studies). Natl. Toxicol. Program Tech. Rep. Ser. 1982, 217, 1–127. Available online: https://pubmed.ncbi.nlm.nih.gov/12778218/ (accessed on 10 January 2023).
- Kim, K.B.; Kwack, S.J.; Lee, J.Y.; Kacew, S.; Lee, B.M. Current opinion on risk assessment of cosmetics. J. Toxicol. Environ. Health-B 2021, 24, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Guideline For Risk Assessment of Cosmetic Products. Available online: https://www.mfds.go.kr/eng/brd/m_28/down.do?brd_id=eng0006&seq=70463&data_tp=A&file_seq=1 (accessed on 10 January 2023).
- Ito, Y.; Kamijima, M.; Nakajima, T. Di (2-ethylhexyl) phthalate-induced toxicity and peroxisome proliferator-activated receptor alpha: A review. Environ. Health Prev. Med. 2019, 24, 47. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Elkhatib, R. Screening of phthalate esters in 47 branded perfumes. Environ. Sci. Pollut. Res. 2016, 23, 455–468. [Google Scholar] [CrossRef]
- Koo, H.J.; Lee, B.M. Estimated exposure to phthalates in cosmetics and risk assessment. J. Toxicol. Environ. Health-A 2004, 67, 1901–1914. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ. Sci. Technol. 2013, 47, 14442–14449. [Google Scholar] [CrossRef]
- Ambe, K.; Sakakibara, Y.; Sakabe, A.; Makino, H.; Ochibe, T.; Tohkin, M. Comparison of the developmental/reproductive toxicity and hepatotoxicity of phthalate esters in rats using an open toxicity data source. J. Toxicol. Sci. 2019, 44, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Hubinger, J.C. A survey of phthalate esters in consumer cosmetic products. J. Cosmet. Sci. 2010, 61, 457–465. [Google Scholar] [PubMed]
- Koniecki, D.; Wang, R.; Moody, R.P.; Zhu, J. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 2011, 111, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Prado, L.; Llompart, M.; Lamas, J.P.; Garcia-Jares, C.; Lores, M. Multicomponent analytical methodology to control phthalates, synthetic musks, fragrance allergens and preservatives in perfumes. Talanta 2011, 85, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Phthalates and Artificial Musks in Perfumes. Available online: https://docplayer.net/20830417-Phthalates-and-artificial-musks-in-perfumes.html. (accessed on 10 January 2023).
- International Agency for Research on Cancer. Agents Classified by the IARC Monographs, Volumes 1–106. 2012. Available online: http://monographs.iarc.fr/ENG/Classification/index.php (accessed on 21 January 2023).
- De Jong, W.H.; Borges, T.; Ion, R.M.; Panagiotakos, D.; Testai, E.; Vermeire, T.; Bernauer, U.; Rousselle, C.; Bégué, S.; Kopperud, H.M.; et al. Guidelines on the benefit-risk assessment of the presence of phthalates in certain medical devices covering phthalates which are carcinogenic, mutagenic, toxic to reproduction (CMR) or have endocrine-disrupting (ED) properties. Regul. Toxicol. Pharmacol. 2020, 111, 104546. [Google Scholar] [CrossRef]
- Kamrin, M.A. Phthalate risks, phthalate regulation, and public health: A review. J. Toxicol. Environ. Health-B 2009, 12, 157–174. [Google Scholar] [CrossRef]
- Fong, J.P.; Lee, F.J.; Lu, I.S.; Uang, S.N.; Lee, C.C. Estimating the contribution of inhalation exposure to di-2-ethylhexyl phthalate (DEHP) for PVC production workers, using personal air sampling and urinary metabolite monitoring. Int. J. Hyg. Environ. Health 2014, 217, 102–109. [Google Scholar] [CrossRef]
- Van Amerongen, C.C.; Ofenloch, R.F.; Cazzaniga, S.; Elsner, P.; Gonçalo, M.; Naldi, L.; Svensson, Å.; Bruze, M.; Schuttelaar, M.L. Skin exposure to scented products used in daily life and fragrance contact allergy in the European general population-The EDEN Fragrance Study. Contact Dermat. 2021, 84, 385–394. [Google Scholar] [CrossRef]
- Pastor-Nieto, M.A.; Gatica-Ortega, M.E. Ubiquity, hazardous effects, and risk assessment of fragrances in consumer products. Curr. Treat. Options Allergy 2021, 8, 21–41. [Google Scholar] [CrossRef]
- Gong, M.; Weschler, C.J.; Zhang, Y. Impact of clothing on dermal exposure to phthalates: Observations and insights from sampling both skin and clothing. Environ. Sci. Technol. 2016, 50, 4350–4357. [Google Scholar] [CrossRef]


| Analyte | Retention Time (Min) | Selected Ions (m/z) | |
|---|---|---|---|
| Quantifier Ion (m/z) | Qualifier Ions (m/z) | ||
| DMP | 6.25 | 163 | 77, 133 |
| DEP | 6.86 | 149 | 177, 105 |
| DBP | 8.83 | 149 | 223, 104 |
| BBP | 13.10 | 149 | 91, 206 |
| DEHP | 16.13 | 149 | 167, 71 |
| Analyte | Linearity Range (µg/mL) | r2 | Recovery (%) | Inter-Day Precision (%RSD) (n = 3 × 3) | Intra-Day Precision (%RSD) (n = 5) | LOD (µg/mL) | LOQ (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Med | High | Low | Med | High | Low | Med | High | |||||
| DMP | 0.010–10 | 0.9990 | 97.5 | 102.1 | 103.1 | 3.01 | 2.25 | 2.22 | 2.94 | 1.86 | 2.09 | 1.9 × 10−3 | 5.8 × 10−3 |
| DEP | 0.010–10 | 0.9996 | 94.9 | 96.9 | 97.5 | 2.99 | 2.46 | 2.67 | 2.75 | 2.04 | 2.25 | 1.5 × 10−3 | 4.6 × 10−3 |
| DBP | 0.007–7 | 0.9999 | 97.8 | 97.4 | 96.6 | 3.37 | 2.46 | 3.01 | 3.65 | 2.22 | 3.11 | 1.2 × 10−3 | 3.6 × 10−3 |
| BBP | 0.012–10 | 0.9997 | 98.2 | 105.6 | 101.9 | 3.21 | 3.06 | 2.99 | 3.20 | 2.68 | 2.94 | 2.1 × 10−3 | 6.5 × 10−3 |
| DEHP | 0.010–10 | 0.9999 | 100.3 | 98.8 | 97.9 | 3.94 | 1.58 | 2.55 | 4.06 | 1.77 | 2.29 | 1.0 × 10−3 | 3.1 × 10−3 |
| Sample Number | DMP | DEP | DBP | BBP | DEHP |
|---|---|---|---|---|---|
| 1 | 5.70 | 0.90 | 19.30 | 0.40 | 85.70 |
| 2 | 27.00 | 2502.00 | nd | nd | 114.00 |
| 3 | 7.00 | 4.17 | 25.50 | 8.83 | 377.67 |
| 4 | nd | 1270.50 | nd | 6.00 | 309.00 |
| 5 | nd | 576.00 | nd | 22.50 | 96.00 |
| 6 | nd | 5766.00 | nd | nd | 186.00 |
| 7 | nd | 1.15 | nd | 1.80 | 48.30 |
| 8 | nd | 32.48 | nd | 0.98 | 37.57 |
| 9 | nd | 0.75 | nd | 0.10 | 49.60 |
| 10 | nd | 39.80 | 5.00 | 1.20 | 92.00 |
| 11 | nd | 13.05 | 3.10 | 0.10 | 46.05 |
| 12 | 20.60 | 1.15 | nd | 1.70 | 44.85 |
| 13 | nd | 1.05 | nd | 1.80 | 44.75 |
| 14 | nd | 0.60 | 3.65 | nd | 43.85 |
| 15 | nd | 874.00 | 2.00 | nd | 57.00 |
| 16 | nd | 26.30 | nd | 0.10 | 0.15 |
| 17 | nd | 12.35 | nd | 2.05 | nd |
| 18 | 12.00 | 3544.00 | 52.00 | nd | 16.00 |
| 19 | nd | 43.20 | 2.85 | 3.15 | 24.65 |
| 20 | nd | 468.50 | nd | 15.00 | 6.00 |
| 21 | nd | 0.35 | nd | 1.57 | 8.21 |
| 22 | 10.50 | 3440.50 | nd | nd | 31.50 |
| 23 | 60.00 | 4668.00 | 66.00 | nd | 6.00 |
| 24 | nd | 6.20 | nd | 0.10 | 44.75 |
| 25 | 44.00 | 3420.00 | nd | nd | nd |
| 26 | nd | 4.80 | nd | 0.10 | 33.50 |
| 27 | nd | 2.80 | nd | nd | 46.70 |
| 28 | nd | 2.45 | 0.95 | nd | 35.10 |
| 29 | nd | 3.25 | nd | nd | 17.15 |
| 30 | nd | 1602.00 | nd | 12.00 | 12.00 |
| 31 | nd | 6.35 | nd | nd | 42.10 |
| 32 | nd | 1.78 | nd | nd | 1.52 |
| 33 | nd | 4.84 | nd | 4.07 | 8.77 |
| 34 | nd | 11.00 | nd | 0.10 | 46.35 |
| 35 | nd | 693.00 | nd | nd | 6.00 |
| 36 | nd | 4.75 | nd | nd | 3.00 |
| 37 | nd | 1170.00 | nd | 10.00 | 10.00 |
| 38 | nd | 83.70 | nd | nd | 72.30 |
| 38 | nd | 20.90 | 1.80 | 1.30 | 14.70 |
| 40 | nd | 543.00 | nd | nd | 6.00 |
| Mean | 20.76 | 771.69 | 15.18 | 4.13 | 55.92 |
| Maximum | 60.00 | 5766.00 | 66.00 | 22.50 | 377.67 |
| Frequency (%) | 20.0 | 100.0 | 27.5 | 57.5 | 95.0 |
| 95 th percentile | 53.60 | 3600.20 | 58.30 | 14.70 | 204.45 |
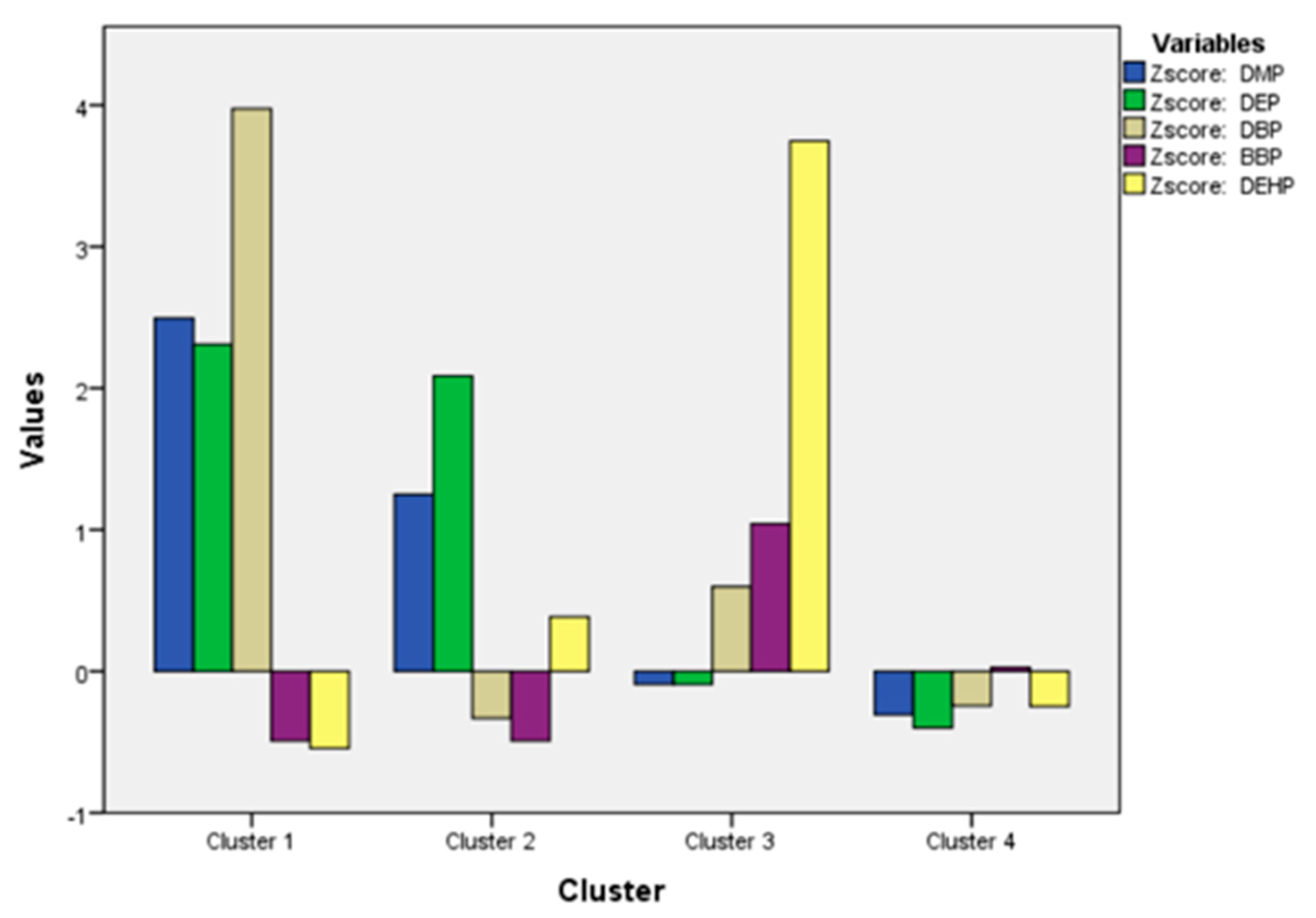
| K-Mean Cluster Analysis | ||||
|---|---|---|---|---|
| Factors | F-Value | Significance | Clusters | Samples |
| Zscore: DMP | 15.081 | 0.000 | 1 | 2 |
| Zscore: DEP | 68.732 | 0.000 | 2 | 4 |
| Zscore: DBP | 95.990 | 0.000 | 3 | 2 |
| Zscore: BBP | 1.238 | 0.310 | 4 | 32 |
| Zscore: DEHP | 48.246 | 0.000 | Total | 40 |
| DMP | DEP | DBP | BBP | DEHP | |
|---|---|---|---|---|---|
| DMP | 1 | ||||
| DEP | 0.612 ** | 1 | |||
| 0.000 | |||||
| DBP | 0.599 ** | 0.459 ** | 1 | ||
| 0.000 | 0.003 | ||||
| DBP | −0.145 | −0.053 | 0.07 | 1 | |
| 0.370 | 0.746 | 0.666 | |||
| DEHP | −0.05 | 0.107 | 0.093 | 0.221 | 1 |
| 0.758 | 0.511 | 0.569 | 0.170 |
| Margin of Safety (MOS) | LCR | |||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||
| Mean | Maximum | Mean | Maximum | Mean | Maximum | Mean | Maximum | |
| DMP | 5.20 × 10−5 | 1.80 × 10−5 | 4.77 × 10−5 | 1.65 × 10−5 | - | - | - | - |
| DEP | 3.49 × 10−3 | 4.68 × 10−2 | 3.21 × 10−3 | 4.29 × 10−2 | - | - | - | - |
| DBP | 5.92 × 10−4 | 1.36 × 10−4 | 5.44 × 10−4 | 1.25 × 10−4 | - | - | - | - |
| BBP | 1.09 × 10−5 | 2.00 × 10−4 | 1.00 × 10−5 | 1.83 × 10−4 | - | - | - | - |
| DEHP | 1.54 × 10−3 | 2.28 × 10−2 | 1.42 × 10−3 | 2.10 × 10−2 | 8.12 × 10−6 | 5.49 × 10−5 | 8.85 × 10−6 | 5.98 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, A.; Shaaban, H. GC-MS Determination of Undeclared Phthalate Esters in Commercial Fragrances: Occurrence, Profiles and Assessment of Carcinogenic and Non-Carcinogenic Risk Associated with Their Consumption among Adult Consumers. Molecules 2023, 28, 1689. https://doi.org/10.3390/molecules28041689
Mostafa A, Shaaban H. GC-MS Determination of Undeclared Phthalate Esters in Commercial Fragrances: Occurrence, Profiles and Assessment of Carcinogenic and Non-Carcinogenic Risk Associated with Their Consumption among Adult Consumers. Molecules. 2023; 28(4):1689. https://doi.org/10.3390/molecules28041689
Chicago/Turabian StyleMostafa, Ahmed, and Heba Shaaban. 2023. "GC-MS Determination of Undeclared Phthalate Esters in Commercial Fragrances: Occurrence, Profiles and Assessment of Carcinogenic and Non-Carcinogenic Risk Associated with Their Consumption among Adult Consumers" Molecules 28, no. 4: 1689. https://doi.org/10.3390/molecules28041689
APA StyleMostafa, A., & Shaaban, H. (2023). GC-MS Determination of Undeclared Phthalate Esters in Commercial Fragrances: Occurrence, Profiles and Assessment of Carcinogenic and Non-Carcinogenic Risk Associated with Their Consumption among Adult Consumers. Molecules, 28(4), 1689. https://doi.org/10.3390/molecules28041689







