A Low Concentration of Citreoviridin Prevents Both Intracellular Calcium Deposition in Vascular Smooth Muscle Cell and Osteoclast Activation In Vitro
Abstract
1. Introduction
2. Results
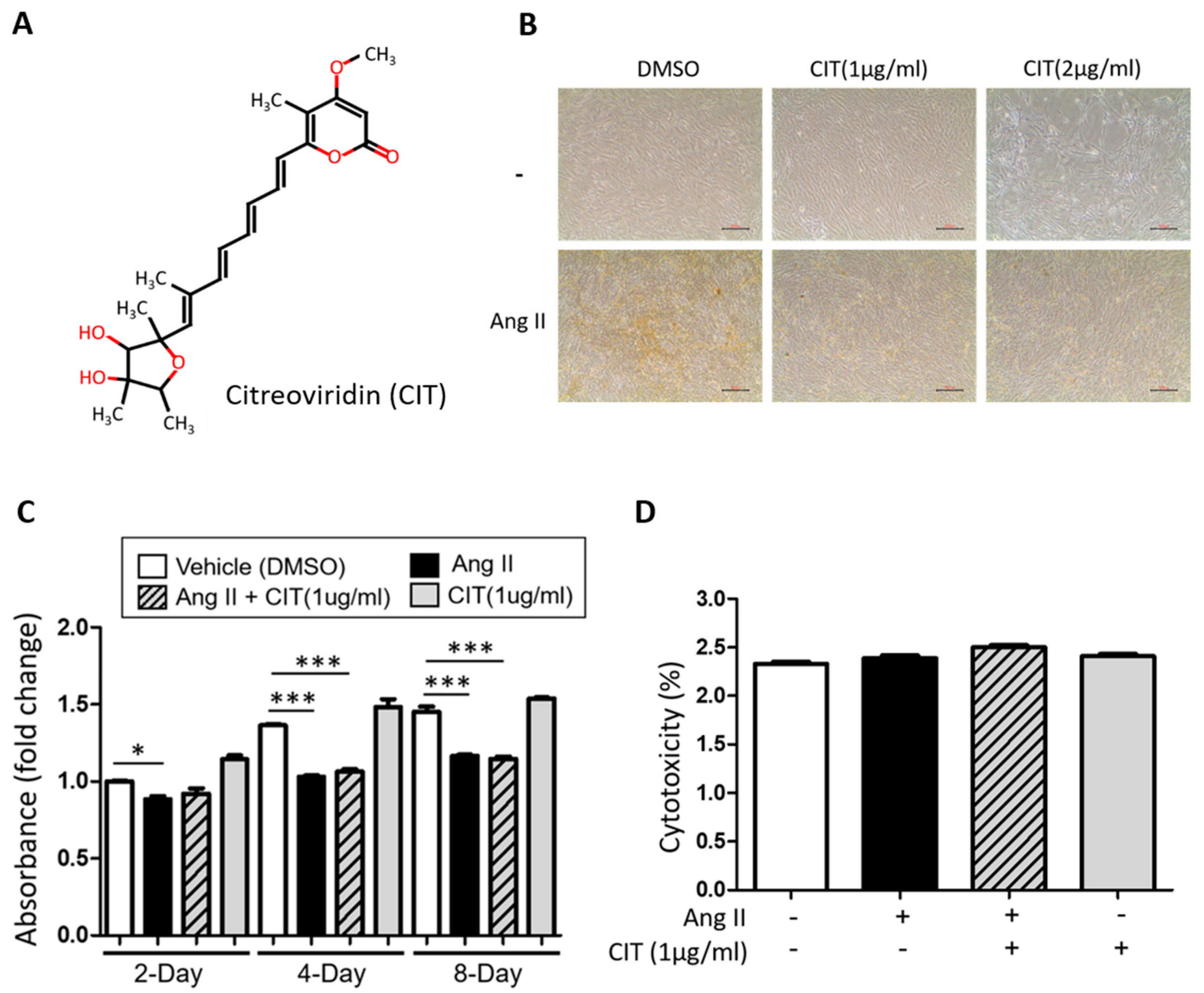
2.1. CIT Attenuates Both Ang II-Induced VC and RANKL-Induced Osteogenic Differentiation
2.2. Effect of CIT on vSMC Viability
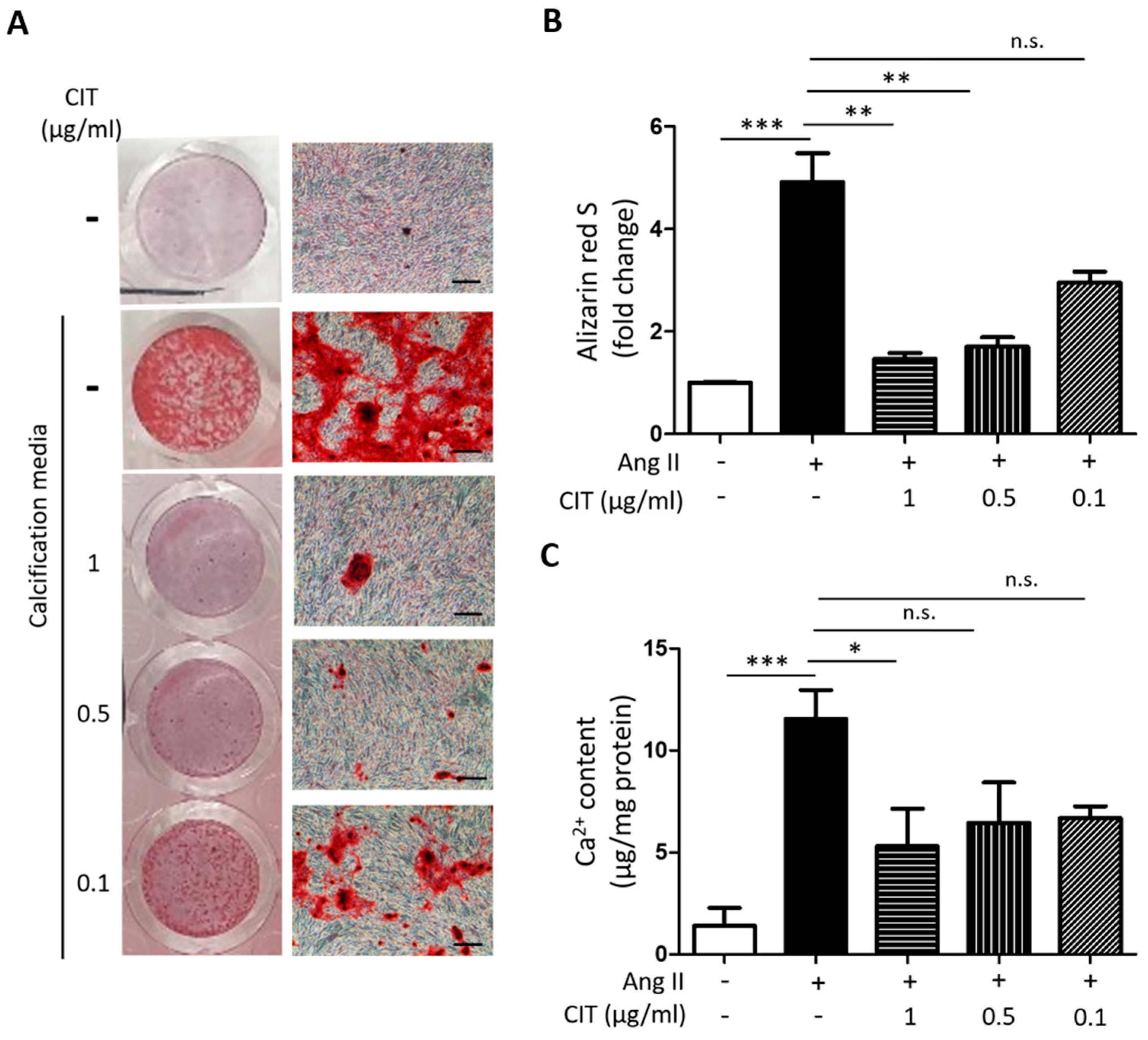
2.3. CIT Inhibits Ang II-Induced Calcium Deposition in vSMCs
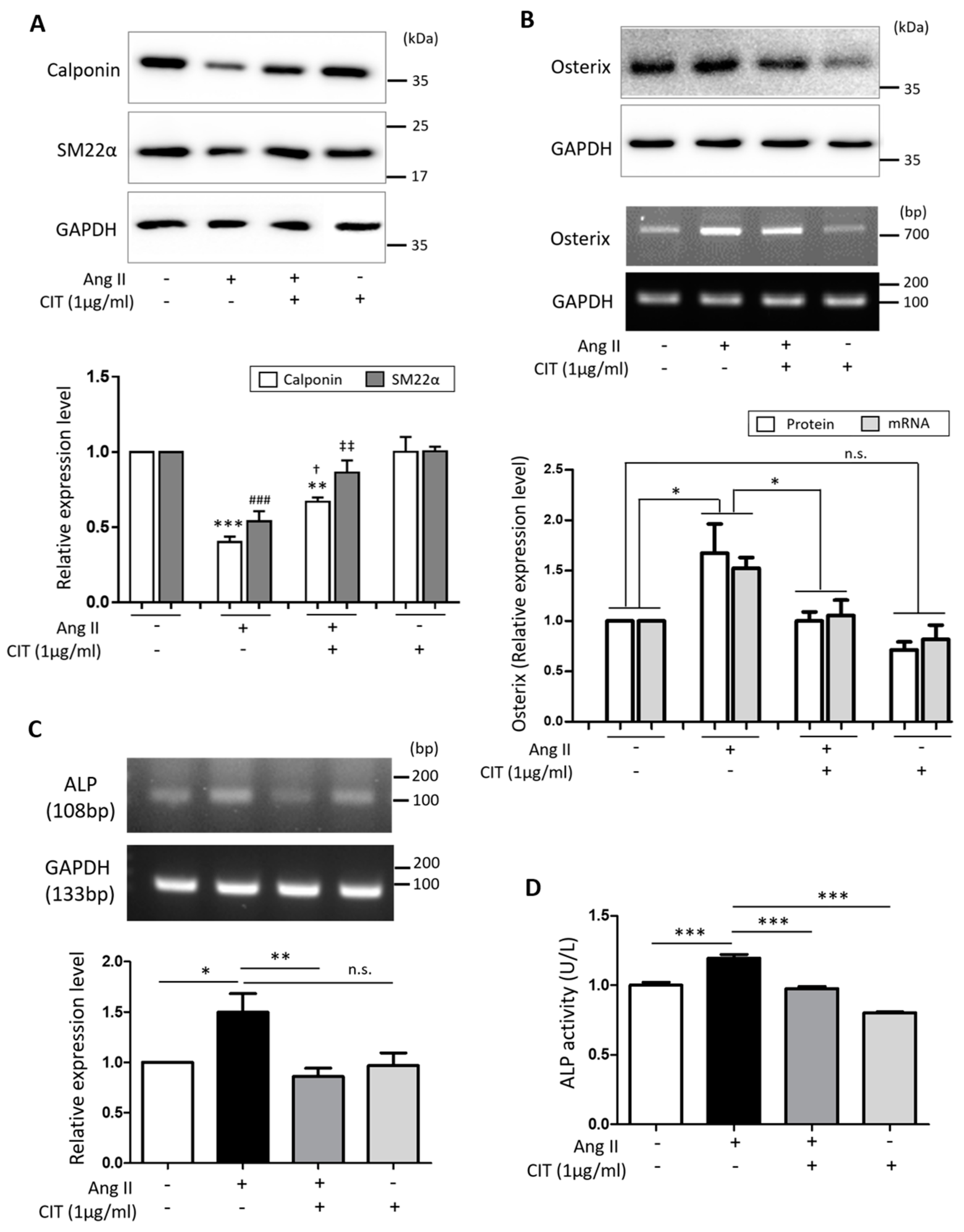
2.4. CIT Attenuates Ang II-Induced Osteogenic Marker Expressions in vSMCs
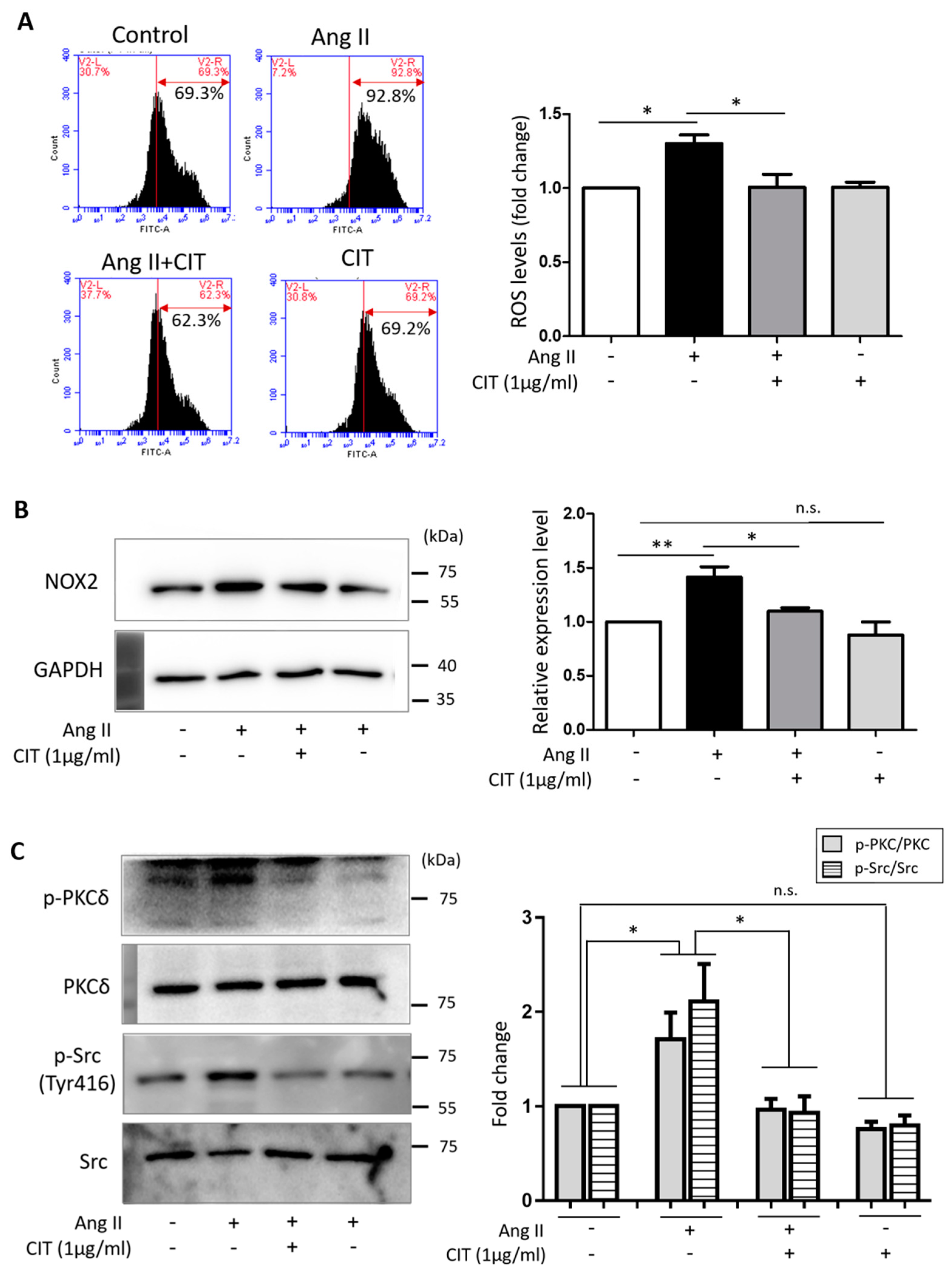
2.5. CIT Inhibits Ang II-Induced ROS Production in vSMCs
2.6. CIT Inhibits Ang II-Induced MAPK Signaling and Subsequent NF-κB and AP-1 Activation
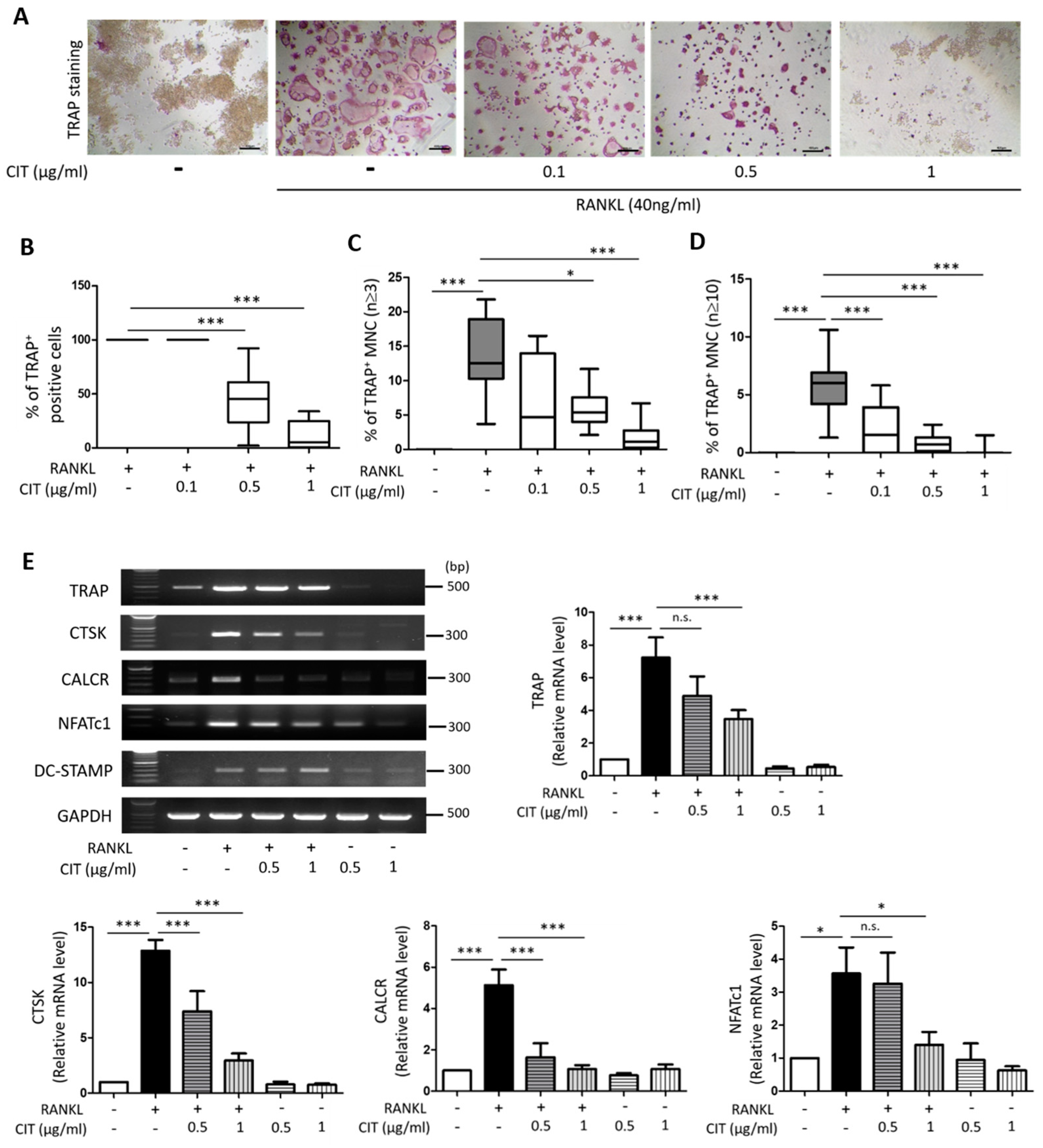
2.7. CIT Inhibits RANKL-Induced Osteoclast Differentiation of Raw264.7 Cells
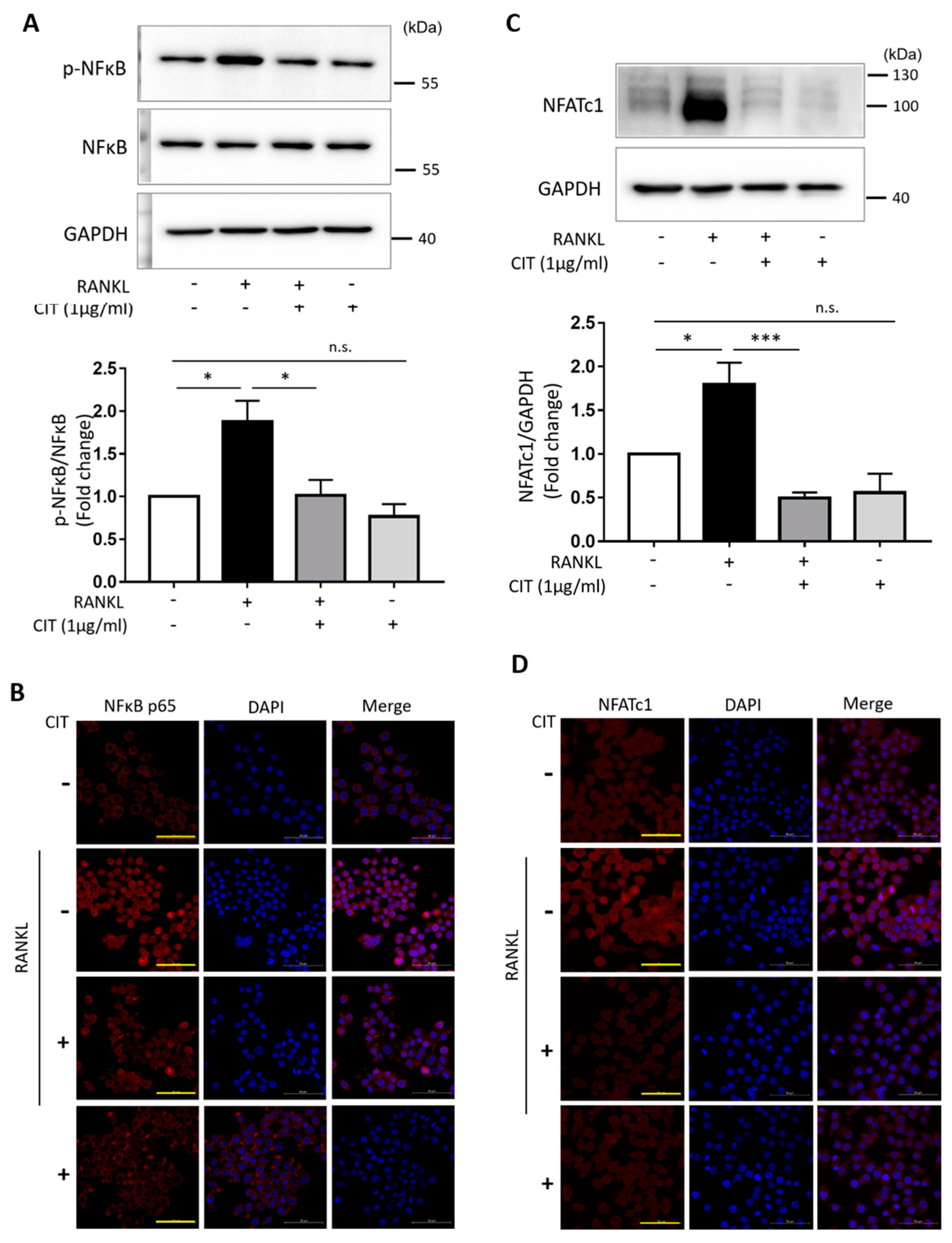
2.8. CIT Inhibits RANKL-Induced Nuclear Translocation of NFκB and NFATc1
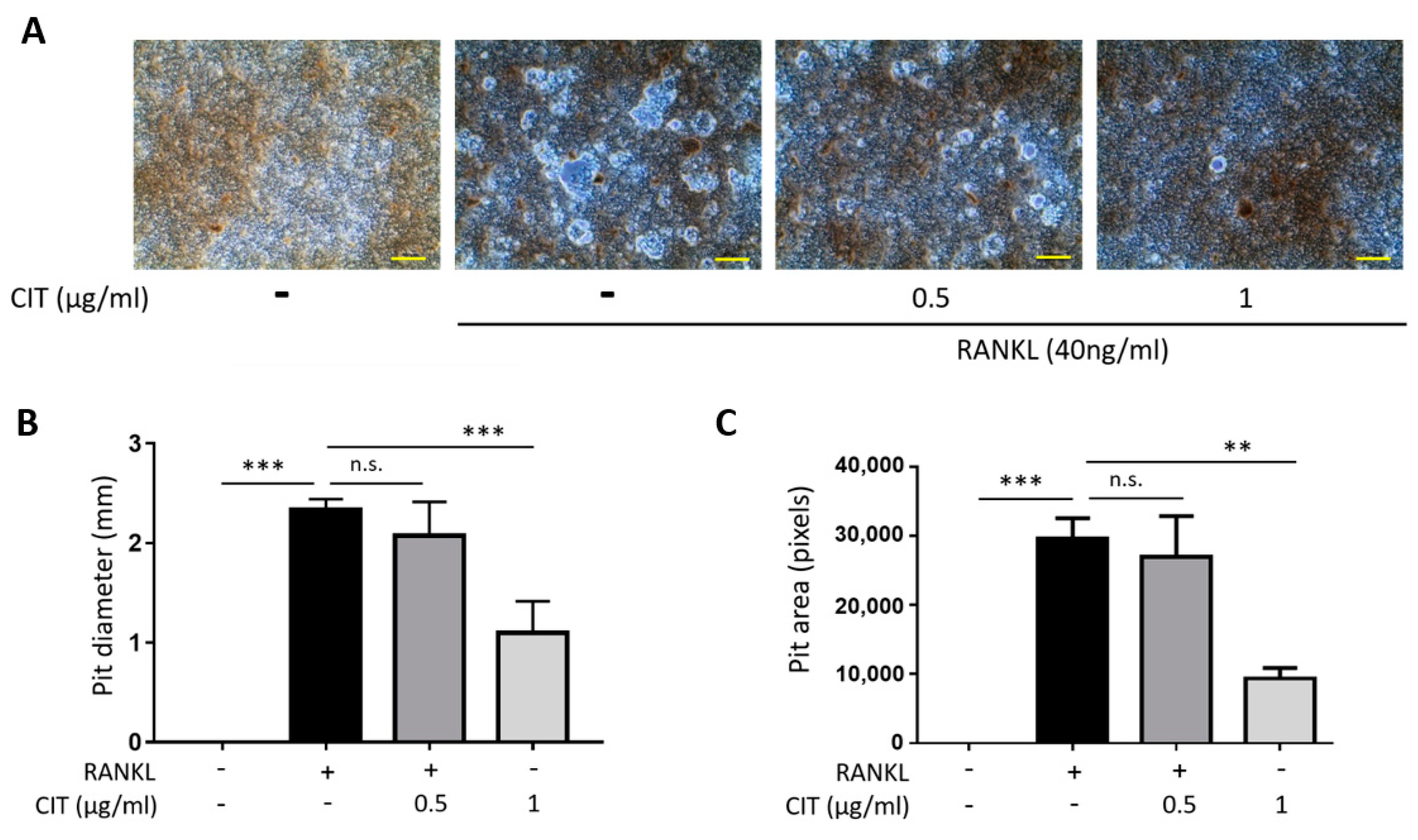
2.9. CIT Inhibits RANKL-Induced Osteoclast Activity
2.10. Ang II-Induced RANKL Secretion from vSMCs May Connect VC and Osteoporosis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.1.1. Culture and Calcium Deposition of Human Aortic Smooth Muscle Cells (HAoSMCs)
4.1.2. Culture and Osteoclast Differentiation of Raw264.7 Cells
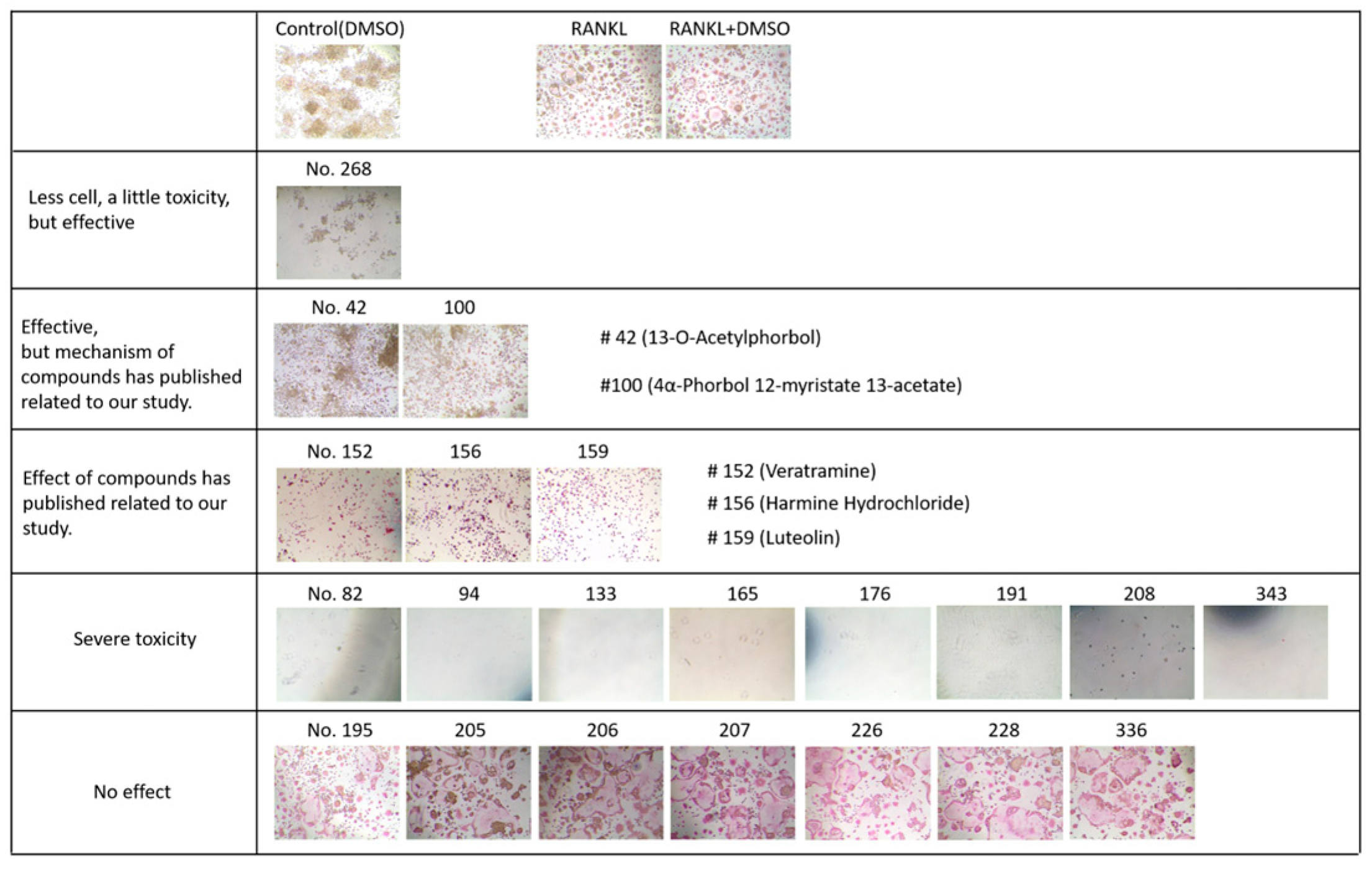
4.2. Screening of Natural Compounds for Suppressing Calcium Deposition of Human Aortic Smooth Muscle Cells (HAoSMCs) Using Alizarin Red Staining
4.3. Evaluation of Cell Viability (WST-1) and Toxicity (LDH)
4.4. Annexin V/PI Staining
4.5. Calcium Assay
4.6. Alkaline Phosphatase (ALP) Assay
4.7. Intracellular ROS Detection
4.8. TRAP Staining
4.9. Pit Assay
4.10. Reverse Transcription PCR (RT-PCR)
4.11. Western Blot
4.12. Immunocytochemistry
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; London, G.M. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001, 38, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Radford, N.B.; DeFina, L.F.; Barlow, C.E.; Lakoski, S.G.; Leonard, D.; Paixao, A.R.; Khera, A.; Levine, B.D. Progression of CAC Score and Risk of Incident CVD. JACC Cardiovasc. Imaging 2016, 9, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Shavelle, D.; Takasu, J.; Lu, B.; Mao, S.S.; Fischer, H.; Budoff, M.J. Valvular and thoracic aortic calcium as a marker of the extent and severity of angiographic coronary artery disease. Am. Heart J. 2003, 146, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc. Med. 2015, 25, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Cannata-Andia, J.B.; Carrillo-Lopez, N.; Messina, O.D.; Hamdy, N.A.T.; Panizo, S.; Ferrari, S.L.; On Behalf of The International Osteoporosis Foundation Iof Working Group On, Bone and Cardiovascular Diseases. Pathophysiology of Vascular Calcification and Bone Loss: Linked Disorders of Ageing? Nutrients 2021, 13, 3835. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Lewiecki, E.M. The Pathophysiology and Treatment of Osteoporosis. Clin. Ther. 2015, 37, 1837–1850. [Google Scholar] [CrossRef]
- O’Brien, W.; Fissel, B.M.; Maeda, Y.; Yan, J.; Ge, X.; Gravallese, E.M.; Aliprantis, A.O.; Charles, J.F. RANK-Independent Oste oclast Formation and Bone Erosion in Inflammatory Arthritis. Arthritis Rheumatol. 2016, 68, 2889–2900. [Google Scholar] [CrossRef]
- Henaut, L.; Massy, Z.A. New insights into the key role of interleukin 6 in vascular calcification of chronic kidney disease. Nephrol. Dial. Transpl. 2018, 33, 543–548. [Google Scholar] [CrossRef]
- Alexander, M.Y. RANKL links arterial calcification with osteolysis. Circ. Res. 2009, 104, 1032–1034. [Google Scholar] [CrossRef]
- Banks, L.M.; Lees, B.; MacSweeney, J.E.; Stevenson, J.C. Effect of degenerative spinal and aortic calcification on bone density measurements in post-menopausal women: Links between osteoporosis and cardiovascular disease? Eur. J. Clin. Investig. 1994, 24, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.A.; Melton, L.J., 3rd; Bryant, S.C.; Fitzpatrick, L.A.; Wahner, H.W.; Schwartz, R.S.; Riggs, B.L. Osteoporosis and calci fication of the aorta. Bone Miner. 1992, 19, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Brueck, C.C.; Shanahan, C.M.; Schoppet, M.; Dobnig, H. Vascular calcification and osteoporosis--from clinical observation towards molecular understanding. Osteoporos. Int. 2007, 18, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gomez, M.C.; Vilahur, G. Osteoporosis and vascular calcification: A shared scenario. Clin. Investig. Arterioscler. 2020, 32, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Crepaldi, G.; Solmi, M.; Cooper, C.; Harvey, N.C.; Reginster, J.Y.; Rizzoli, R.; Civitelli, R.; Schofield, P.; et al. Relationship Between Low Bone Mineral Density and Fractures with Incident Cardiovascular Disease: A Systematic Review and Meta-Analysis. J. Bone Miner. Res. 2017, 32, 1126–1135. [Google Scholar] [CrossRef]
- Shimizu, H.; Nakagami, H.; Morishita, R. [Bone metabolism and cardiovascular function update. Cross link of hypertension, bone loss and vascular calcification—Common back grounds in renin angiotensin system with anti-aging aspect -]. Clin. Calcium 2014, 24, 53–62. [Google Scholar]
- Sato, H.; Sato, M.; Kanai, H.; Uchiyama, T.; Iso, T.; Ohyama, Y.; Sakamoto, H.; Tamura, J.; Nagai, R.; Kurabayashi, M. Mito chondrial reactive oxygen species and c-Src play a critical role in hypoxic response in vascular smooth muscle cells. Cardiovasc. Res. 2005, 67, 714–722. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-kappabeta: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef]
- Jia, G.; Stormont, R.M.; Gangahar, D.M.; Agrawal, D.K. Role of matrix Gla protein in angiotensin II-induced exacerbation of vascular calcification. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H523–H532. [Google Scholar] [CrossRef]
- Das, S.; Senapati, P.; Chen, Z.; Reddy, M.A.; Ganguly, R.; Lanting, L.; Mandi, V.; Bansal, A.; Leung, A.; Zhang, S.; et al. Regu lation of angiotensin II actions by enhancers and super-enhancers in vascular smooth muscle cells. Nat. Commun. 2017, 8, 1467. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kadono, Y.; Naito, A.; Matsumoto, K.; Yamamoto, T.; Tanaka, S.; Inoue, J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001, 20, 1271–1280. [Google Scholar] [CrossRef]
- Yagi, M.; Ninomiya, K.; Fujita, N.; Suzuki, T.; Iwasaki, R.; Morita, K.; Hosogane, N.; Matsuo, K.; Toyama, Y.; Suda, T.; et al. Induction of DC-STAMP by alternative activation and downstream signaling mechanisms. J. Bone Miner. Res. 2007, 22, 992–1001. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kogawa, M.; Wada, S.; Takayanagi, H.; Tsujimoto, M.; Katayama, S.; Hisatake, K.; Nogi, Y. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J. Biol. Chem. 2004, 279, 45969–45979. [Google Scholar] [CrossRef] [PubMed]
- Byon, C.H.; Sun, Y.; Chen, J.; Yuan, K.; Mao, X.; Heath, J.M.; Anderson, P.G.; Tintut, Y.; Demer, L.L.; Wang, D.; et al. Runx2-upregulated receptor activator of nuclear factor kappaB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Osako, M.K.; Nakagami, H.; Shimamura, M.; Koriyama, H.; Nakagami, F.; Shimizu, H.; Miyake, T.; Yoshizumi, M.; Rakugi, H.; Morishita, R. Cross-talk of receptor activator of nuclear factor-kappaB ligand signaling with renin-angiotensin system in vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1287–1296. [Google Scholar] [CrossRef]
- Asaba, Y.; Ito, M.; Fumoto, T.; Watanabe, K.; Fukuhara, R.; Takeshita, S.; Nimura, Y.; Ishida, J.; Fukamizu, A.; Ikeda, K. Activation of renin-angiotensin system induces osteoporosis independently of hypertension. J. Bone Miner. Res. 2009, 24, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, A.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodama, T.; Akira, S.; Iwakura, Y.; et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006, 203, 2673–2682. [Google Scholar] [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, Z.B.; Boughner, D.R.; Drangova, M.; Rogers, K.A. Angiotensin II type 1 receptor blocker inhibits arterial calcification in a pre-clinical model. Cardiovasc. Res. 2011, 90, 165–170. [Google Scholar] [CrossRef]
- Ruths, S.; Bakken, M.S.; Ranhoff, A.H.; Hunskaar, S.; Engesaeter, L.B.; Engeland, A. Risk of hip fracture among older people using antihypertensive drugs: A nationwide cohort study. BMC Geriatr. 2015, 15, 153. [Google Scholar] [CrossRef]
- Jilka, R.L.; Hangoc, G.; Girasole, G.; Passeri, G.; Williams, D.C.; Abrams, J.S.; Boyce, B.; Broxmeyer, H.; Manolagas, S.C. Increased osteoclast development after estrogen loss: Mediation by interleukin-6. Science 1992, 257, 88–91. [Google Scholar] [CrossRef]
- Zhao, R. Immune regulation of osteoclast function in postmenopausal osteoporosis: A critical interdisciplinary perspective. Int. J. Med. Sci. 2012, 9, 825–832. [Google Scholar] [CrossRef]
- Toth, A.; Balogh, E.; Jeney, V. Regulation of Vascular Calcification by Reactive Oxygen Species. Antioxidants 2020, 9, 963. [Google Scholar] [CrossRef]
- Chao, C.T.; Yeh, H.Y.; Tsai, Y.T.; Chuang, P.H.; Yuan, T.H.; Huang, J.W.; Chen, H.W. Natural and non-natural antioxidative compounds: Potential candidates for treatment of vascular calcification. Cell Death Discov. 2019, 5, 145. [Google Scholar] [CrossRef]
- Hou, H.F.; Yuan, N.; Guo, Q.; Sun, T.; Li, C.; Liu, J.B.; Li, Q.W.; Jiang, B.F. Citreoviridin Enhances Atherogenesis in Hypercho lesterolemic ApoE-Deficient Mice via Upregulating Inflammation and Endothelial Dysfunction. PLoS ONE 2015, 10, e0125956. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.I.; Almeida, N.G.; Carvalho, K.L.; Goncalves, G.A.; Silva, C.N.; Santos, E.A.; Garcia, J.C.; Vargas, E.A. Co-occur rence of aflatoxins B(1), B(2), G(1) and G(2), ochratoxin A, zearalenone, deoxynivalenol, and citreoviridin in rice in Brazil. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Sun, S. Chronic exposure to cereal mycotoxin likely citreoviridin may be a trigger for Keshan disease mainly through oxidative stress mechanism. Med. Hypotheses 2010, 74, 841–842. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, X.; Jiang, L.; Yang, G.; Sun, X.; Geng, C.; Li, Q.; Yao, X.; Chen, M. Citreoviridin Induces Autophagy-Dependent Apoptosis through Lysosomal-Mitochondrial Axis in Human Liver HepG2 Cells. Toxins 2015, 7, 3030–3044. [Google Scholar] [CrossRef]
- Hou, H.; Zhou, R.; Jia, Q.; Li, Q.; Kang, L.; Jiao, P.; Li, D.; Jiang, B. Citreoviridin enhances tumor necrosis factor-alpha-induced adhesion of human umbilical vein endothelial cells. Toxicol. Ind. Health 2015, 31, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Li, D.; Chen, M.; Jiang, L.; Liu, X.; Li, Q.; Geng, C.; Sun, X.; Yang, G.; Zhang, L.; et al. Citreoviridin induces myocardial apoptosis through PPAR-gamma-mTORC2-mediated autophagic pathway and the protective effect of thiamine and selenium. Chem. Biol. Interact. 2019, 311, 108795. [Google Scholar] [CrossRef] [PubMed]
- Heussner, A.H.; Dietrich, D.R.; O’Brien, E. In vitro investigation of individual and combined cytotoxic effects of ochratoxin A and other selected mycotoxins on renal cells. Toxicol. In Vitro 2006, 20, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Johnson, E.A. Botulinum toxin therapy: Past, present and future developments. J. Neural. Transm. 2022, 129, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, F.; Henze, L.A.; Estepa, M.; Moser, B.; Pieske, B.; Lang, F.; Eckardt, K.U.; Alesutan, I.; Voelkl, J. Role of SGK1 in the Osteogenic Transdifferentiation and Calcification of Vascular Smooth Muscle Cells Promoted by Hyperglycemic Conditions. Int. J. Mol. Sci. 2020, 21, 7207. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Nazarewicz, R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: Potential mecha nisms and relevance for cardiovascular disease. Antioxid. Redox Signal. 2013, 19, 1085–1094. [Google Scholar] [CrossRef]
- Jeong, J.; Cho, S.; Seo, M.; Lee, B.S.; Jang, Y.; Lim, S.; Park, S. Soluble RAGE attenuates Ang II-induced arterial calcification via inhibiting AT1R-HMGB1-RAGE axis. Atherosclerosis 2022, 346, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Burley, G.; Lin, S.; Shi, Y.C. Osteoporosis pathogenesis and treatment: Existing and emerging avenues. Cell. Mol. Biol. Lett. 2022, 27, 72. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in Osteoclast Differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef]
- Hsiao, C.Y.; Chen, T.H.; Chu, T.H.; Ting, Y.N.; Tsai, P.J.; Shyu, J.F. Calcitonin Induces Bone Formation by Increasing Expression of Wnt10b in Osteoclasts in Ovariectomy-Induced Osteoporotic Rats. Front. Endocrinol. 2020, 11, 613. [Google Scholar] [CrossRef]
- Tseng, W.; Graham, L.S.; Geng, Y.; Reddy, A.; Lu, J.; Effros, R.B.; Demer, L.; Tintut, Y. PKA-induced receptor activator of NF-kappaB ligand (RANKL) expression in vascular cells mediates osteoclastogenesis but not matrix calcification. J. Biol. Chem. 2010, 285, 29925–29931. [Google Scholar] [CrossRef]
- Maranduca, M.A.; Tanase, D.M.; Branisteanu, D.C.; Serban, D.N.; Branisteanu, D.E.; Serban, I.L. Involvement of proinflamma tory cytokines in angiotensin II-induced hypertension in rat. Exp. Ther. Med. 2020, 20, 3541–3545. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lu, H.; Chen, R.; Tian, Y.; Jiang, Y.; Zhang, S.; Ni, D.; Su, Z.; Shao, X. Angiotensin II enhances the acetylation and release of HMGB1 in RAW264.7 macrophage. Cell. Biol. Int. 2018, 42, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Lee, M.E.; Jeong, J.; Lee, J.; Cho, S.; Seo, M.; Park, S. sRAGE attenuates angiotensin II-induced cardiomyocyte hyper trophy by inhibiting RAGE-NFkappaB-NLRP3 activation. Inflamm. Res. 2018, 67, 691–701. [Google Scholar] [CrossRef]
- Bidwell, J.P.; Yang, J.; Robling, A.G. Is HMGB1 an osteocyte alarmin? J. Cell. Biochem. 2008, 103, 1671–1680. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Okui, T.; Yoneda, T.; Ryumon, S.; Nakamura, T.; Kawai, H.; Kunisada, Y.; Ibaragi, S.; Masui, M.; Ono, K.; et al. High-mobility group box 1 induces bone destruction associated with advanced oral squamous cancer via RAGE and TLR4. Biochem. Biophys. Res. Commun. 2020, 531, 422–430. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Lee, B.-S.; Jung, S.E.; Yoon, Y.; Song, B.-W.; Kim, I.-K.; Choi, J.-W.; Kim, S.W.; Lee, S.; Lim, S. A Low Concentration of Citreoviridin Prevents Both Intracellular Calcium Deposition in Vascular Smooth Muscle Cell and Osteoclast Activation In Vitro. Molecules 2023, 28, 1693. https://doi.org/10.3390/molecules28041693
Jeong S, Lee B-S, Jung SE, Yoon Y, Song B-W, Kim I-K, Choi J-W, Kim SW, Lee S, Lim S. A Low Concentration of Citreoviridin Prevents Both Intracellular Calcium Deposition in Vascular Smooth Muscle Cell and Osteoclast Activation In Vitro. Molecules. 2023; 28(4):1693. https://doi.org/10.3390/molecules28041693
Chicago/Turabian StyleJeong, Seongtae, Bok-Sim Lee, Seung Eun Jung, Yoojin Yoon, Byeong-Wook Song, Il-Kwon Kim, Jung-Won Choi, Sang Woo Kim, Seahyoung Lee, and Soyeon Lim. 2023. "A Low Concentration of Citreoviridin Prevents Both Intracellular Calcium Deposition in Vascular Smooth Muscle Cell and Osteoclast Activation In Vitro" Molecules 28, no. 4: 1693. https://doi.org/10.3390/molecules28041693
APA StyleJeong, S., Lee, B.-S., Jung, S. E., Yoon, Y., Song, B.-W., Kim, I.-K., Choi, J.-W., Kim, S. W., Lee, S., & Lim, S. (2023). A Low Concentration of Citreoviridin Prevents Both Intracellular Calcium Deposition in Vascular Smooth Muscle Cell and Osteoclast Activation In Vitro. Molecules, 28(4), 1693. https://doi.org/10.3390/molecules28041693







