An Eco-Benign Biomimetic Approach for the Synthesis of Ni/ZnO Nanocomposite: Photocatalytic and Antioxidant Activities
Abstract
:1. Introduction
2. Results and Discussions
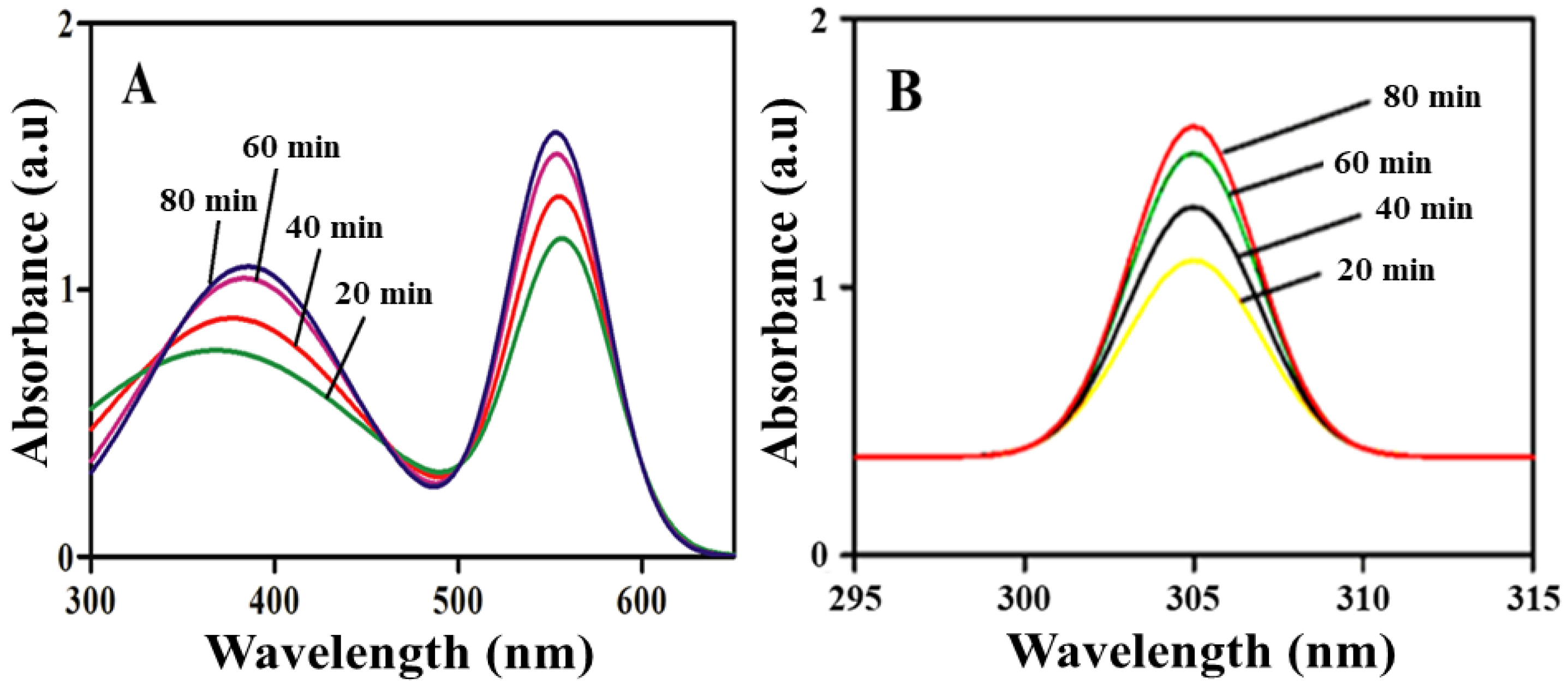
2.1. Optical Study
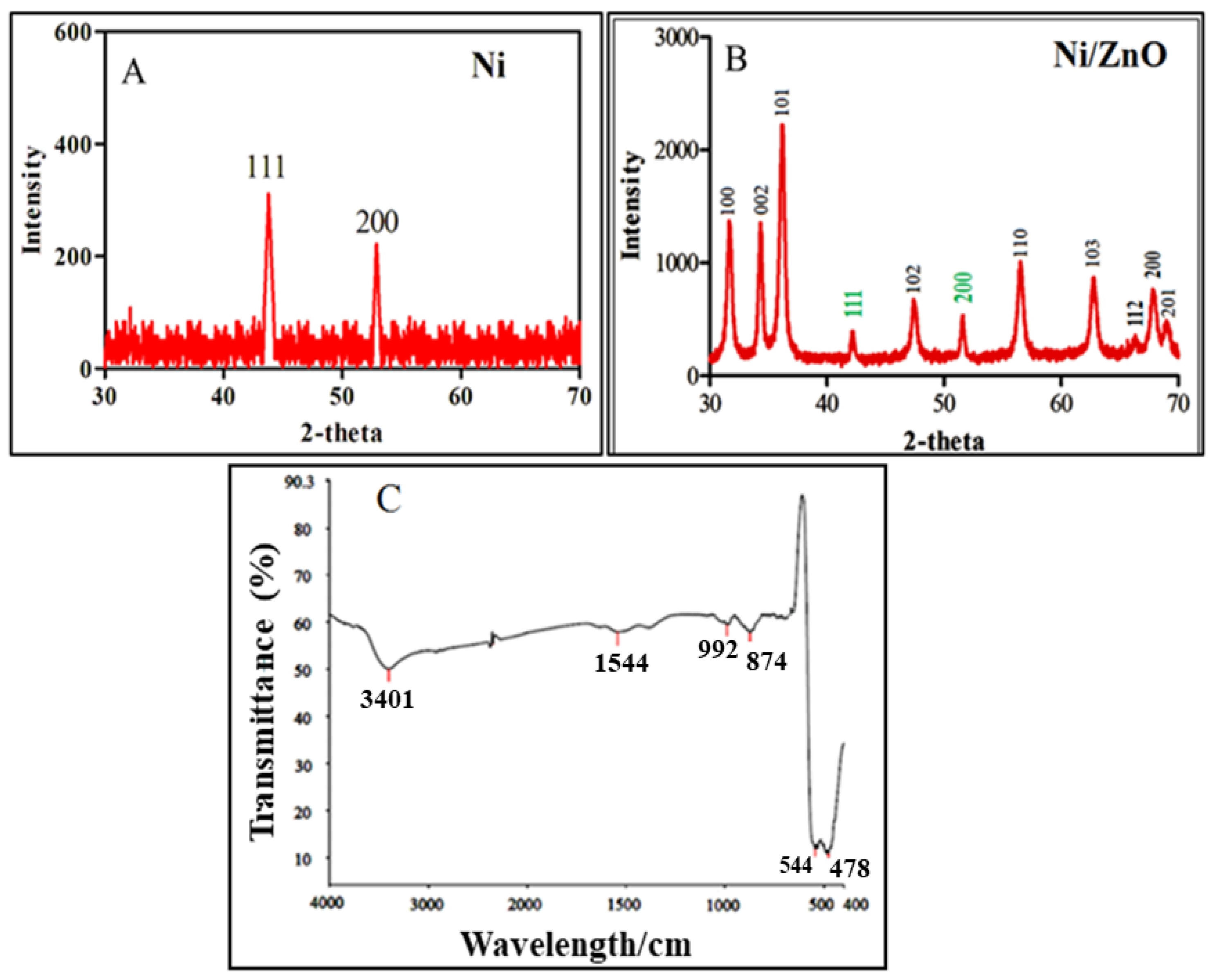
2.2. XRD Analysis
2.3. FTIR Analysis
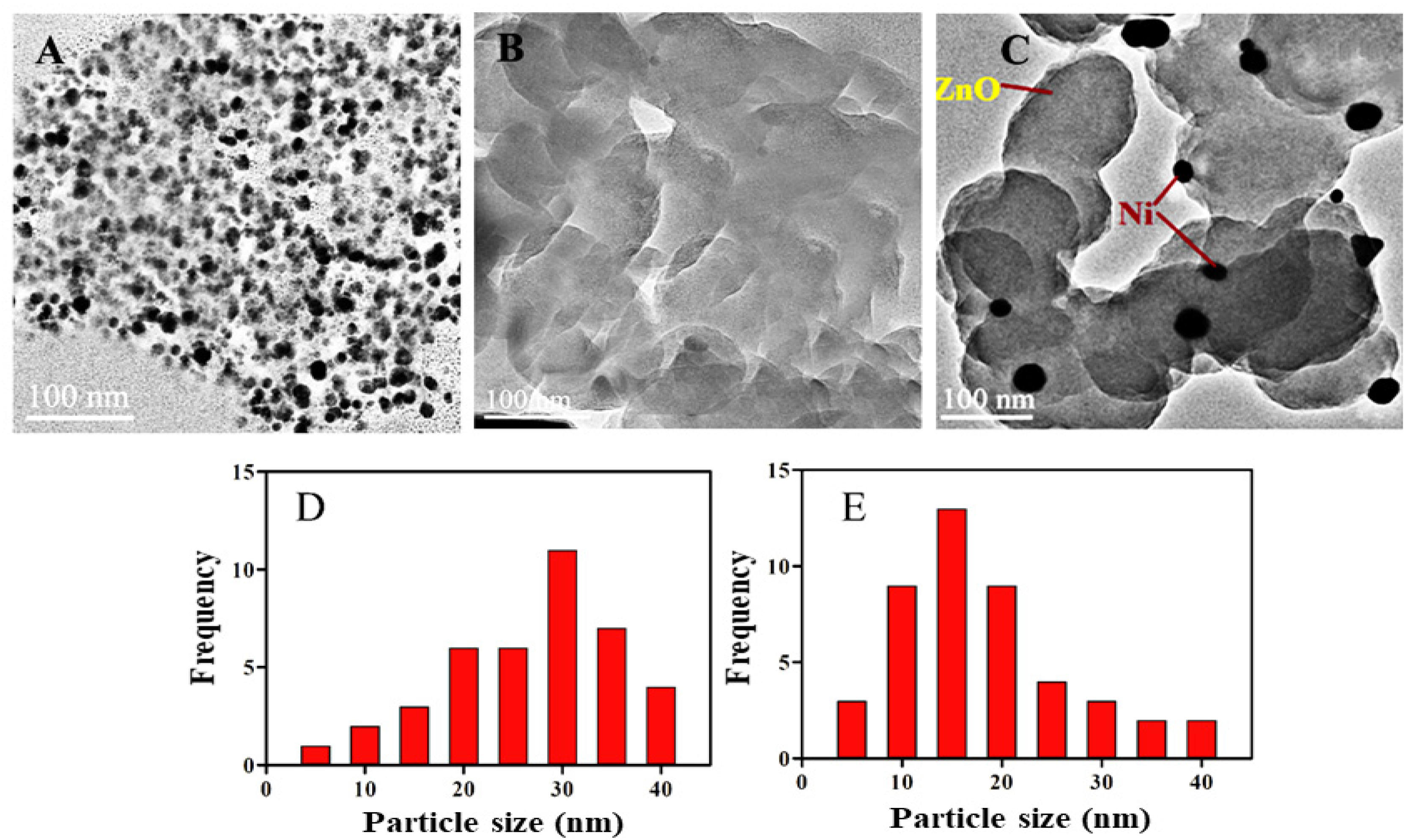
2.4. HRTEM Analysis and Particle Size and Morphology
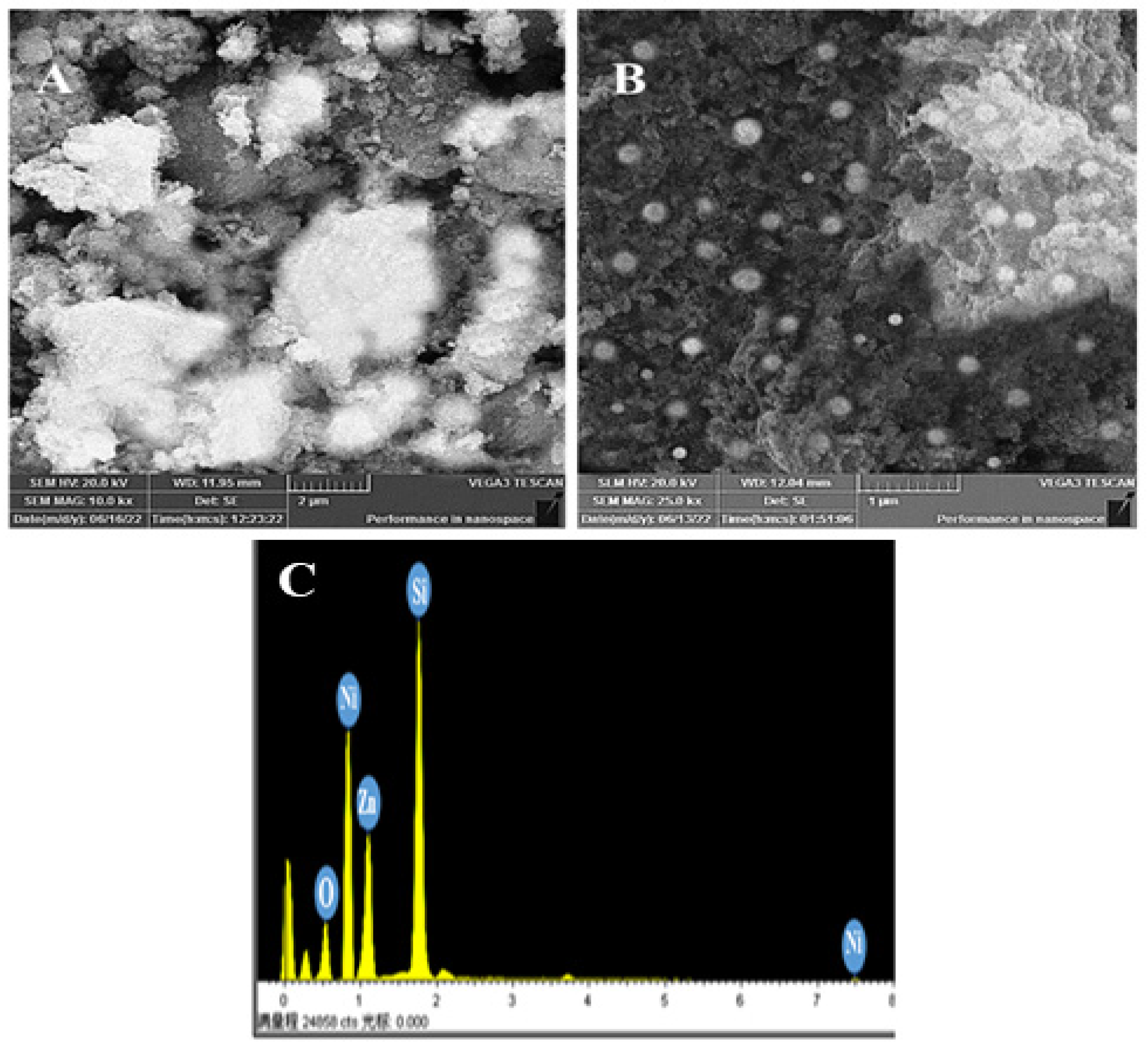
2.5. SEM and EDX Analysis
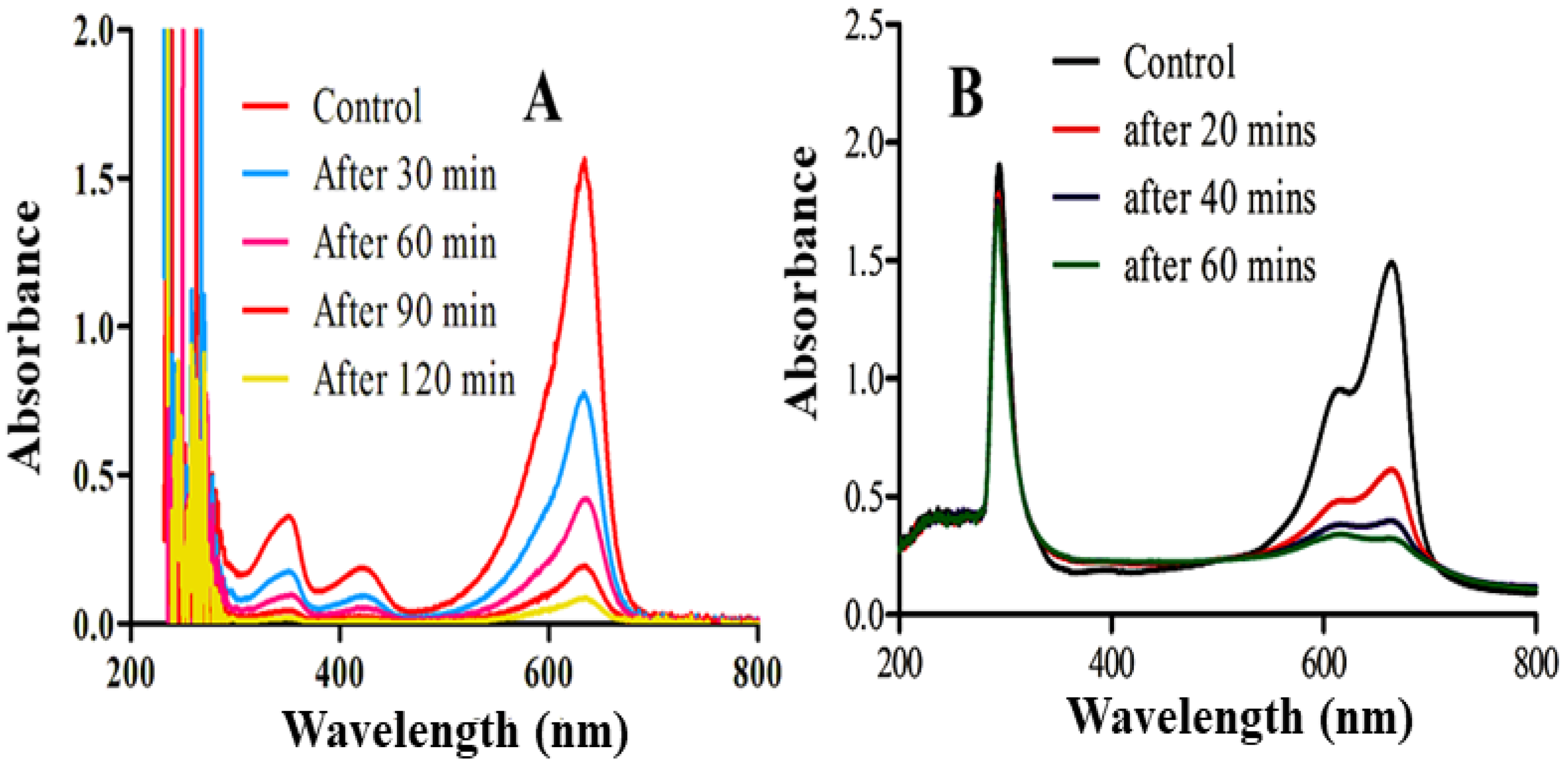
2.6. Photocatalytic Activity
2.6.1. Factors Affecting the Photodegradation of MB
2.6.2. Effect of Catalyst Dosage
2.6.3. Effect of Initial Dye Concentration
2.6.4. Effect of Solution pH
2.7. Scavenging Test
2.8. Antibacterial Test
2.9. MIC of Ni/ZnO Nanocomposite
2.10. Determination of ROS
Proposed Mechanism for Antimicrobial Test
2.11. Hemolytic Activity
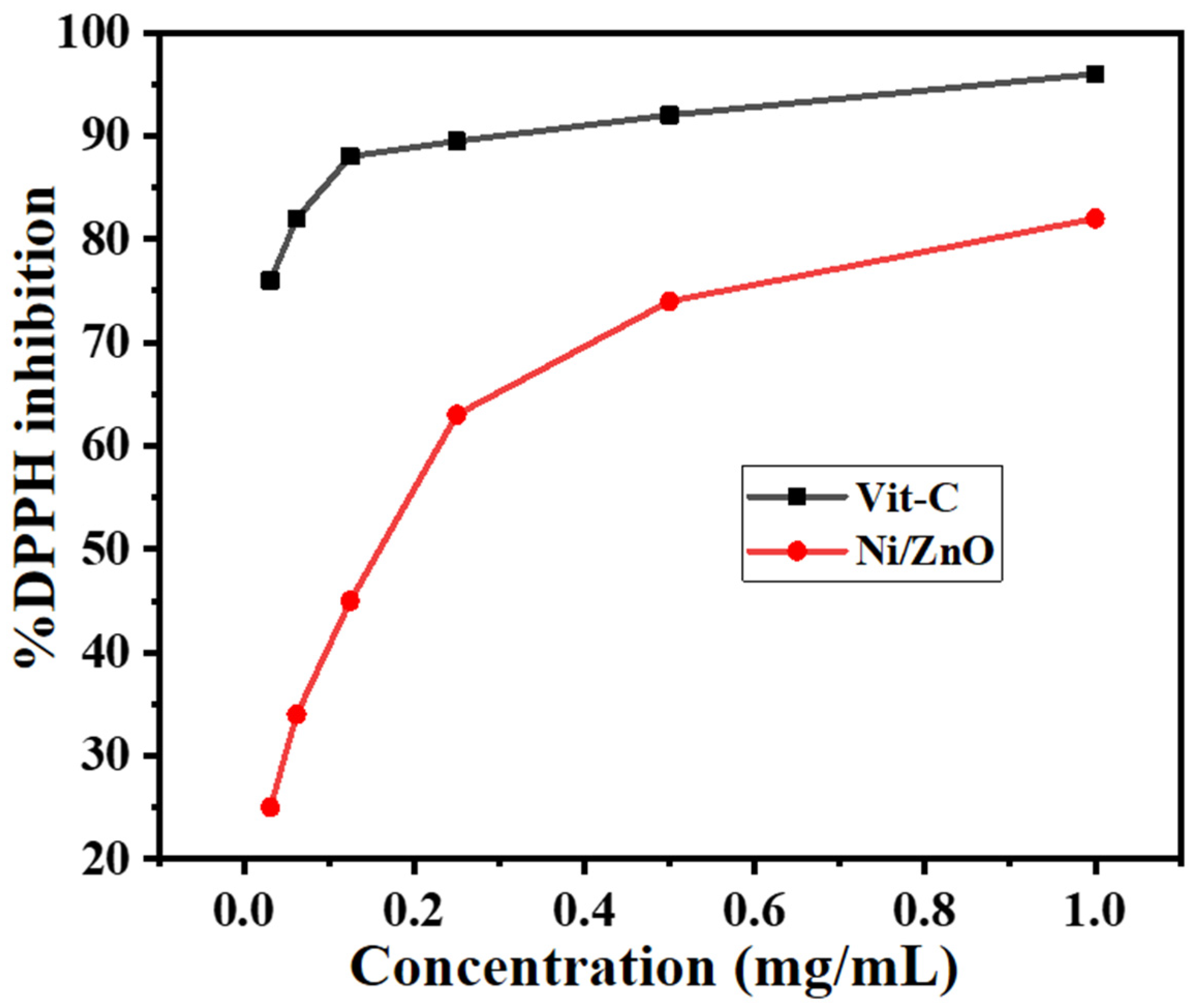
2.12. Antioxidant Activity
3. Experimental
3.1. Synthesis of ZnO
3.2. Post-Synthesis of Ni/ZnO Nanocomposite
3.3. Photocatalytic Activity Test
3.4. Antibacterial Activity of Ni/ZnO Nanocomposite
3.5. Reactive Oxygen Species (ROS) Test
3.6. Minimum Inhibitory Concentration (MIC)
3.7. Hemolytic Activity
3.8. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daud, M.; Nafees, M.; Ali, S.; Rizwan, M.; Bajwa, R.A.; Shakoor, M.B.; Arshad, M.U.; Chatha, S.A.S.; Deeba, F.; Murad, W. Drinking water quality status and contamination in Pakistan. BioMed Res. Int. 2017, 2017, 7908183. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, I.; Lo Porto, C.; Dell’Edera, M.; Petronella, F.; Agostiano, A.; Curri, M.L.; Comparelli, R. Photocatalytic TiO2—Based Nanostructured Materials for Microbial Inactivation. Catalysts 2020, 10, 1382. [Google Scholar] [CrossRef]
- da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomed. 2019, 14, 9395. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, Y.; You, Q.; Huang, P.; Wang, Y.; Huang, Z.N.; Ge, Y.; Wu, L.; Dong, Z.; Dai, X.; et al. Enhanced Photodetection Properties of Tellurium@Selenium Roll-to-Roll Nanotube Heterojunctions. Small 2019, 15, 1900902. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hu, L.; Tang, Y.; Xie, Z.; Zhang, H. Recent Advances in Functional 2D MXene—Based Nanostructures for Next-Generation Devices. Adv. Funct. Mater. 2020, 30, 2005223. [Google Scholar] [CrossRef]
- Doustkhah, E.; Esmat, M.; Fukata, N.; Ide, Y.; Hanaor, D.A.H.; Assadi, M.H.N. MOF—derived nanocrystalline ZnO with controlled orientation and photocatalytic activity. Chemosphere 2022, 303, 134932. [Google Scholar] [CrossRef]
- Cross, S.E.; Innes, B.; Roberts, M.S.; Tsuzuki, T.; Robertson, T.A.; McCormick, P. Human skin penetration of sunscreen nanoparticles: In-vitro assessment of a novel micronized zinc oxide formulation. Ski. Pharmacol. Physiol. 2007, 20, 148–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Gong, F.; Jiu, B.; Li, F. Large scale synthesis of hexagonal simonkolleit nanosheets for ZnO gas sensors with enhanced performances. Mater. Lett. 2017, 186, 7–11. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Bakrudeen, H.B.; Sugunalakshmi, M.; Reddy, B.S. Auto-fluorescent mesoporous ZnO nanospheres for drug delivery carrier application. Mater. Sci. Eng. C 2015, 56, 335–340. [Google Scholar] [CrossRef]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185, 1–22. [Google Scholar] [CrossRef]
- Hassanpour, M.; Safardoust-Hojaghan, H.; Salavati-Niasari, M. Degradation of methylene blue and Rhodamine B as water pollutants via green synthesized Co3O4/ZnO nanocomposite. J. Mol. Liq. 2017, 229, 293–299. [Google Scholar] [CrossRef]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Wehrschetz-Sigl, E.; Hasmann, A.; Guebitz, G.; Gedanken, A. Antibacterial properties of an in situ generated and simultaneously deposited nanocrystalline ZnO on fabrics. ACS Appl. Mater. Interfaces 2009, 1, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.; Mishra, A.; Yun, S.-I.; Kim, Y.-S.; Shin, H.-S. Antibacterial activity of ZnO nanoparticles prepared via non-hydrolytic solution route. Appl. Microbiol. Biotechnol. 2010, 87, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-E.; Jin, H.-E. Synthesis, characterization, and three-dimensional structure generation of zinc oxide-based nanomedicine for biomedical applications. Pharmaceutics 2019, 11, 575. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Van Giau, V.; An, S.S.A.; Hulme, J. Recent advances in the treatment of pathogenic infections using antibiotics and nano-drug delivery vehicles. Drug Des. Dev. Ther. 2019, 13, 327. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Gun’Ko, Y.; Vallet-Regí, M. ZnO nanostructures for drug delivery and theranostic applications. Nanomaterials 2018, 8, 268. [Google Scholar] [CrossRef]
- Khan, S.T.; Musarrat, J.; Al-Khedhairy, A.A. Countering drug resistance, infectious diseases, and sepsis using metal and metal oxides nanoparticles: Current status. Colloids Surf. B Biointerfaces 2016, 146, 70–83. [Google Scholar] [CrossRef]
- Guo, L.; Cao, H.; Cao, L.; Yang, Y.; Wang, M. SERS study of wheat leaves substrates with two different structures. Opt. Commun. 2022, 510, 127921. [Google Scholar] [CrossRef]
- Salehi, R.; Arami, M.; Mahmoodi, N.M.; Bahrami, H.; Khorramfar, S. Novel biocompatible composite (chitosan–zinc oxide nanoparticle): Preparation, characterization and dye adsorption properties. Colloids Surf. B Biointerfaces 2010, 80, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.K.; Kataria, J.; Sharma, S. Novel green biomimetic approach for preparation of highly stable Au-ZnO heterojunctions with enhanced photocatalytic activity. ACS Appl. Nano Mater. 2018, 1, 1870–1878. [Google Scholar] [CrossRef]
- Khan, A.U.; Arooj, A.; Tahir, K.; Ibrahim, M.M.; Jevtovic, V.; Al-Abdulkarim, H.A.; Saleh, E.A.M.; Al-Shehri, H.S.; Amin, M.A.; Li, B. Facile fabrication of novel Ag2S-ZnO/GO nanocomposite with its enhanced photocatalytic and biological applications. J. Mol. Struct. 2021, 1251, 131991. [Google Scholar] [CrossRef]
- Assadi, M.H.N.; Zhang, Y.B.; Li, S. Predominant role of defects in magnetic interactions in codoped ZnO:Co. J. Phys. Condens. Matter 2010, 22, 296004. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Feng, L.; Zhi-Ming, J.; Xiao-Bo, S.; Peng-Hui, Y.; Xue-Ren, W.; Cheng, S.; Zhan-Qi, G.; Liang-Sheng, L. Efficient plasmonic photocatalytic activity on silver-nanoparticle-decorated AgVO3 nanoribbons. J. Mater. Chem. A 2014, 2, 13226–13231. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Rodríguez, H.B.; San Román, E.; Feldhoff, A.; Grela, M.A. Ag@ZnO core—Shell nanoparticles formed by the timely reduction of Ag+ ions and zinc acetate hydrolysis in N, N-dimethylformamide: Mechanism of growth and photocatalytic properties. J. Phys. Chem. C 2011, 115, 24967–24974. [Google Scholar] [CrossRef]
- Khatami, M.; Alijani, H.Q.; Sharifi, I. Biosynthesis of bimetallic and core-shell nanoparticles: Their biomedical applications—A review. IET Nanobiotechnol. 2018, 12, 879–887. [Google Scholar] [CrossRef]
- Ahmad, M.; Zhao, J.; Iqbal, J.; Miao, W.; Xie, L.; Mo, R.; Zhu, J. Conductivity enhancement by slight indium doping in ZnO nanowires for optoelectronic applications. J. Phys. D Appl. Phys. 2009, 42, 165406. [Google Scholar] [CrossRef]
- Hao, Z.; Li, N.; Cao, H.; Guo, L.; Cao, H.; Li, N.; Cao, L.; Liu, H.L.; Jiao, T.; Wang, M. Modified Ag nanoparticles on the regular array structure to improve the optical properties. J. Lumin. 2022, 243, 118684. [Google Scholar] [CrossRef]
- Munir, S.; Warsi, M.F.; Zulfiqar, S.; Ayman, I.; Haider, S.; Alsafari, I.A.; Agboola, P.O.; Shakir, I. Nickel Ferrite/Zinc Oxide Nanocomposite Photocatalytic and Antibacterial Properties. J. Saudi Chem. Soc. 2021, 25, 101388. [Google Scholar] [CrossRef]
- Khan, F.U.; Khan, Z.U.H.; Ma, J.; Khan, A.U.; Sohail, M.; Chen, Y.; Yang, Y.; Pan, X. An Astragalus membranaceus based eco-friendly biomimetic synthesis approach of ZnO nanoflowers with an excellent antibacterial, antioxidant and electrochemical sensing effect. Mater. Sci. Eng. C 2021, 118, 111432. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-y.; Cao, Z.-m.; Hua, Y.; Wei, G.; Yu, X.-z.; Shang, W.-b.; Lian, H.-z. Solvothermal synthesis of novel magnetic nickel based iron oxide nanocomposites for selective capture of global-and mono-phosphopeptides. Anal. Chem. 2019, 92, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Tahir, K.; El-Zahhar, A.A.; Arooj, A.; Al-Abdulkarim, H.A.; Saleh, E.A.M.; Nazir, S.; Al-Shehri, H.S.; Husain, K.; Khan, A.U. Facile synthesis of silver modified zinc oxide nanocomposite: An efficient visible light active nanomaterial for bacterial inhibition and dye degradation. Photodiagn. Photodyn. Ther. 2021, 36, 102619. [Google Scholar] [CrossRef] [PubMed]
- Chequer, F.D.; De Oliveira, G.R.; Ferraz, E.A.; Cardoso, J.C.; Zanoni, M.B.; de Oliveira, D.P. Textile dyes: Dyeing process and environmental impact. Eco-Friendly Text. Dye. Finish. 2013, 6, 151–176. [Google Scholar]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Balazs, N.; Mogyorosi, K.; Sranko, D.F.; Pallagi, A.; Alapi, T.; Oszko, A.; Dombi, A.; Sipos, P. The effect of particle shape on the activity of nanocrystalline TiO2 photocatalysts in phenol decomposition. Appl. Catal. B Environ. 2008, 84, 356–362. [Google Scholar] [CrossRef]
- Basu, M.; Sinha, A.K.; Pradhan, M.; Sarkar, S.; Negishi, Y.; Pal, T. Evolution of hierarchical hexagonal stacked plates of CuS from liquid-liquid interface and its photocatalytic application for oxidative degradation of different dyes under indoor lighting. Environ. Sci. Technol. 2010, 44, 6313–6318. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M. Synthesis and characterization of titanium dioxide nanoparticles using Euphorbia heteradena Jaub root extract and evaluation of their stability. Ceram. Int. 2015, 41, 14435–14439. [Google Scholar] [CrossRef]
- Hassandoost, R.; Kotb, A.; Movafagh, Z.; Esmat, M.; Guegan, R.; Endo, S.; Jevasuwan, W.; Fukata, N.; Sugahara, Y.; Khataee, A.; et al. Nanoarchitecturing bimetallic manganese cobaltite spinels for sonocatalytic degradation of oxytetracycline. Chem. Eng. J. 2022, 431, 133851. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, Q.U.; Tahir, K.; Ullah, S.; Arooj, A.; Li, B.; ur Rehman, K.; Nazir, S.; Khan, M.U.; Ullah, I. A Tagetes minuta based eco-benign synthesis of multifunctional Au/MgO nanocomposite with enhanced photocatalytic, antibacterial and DPPH scavenging activities. Mater. Sci. Eng. C 2021, 126, 112146. [Google Scholar] [CrossRef]
- Alam, N.; Tahir, K.; Nazir, S.; Khan, A.U.; Albalawi, K.; Refat, M.S.; Almarhoon, Z.M.; Jevtovic, V.; Al-Shehri, H.S.; Aldawsari, A.M. Effect of light-dark conditions on inhibition of Gram positive and gram negative bacteria and dye decomposition in the presence of photocatalyst Co/ZnO nanocomposite synthesized by ammonia evaporation method. Photodiagn. Photodyn. Ther. 2022, 38, 102853. [Google Scholar] [CrossRef] [PubMed]
- Anitha, S.; Muthukumaran, S. Structural, optical and antibacterial investigation of La, Cu dual doped ZnO nanoparticles prepared by co-precipitation method. Mater. Sci. Eng. C 2020, 108, 110387. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Irshad, R.; Tahir, K.; Li, B.; Ahmad, A.; Siddiqui, A.R.; Nazir, S. Antibacterial activity of biochemically capped iron oxide nanoparticles: A view towards green chemistry. J. Photochem. Photobiol. B Biol. 2017, 170, 241–246. [Google Scholar] [CrossRef] [PubMed]
- El-Bindary, A.A.; Toson, E.A.; Shoueir, K.R.; Aljohani, H.A.; Abo-Ser, M.M. Metal–organic frameworks as efficient materials for drug delivery: Synthesis, characterization, antioxidant, anticancer, antibacterial and molecular docking investigation. Appl. Organomet. Chem. 2020, 34, e5905. [Google Scholar] [CrossRef]
- Khan, A.U.; Yuan, Q.; Wei, Y.; Khan, G.M.; Khan, Z.U.H.; Khan, S.; Ali, F.; Tahir, K.; Ahmad, A.; Khan, F.U. Photocatalytic and antibacterial response of biosynthesized gold nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 162, 273–277. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, A.U.; Li, B.; Mahnashi, M.H.; Alyami, B.A.; Alqahtani, Y.S.; Alqarni, A.O.; Khan, Z.U.H.; Ullah, S.; Wasim, M. Biosynthesis of silver capped magnesium oxide nanocomposite using Olea cuspidata leaf extract and their photocatalytic, antioxidant and antibacterial activity. Photodiagn. Photodyn. Ther. 2021, 33, 102153. [Google Scholar] [CrossRef]

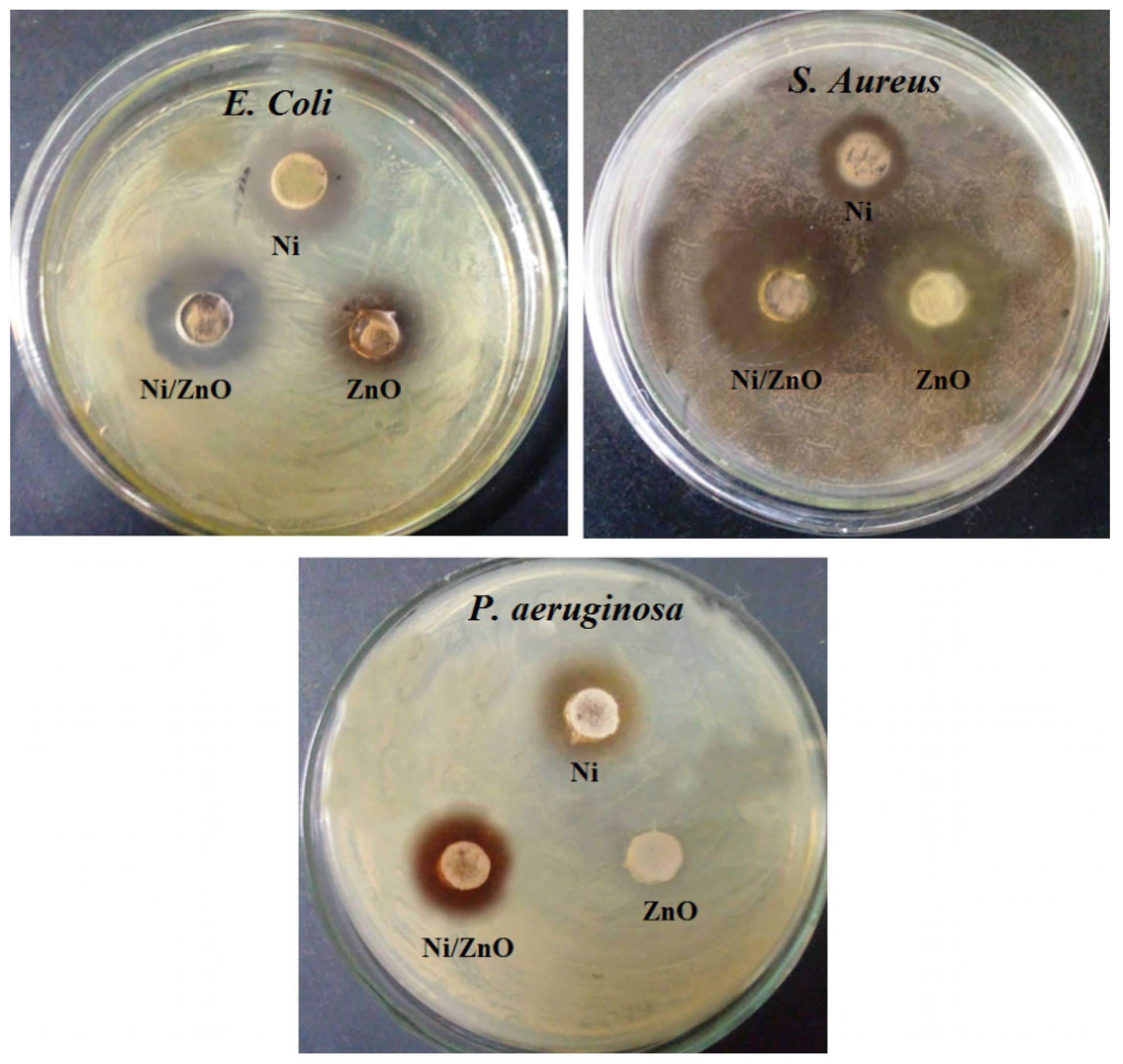
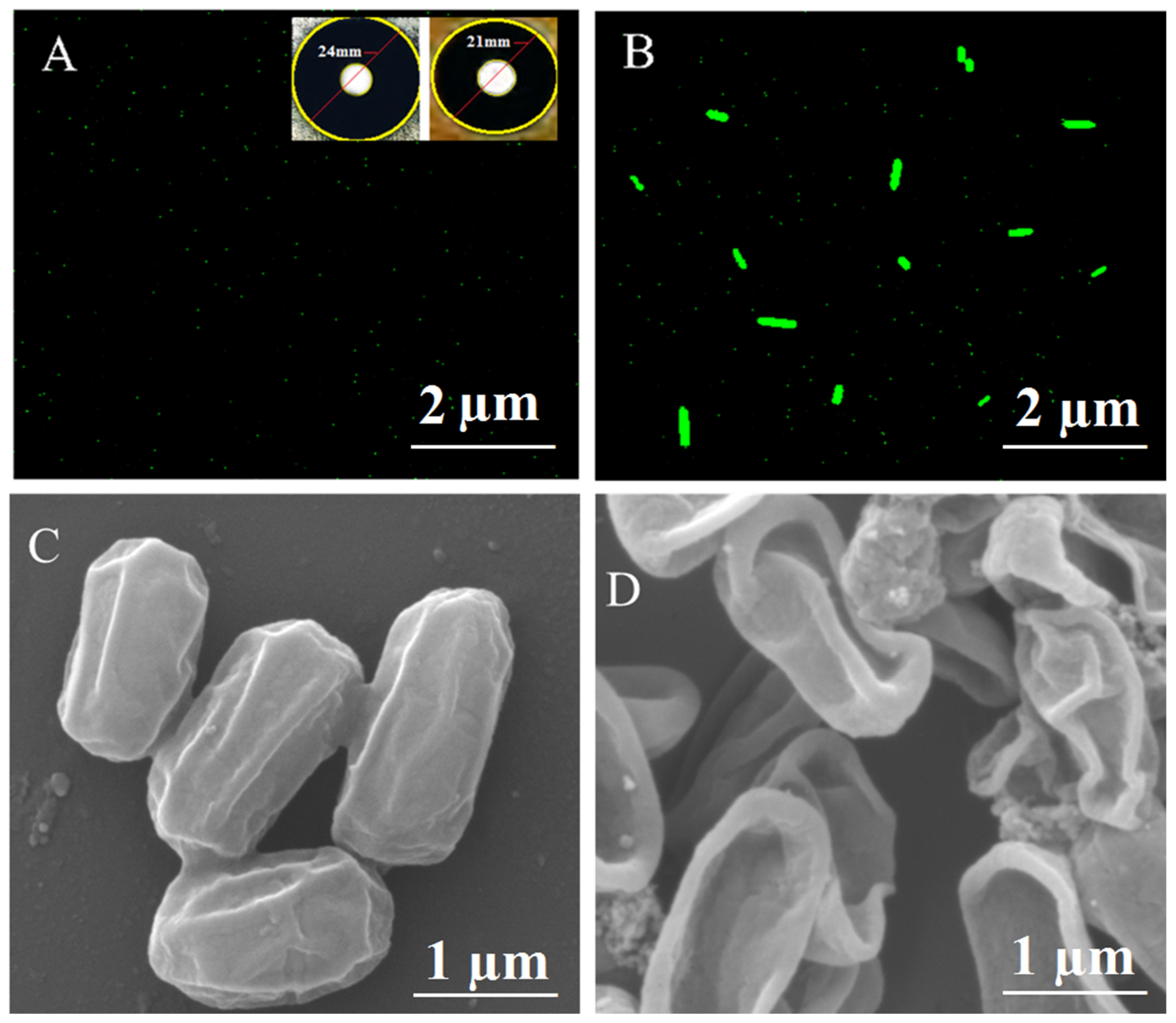
| Bacterial Strains | Zone of Inhibition (mm) | ||||
|---|---|---|---|---|---|
| Ni | ZnO | Irradiated Ni/ZnO | Dark Ni/ZnO | Positive Control | |
| E. coli | 11 ± 0.4 | 13 ± 0.3 | 16 ± 0.3 | 8 ± 0.4 | Zero inhibition |
| P. aeruginosa | 9 ± 0.3 | 14 ± 0.4 | 19 ± 0.4 | 10 ± 0.4 | Zero inhibition |
| S. aureus | 9 ± 0.2 | 5 ± 0.3 | 23 ± 0.5 | 14 ± 0.5 | Zero inhibition |
| Bacteria | Ni/ZnO Nanocomposite (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Control | 70 | 60 | 50 | 40 | 30 | 20 | |
| E. coli | + | − | − | − | − | − | + |
| P. aeruginosa | + | − | − | − | − | − | + |
| S. aureus | + | − | − | − | − | − | − |
| Sample (n = 3) (μg) | Hemolytic Activity (%) (OD540 nm) |
|---|---|
| Control 1% Triton X-100 Ni/ZnO (12.5) Ni/ZnO (25) Ni/ZnO (50) Ni/ZnO (75) Ni/ZnO (100) Ni/ZnO (125) | 1.18 ± 0.11 99.9 ± 0.3 1.23 ± 0.8 1.28 ± 0.10 1.28 ± 0.11 1.30 ± 0.10 1.31 ± 0.9 1.33 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhar, M.S.O.; Muhammad, D.; Tahir, K.; Zaki, M.E.A.; Urooj, M.; Nazir, S.; Albalawi, K.; Al-Shehri, H.S.; Saleh, E.A.M.; Khan, A.U. An Eco-Benign Biomimetic Approach for the Synthesis of Ni/ZnO Nanocomposite: Photocatalytic and Antioxidant Activities. Molecules 2023, 28, 1705. https://doi.org/10.3390/molecules28041705
Alhar MSO, Muhammad D, Tahir K, Zaki MEA, Urooj M, Nazir S, Albalawi K, Al-Shehri HS, Saleh EAM, Khan AU. An Eco-Benign Biomimetic Approach for the Synthesis of Ni/ZnO Nanocomposite: Photocatalytic and Antioxidant Activities. Molecules. 2023; 28(4):1705. https://doi.org/10.3390/molecules28041705
Chicago/Turabian StyleAlhar, Munirah Sulaiman Othman, Dost Muhammad, Kamran Tahir, Magdi E. A. Zaki, Muniba Urooj, Sadia Nazir, Karma Albalawi, Hamza S. Al-Shehri, Ebraheem Abdu Musad Saleh, and Afaq Ullah Khan. 2023. "An Eco-Benign Biomimetic Approach for the Synthesis of Ni/ZnO Nanocomposite: Photocatalytic and Antioxidant Activities" Molecules 28, no. 4: 1705. https://doi.org/10.3390/molecules28041705
APA StyleAlhar, M. S. O., Muhammad, D., Tahir, K., Zaki, M. E. A., Urooj, M., Nazir, S., Albalawi, K., Al-Shehri, H. S., Saleh, E. A. M., & Khan, A. U. (2023). An Eco-Benign Biomimetic Approach for the Synthesis of Ni/ZnO Nanocomposite: Photocatalytic and Antioxidant Activities. Molecules, 28(4), 1705. https://doi.org/10.3390/molecules28041705








