Flavonoid Components, Distribution, and Biological Activities in Taxus: A review
Abstract
:1. Introduction
2. Chemical Structure
3. Results
3.1. Chemical Components
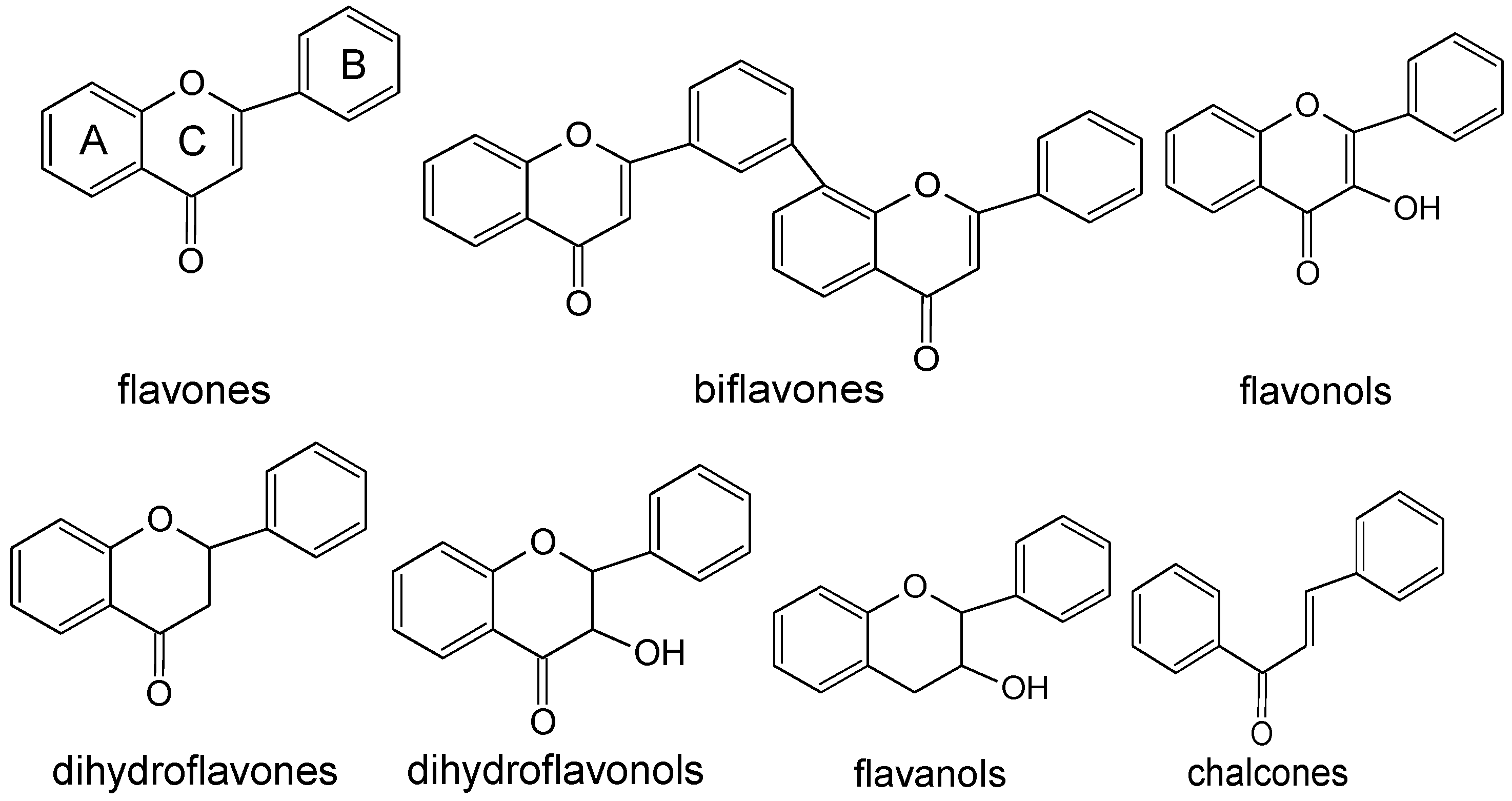
3.1.1. Flavones
3.1.2. Biflavones
3.1.3. Flavonols or Flavonol Glycosides
3.1.4. Dihydroflavones, Dihydroflavonols, and Dihydroflavonol Glycosides
3.1.5. Flavanols and Biflavanols
3.1.6. Chalcones
3.2. Flavonoid Properties, Extraction, and Isolation
3.2.1. Physico-Chemical Properties of Flavonoids
3.2.2. Extraction and Isolation Methods
3.3. Flavonoid Distribution
3.4. Flavonoid Bioactivities
3.4.1. Antibacterial Activities
3.4.2. Antioxidant and Antiaging Activities
3.4.3. Anti-Alzheimer’s Activities
3.4.4. Antidiabetes Activities
3.4.5. Anticancer Activities
3.4.6. Antidepressant Activities
3.4.7. Neuronal Protective Activities
3.4.8. Antileishmaniasis Activities
3.4.9. Anti-Inflammatory, Antinociceptive, and Antiallergic Activities
3.4.10. Antivirus Activities
3.4.11. Antilipase Activities
3.4.12. Promotion of Melanogenesis
3.4.13. Hepatic-Protective Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spjut, R.W. Taxonomy and nomenclature of Taxus (Taxaceae). J. Bot. Res. Inst. Texas. 2007, 1, 203–289. [Google Scholar]
- Swamy, M.K.; Pullaiah, T.; Chen, Z.S. Paclitaxel: Sources, Chemistry, Anticancer Actions and Current Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 33–34. [Google Scholar]
- Vue, B.; Zhang, S.; Chen, Q.H. Flavonoids with therapeutic potential in prostate cancer. Anticancer Agents Med. Chem. 2016, 16, 1205–1229. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.P.; Li, J.; Pu, S.B. Chemical constituents of leaves of Taxus chinensis. Chem. Nat. Compd. 2018, 54, 841–845. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.C.; et al. Dietary polyphenol intake in Europe: The Europeanprospective investigation into cancer and nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Fang, X.; Yang, J.; Yao, Y.; Nandakumar, K.S.; Salem, M.L.; Cheng, K. Recent research on flavonoids and their biomedical applications. Curr. Med. Chem. 2021, 28, 1042–1066. [Google Scholar] [CrossRef]
- Yu, C.; Luo, X.J.; Zhan, X.R.; Hao, J.; Zhang, L.; Song, Y.B.; Shen, C.J.; Dong, M. Comparative metabolomics reveals the metabolic variations between two endangered Taxus Species (T. Fuana and T. Yunnanensis) in the Himalayas. BMC Plant Biol. 2018, 18, 197. [Google Scholar] [CrossRef]
- Bekhouche, M.; Benyammi, R.; Slaoui, M.K.; Khelifi, L.; Morsli, A. Free radical scavenging activity and detailed flavonoid profiling of Algerian yew (Taxus Baccata L.) by LC–ESI–MS/MS. Int. J. Pharmaceut. 2021, 12, 2613–2619. [Google Scholar]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Jang, C.H.; Moon, N.; Lee, J.; Kwon, M.J.; Oh, J.; Kim, J.S. Luteolin synergistically enhances antitumor activity of Oxaliplatin in clorectal carcinoma via AMPK inhibition. Antioxidants 2022, 11, 626. [Google Scholar] [CrossRef]
- Al-Yamani, M.J.; Asdaq, S.M.B.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Alsalman, A.J.; Al Hawaj, M.A.; Alanazi, A.A.; Alanzi, K.D.; Imran, M. The role of serotonergic and catecholaminergic systems for possible antidepressant activity of apigenin. Saudi J. Biol. Sci. 2022, 29, 11–17. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Wiwart, M. Antifungal activity of biflavones from Taxus Baccata and Ginkgo Biloba. Z. Naturforsch. C 2003, 58, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Gai, Q.Y.; Jiao, J.; Wang, X.; Liu, J.; Fu, Y.J.; Lu, Y.; Wang, Z.Y.; Xu, X.J. Simultaneous determination of taxoids and flavonoids in twigs and leaves of three Taxus species by UHPLC-MS/MS. J. Pharmaceut. Biomed. 2020, 189, 113456. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M. Flavonoids from thegenus Taxus. Z. Nat. C 2004, 59, 43–47. [Google Scholar] [CrossRef]
- Yeh, P.H.; Shieh, Y.D.; Hsu, L.C.; Kuo, L.M.Y.; Lin, J.H.; Liaw, C.C.; Kuo, Y.H. Naturally occurring cytotoxic [3′→ 8″-biflavonoids from Podocarpus nakaii. J. Tradit. Compl. Med. 2012, 2, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.Y.; Kumar, I.; Prasad, J.S.; Nagarajan, G.R.; Parthasarathy, M.R.; Krishnamurty, H.G. Phenolic constituents of Taxus baccata leaves. Planta Med. 1976, 30, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Rao, S.P.; Srinivas, K.V.N.S.; Yadav, J.S. Lignans, biflavones and taxoids from HimalayanTaxus baccata. Phytochemistry 1995, 38, 715–717. [Google Scholar] [CrossRef]
- Tachibana, S.; Matsuo, A.; Itoh, K.; Oki, T. Extractives in the leaves and bark of Taxus cuspidata Sieb. et. Zucc. var. Nana Rehder. J. Japan Wood Res. Soc. 1994, 40, 1008–1013. [Google Scholar]
- Tatsuo, K.; Tokunosuk, S. Studies on Flavonoids of the leaves of Coniferae and allied plants. I: On theflavonoid from the leaves of Torreya Nucifera SIEB. et ZUCC. J. Pharm. Soc. Japan 1958, 78, 1010–1013. [Google Scholar] [CrossRef]
- Konda, Y.; Sasaki, T.; Kagawa, T.; Takayanagi, H.; Harigaya, Y.; Sun, X.-L.; Li, X.; Onda, M. Conformational analysis of C3′-C8connected biflavones. J. Hetrocycl. Chem. 1995, 32, 1531–1535. [Google Scholar] [CrossRef]
- Tachibana, S.; Itoh, K.; Ohkubo, K.; Neil Towers, G.H.N. Leaf flavonoids of Taxus Brevifolia. J. Japan Wood Res. Soc. 1994, 40, 1394–1397. [Google Scholar]
- Olsen, C.E.; Singh, R.; Gupta, S.; Bisht, K.S.; Parmar, V.S. Chemical constituents of Taxus canadensis. Indian J. Chem. 1998, 37, 828–831. [Google Scholar] [CrossRef]
- Parveen, N.; Taufeeq, H.M.; Khan, N.U.D. Biflavones from the leaves of Himalayan yew: Taxus wallichiana. J. Nat. Prod. 1985, 48, 994. [Google Scholar] [CrossRef]
- Qiu, L.G.; Lian, M. Biflavones of Taxus wallichiana Zucc. J. Integr. Plant Biol. 1989, 31, 54–56. [Google Scholar] [CrossRef]
- Zhang, M.L.; Huo, C.H.; Dong, M.; Liang, C.H.; Gu, Y.C.; Shi, Q.W. Non-taxoid chemical constituents from leaves of Taxus mairei. China J. Chin. Mater. Med. 2007, 32, 1421–1425. [Google Scholar]
- Tachibana, S.; Watanabe, E.; Ueno, J.; Tokubuchi, K.; Itoh, K. Isolation of phenylisoserine methyl ester from the leaves of Taxus cuspidatavar. Nana. J. Wood Sci. 2005, 51, 176–180. [Google Scholar] [CrossRef]
- Suh, K.S.; Chon, S.; Jung, W.W.; Choi, E.M. Protective effects of sciadopitysin against methylglyoxal-induced degeneration in neuronal SK-N-MC cells. J. Appl. Toxicol. 2022, 42, 274–284. [Google Scholar] [CrossRef]
- Li, N.; Pan, Z.; Zhang, D.; Wang, H.X.; Yu, B.; Zhao, S.P.; Guo, J.J.; Wang, J.W.; Yao, L.; Cao, W.G. Chemical components, biological activities, and toxicological evaluation of the fruit (Aril) of two precious plant species fromgenus Taxus. Chem. Biodivers. 2017, 14, e1700305. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, L.; Yin, W.; Nie, Y.; Zeng, P.; Yang, X. Anti-tumor effect of ginkgetin on human hepatocellular carcinoma cell lines by inducing cell cycle arrest and promoting cell apoptosis. Cell Cycle 2022, 21, 74–85. [Google Scholar] [CrossRef]
- Rizk, Y.S.; de Jesus Hardoim, D.; Santos, K.B.A.; Zaverucha-do-Valle, T.; Taniwaki, N.N.; Almeida-Souza, F.; Carollo, C.A.; Vannier-Santos, M.A.; de Arruda, C.C.P.; da Silva Calabrese, K. Amentoflavone isolated from Selaginella sellowii Hieron induces mitochondrial dysfunction in Leishmania amazonensis promastigotes. Parasitol. Int. 2022, 86, 102458. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Rabeh, M.A.; Raey, M.A.E.; El-Kadder, E.M.A.; Sobeh, M.; Abdelmohsen, U.R.; Albohy, A.; Andrianov, A.M.; Bosko, I.P.; Al-Sanea, M.M.; et al. Metabolomic profiling of three Araucaria species, and their possible potential role against COVID-19. J. Biomol. Struct. Dyn. 2022, 40, 6426–6438. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J.S.; Kong, L.Y. Chemical constituents from the needles of Taxus canadensis. Chin. J. Nat. Med. 2011, 9, 188–190. [Google Scholar]
- Wei, Q.; Li, S.; Huang, S. Flavonoids of stems of Taxus chinensis var. mairei. Chem. Nat. Compd. 2021, 57, 523–524. [Google Scholar] [CrossRef]
- Shao, N.; Feng, Z.; Li, N. Isoginkgetin inhibits inflammatory response in the fibroblast-like synoviocytes of rheumatoid arthritis by suppressing matrix metallopeptidase 9 expression. Chem. Biol. Drug Des. 2022, 99, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Mijares, M.R.; De Sanctis, J.B. Effects of flavonoids and its derivatives on immune cell responses. Recent Pat. Infla. 2019, 13, 84–104. [Google Scholar] [CrossRef]
- Parmar, V.S.; Jha, A.; Bisht, K.S.; Taneja, P.; Singh, S.K.; Kumar, A.; Poonam, J.R.; Olsen, C.E. Constituents of the yew trees. Phytochemistry 1999, 50, 1267–1304. [Google Scholar] [CrossRef]
- Wang, S.; Yan, Y.; Cheng, Z.; Hu, Y.; Liu, T. Sotetsuflavone suppresses invasion and metastasis in non-small-cell lung cancer A549 cells by reversing EMT via the TNF-α/NF-κB and PI3K/AKT signaling pathway. Cell Death Discov. 2018, 4, 26. [Google Scholar] [CrossRef]
- Parmar, V.S.; Vardhan, A.; Bisht, K.S.; Sharma, N.K.; Jain, R.; Taneja, R.; Tyagi, O.D.; Boll, P.M. A rare biflavone from Taxus baccata. Indian J. Chem. B 1993, 32, 601–603. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J.S.; Kong, L.Y. A new biflavone from needles of Taxus canadensis. China J. Chin. Mater. Med. 2016, 41, 443–445. [Google Scholar] [CrossRef]
- Yang, S.J.; Fang, J.M.; Cheng, Y.S. Lignans, Flavonoids and phenolic derivatives from Taxus Mairei. J. Chin. Chem. Soc. 1999, 46, 811–818. [Google Scholar] [CrossRef]
- Behbahani, M.; Sayedipour, S.; Pourazar, A.; Shanehsazzadeh, M. In vitro anti-HIV-1 activities of kaempferol and kaempferol-7-O-glucoside isolated from Securigera securidaca. Res. Pharm. Sci. 2014, 9, 463. [Google Scholar]
- Vignolini, P.; Gehrmann, B.; Melzig, M.F.; Borsacchi, L.; Scardigli, A.; Romani, A. quality control and analytical test method for Taxus baccata tincture preparation. Nat. Prod. Commun. 2012, 7, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Liana, L.; Rizal, R.; Widowati, W.; Fioni, F.; Akbar, K.; Fachrial, E.; Lister, I.N.E. Antioxidant and anti-hyaluronidase activities of dragon fruit peel extract and kaempferol-3-o-rutinoside. J. Kedokt. Brawijaya 2019, 30, 247–252. [Google Scholar] [CrossRef]
- Peng, S.; Fang, C.; He, H.; Song, X.; Zhao, X.; Zou, Y.; Li, L.; Jia, R.; Yin, Z. Myricetin exerts its antiviral activity against infectious bronchitis virus by inhibiting the deubiquitinating activity of papain-like protease. Poultry Sci. 2022, 101, 101626. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Ferrer, E.; Queralt Regue, J.; Garcia-Sala, X.; Boix Montanes, A.; Lamuela-Raventos, R.M. In vivo anti-inflammatory and antiallergic activity of pure naringenin, naringenin chalcone, and quercetin in mice. J. Nat. Prod. 2019, 82, 177–182. [Google Scholar] [CrossRef]
- Sharma, S.; Dahiya, A.; Kumar, S.; Verma, Y.K.; Dutta, A. Quercetin 3-O-rutinoside prevents radiation induced oxidative damage and inflammation by coordinated regulation of Nrf2/NF-κB/NLRP3-inflammasome signaling in gastrointestine. Phytomed. Plus 2023, 3, 100385. [Google Scholar] [CrossRef]
- Gansukh, E.; Kazibwe, Z.; Pandurangan, M.; Judy, G.; Kim, D.H. Probing the impact of quercetin-7-O-glucoside on influenza virus replication influence. Phytomedicine 2016, 23, 958–967. [Google Scholar] [CrossRef]
- Zhan, Y.; Ta, W.; Tang, W.; Hua, R.; Wang, J.; Wang, C.; Lu, W. Potential antiviral activity of isorhamnetin against SARS-CoV-2 spike pseudotyped virus in vitro. Drug Develop. Res. 2021, 82, 1124–1130. [Google Scholar] [CrossRef]
- Ham, Y.H.; Park, W.G.; Han, S.S.; Bae. Y.S. Flavonoid glycosides from needles of Taxus cuspidata (Taxaceae). J. Korean Wood Sci. Technol. 1997, 25, 45–51. [Google Scholar]
- Tang, P.; Tang, Y.; Liu, Y.; He, B.; Shen, X.; Zhang, Z.J.; Qin, D.L.; Tian, J. Quercetin-3-O-α-L-arabinopyranosyl-(1→2)-β-D-glucopyra-noside isolated from Eucommia ulmoides oliver leaf relieves insulin resistance in HepG2 cells via the IRS-1/PI3K/Akt/GSK-3β pathway. Biol. Pharm. Bull. 2022, b22, 00597. [Google Scholar]
- Jiang, P.; Zhao, Y.; Xiong, J.; Wang, F.; Xiao, L.; Bao, S.; Yu, X. Extraction, purification, and biological activities of flavonoids from branches and leaves of Taxus cuspidata S. et Z. BioResources 2021, 16, 2655–2682. [Google Scholar] [CrossRef]
- Wu, D.; Duan, R.; Tang, L.; Hu, X.; Geng, F.; Sun, Q.; Zhang, Y.; Li, H. Binding mechanism and functional evaluation of quercetin 3-rhamnoside on lipase. Food Chem. 2021, 15, 129960. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Luo, X.J.; Zhang, C.C.; Xu, X.Y.; Yu, C.N.; Jiang, Z.F.; Zhang, L.; Yuan, H.W.; Zheng, B.S.; Pi, E.X.; et al. Comparative metabolomic analysis reveals the variations in taxoids and flavonoids among three Taxus species. BMC Plant Biol. 2019, 19, 529. [Google Scholar] [CrossRef] [PubMed]
- Le Lee, J.; Loe, M.W.; Lee, R.C.; Chu, J.J. Antiviral activity of pinocembrin against Zika virus replication. Antivir. Res. 2019, 167, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.B.; Dalhat, M.H.; Khan, M.K.; Afzal, O.; Altamimi, A.S.; Alzarea, S.I.; Almalki, W.H.; Kazmi, I. Butin mitigates memory impairment in Streptozotocin-Induced diabetic rats by inhibiting oxidative stress and inflammatory responses. Metabolites 2022, 12, 1050. [Google Scholar] [CrossRef]
- Wadhwa, R.; Paudel, K.R.; Chin, L.H.; Hon, C.M.; Madheswaran, T.; Gupta, G.; Panneerselvam, J.; Lakshmi, T.; Singh, S.K.; Gulati, M.; et al. Anti-inflammatory and anticancer activities of Naringenin-loaded liquid crystalline nanoparticles in vitro. J. Food Biochem. 2021, 45, e13572. [Google Scholar] [CrossRef]
- Yoon, J.H.; Youn, K.; Jun, M. Discovery of pinostrobin as a melanogenic agent in cAMP/PKA and p38 MAPK signaling pathway. Nutrients 2022, 14, 3713. [Google Scholar] [CrossRef]
- Rehman, K.; Chohan, T.A.; Waheed, I.; Gilani, Z.; Akash, M.S.H. Taxifolin prevents postprandial hyperglycemia by regulating the activity of α-amylase: Evidence from an in vivo and in silico studies. J. Cell. Biochem. 2019, 120, 425–438. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, E.N.; Jeong, G.S. Aromadendrin protects neuronal cells from methamphetamine-induced neurotoxicity by regulating endoplasmic reticulum stress and PI3K/Akt/mTOR signaling pathway. Int.J. Mol. Sci. 2021, 22, 2274. [Google Scholar] [CrossRef] [Green Version]
- Veselova, M.V.; Fedoreev, S.A.; Vasilevskaya, N.A.; Denisenko, V.A.; Gerasimenko, A.V. Antioxidant activity of polyphenols from the far-east plant Taxus cuspidata. Pharm. Chem. J. 2007, 41, 88–93. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Shen, Z.; Zhou, Q.; Wang, M.F. Identification of the antiglycative components of Hong Dou Shan (Taxus chinensis) leaf tea. Food Chem. 2019, 297, 124942. [Google Scholar] [CrossRef]
- Elbaz, H.A.; Lee, I.; Antwih, D.A.; Liu, J.; Hüttemann, M.; Zielske, S.P. Epicatechin stimulates mitochondrial activity and selectively sensitizes cancer cells to radiation. PLoS ONE 2014, 9, e88322. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.A.; Shin, Y.S.; Kang, S.U.; Kim, J.H.; Oh, Y.T.; Park, K.H.; Lee, B.H.; Kim, C.H. Radioprotective effect of epicatechin in cultured human fibroblasts and zebrafish. J. Radiat. Res. 2014, 55, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Cui, M.; Zheng, C.; Zhang, P.; Ren, S.; Bao, J.; Gao, D.; Sun, R.; Wang, M.; Lin, J.; et al. Both baicalein and gallocatechin gallate effectively inhibit SARS-CoV-2 replication by targeting M pro and sepsis in mice. Inflammation 2022, 45, 1076–1088. [Google Scholar] [CrossRef]
- Fan, J.; Liu, H.; Wang, J.; Zeng, J.; Tan, Y.; Wang, Y.; Yu, X.; Li, W.; Wang, P.; Yang, Z.; et al. Procyanidin B2 improves endothelial progenitor cell function and promotes wound healing in diabetic mice via activating Nrf2. J. CellMol. Med. 2021, 25, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, F.; Ohira, T.; Kikuchi, Y. Constituents from the roots of Taxus cuspidata. J. Wood Sci. 2004, 50, 548–551. [Google Scholar] [CrossRef]
- Ruddock, P.S.; Charland, M.; Ramirez, S.; López, A.; Towers, G.N.; Arnason, J.T.; Liao, M.; Dillon, J.A.R. Antimicrobial activity of flavonoids from Piper lanceaefolium and other Colombian medicinal plants against antibiotic susceptible and resistant strains of Neisseria gonorrhoeae. Sex. Transm. Dis. 2011, 38, 82–88. [Google Scholar] [CrossRef]
- Al-Qahtani, W.H.; Alshammari, G.M.; Ajarem, J.S.; Al-Zahrani, A.Y.; Alzuwaydi, A.; Eid, R.; Yahya, M.A. Isoliquiritigenin prevents Doxorubicin-induced hepatic damage in rats by upregulating and activating SIRT1. Biomed. Pharmacother. 2022, 146, 112594. [Google Scholar] [CrossRef]
- Gao, L.; Cui, S.; Huang, Z.; Cui, H.; Alahmadi, T.A.; Manikandan, V. Antinociceptive and anti-inflammatory activities of butein in different nociceptive and inflammatory mice models. Saudi J. Biol. Sci. 2021, 28, 7090–7097. [Google Scholar] [CrossRef]
- Messi, B.B.; Ndjoko-Ioset, K.; Hertlein-Amslinger, B.; Lannang, A.M.; Nkengfack, A.E.; Wolfender, J.L.; Hostettmann, K.; Bringmann, G. Preussianone, Anew flavanone-chromone biflavonoid from Garcinia preussii. Engl. Mol. 2021, 17, 6014–6025. [Google Scholar] [CrossRef] [Green Version]
- Hollman, P.C.; Arts, I.C. Flavonols, Flavones and flavanols-nature, occurrence and dietary burden. J. Sci. Food Agr. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Raut, N.A.; Dhore, P.W.; Saoji, S.D.; Kokare, D.M. Selected Bioactive Natural Products for Diabetes Mellitus. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 48, pp. 287–322. [Google Scholar]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Stobiecki, M.; Kachlicki, P. Isolation and identification of flavonoids. In The Science of Flavonoids; Grotewold, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 47–69. [Google Scholar]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.Y.; Shi, F.X.; Gao, X. Preliminary phytochemical analysis of Acanthopanan trifoliatus (L.) Merr. J. Med. Plants Res. 2011, 5, 4059–4064. [Google Scholar]
- Dai, X.; Shi, X.; Yang, C.; Zhao, X.; Zhuang, J.; Liu, Y.; Gao, L.; Xia, T. Two UDP-glycosyltransferases catalyze the biosynthesis of bitter flavonoid 7-o-neohesperidoside through sequential glycosylation in tea plants. J. Agr. Food Chem. 2022, 70, 2354–2365. [Google Scholar] [CrossRef]
- Du Preez, B.V.P. Cyclopia Maculata: Source of Flavanone Glycosides as Precursors for Taste Modulating Aglycones. Doctoral dissertation, Stellenbosch University, Stellenbosch, South Africa, 2014.
- Roselló-Soto, E.; Barba, F.J.; Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Moltó, J.C. Phenolic profile of oils obtained from “horchata” by-products assisted by supercritical-CO2 and its relationship with antioxidant and lipid oxidation parameters: Triple TOF-LC-MS-MS characterization. Food Chem. 2019, 274, 865–871. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Tonato, D.; Quadros, M.M.; Boeira, C.P.; Cichoski, A.J.; Terra, L.M.; Kuhn, R.C. Ultrasound extraction of bioactive compounds from Citrus reticulata peel using electrolyzed water. J. Food Proc. Preserv. 2019, 43, e14236. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of current extraction techniques for flavonoids from plant materials. Processes 2020, 8, 1222. [Google Scholar] [CrossRef]
- Fomo, G.; Madzimbamuto, T.N.; Ojumu, T.V. Applications of nonconventional green extraction technologies in process industries: Challenges, limitations and perspectives. Sustainability 2020, 12, 5244. [Google Scholar] [CrossRef]
- Henrique, P.; Helena, D.; Ribeiro, B.; Amadeu, G.; Vitali, L.; Hense, H. Extraction of bioactive compounds from feijoa (Acca sellowiana (O. Berg) Burret) peel by low and high-pressure techniques. J. Supercrit. Fluids. 2019, 145, 219–227. [Google Scholar] [CrossRef]
- Ekalu, A.; Habila, J.D. Flavonoids: Isolation, characterization, and health benefits. Beni-Suef U. J. Basic 2020, 9, 45. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Contents and antibacterial activity of flavonoids extracted from leaves of Psidium guajava. J. Med. Plant Res. 2010, 4, 393–396. [Google Scholar] [CrossRef]
- Lobo, R.O.; Dias, F.O.; Shenoy, C.K. Kombucha for healthy living: Evaluation of antioxidant potential and bioactive compounds. Int. Food Res. J. 2017, 24, 541–546. [Google Scholar]
- Chen, C.; Liu, F.; Zhao, J.; Chen, T.; Li, Y.; Zhang, D. Efficient separation of five flavonoids from Oxytropis falcata Bunge by high-speed counter-current chromatography and their anticancer activity. Acta Chromatogr. 2020, 32, 189–193. [Google Scholar] [CrossRef]
- Süzgeç-Selçuk, S.; Birteksöz, A.S. Flavonoids of Helichrysum chasmolycicum and its antioxidant and antimicrobial activities. S. Afr. J. Bot. 2011, 77, 170–174. [Google Scholar] [CrossRef]
- Yang, L.; Zheng, Z.S.; Cheng, F.; Ruan, X.; Jiang, D.A.; Pan, C.-D.; Wang, Q. Seasonal dynamics of metabolites in needles of Taxus wallichiana var. mairei. Molecules 2016, 21, 1403. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.Q.; Yu, Z.Y. Comparison study on total flavonoid content and anti-free redical activity of the leaves of bamboo, Phyllostachys nigra, and Ginkgo bilabo. China J. Chin. Mater. Med. 2002, 27, 254–257. [Google Scholar]
- Tang, W.; Hazebroek, J.; Zhong, C.; Harp, T.; Vlahakis, C.; Baumhover, B.; Asiago, V. Effect of genetics, environment, and phenotype on the metabolome of maize hybrids using GC/MS and LC/MS. J. Agric. Food Chem. 2017, 65, 5215–5225. [Google Scholar] [CrossRef]
- Aversano, R.; Contaldi, F.; Adelfi, M.G.; D’Amelia, V.; Diretto, G.; De Tommasi, N.; Vaccaro, C.; Vassallo, A.; Carputo, D. Comparative metabolite and genome analysis of tuber-bearing potato species. Phytochemistry 2017, 137, 42–51. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, F.J.; Zhuang, W.B.; Shu, X.C.; Wang, Z. Metabolic variations of flavonoids in leaves of T. media and T. mairei obtained byUPLC-ESI-MS/MS. Molecules 2019, 24, 3323. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Cisowski, W.; Wiwart, M.; Madziar, B. Antifungal biflavones from Cupressocyparis leylandii. Planta Med. 1999, 65, 572–573. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, P. Les Cupressales: Une définitionchimiosystématique. Candollea 1982, 37, 243–256. [Google Scholar]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in metabolic diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef]
- Gu, Q.; Li, Y.P.; Chen, Y.C.; Yao, P.F.; Ou, T.M. Sciadopitysin: Active component from Taxus Chinensis for anti-alzheimer’s disease. Nat. Prod. Res. 2013, 27, 2157–2160. [Google Scholar] [CrossRef]
- Sarmah, S.; Roy, A.S. A Review on Prevention of Glycation of proteins: Potential therapeuticsubstances to mitigate the severity of diabetes complications. Int. J. Biol. Macromol. 2022, 195, 565–588. [Google Scholar] [CrossRef]
- Maher, P.; Dargusch, R.; Ehren, J.L.; Okada, S.; Sharma, K.; Schubert, D. Fisetin lowers methylglyoxal dependent protein glycation and limits the complications of diabetes. PLoS ONE 2011, 6, e21226. [Google Scholar] [CrossRef]
- Liu, J.C.; Jiao, Z.G.; Wang, S.X. The Inhibitory effect of polyphenols extract from apple on α-amylase and α-glucosidase. J. Fruit Sci. 2011, 28, 553–557. [Google Scholar] [CrossRef]
- Lv, P.; Yu, J.; Xu, X.; Lu, T.; Xu, F. Eriodictyol inhibits high glucose-induced oxidative stress and inflammation in retinal ganglial cells. J. Cell. Biochem. 2019, 120, 5644–5651. [Google Scholar] [CrossRef]
- Li, J.F.; Cai, D.K.; Bi, H.C.; Jin, J.; Huang, M. Effects of flavonoids derived from Taxus yunnanensis on p-glycoprotein and cytochrome P450 3A4. Asian J. Pharm. Sci. 2013, 8, 168–173. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Alsahli, M.A.; Almatroudi, A.; Almogbel, M.A.; Khan, A.A.; Anwar, S.; Almatroodi, S.A. The potential role of apigenin in cancer prevention and treatment. Molecules 2022, 27, 6051. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kumar, V.; Tiwari, R.K.; Ravidas, V.; Pandey, K.; Kumar, A. Amphotericin B: A drug of choice for Visceral Leishmaniasis. Acta Tropica 2022, 2022, 106661. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Yang, N.; Wang, F.; Zhao, G.; Gao, H.; Li, Y. Epidemiology of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Disaster Med. Public. 2020, 14, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhu, J.; Wang, L.; Jing, H.; Ma, C.; Kou, X.; Wang, H. Inhibitory mechanisms and interaction of tangeretin, 5-demethyltangeretin, nobiletin, and 5- demethylnobiletin from citrus peels on pancreatic lipase: Kinetics, spectroscopies, and molecular dynamics simulation. Int. J. Biol. Macromol. 2020, 164, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
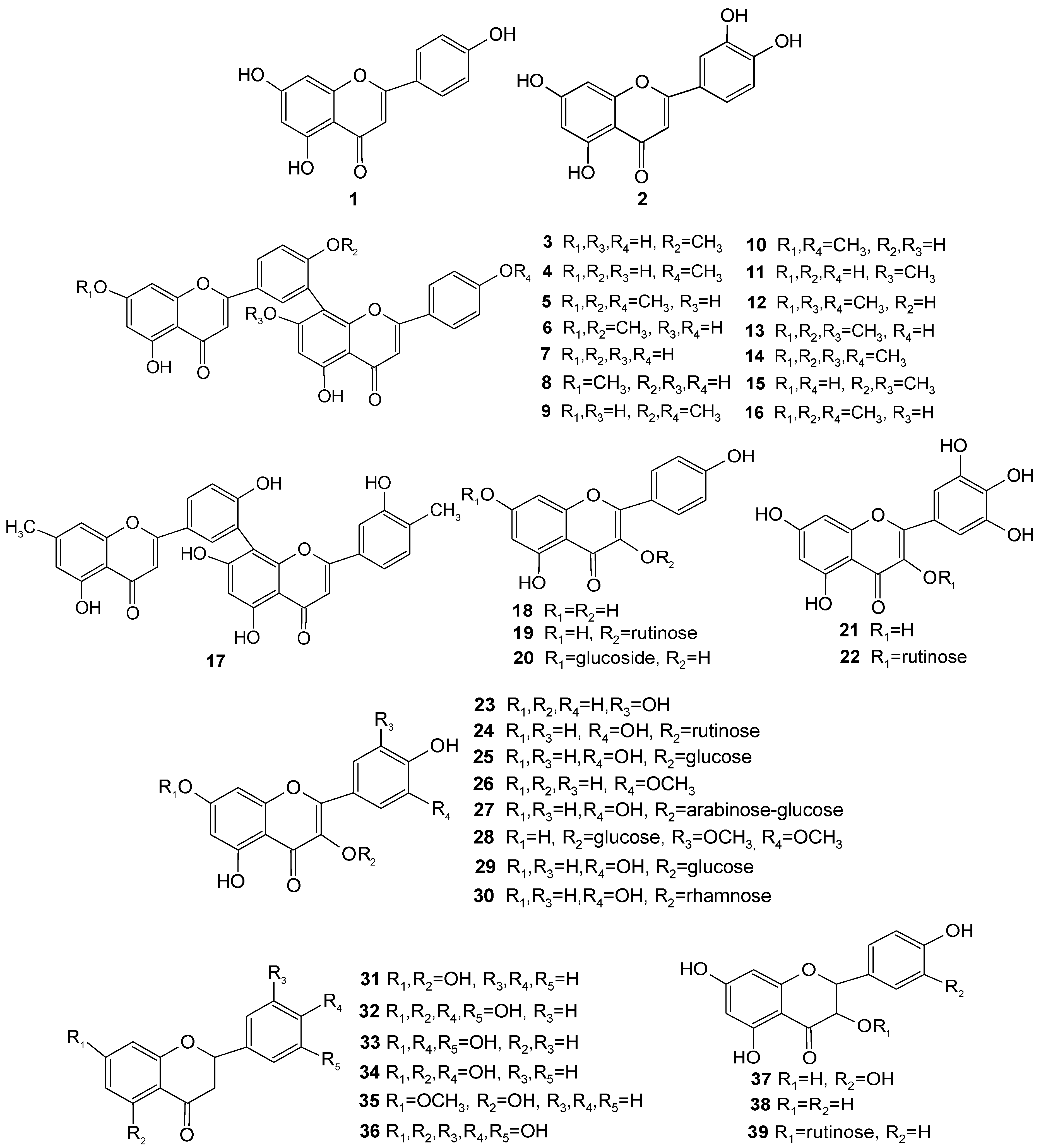

| No. | Type | Compound | Molecular Formula | Molecular Weight(Da) | Species (Part) | Bioactivity |
|---|---|---|---|---|---|---|
| 1 | Flavone | Apigenin | C15H10O5 | 270.24 | T. fuana, T. yunnanensis (twigs); T. baccata (needles) [7,8] | Antioxidant, anticancer, antidepressant, anti-inflammatory activities [9,10,11] |
| 2 | Flavone | Luteolin | C15H10O6 | 286.24 | T. fuana, T. yunnanensis (twigs) [7] | Antioxidant, anticancer, anti-inflammatory activities [9,10] |
| 3 | Biflavone | Bilobetin | C31H20O10 | 552.48 | T. baccata, T. celebica (needles); T. chinensis, T. cuspidata, T. media (twigs, leaves) [12,13] | Antibacterial activities [12] |
| 4 | Biflavone | 4′′′-O-Methylamentoflavone(or podocarpusflavone-A) | C31H20O10 | 552.48 | T. baccata, T. media (needles) [9,14] | Anticancer activities [15] |
| 5 | Biflavone | Sciadopitysin | C33H24O10 | 580.54 | T. baccata, T. media, T. celebica (needles, leaves); T. cuspidata (twigs, bark, leaves, branches); T. brevifolia (leaves); T. canadensis (leaves, twigs); T. wallichiana (leaves); T. mairei (leaves); T. chinensis, T.media (twigs, leaves); T. cuspidate var. nana (leaves) [4,12,13,16,17,18,19,20,21,22,23,24,25,26] | Anti-Alzheimer’s disease, antibacterial, neuronal protective activities [12,27] |
| 6 | Biflavone | Ginkgetin | C32H22O10 | 566.51 | T. baccata (needles, leaves), T. cuspidata (twigs, bark, leaves, branches); T. canadensis (leaves, twigs); T. chinensis var. mairei, T. media (fruits); T. chinensis, T. media (twigs, leaves, fruits); T. wallichiana (leaves); T. fuana, T. yunnanensis (twigs); T. cuspidate var. nana (leaves) [4,7,12,13,16,17,18,19,20,22,23,24,26,28] | Antibacterial, anticancer activities [12,29] |
| 7 | Biflavone | Amentoflavone | C30H18O10 | 538.46 | T. baccata (needles); T. wallichiana(leaves); T. fuana, T. yunnanensis (twigs) [7,12,23,24] | Antibacterial, antileishmaniasis, antivirus activities [12,30,31] |
| 8 | Biflavone | Sequoiaflavone | C31H20O10 | 552.48 | T. baccata (needles, leaves); T. media (needles); T. wallichiana (leaves); T. canadensis (needles); T. mairei, T. chinensis (leaves) [4,12,14,16,23,24,25,32] | Antibacterial activities [12] |
| 9 | Biflavone | Isoginkgetin | C32H22O10 | 566.51 | T. chinensis, T. cuspidata, T. media (twigs, leaves); T. chinensis var. mairei (twigs) [13,33] | Anti-inflammatory activities [34] |
| 10 | Biflavone | Putraflavone | C32H22O10 | 566.51 | T. canadensis (needles); T. chinensis var. mairei (twigs) [32,33] | Anti-inflammatory activities [35] |
| 11 | Biflavone | Sotetsuflavone | C31H20O10 | 552.48 | T. baccata (not mentioned); T. cuspidata (leaves) [19,36] | Anticancer activities [37] |
| 12 | Biflavone | Kayaflavone | C33H24O10 | 580.54 | T. cuspidata (leaves); T. baccata (needles) [17,19,36] | Antivirus activities [31] |
| 13 | Biflavone | 4′,7,7″-Tri-O-methyl amentoflavone | C33H24O10 | 580.54 | T. baccata (leaves) [36,38] | Not reported |
| 14 | Biflavone | 4′,4″,7,7″-Tetra-O-methyl amentoflavone | C34H26O10 | 594.56 | T. baccata (needles, leaves) [36,38] | Not reported |
| 15 | Biflavone | 4′,7″-Di-O-methyl amentoflavone | C32H22O10 | 566.51 | T. baccata (needles) [36] | Not reported |
| 16 | Biflavone | 4″-O-methyl ginkgetin | C33H24O10 | 580.54 | T. chinensis var. mairei, T. media (fruits) [28] | Not reported |
| 17 | Biflavone | 3″-hydroxy-4″,7-dimethyl amentoflavone | C32H22O9 | 550.51 | T. canadensis (needles) [39] | Not reported |
| 18 | Flavonol | Kaempferol | C15H10O6 | 286.24 | T. brevifolia (leaves); T. baccata (needles); T. fuana, T. yunnanensis (twigs); T. mairei (twigs) [7,14,21,40] | Antioxidant, antivirus, anti-inflammatory activities [9,41] |
| 19 | Flavonol glycoside | Kaempferol-3-O-rutinoside | C27H30O15 | 594.52 | T. baccata (needles, twigs); T. chinensis var. mairei (twigs) [14,33,42] | Antioxidant activities [43] |
| 20 | Flavonol glycoside | Kaempferol-7-O-glucoside | C21H20O11 | 448.38 | T. baccata (needles); T. chinensis var. mairei (twigs) [14,33] | Antivirus activities [41] |
| 21 | Flavonol | Myricetin | C15H10O8 | 318.23 | T. baccata (needles) [14] | Antivirus activities [44] |
| 22 | Flavonol glycoside | Myricetin-3-O-rutinoside | C27H30O17 | 626.52 | T. baccata (needles) [14] | Not reported |
| 23 | Flavonol | Quercetin | C15H10O7 | 302.24 | T. brevifolia (leaves); T. cuspidate (bark, leaves); T. baccata (needles, twigs); T. fuana, T. yunnanensis (twigs); T. chinensis, T. cuspidata, T. media (twigs, leaves); T. mairei (twigs); T. chinensis var. mairei (twigs); T. cuspidate var. nana (leaves) [7,13,14,18,21,26,33,40,42] | Antioxidant, anti-inflammatory, antiallergic activities [9,45] |
| 24 | Flavonol glycoside | Quercetin-3-O-rutinoside (or rutin) | C27H30O16 | 610.52 | T. baccata (needles or leaves, twigs); T. chinensis var. mairei (twigs) [14,33,42] | Anticancer activities [46] |
| 25 | Flavonol glycoside | Quercetin-7-O-glucoside | C21H20O12 | 464.38 | T. baccata (needles) [14] | Antivirus activities [47] |
| 26 | Flavonol | Isorhamnetin | C16H12O7 | 316.26 | T. brevifolia (leaves); T. cuspidate (bark, leaves); T. cuspidate var. nana (leaves); T. baccata (needles) [8,18,21,26] | Antivirus activities [48] |
| 27 | Flavonol glycoside | Quercetin-3-O-α-L-arabinopyranosyl-(1′′′→6”)-β-D-glucopyranoside | C26H28O16 | 596.49 | T. cuspidata (needles) [49] | Antidiabetes activities [50] |
| 28 | Flavonol glycoside | Tricin-3-O-glucoside | C23H24O13 | 508.43 | T. chinensis var. mairei, T. media (fruits) [28] | Not reported |
| 29 | Flavonol glycoside | Quercetin-3-O-glucoside | C21H20O12 | 464.38 | T. cuspidata (branches, leaves); T. chinensis, T. cuspidata, T. media (twigs, leaves) [13,51] | Anticancer activities [51] |
| 30 | Flavonol glycoside | Quercetin 3-rhamnoside | C21H20O11 | 448.38 | T. chinensis, T. cuspidata, T. media (twigs, leaves) [13] | Antilipase activities [52] |
| 31 | Dihydroflavone | Pinocembrin | C15H12O4 | 256.25 | T. mairei (twigs) [53] | Antivirus activities [54] |
| 32 | Dihydroflavone | Eriodictyol | C15H12O6 | 288.25 | T. mairei (twigs) [53] | Antidiabetes activities [55] |
| 33 | Dihydroflavone | Butin | C15H12O5 | 272.25 | T. mairei (twigs) [53] | Antidiabetes activities [55] |
| 34 | Dihydroflavone | Naringenin | C15H12O5 | 272.25 | T. media, T. cuspidata (twigs); T. chinensis var. mairei (twigs) [33,53] | Anticancer, anti-inflammatory, antiallergic activities [45,56] |
| 35 | Dihydroflavone | Pinostrobin | C16H14O4 | 270.28 | T. media, T. cuspidata (twigs) [53] | Promotion of melanogenesis [57] |
| 36 | Dihydroflavone | Dihydrotricetin | C15H12O7 | 304.25 | T. media, T. cuspidata (twigs) [53] | Not reported |
| 37 | Dihydroflavonol | Taxifolin | C15H12O7 | 304.25 | T. baccata (needles) [8] | Antidiabetes activities [58] |
| 38 | Dihydroflavonol | Aromadendrin | C15H12O6 | 288.25 | T. chinensis var. mairei, T. media (fruits) [28] | Neuronal protective activities [59] |
| 39 | Dihydroflavonol glycoside | Aromadendrin-3-O-rutinoside | C27H32O15 | 596.53 | T. chinensis var. mairei, T. media (fruits) [28] | Not reported |
| 40 | Flavanol | 5-deoxyleucopelargonidin | C15H14O5 | 274.27 | T. media (twigs) [53] | Not reported |
| 41 | Flavanol | Leucopelargonidin | C15H14O6 | 290.27 | T. media (twigs); T. chinensis var. mairei, T. media (fruits) [24,53] | Not reported |
| 42 | Flavanol | Leucocyanidin | C15H14O7 | 306.27 | T. media (twigs) [53] | Not reported |
| 43 | Flavanol | (+)-Catechin | C15H14O6 | 290.27 | T. cuspidata (needles, wood, roots); T. fuana, T. yunnanensis (twigs); T. chinensis (leaves) [7,49,60,61] | Antioxidant, antidiabetes, anticanceractivities [3,51,60,61] |
| 44 | Flavanol | (-)-Epicatechin | C15H14O6 | 290.27 | T. cuspidata (needles, wood, roots); T. fuana, T. yunnanensis (twigs); T. chinensis (leaves) [7,49,60,61] | Antioxidant, antidiabetes, anticanceractivities [51,60,61,62,63] |
| 45 | Flavanol | Gallocatechin | C15H14O7 | 306.27 | T. chinensis (leaves) [61] | Antidiabetes, antivirus activities [61,64] |
| 46 | Flavanol | Epigallocatechin | C15H14O7 | 306.27 | T. chinensis (leaves) [61] | Antidiabetes activities [61] |
| 47 | Flavanol | (+)-Catechin pentaacetate | C25H24O11 | 500.45 | T. mairei (twigs) [40] | Not reported |
| 48 | Flavanol | (-)-Epicatechin pentaacetate | C25H24O11 | 500.45 | T. mairei (twigs) [40] | Not reported |
| 49 | Flavanol | Procyanidin B2 | C30H26O12 | 578.52 | T. chinensis (leaves) [61] | Antidiabetes activities [51,65] |
| 50 | Biflavanol | Procyanidin B-2 decaacetate | C50H46O22 | 998.89 | T. mairei (twigs) [40] | Not reported |
| 51 | Biflavanol | Procyanidin B-3-decaacetate | C50H46O22 | 998.89 | T. mairei (twigs) [40] | Not reported |
| 52 | Biflavanol | Procyanidin B-4-decaacetate | C50H46O22 | 998.89 | T. mairei (twigs) [40] | Not reported |
| 53 | Biflavanol | Afzelechin-(4α→8)-afzelechin | C30H26O10 | 546.52 | T. cuspidata (roots) [66] | Not reported |
| 54 | Biflavanol | Afzelechin-(4α→8)-afzelechin octaacetate | C46H42O18 | 882.81 | T. cuspidata (roots) [66] | Not reported |
| 55 | Chalcone | Pinocembrin chalcone(or 2′,4′,6′-trihydroxychalcone) | C15H12O4 | 256.25 | T. mairei (twigs) [53] | Antibacterial activities [67] |
| 56 | Chalcone | Isoliquiritigenin(or 2′,4′,4′-trihydroxy chalcone) | C15H12O4 | 256.25 | T. media (twigs) [53] | Hepatic-protective activities [68] |
| 57 | Chalcone | Butein | C15H12O5 | 272.25 | T. media (twigs) [53] | Anti-inflammatory, antinociceptive activities [69] |
| 58 | Chalcone | Homoeriodictyol chalcone | C16H14O6 | 302.28 | T. media (twigs) [53] | Not reported |
| 59 | Chalcone | Naringenin chalcone | C15H12O5 | 272.25 | T. media, T. cuspidata (twigs) [53] | Anti-inflammatory activities [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Li, Q.-Z.; Wang, R.-L. Flavonoid Components, Distribution, and Biological Activities in Taxus: A review. Molecules 2023, 28, 1713. https://doi.org/10.3390/molecules28041713
Wei Q, Li Q-Z, Wang R-L. Flavonoid Components, Distribution, and Biological Activities in Taxus: A review. Molecules. 2023; 28(4):1713. https://doi.org/10.3390/molecules28041713
Chicago/Turabian StyleWei, Qiang, Qi-Zhao Li, and Rui-Lin Wang. 2023. "Flavonoid Components, Distribution, and Biological Activities in Taxus: A review" Molecules 28, no. 4: 1713. https://doi.org/10.3390/molecules28041713
APA StyleWei, Q., Li, Q.-Z., & Wang, R.-L. (2023). Flavonoid Components, Distribution, and Biological Activities in Taxus: A review. Molecules, 28(4), 1713. https://doi.org/10.3390/molecules28041713





