Enhanced Activity of Alkali-Treated ZSM-5 Zeolite-Supported Pt-Co Catalyst for Selective Hydrogenation of Cinnamaldehyde
Abstract
:1. Introduction
2. Results and Discussion
2.1. Textural and Structural Properties of the Alkali-Treated ZSM-5-Supported Catalysts
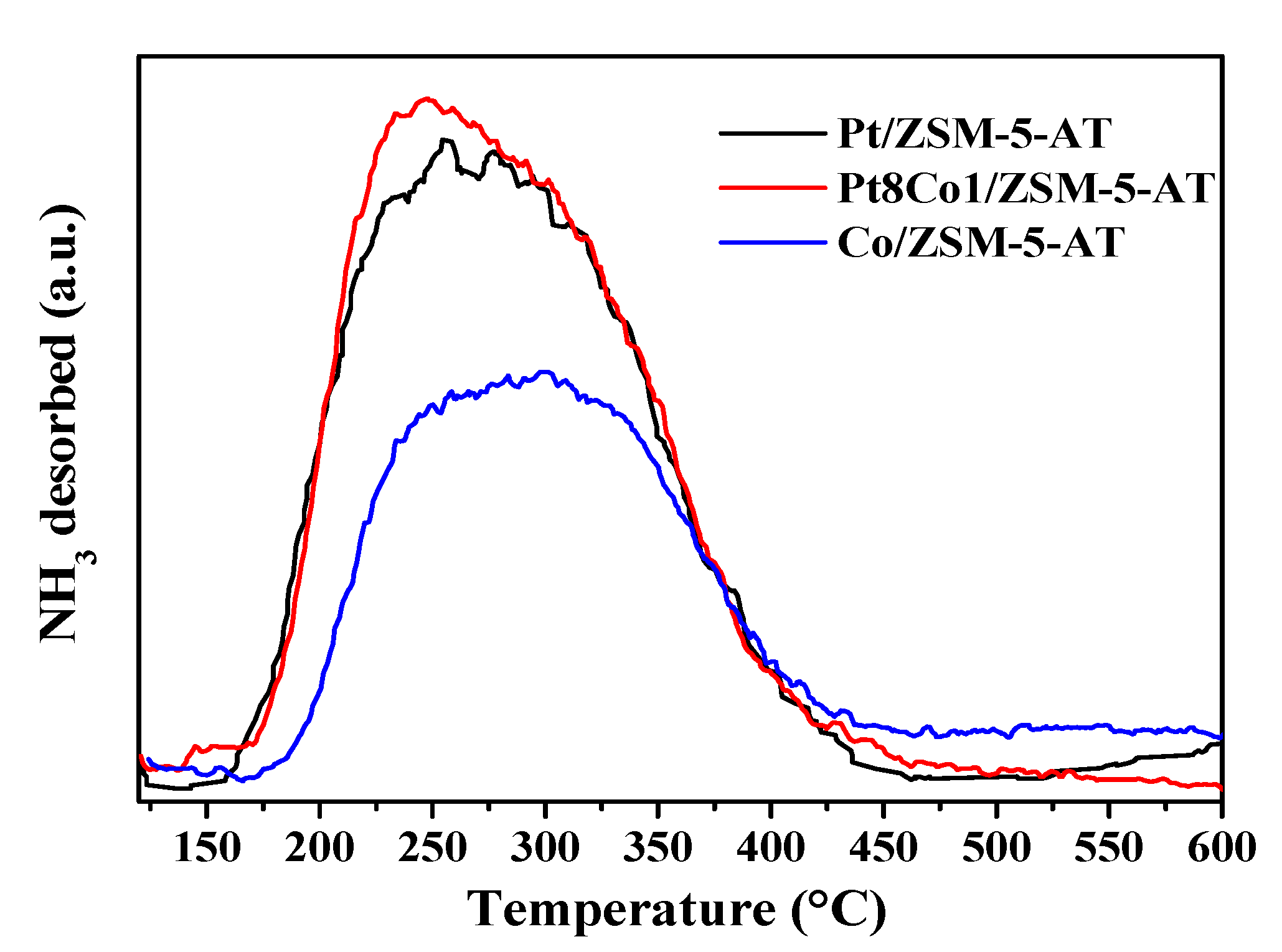
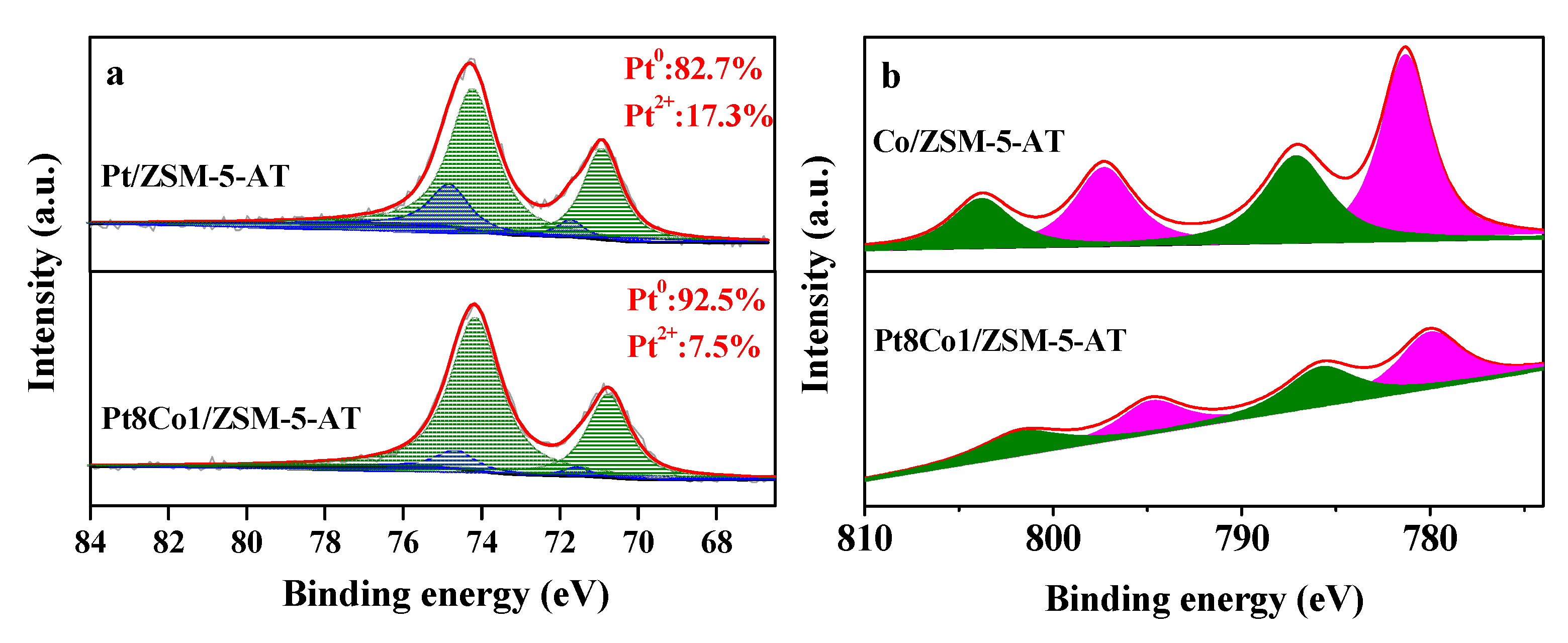
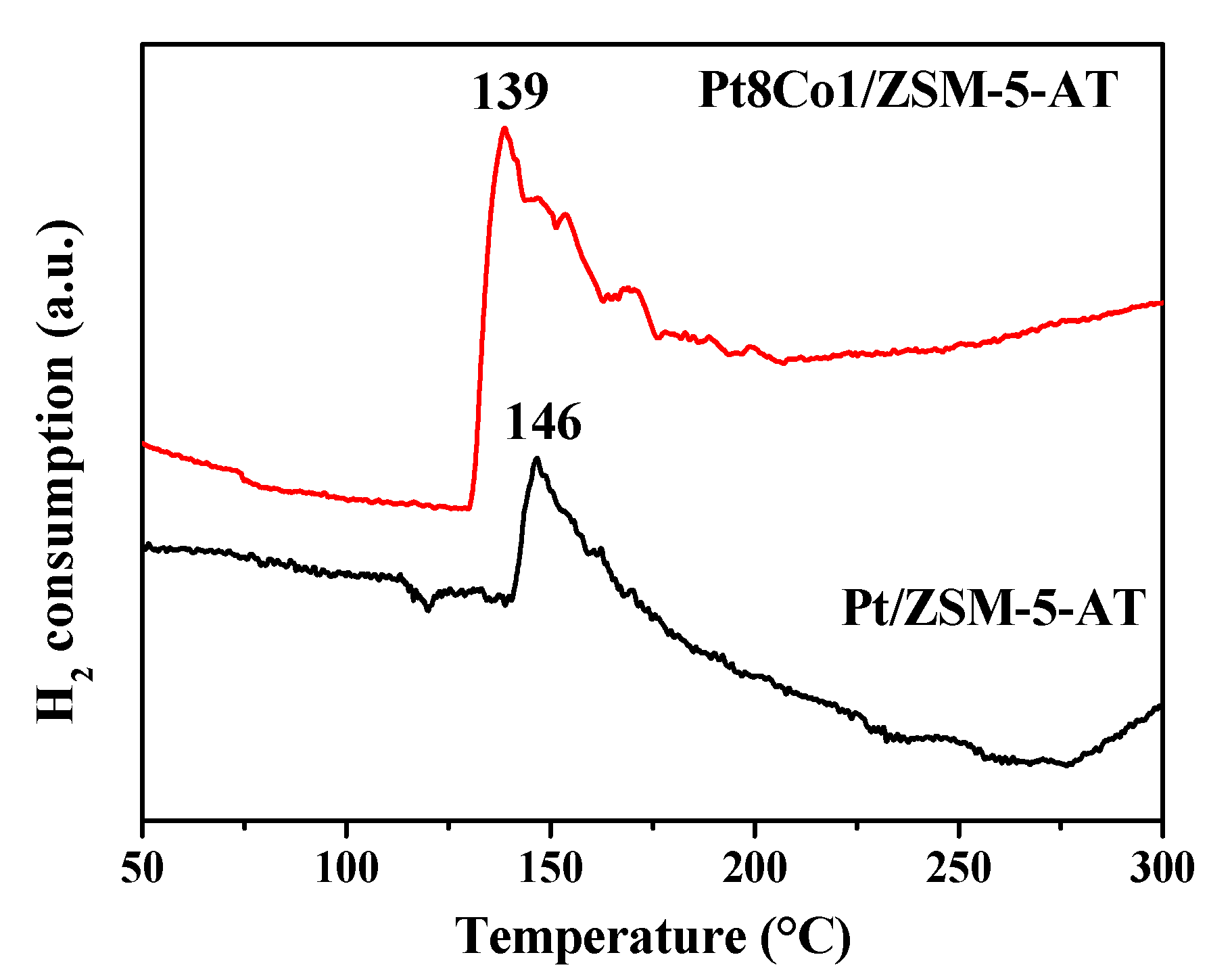
2.2. Surface Properties
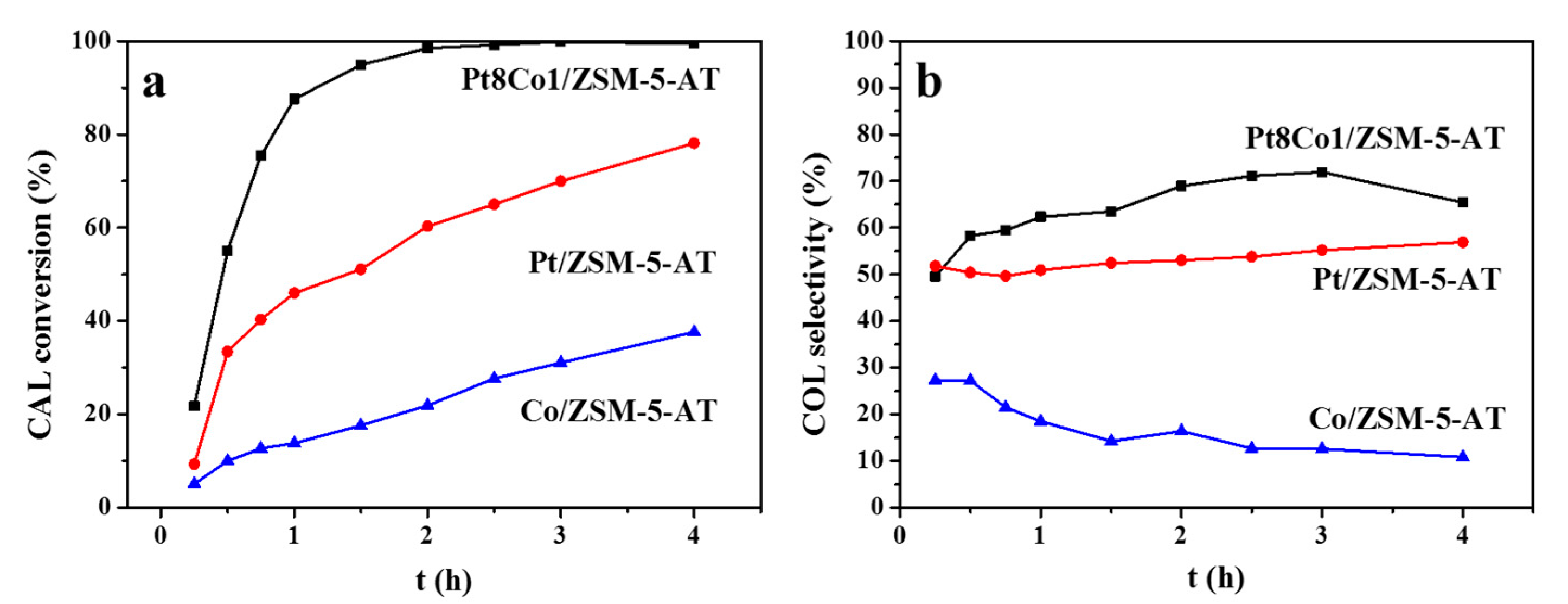
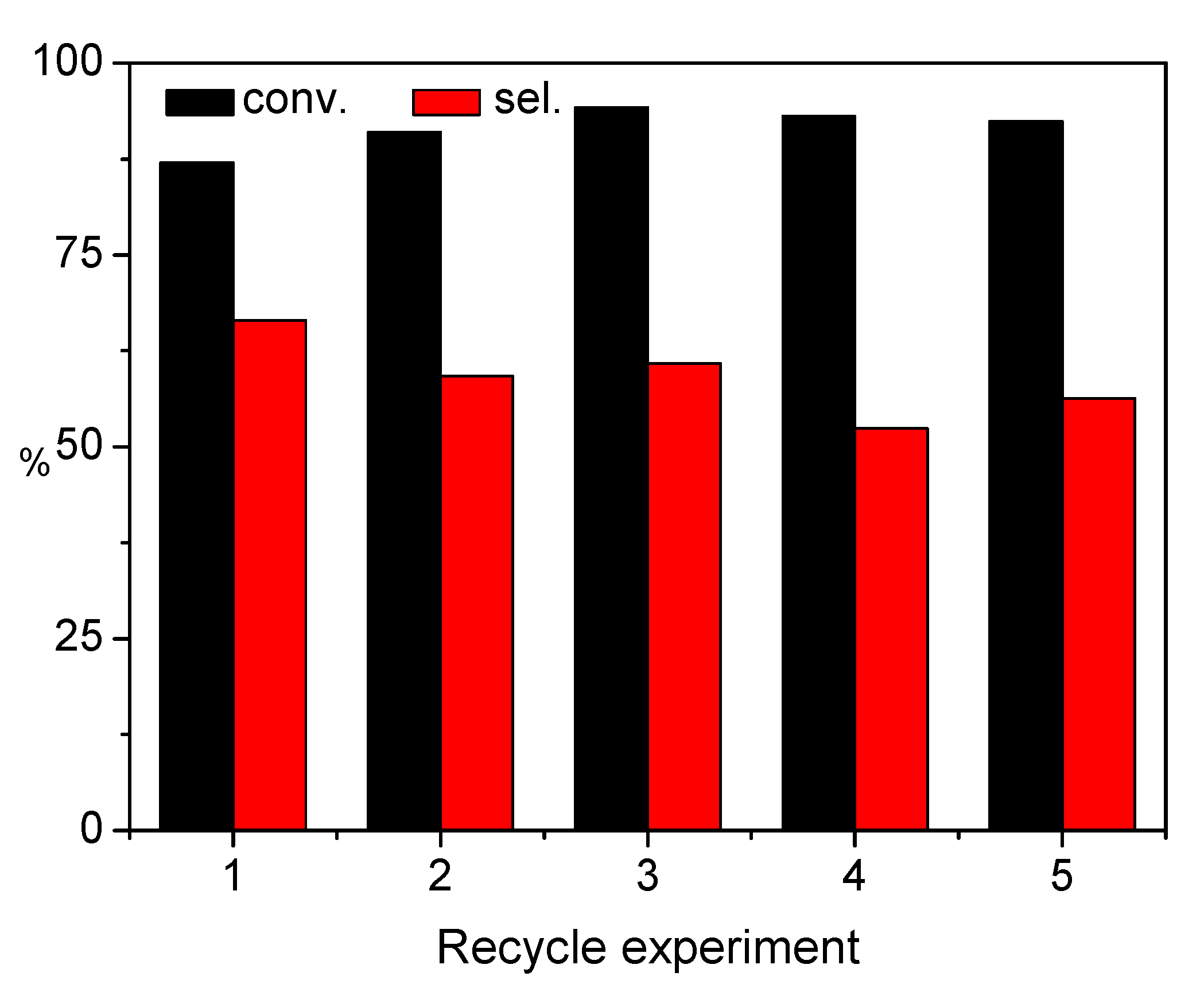
2.3. Chemoselective Hydrogenation of COL
2.4. Tentative Reaction Mechanism
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalyst Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gallezot, P.; Richard, D. Selective hydrogenation of α,β-unsaturated aldehydes. Catal. Rev. 1998, 40, 81–126. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, Z.; Sharma, S.; Li, G. Recent progress in heterogeneous catalysis over atomically and structurally precise metal nanoclusters. Chem. Rec. 2021, 21, 879–892. [Google Scholar] [CrossRef]
- Luneau, M.; Lim, J.S.; Patel, D.A.; Sykes, E.C.H.; Friend, C.M.; Sautet, P. Guidelines to achieving high selectivity for the hydrogenation of α,β-unsaturated aldehydes with bimetallic and dilute alloy catalysts: A review. Chem. Rev. 2020, 120, 12834–12872. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, X.; Geng, P.; Li, Q. Recent advances in selective hydrogenation of cinnamaldehyde over supported metal-based catalysts. ACS Catal. 2020, 10, 2395–2412. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, S.; Xu, Y.; Yu, D. Catalytic selective hydrogenation of cinnamaldehyde to hydrocinnamaldehyde. Appl. Catal. A 2000, 192, 247–251. [Google Scholar] [CrossRef]
- Lan, X.; Wang, T. Highly selective catalysts for the hydrogenation of unsaturated aldehydes: A review. ACS Catal. 2020, 10, 2764–2790. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Zhou, Y.; Li, G.; Li, Z.; Liu, C.; Bao, M.; Shen, W. Selective Hydrogenation of C=C bond in α, β–Unsaturated Aldehydes and Ketones over Pd-Au Clusters of 1.8 nm. Nanoscale 2016, 8, 18626–18629. [Google Scholar] [CrossRef]
- Lv, Y.; Han, M.; Gong, W.; Wang, D.; Chen, C.; Wang, G.; Zhang, H.; Zhao, H. Fe-Co Alloyed Nanoparticles Catalyzing Efficient Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol in Water. Angew. Chem. Int. Ed. 2020, 59, 23521–23526. [Google Scholar] [CrossRef]
- Wang, L.; Han, R.; Ma, Y.; Duyar, M.S.; Liu, W.; Liu, J. Spatial location and microenvironment engineering of Pt-CeO2 nanoreactors for selective hydrogenation of cinnamaldehyde to cinnamyl alcohol. J. Phys. Chem. C 2021, 125, 22603–22610. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.; Zhang, L.; Liu, F.; Wang, A.; Zhang, T. Producing of cinnamyl alcohol from cinnamaldehyde over supported gold nanocatalyst. Chin. J. Catal. 2021, 42, 470–481. [Google Scholar] [CrossRef]
- Zhao, J.; Malgras, V.; Na, J.; Liang, R.; Cai, Y.; Kang, Y.; Alshehri, A.A.; Alzahranie, K.A.; Alghamdi, Y.G.; Asahi, T.; et al. Magnetically induced synthesis of mesoporous amorphous CoB nanochains for efficient selective hydrogenation of cinnamaldehyde to cinnamyl alcohol. Chem. Eng. J. 2020, 398, 125564. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Lei, H.; Chen, J.; Wang, S.; Chen, C. Combining shell confinement and outside decoration to boost the selectivity of Pt nanocatalysts in hollow-flower like Zr-MOFs double shell micro-reactor for hydrogenation cinnamaldehyde to unsaturated alcohol. Appl. Surf. Sci. 2022, 599, 153899. [Google Scholar] [CrossRef]
- Geng, R.; Jia, H.; Xie, Y.; Pan, D.; Yu, F.; Fan, B. Influence of the defects on selective hydrogenation of cinnamaldehyde to cinnamyl alcohol over UiO-66 supported Pt catalysts. Micro. Meso. Mat. 2022, 338, 111968. [Google Scholar] [CrossRef]
- Tao, R.; Shan, B.Q.; Sun, H.D.; Ding, M.; Xue, Q.; Jiang, J.; Wu, P.; Zhang, K. Surface Molecule Manipulated Pt/TiO2 Catalysts for Selective Hydrogenation of Cinnamaldehyde. J. Phys. Chem. C 2021, 125, 13304–13312. [Google Scholar] [CrossRef]
- Giroir-Fendler, A.; Richard, D.; Gallezot, P. Chemioselectivity in the catalytic hydrogenation of cinnamaldehyde: Effect of metal particle morphology. Catal. Lett. 1990, 5, 175–181. [Google Scholar] [CrossRef]
- Zhu, Y.; Zaera, F. Selectivity in the catalytic hydrogenation of cinnamaldehyde promoted by Pt/SiO2 as a function of metal nanoparticle size. Catal. Sci. Technol. 2014, 4, 955–962. [Google Scholar] [CrossRef]
- Durndell, L.J.; Parlett, C.; Hondow, N.S.; Isaacs, M.A.; Wilson, K.; Lee, A.F. Selectivity control in Pt-catalyzed cinnamaldehyde hydrogenation. Sci. Rep. 2015, 5, 9425. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Chen, W.; Xu, W.; Han, Z.; Waheed, A.; Ye, Z.; Li, G.; Baiker, A. Continuous Dimethyl Carbonate Synthesis from CO2 and Methanol over BixCe1-xOδ Monoliths: Effect of Bismuth Doping on Population of Oxygen Vacancies, Activity, and Reaction Pathway. Nano Res. 2022, 15, 1366–1374. [Google Scholar] [CrossRef]
- Wang, G.; Xin, H.; Wang, Q.; Wu, P.; Li, X. Efficient liquid-phase hydrogenation of cinnamaldehyde to cinnamyl alcohol with a robust PtFe/HPZSM-5 catalyst. J. Catal. 2020, 382, 1–12. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, X.; Shi, Q.; Li, J.; Ping, G.; Xu, H.; Ding, H.; Li, G. Synergistic Effects of PtFe/CeO2 Catalyst afford high Catalytic Performance in selective hydrogenation of cinnamaldehyde. J. Rare Earths 2022, 40. [Google Scholar] [CrossRef]
- Zhang, N.; Shao, Q.; Wang, P.; Zhu, X.; Huang, X. Porous Pt-Ni Nanowires within In Situ Generated Metal-Organic Frameworks for Highly Chemoselective Cinnamaldehyde Hydrogenation. Small 2018, 14, 1704318. [Google Scholar] [CrossRef] [PubMed]
- Toebes, M.L.; Zhang, Y.; Hájek, J.; Nijhuis, T.A.; Bitter, J.H.; Dillen, A.J.; Murzin, D.Y.; Koningsberger, D.C.; Jong, K.P. Support effects in the hydrogenation of cinnamaldehyde over carbon nanofiber-supported platinum catalysts: Characterization and catalysis. J. Catal. 2004, 226, 215–225. [Google Scholar] [CrossRef]
- Wang, F.; Yu, Z.; Wei, X.; Wu, Z.; Liu, N.; Xu, J.; Xue, B.; Li, G. Pt/Ce-La Nanocomposite for Hydrogenation Promoted by a Synergistic Effect of Support with Redox and Basic Property. Catal. Lett. 2022, 152, 3669–3678. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, C.; Zhang, B.; Li, X.; Ying, Z.; Liu, T.; Lin, W.; Yu, Y.; Cheng, H.; Zhao, F. Highly selective Pt/ordered mesoporous TiO2-SiO2 catalysts for hydrogenation of cinnamaldehyde: The promoting role of Ti2+. J. Colloid Interface Sci. 2016, 463, 75–82. [Google Scholar] [CrossRef]
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Blackmond, D.G.; Oukaci, R.; Blanc, B.; Gallezot, P. Geometric and electronic effects in the selective hydrogenation of α,β-unsaturated aldehydes over zeolite-supported metals. J. Catal. 1991, 131, 401–411. [Google Scholar] [CrossRef]
- Wang, F.; Wen, Z.; Fang, Q.; Ge, Q.; Sun, J.; Li, G. Manganese Cluster Induce the Control Synthesis of RHO- and CHA-Type Silicoaluminaphosphates for Dimethylether to Light Olefin Conversion. Fuel 2019, 244, 104–109. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, X.; Xiong, G.; Nie, B.; Liu, C.; He, N.; Liu, J. Towards the preparation of binderless ZSM-5 zeolite catalysts: The crucial role of silanol nests. Catal. Sci. Technol. 2020, 10, 7829–7841. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Li, J.; Gao, Y.; Yu, R.; Jiang, R. One-pot synthesis of phosphorus-modified ZSM-5 zeolite by solid-state method and its MTO Catalytic performance. Chem.-Eur. J. 2022, e202203095. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Wang, Y.; Cao, Q.; Xie, X.; Fang, W. Hydrogenation of levulinic acid to γ-valerolactone over bifunctional Ru/(AlO)(ZrO)n catalyst: Effective control of Lewis acidity and surface synergy. Mole. Catal. 2020, 493, 111097. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Y.; Yang, D.P.; Dai, J.; Liu, Z.; Chen, Y.; Huang, J.; Li, Q. Biogenic Pt/CaCO3 nanocomposite as a robust catalyst toward benzene oxidation. ACS Appl. Mater. Interf. 2019, 12, 2469–2480. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, S.; Yu, C.; Zhang, J.; Pan, X.; Li, G. Ionic liquid-assisted one-step preparation of ultrafine amorphous metallic hydroxide nanoparticles for the highly efficient oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 15767–15773. [Google Scholar] [CrossRef]
- Rocco, A.M.; Melo, K.V.; Mota, C.J.A.; David, M.V.; Souza, I.N. Synthesis of PtCo/ZSM-5/C electrocatalyst and electrochemical activity. Ionics 2019, 25, 253–264. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Pei, W.; Li, G.; Liu, W.; Du, P.; Wang, Z.; Qin, Z.; Qi, H.; Liu, X.; et al. Crystal Phase Mediated Restructuring of Pt on TiO2 with Tunable Reactivity: Redispersion versus Reshaping. ACS Catal. 2022, 12, 3634–3643. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, Y.; Sun, X.; Jiang, F.; Zhao, J.; Wu, Z.; Wang, F.; Li, G. Hydrogenation of pentenal over supported Pt nanoparticles: Influence of Lewis-acid sites in the conversion pathway. New J. Chem. 2021, 45, 18881–18887. [Google Scholar] [CrossRef]





| Catalyst | Metal Loaded (%) a | Composition (%) a | Specific Area (m2·g−1) b | Pore Volume (cm3·g−1) c | Pore Diameter (nm) d | Total Acid Content (µmol·g−1) e | |
|---|---|---|---|---|---|---|---|
| Pt | Co | ||||||
| Pt/ZSM-5 | 0.98 | 100 | 0 | 247.4 | 0.16 | 0.48 | 263.9 |
| Pt8Co1/ZSM-5 | 0.97 | 88.9 | 11.1 | 227.8 | 0.14 | 0.47 | 284.3 |
| Co/ZSM-5 | 0.97 | 0 | 100 | 242.1 | 0.17 | 0.47 | 173.8 |
| Entry | Catalyst | CAL Conversion (%) | Product Selectivity (%) | |||
|---|---|---|---|---|---|---|
| COL | HCAL | HCOL | Other | |||
| 1 | Pt8Co1/ZSM-5-AT | 93.7 | 68.9 | 7.7 | 12.7 | 10.7 |
| 2 | Pt/ZSM-5-AT | 60.3 | 53.0 | 15.6 | 4.2 | 27.2 |
| 3 | Co/ZSM-5-AT | 21.9 | 16.4 | 44.3 | 2.8 | 36.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Lu, S.; Liu, X.; Li, G.; Wang, F. Enhanced Activity of Alkali-Treated ZSM-5 Zeolite-Supported Pt-Co Catalyst for Selective Hydrogenation of Cinnamaldehyde. Molecules 2023, 28, 1730. https://doi.org/10.3390/molecules28041730
Cheng S, Lu S, Liu X, Li G, Wang F. Enhanced Activity of Alkali-Treated ZSM-5 Zeolite-Supported Pt-Co Catalyst for Selective Hydrogenation of Cinnamaldehyde. Molecules. 2023; 28(4):1730. https://doi.org/10.3390/molecules28041730
Chicago/Turabian StyleCheng, Shibo, Shan Lu, Xiang Liu, Gao Li, and Fei Wang. 2023. "Enhanced Activity of Alkali-Treated ZSM-5 Zeolite-Supported Pt-Co Catalyst for Selective Hydrogenation of Cinnamaldehyde" Molecules 28, no. 4: 1730. https://doi.org/10.3390/molecules28041730





