The Anti-Obesity and Anti-Steatotic Effects of Chrysin in a Rat Model of Obesity Mediated through Modulating the Hepatic AMPK/mTOR/lipogenesis Pathways
Abstract
1. Introduction
2. Results
2.1. Body Weight Change during Treatment Period
2.2. Glucose Homeostasis Parameters
2.3. Serum Lipids Profile and Hepatic Triglyceride Content
2.4. Liver Function Tests
2.5. Adipocytokines Levels
2.6. Hepatic Redox Parameters
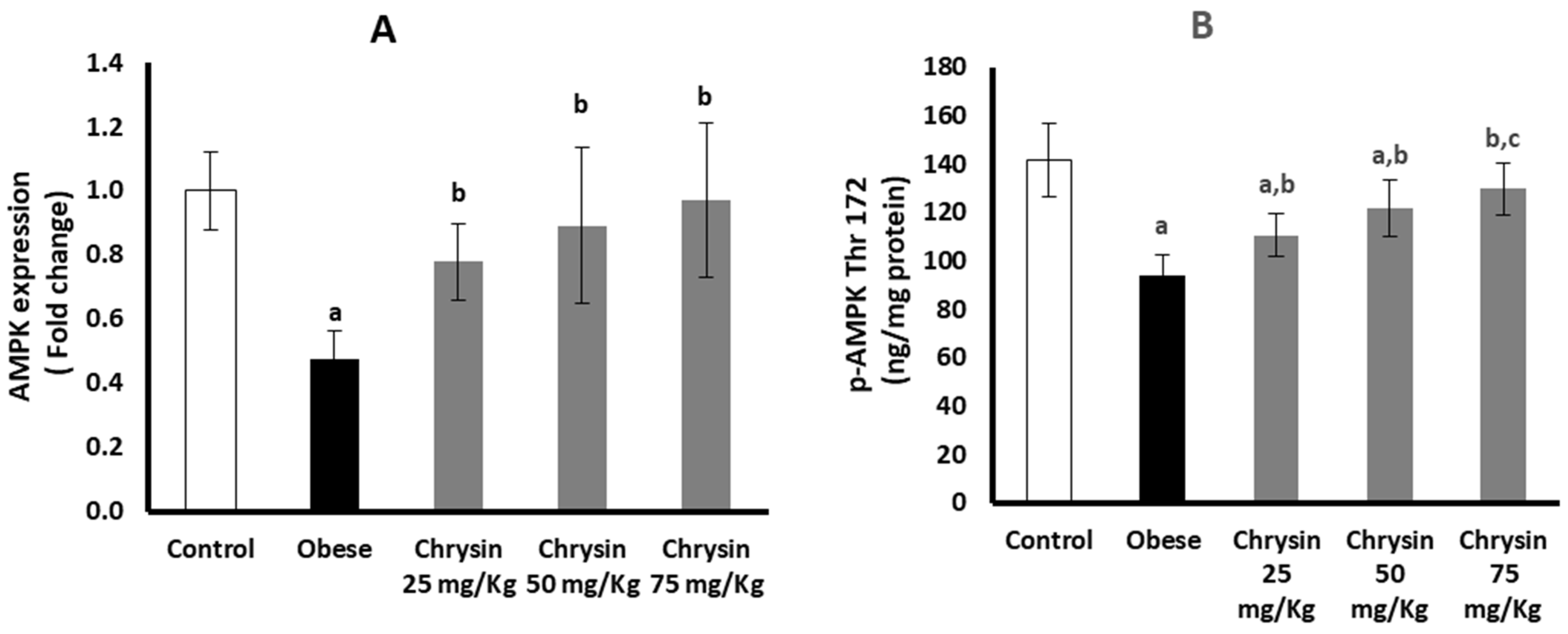
2.7. Hepatic Expression of AMPK at mRNA and Protein Levels
2.8. Hepatic Expression of LKB 1 at mRNA and Protein Levels
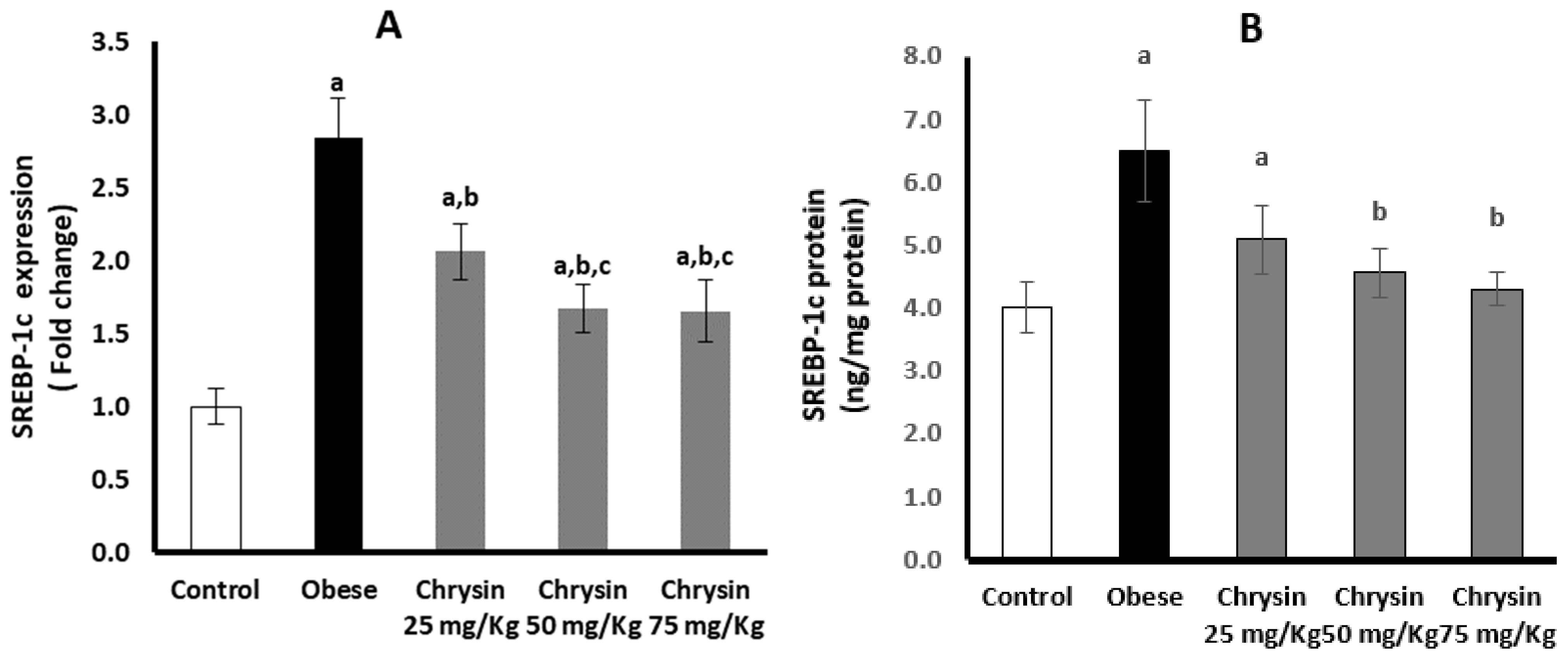
2.9. Hepatic Expression of SREBP-1c at mRNA and Protein Levels
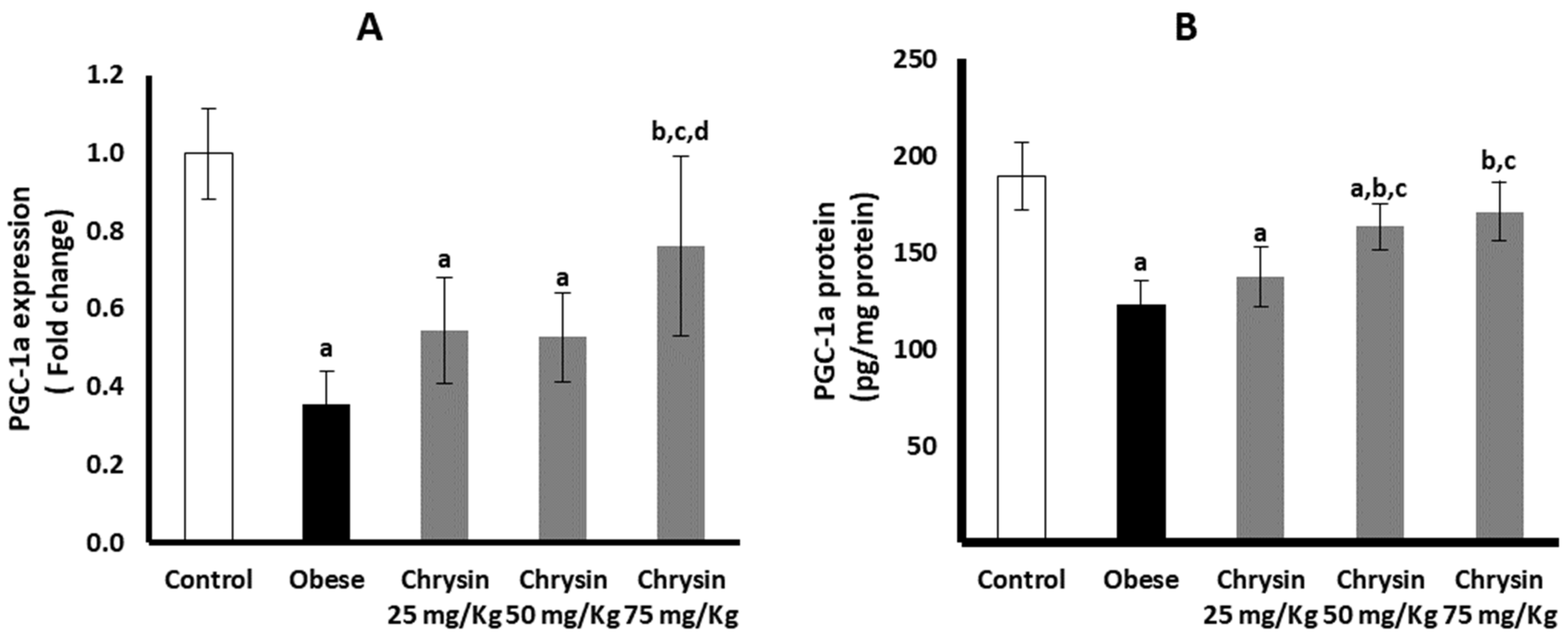
2.10. Hepatic Expression of PGC-1a at mRNA and Protein Levels
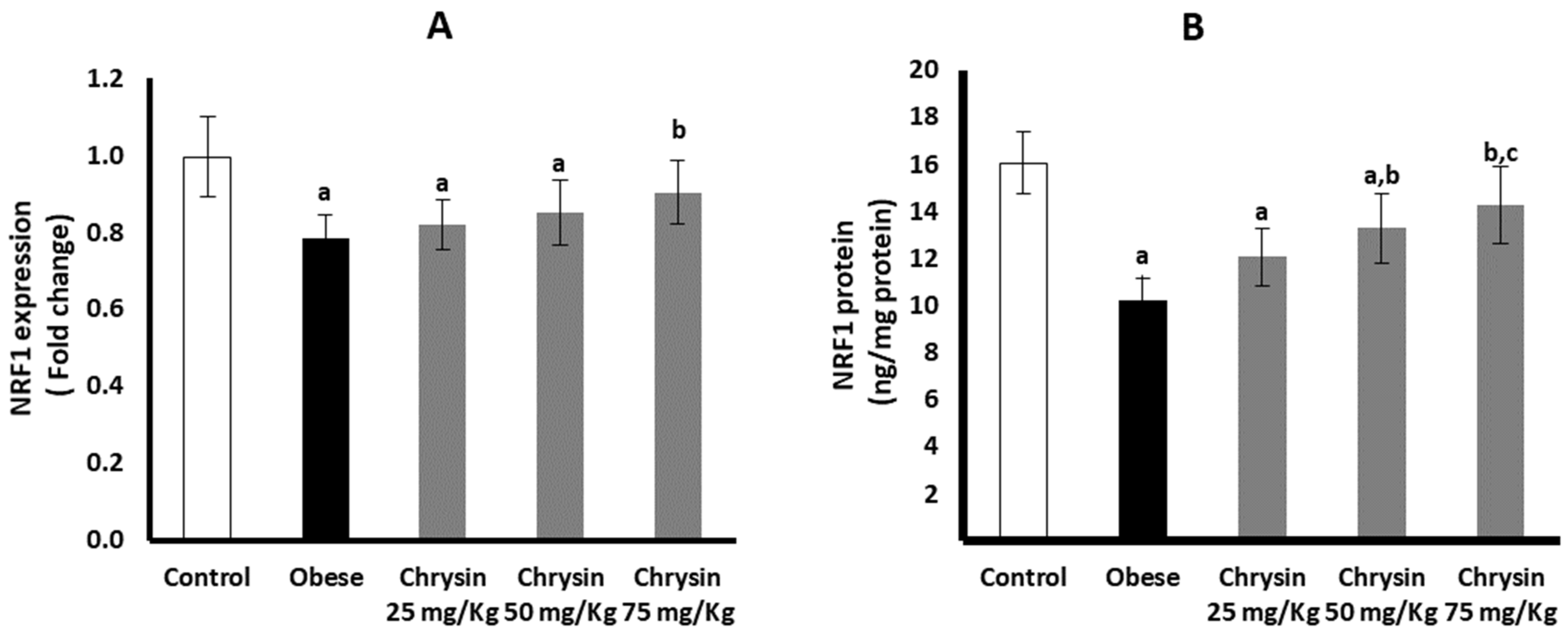
2.11. Hepatic Expression of NRF 1 at mRNA and Protein Levels
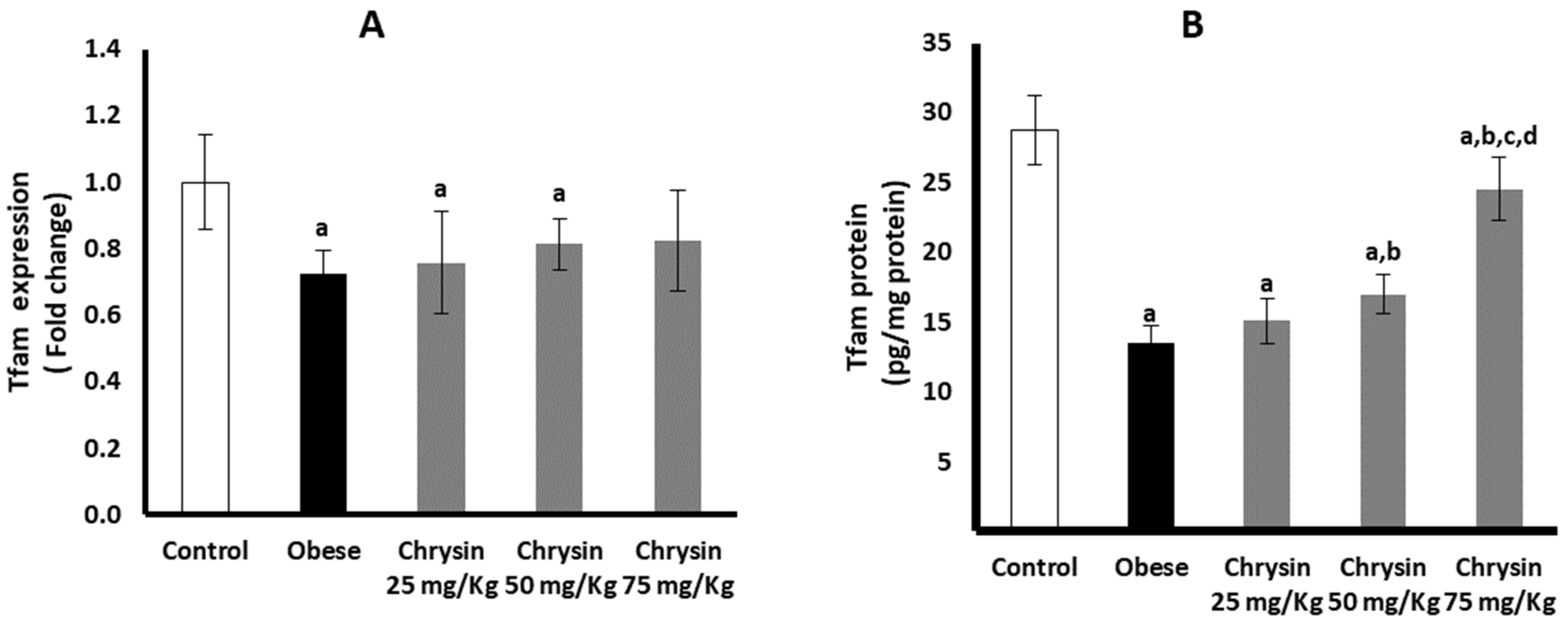
2.12. Hepatic Expression of Tfam at mRNA and Protein Levels
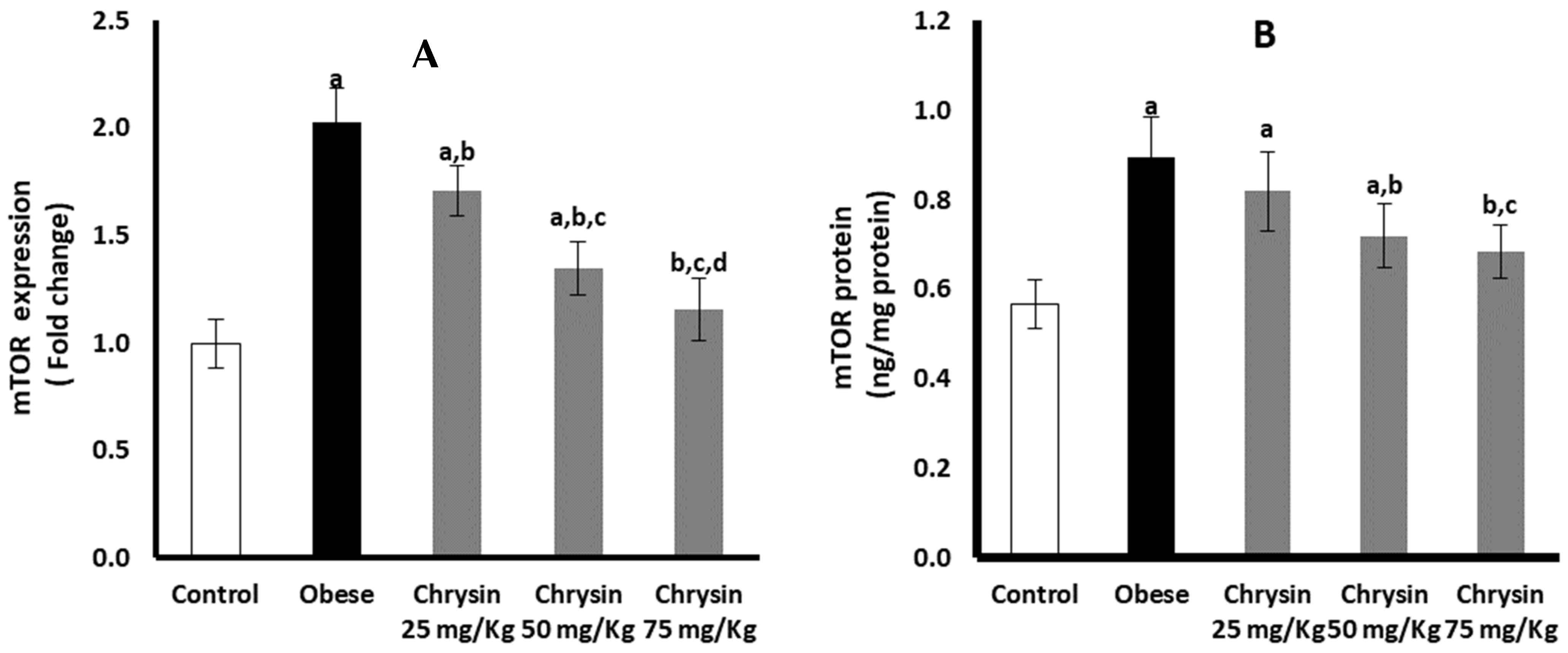
2.13. Hepatic Expression of mTOR at mRNA and Protein Levels
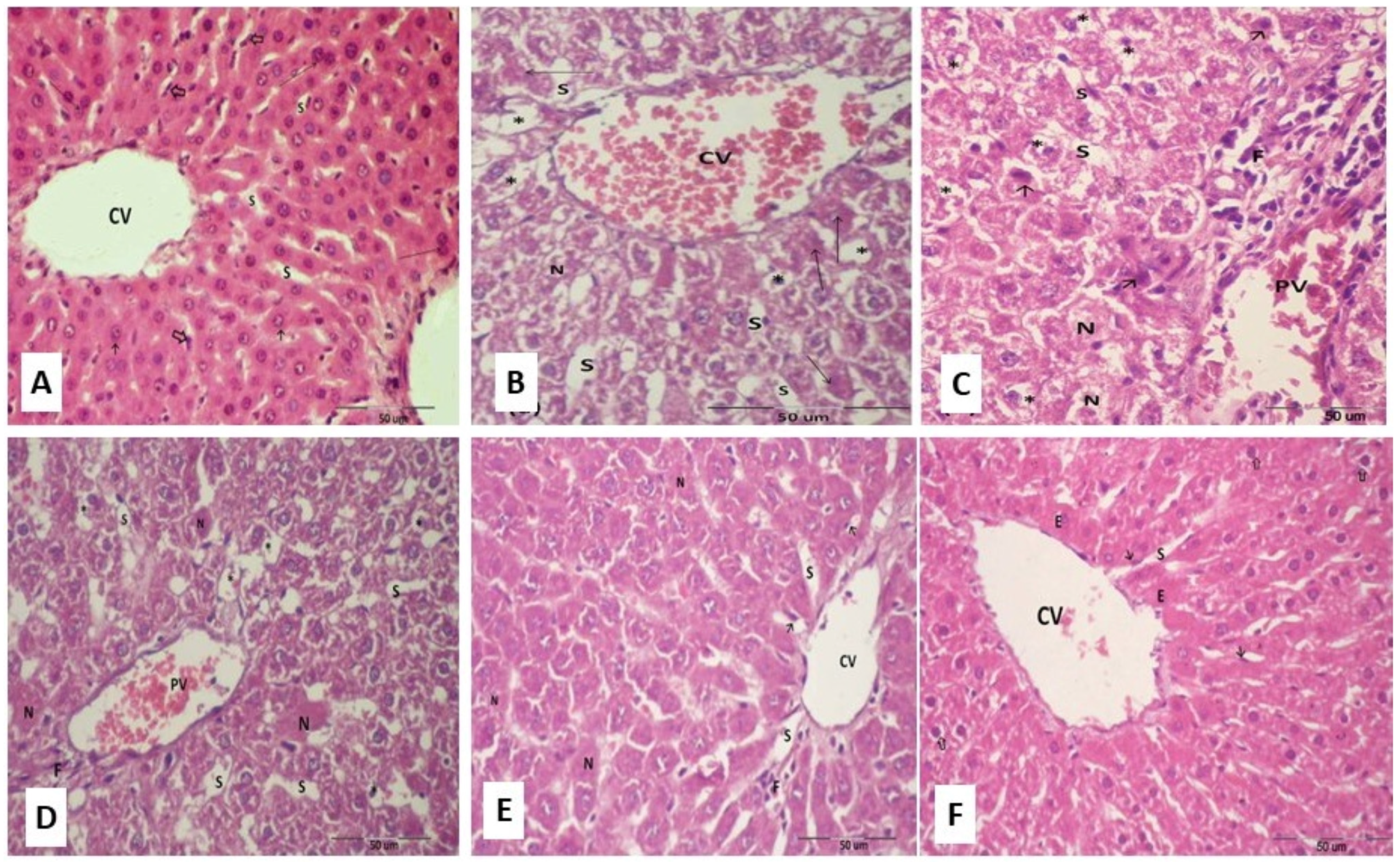
2.14. Histopathological Examinations of Hepatic Tissues
3. Materials and Methods
3.1. Materials
3.2. Experimental Animals
3.3. Ethical Statement
3.4. Induction of Obesity
3.5. Experimental Design
3.6. Collection of Samples
3.7. Serum Parameters
3.8. Determination of Hepatic Protein Contents of p-AMPK Thr172, LKB1, PGC-1α, Tfam, NRF1, SREBP-1c and mTOR Using ELISA
3.9. Tissues Contents of Total Reduced and Oxidized Glutathione
3.10. Determination of Malondialdehyde (MDA) as Thiobarbituric Acid Reactive Substances (TBARS)
3.11. Determination of Triglyceride Content
3.12. Hepatic Expression of AMPK, LKB1, mTOR, PGC-1α, Tfam, NRF1, and SREB-1c
3.13. Histopathological Examination
3.14. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woolsey, S.J.; Gong, I.; Kim, R.; Levstik, M.; Beaton, M.; Tirona, R. Regulation of Cytochrome P450 3A4 in Non-Alcoholic Fatty Liver Disease by Fibroblast Growth Factor 21; Wiley Online Library: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Uthayakumar, C.; Kotalawala, S. Impact of Obesity in Non-Alcoholic Fatty Liver Disease. J. Biomed. Sci. Eng. 2021, 14, 21. [Google Scholar] [CrossRef]
- Hanafi, M.Y.; Zaher, E.L.; El-Adely, S.E.; Sakr, A.; Ghobashi, A.H.; Hemly, M.H.; Kazem, A.H.; Kamel, M.A. The therapeutic effects of bee venom on some metabolic and antioxidant parameters associated with HFD-induced non-alcoholic fatty liver in rats. Exp. Ther. Med. 2018, 15, 5091–5099. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Adipokines in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1062–1079. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Perakakis, N.; Mantzoros, C.S. Association of Adipokines with Development and Progression of Nonalcoholic Fatty Liver Disease. Endocrinol. Metab. 2018, 33, 33–43. [Google Scholar] [CrossRef]
- López, M. EJE PRIZE 2017: Hypothalamic AMPK: A golden target against obesity? Eur. J. Endocrinol. 2017, 176, R235–R246. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling pathways in obesity: Mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J.; et al. AMPK Phosphorylates and Inhibits SREBP Activity to Attenuate Hepatic Steatosis and Atherosclerosis in Diet-Induced Insulin-Resistant Mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef]
- Gauthier, M.-S.; O’Brien, E.L.; Bigornia, S.; Mott, M.; Cacicedo, J.M.; Xu, X.J.; Gokce, N.; Apovian, C.; Ruderman, N. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem. Biophys. Res. Commun. 2011, 404, 382–387. [Google Scholar] [CrossRef]
- Xu, X.J.; Gauthier, M.-S.; Hess, D.T.; Apovian, C.M.; Cacicedo, J.M.; Gokce, N.; Farb, M.; Valentine, R.J.; Ruderman, N.B. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J. Lipid Res. 2012, 53, 792–801. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Q.; Xie, B.; Quan, C.; Sheng, Y.; Zhu, S.; Rong, P.; Zhou, S.; Sakamoto, K.; MacKintosh, C.; et al. Disruption of the AMPK–TBC1D1 nexus increases lipogenic gene expression and causes obesity in mice via promoting IGF1 secretion. Proc. Natl. Acad. Sci. USA 2016, 113, 7219–7224. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Y. mTORC1 signaling in hepatic lipid metabolism. Protein Cell 2018, 9, 145–151. [Google Scholar] [CrossRef]
- Reis-Barbosa, P.H.; Marcondes-de-Castro, I.A.; de Souza Marinho, T.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. The mTORC1/AMPK pathway plays a role in the beneficial effects of semaglutide (GLP-1 receptor agonist) on the liver of obese mice. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101922. [Google Scholar] [CrossRef]
- Zhao, R.; Zhu, M.; Zhou, S.; Feng, W.; Chen, H. Rapamycin-loaded mPEG-PLGA nanoparticles ameliorate hepatic steatosis and liver injury in non-alcoholic fatty liver disease. Front. Chem. 2020, 8, 407. [Google Scholar] [CrossRef]
- Bournat, J.C.; Brown, C.W. Mitochondrial dysfunction in obesity. Curr. Opin. Endocrinol. Diabetes 2010, 17, 446–452. [Google Scholar] [CrossRef]
- Wang, S.-W.; Sheng, H.; Bai, Y.-F.; Weng, Y.-Y.; Fan, X.-Y.; Lou, L.-J.; Zhang, F. Neohesperidin enhances PGC-1α-mediated mitochondrial biogenesis and alleviates hepatic steatosis in high fat diet fed mice. Nutr. Diabetes 2020, 10, 27. [Google Scholar] [CrossRef]
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; le Roux, C.W. Obesity management as a primary treatment goal for type 2 diabetes: Time to reframe the conversation. Lancet 2021, 399, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef]
- Pai, S.A.; Munshi, R.P.; Panchal, F.H.; Gaur, I.-S.; Juvekar, A.R. Chrysin ameliorates nonalcoholic fatty liver disease in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.A.; Martis, E.A.; Munshi, R.P.; Gursahani, M.S.; Mestry, S.N.; Juvekar, A.R. Chrysin mitigated obesity by regulating energy intake and expenditure in rats. J. Tradit. Complement. Med. 2020, 10, 577–585. [Google Scholar] [CrossRef]
- Kamel, M.A.; Helmy, M.H.; Hanafi, M.Y.; Mahmoud, S.A.; Elfetooh, H.A.; Badr, M.S. Maternal Obesity and Malnutrition in Rats Differentially Affect Glucose Sensing in the Muscles and Adipose Tissues in the Offspring. Int. J. Biochem. Res. Rev. 2014, 4, 440–469. [Google Scholar] [CrossRef]
- Caumo, A.; Perseghin, G.; Brunani, A.; Luzi, L. New Insights on the Simultaneous Assessment of Insulin Sensitivity and β-Cell Function With the HOMA2 Method. Diabetes Care 2006, 29, 2733–2734. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.; Hadley, M. [43] Malondialdehyde determination as index of lipid Peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1990; pp. 421–431. [Google Scholar] [CrossRef]
- Minniti, M.E.; Ahmed, O.; Pedrelli, M. Enzymatic Quantification of Liver Lipids After Folch Extraction. Methods Mol. Biol. 2020, 2164, 101–108. [Google Scholar] [CrossRef]
- Su, X.; Peng, D. The exchangeable apolipoproteins in lipid metabolism and obesity. Clin. Chim. Acta 2020, 503, 128–135. [Google Scholar] [CrossRef]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Bloc’h, J.L.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Parsanathan, R.; Jain, S.K. Glutathione deficiency induces epigenetic alterations of vitamin D metabolism genes in the livers of high-fat diet-fed obese mice. Sci. Rep. 2019, 9, 14784. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Kamel, M.A.; El-Nabi, S.E.H.; Ismail, A.H.A.; Ullah, S.; Al-Ghamdi, A.; Hathout, H.M.R.; El-Garawani, I.M. The regulation of HBP1, SIRT1, and SREBP-1c genes and the related microRNAs in non-alcoholic fatty liver rats: The association with the folic acid anti-steatosis. PLoS ONE 2022, 17, e0265455. [Google Scholar] [CrossRef] [PubMed]
- Bakir, M.B.; Salama, M.A.; Refaat, R.; Ali, M.A.; Khalifa, E.A.; Kamel, M.A. Evaluating the therapeutic potential of one-carbon donors in nonalcoholic fatty liver disease. Eur. J. Pharmacol. 2019, 847, 72–82. [Google Scholar] [CrossRef]
- Gross, J.D.; Pears, C.J. Possible Involvement of the Nutrient and Energy Sensors mTORC1 and AMPK in Cell Fate Diversification in a Non-Metazoan Organism. Front. Cell Dev. Biol. 2021, 9, 758317. [Google Scholar] [CrossRef]
- Van Nostrand, J.L.; Hellberg, K.; Luo, E.-C.; Van Nostrand, E.L.; Dayn, A.; Yu, J.; Shokhirev, M.N.; Dayn, Y.; Yeo, G.W.; Shaw, R.J. AMPK regulation of Raptor and TSC2 mediate metformin effects on transcriptional control of anabolism and inflammation. Genes Dev. 2020, 34, 1330–1344. [Google Scholar] [CrossRef]
- Lamming, D.W.; Sabatini, D.M. A Central Role for mTOR in Lipid Homeostasis. Cell Metab. 2013, 18, 465–469. [Google Scholar] [CrossRef]
- Chen, K.-G.; Kang, R.-R.; Sun, Q.; Liu, C.; Ma, Z.; Liu, K.; Deng, Y.; Liu, W.; Xu, B. Resveratrol ameliorates disorders of mitochondrial biogenesis and mitophagy in rats continuously exposed to benzo(a)pyrene from embryonic development through adolescence. Toxicology 2020, 442, 152532. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Tian, S.; Teng, C.; Huang, L.; Liu, X.; Wang, J.; Zhang, Y.; Li, B.; Shan, Y. Sulforaphane improves lipid metabolism by enhancing mitochondrial function and biogenesis in vivo and in vitro. Mol. Nutr. Food Res. 2019, 63, 1800795. [Google Scholar] [CrossRef]
- Nassir, F.; Ibdah, J.A. Role of Mitochondria in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 8713–8742. [Google Scholar] [CrossRef]
- Stompor-Gorący, M.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on Contemporary Status and Future Possibilities as Pro-Health Agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Kopustinskiene, D.M.; Simal-Gandara, J.; Bernatoniene, J.; Samarghandian, S. An updated review on the versatile role of chrysin in neurological diseases: Chemistry, pharmacology, and drug delivery approaches. Biomed. Pharmacother. 2021, 141, 111906. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, M.; Rezaee, S.A.; Hosseinzadeh, H. An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomed. Pharmacother. 2017, 92, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, J.; Ju, Z.; Huang, W.; Wang, Z.; Yang, L.; Ding, L. Danning tablets alleviate high fat diet-induced obesity and fatty liver in mice via modulating SREBP pathway. J. Ethnopharmacol. 2021, 279, 114320. [Google Scholar] [CrossRef]
- Shang, J.; Chen, L.-L.; Xiao, F.-X.; Sun, H.; Ding, H.-C.; Xiao, H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol. Sin. 2008, 29, 698–706. [Google Scholar] [CrossRef]
- Quan, H.Y.; Kim, D.Y.; Kim, S.J.; Jo, H.K.; Kim, G.W.; Chung, S.H. Betulinic acid alleviates non-alcoholic fatty liver by inhibiting SREBP1 activity via the AMPK–mTOR–SREBP signaling pathway. Biochem. Pharmacol. 2013, 85, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Chen, H.; Wu, S.; Tian, H. LRG ameliorates steatohepatitis by activating the AMPK/mTOR/SREBP1 signaling pathway in C57BL/6J mice fed a high-fat diet. Mol. Med. Rep. 2019, 20, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwivedi, U.N.; Kakkar, P. Berbamine induced AMPK activation regulates mTOR/SREBP-1c axis and Nrf2/ARE pathway to allay lipid accumulation and oxidative stress in steatotic HepG2 cells. Eur. J. Pharmacol. 2020, 882, 173244. [Google Scholar] [CrossRef]
- Zarzecki, M.S.; Araujo, S.M.; Bortolotto, V.C.; de Paula, M.T.; Jesse, C.R.; Prigol, M. Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Toxicol. Rep. 2014, 1, 200–208. [Google Scholar] [CrossRef]
- Kicinska, A.; Jarmuszkiewicz, W. Flavonoids and Mitochondria: Activation of Cytoprotective Pathways? Molecules 2020, 25, 3060. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S. Is the adiponectin-AMPK-mitochondrial axis involved in progression of nonalcoholic fatty liver disease? Hepatology 2014, 60, 22. [Google Scholar] [CrossRef]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.-L.; Schulze, A. SREBP Activity Is Regulated by mTORC1 and Contributes to Akt-Dependent Cell Growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Liu, Y.; Zhu, D.; Yu, J.; Li, G.; Sun, Z.; Wang, W.; Jiang, H.; Hong, Z. MiR-27a promotes insulin resistance and mediates glucose metabolism by targeting PPAR-γ-mediated PI3K/AKT signaling. Aging 2019, 11, 7510–7524. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Samini, F.; Farkhondeh, T. Chrysin treatment improves diabetes and its complications in liver, brain, and pancreas in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2015, 94, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Eldutar, E.; Kandemir, F.M.; Kucukler, S.; Caglayan, C. Restorative effects of Chrysin pretreatment on oxidant–antioxidant status, inflammatory cytokine production, and apoptotic and autophagic markers in acute paracetamol-induced hepatotoxicity in rats: An experimental and biochemical study. J. Biochem. Mol. Toxicol. 2017, 31, e21960. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, K.L.; Salmon, A.B.; Hohnen-Behrens, C.; Turner, N.; Hoy, A.J.; Maghzal, G.J.; Stocker, R.; Van Remmen, H.; Kraegen, E.W.; Cooney, G.J.; et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 17787–17792. [Google Scholar] [CrossRef]

| Control Rats | Obese Rats | ||||
|---|---|---|---|---|---|
| Untreated | Chrysin-Treated | ||||
| 25 mg/Kg | 50 mg/Kg | 75 mg/Kg | |||
| Initial weight (g) | 206.3 ±13.4 | 388.2 ± 27.9 a | 372.6 ± 32.8 a | 367.3 ± 27.9 a | 365.2 ± 23.1 a |
| Final weight (g) | 232.6 ± 15.5 | 456.1 ± 35.5 a | 427.1 ± 36.0 a | 410.7 ± 30.9 a,b | 399.9 ± 24.3 a,b |
| Weight gain (g) | 26.3 ± 2.6 | 67.9 ± 8.7 a | 54.6 ± 6.8 a,b | 43.4 ± 5.9 a,b,c | 34.7± 2.6 b,c,d |
| Control Rats | Obese Rats | ||||
|---|---|---|---|---|---|
| Untreated | Chrysin-Treated | ||||
| 25 mg/Kg | 50 mg/Kg | 75 mg/Kg | |||
| FBS (mg/dL) | 86.7 ± 7.4 | 184.0 ± 12.7 a | 156.1 ± 10.6 a,b | 137.4 ± 11.3 a,b,c | 128.7 ± 8.9 a,b,c |
| Insulin (mIU/mL) | 6.4 ± 1.8 | 13.3 ± 1.4 a | 11.9 ± 0.9 a | 9.7 ± 0.8 a,b,c | 9.6 ± 0.8 a,b,c |
| HOMA-IR | 1.3 ± 0.3 | 6.0 ± 0.5 a | 4.6 ± 0.3 a,b | 3.3 ± 0.3 a,b,c | 3.1 ± 0.3 a,b,c |
| TG (mg/dL) | 45.8 ± 4.2 | 70.5 ± 12.9 a | 58.4 ± 4.3 a,b | 51.5 ± 3.6 b | 49.99 ± 6.1 b |
| TC (mg/dL) | 156.4 ± 11.1 | 196.8 ± 12.6 a | 170.9 ± 10.6 b | 161.6 ± 11.3 b | 157.7 ± 12.3 b |
| HDL-c (mg/dL) | 47.0 ± 4.7 | 35.4 ± 2.8 a | 39.2 ± 3.1 a | 42.5 ± 4.2 b | 44.3 ± 3.7 b |
| LDL-c (mg/dL) | 100.0 ± 7.8 | 147.3 ± 12.2 a | 120.0 ± 10.0 a,b | 108.8 ± 11.0 b | 103.4 ± 11.2 b,c |
| ALT (U/L) | 37.3 ± 4.9 | 75.1 ± 16.7 a | 58.6 ± 6.0 a,b | 40.4 ± 5.6 b,c | 39.3± 4.3 b,c |
| AST (U/L) | 124.5 ± 21.2 | 190.1 ± 23.7 a | 168.6 ± 22.4 a | 142.8 ± 9.9 b | 141.0 ± 10.4 b,c |
| Bil (mg/dL) | 0.44 ± 0.06 | 1.12 ± 0.15 a,c | 0.86 ± 0.11 a,b | 0.54 ± 0.07 b,c | 0.53 ± 0.07 b,c |
| Hepatic TG (mg/g tissues) | 33.7 ± 3.5 | 99.3 ± 9.1 a | 75.2 ± 6.3 a,b | 55.4 ± 5.8 a,b,c | 53.5 ± 5.4 a,b,c |
| Control Rats | Obese Rats | ||||
|---|---|---|---|---|---|
| Untreated | Chrysin-Treated | ||||
| 25 mg/Kg | 50 mg/Kg | 75 mg/Kg | |||
| TNF-α (pg/mL) | 5.5 ± 1.0 | 63.2 ± 6.7 a | 47.4 ± 6.9 a,b | 41.8 ± 6.0 a,b | 32.9 ± 5 a,b,c,d |
| Adiponectin (pg/mL) | 81.5 ± 7.9 | 36.4 ± 6.5 a | 47.1 ± 5.8 a,b | 74.3 ± 6.2 b,c | 77.8 ± 6.1 b,c |
| Leptin (pg/mL) | 6.7 ± 0.5 | 14.5± 1.1 a | 11.6 ± 1.0 a,b | 9.6 ± 0.8 a,b,c | 9.0 ± 0.9 a,b,c |
| Control Rats | Obese Rats | ||||
|---|---|---|---|---|---|
| Untreated | Chrysin-Treated | ||||
| 25 mg/Kg | 50 mg/Kg | 75 mg/Kg | |||
| MDA (nmol/g tissues) | 28.1 ± 2.9 | 71.7 ± 5.6 a | 51.8 ± 4.3 a,b | 43.6 ± 3.8 a,b,c | 42.6 ± 4.0 a,b,c |
| tGSH (nmol/mg protein) | 27.7 ± 1.7 | 21.4 ± 1.8 a | 23.7 ± 1.9 a | 24.7 ± 1.9 a,b | 25.0 ± 1.7 a,b |
| GSSG (nmol/mg protein) | 1.1 ± 0.1 | 2.0 ± 0.2 a | 1.5 ± 0.2 a,b | 1.2 ± 0.2 b,c | 1.2 ± 0.1 b,c |
| GSH (nmol/mg protein) | 25.6 ± 1.6 | 17.5 ± 1.5 a | 20.8 ± 1.7 a,b | 22.3 ± 1.8 a,b | 22.7 ± 1.6 a,b |
| GSH/GSSG ratio | 23.6 ± 2.0 | 8.9 ± 0.6 a | 14.1 ± 1.2 a,b | 19.0 ± 2.5 a,b,c | 19.7 ± 1.9 a,b,c |
| Gene | Accession Number | Primer Sequence | |
|---|---|---|---|
| 18S rRNA | NR_046237.2 | F: | GTAACCCGTTGAACCCCATT |
| R: | CAAGCTTATGACCCGCACTT | ||
| PGC-1α | NM_031347.1 | F: | GTGCAGCCAAGACTCTGTATGG |
| R: | GTCCAGGTCATTCACATCAAGTTC | ||
| NRF-1 | NM_001100708.1 | F: | TTACTCTGCTGTGGCTGATGG |
| R: | CCTCTGATGCTTGCGTCGTCT | ||
| Tfam | NM_031326.2 | F: | CCCACAGAGAACAGAAACAG |
| R: | CCCTGGAAGCTTTCAGATACG | ||
| AMPK | NM_023991.1 | F: | GTGGTGTTATCCTGTATGCCCTTCT |
| R: | CTGTTTAAACCATTCATGCTCTCGT | ||
| LKB1 | NM_001108069.2 | F: | GAGGAAGTGGGTCAGAATGGA |
| R: | CCGGCCTTCTGGCTTCA | ||
| SREB1C | NM_001276708.1 | F: | GACGACGGAGCCATGGATT |
| R: | GGGAAGTCACTGTCTTGGTTGTT | ||
| mTOR | NM_019906.2 | F: | TTGGAGTGGCTGGGTGCTGA |
| R: | AAGGGCTGAACTTGCTGGAA | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oriquat, G.; Masoud, I.M.; Kamel, M.A.; Aboudeya, H.M.; Bakir, M.B.; Shaker, S.A. The Anti-Obesity and Anti-Steatotic Effects of Chrysin in a Rat Model of Obesity Mediated through Modulating the Hepatic AMPK/mTOR/lipogenesis Pathways. Molecules 2023, 28, 1734. https://doi.org/10.3390/molecules28041734
Oriquat G, Masoud IM, Kamel MA, Aboudeya HM, Bakir MB, Shaker SA. The Anti-Obesity and Anti-Steatotic Effects of Chrysin in a Rat Model of Obesity Mediated through Modulating the Hepatic AMPK/mTOR/lipogenesis Pathways. Molecules. 2023; 28(4):1734. https://doi.org/10.3390/molecules28041734
Chicago/Turabian StyleOriquat, Ghaleb, Inas M. Masoud, Maher A. Kamel, Hebatallah Mohammed Aboudeya, Marwa B. Bakir, and Sara A. Shaker. 2023. "The Anti-Obesity and Anti-Steatotic Effects of Chrysin in a Rat Model of Obesity Mediated through Modulating the Hepatic AMPK/mTOR/lipogenesis Pathways" Molecules 28, no. 4: 1734. https://doi.org/10.3390/molecules28041734
APA StyleOriquat, G., Masoud, I. M., Kamel, M. A., Aboudeya, H. M., Bakir, M. B., & Shaker, S. A. (2023). The Anti-Obesity and Anti-Steatotic Effects of Chrysin in a Rat Model of Obesity Mediated through Modulating the Hepatic AMPK/mTOR/lipogenesis Pathways. Molecules, 28(4), 1734. https://doi.org/10.3390/molecules28041734







