Investigation into Red Emission and Its Applications: Solvatochromic N-Doped Red Emissive Carbon Dots with Solvent Polarity Sensing and Solid-State Fluorescent Nanocomposite Thin Films
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of 813-CD-600 PEI
2.3. Synthesis of 813-CD-25K PEI
2.4. Synthesis of 813-CD-600 PEI/PVP Nanocomposite Thin Film
2.5. Characterization
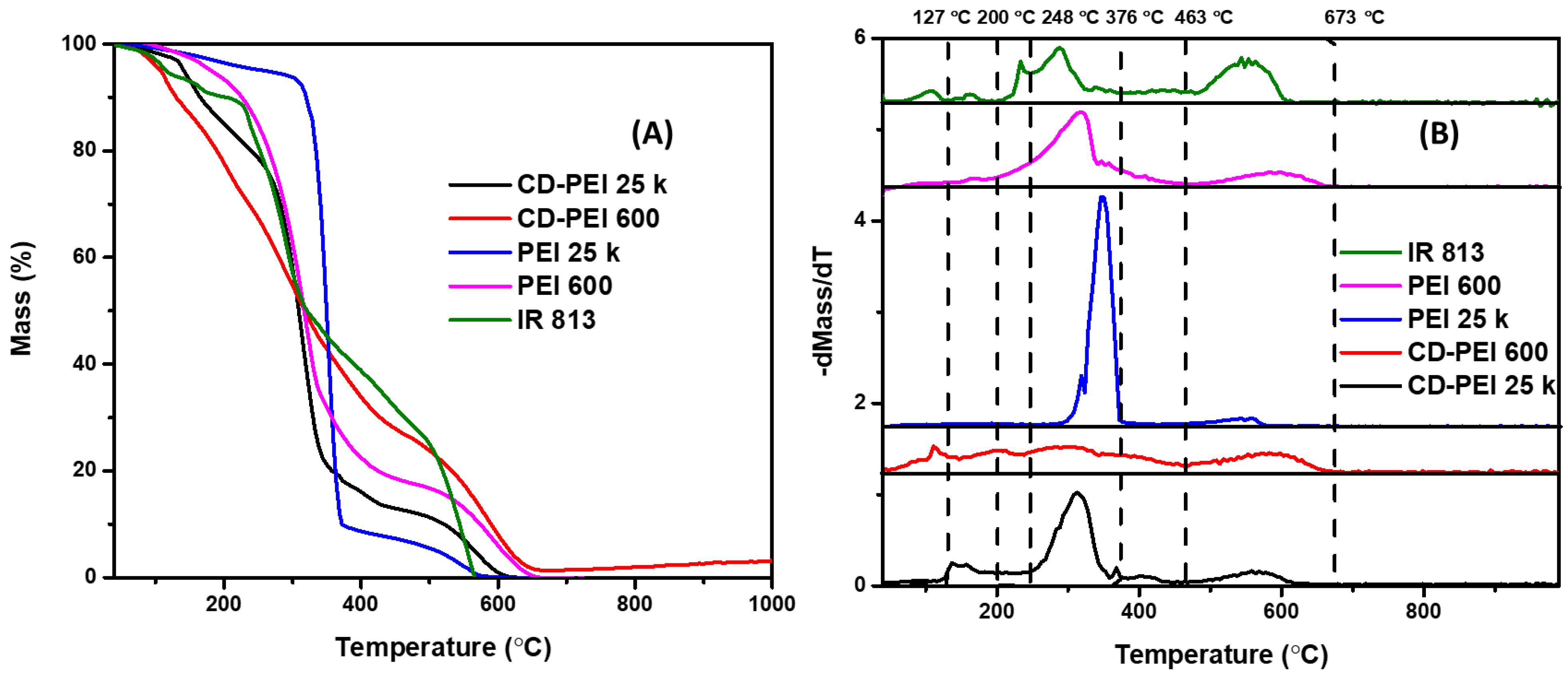
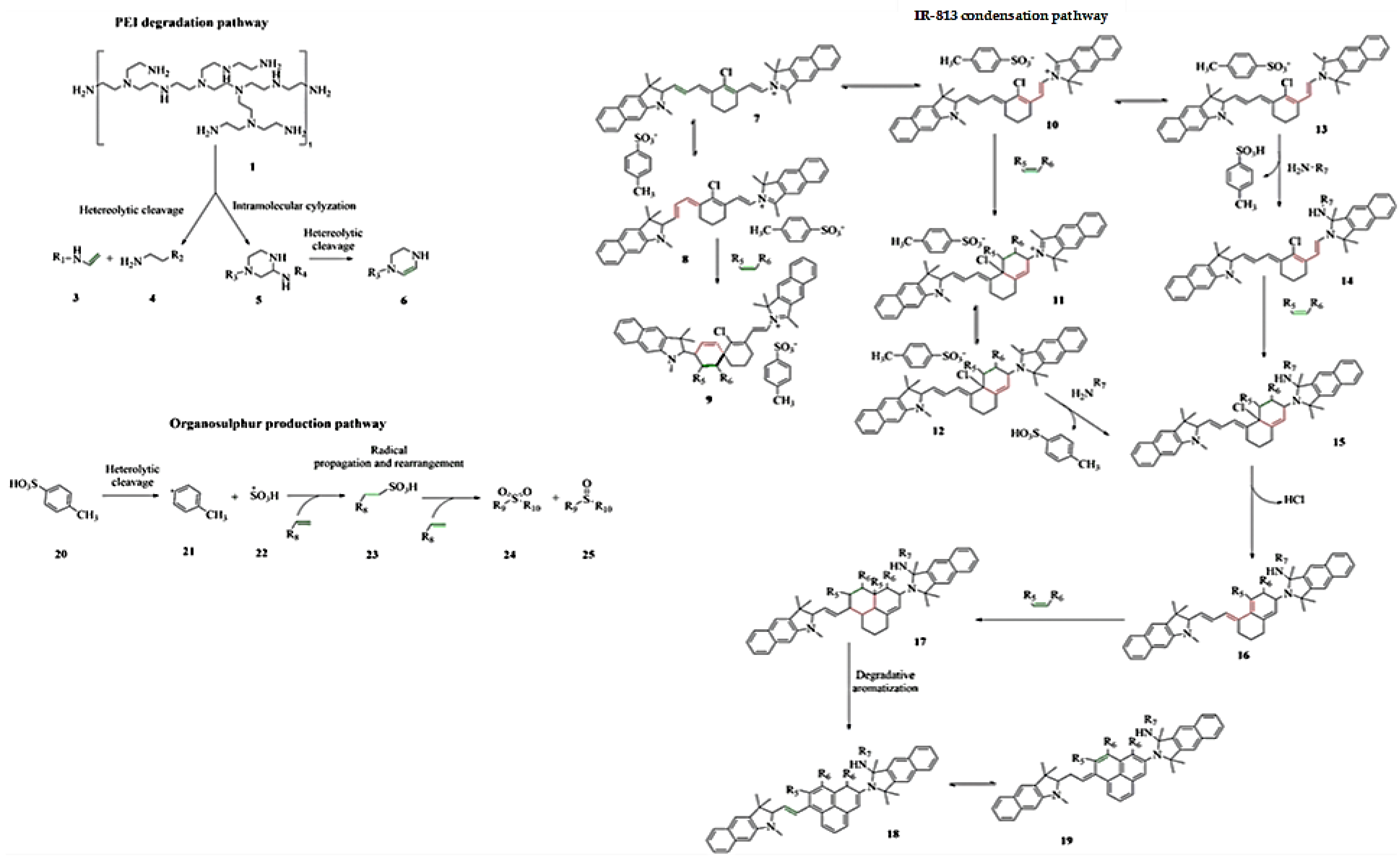
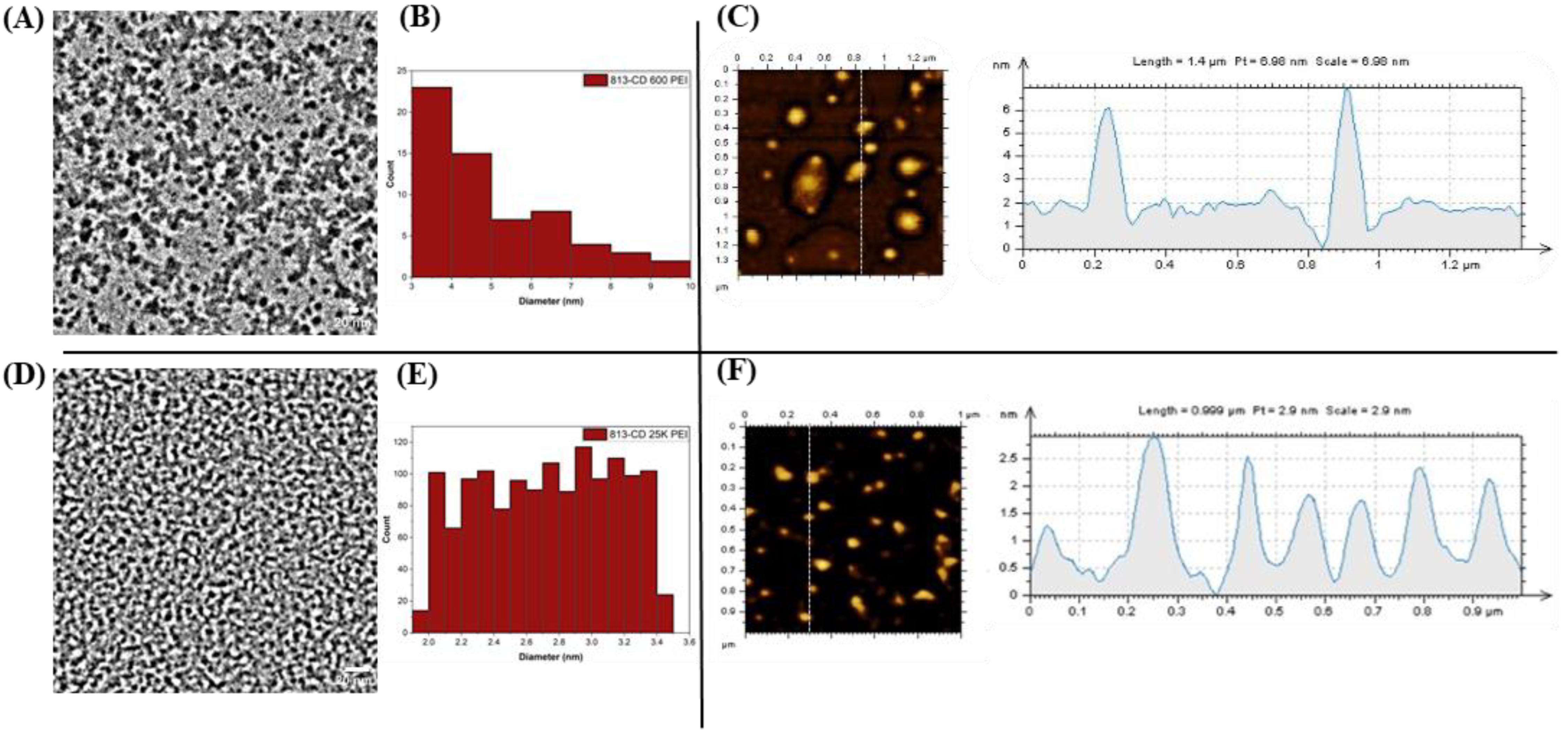
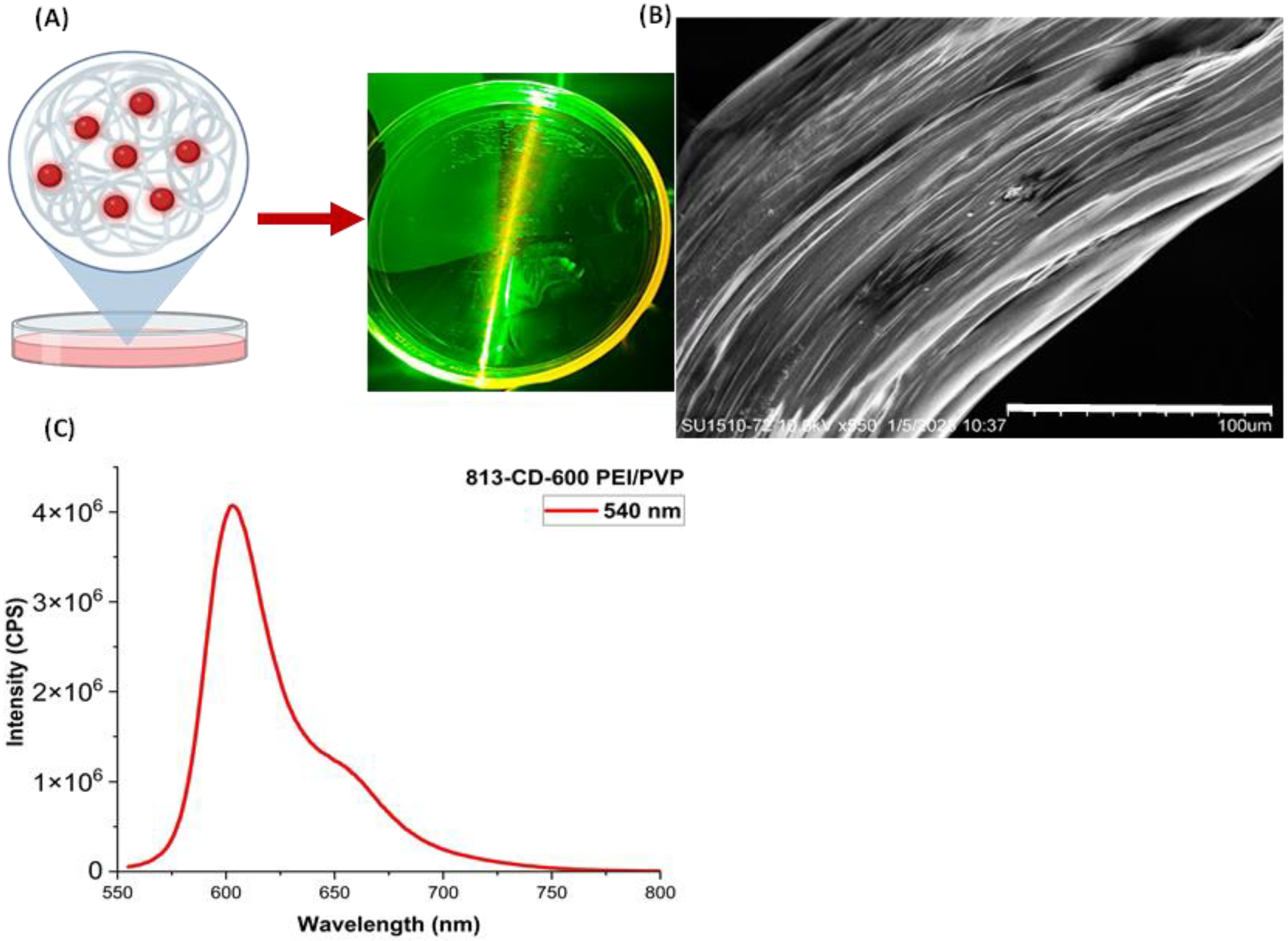
3. Results and Discussion
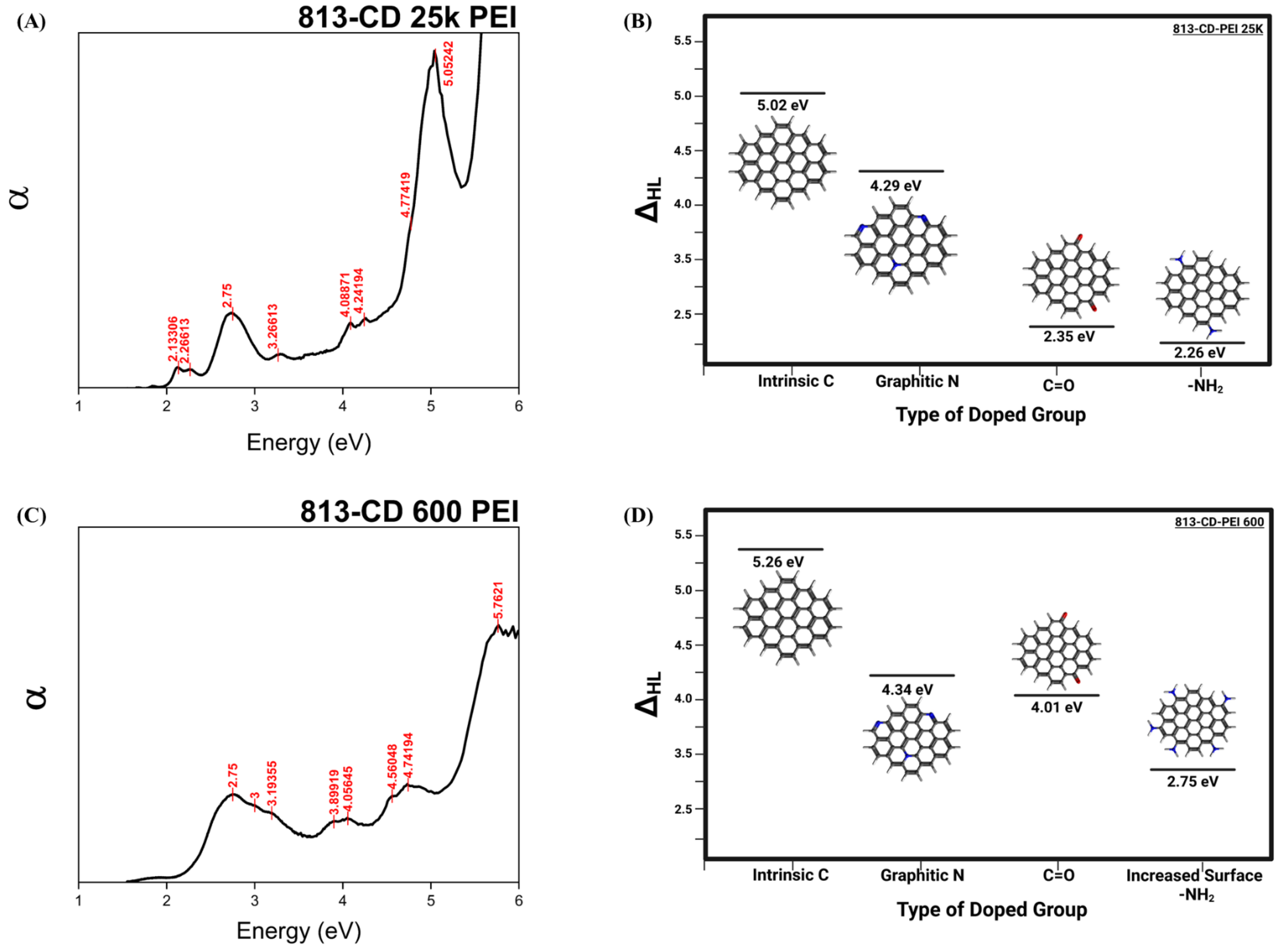
3.1. UV-Vis Analysis of 813-CD 600 PEI
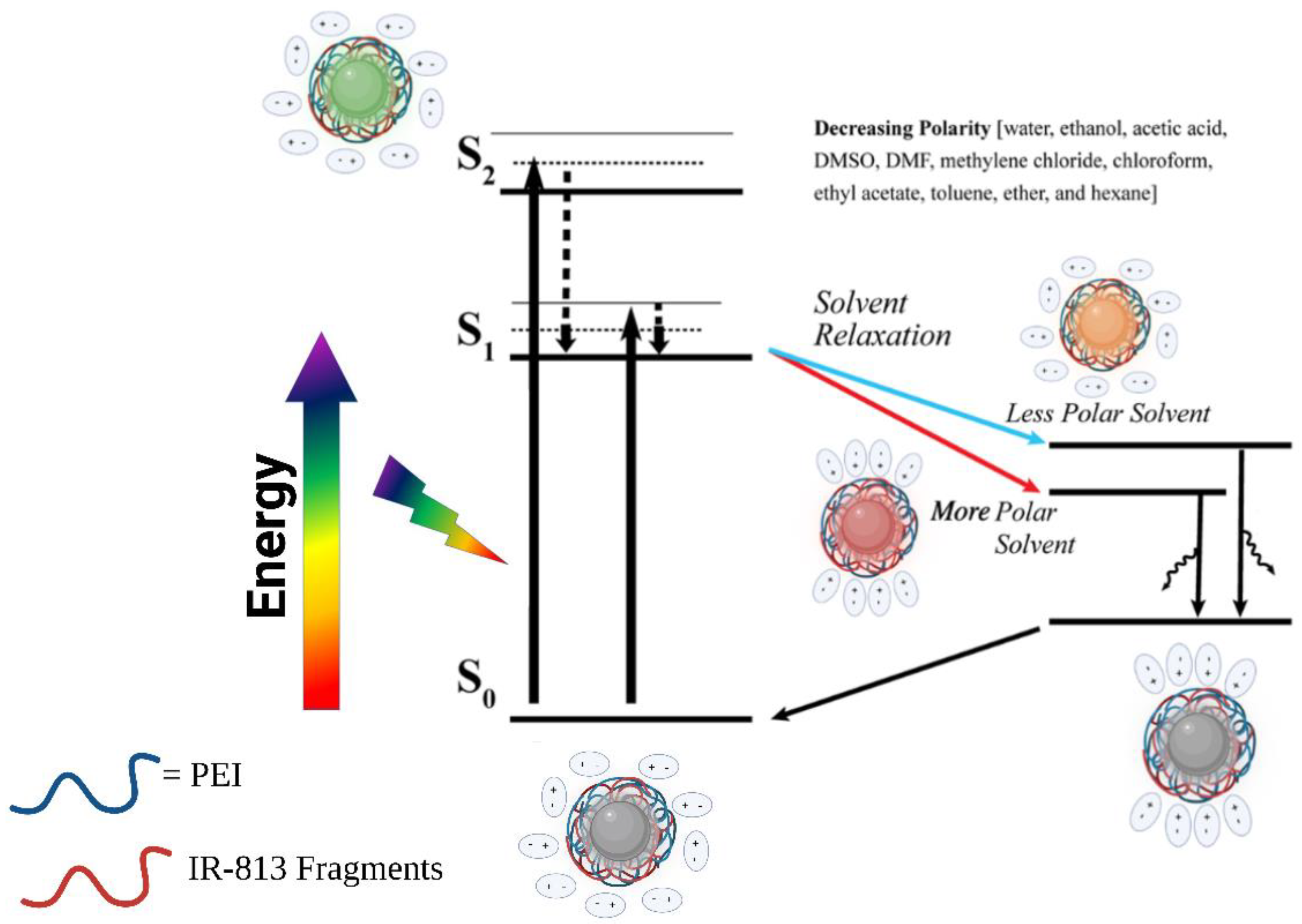
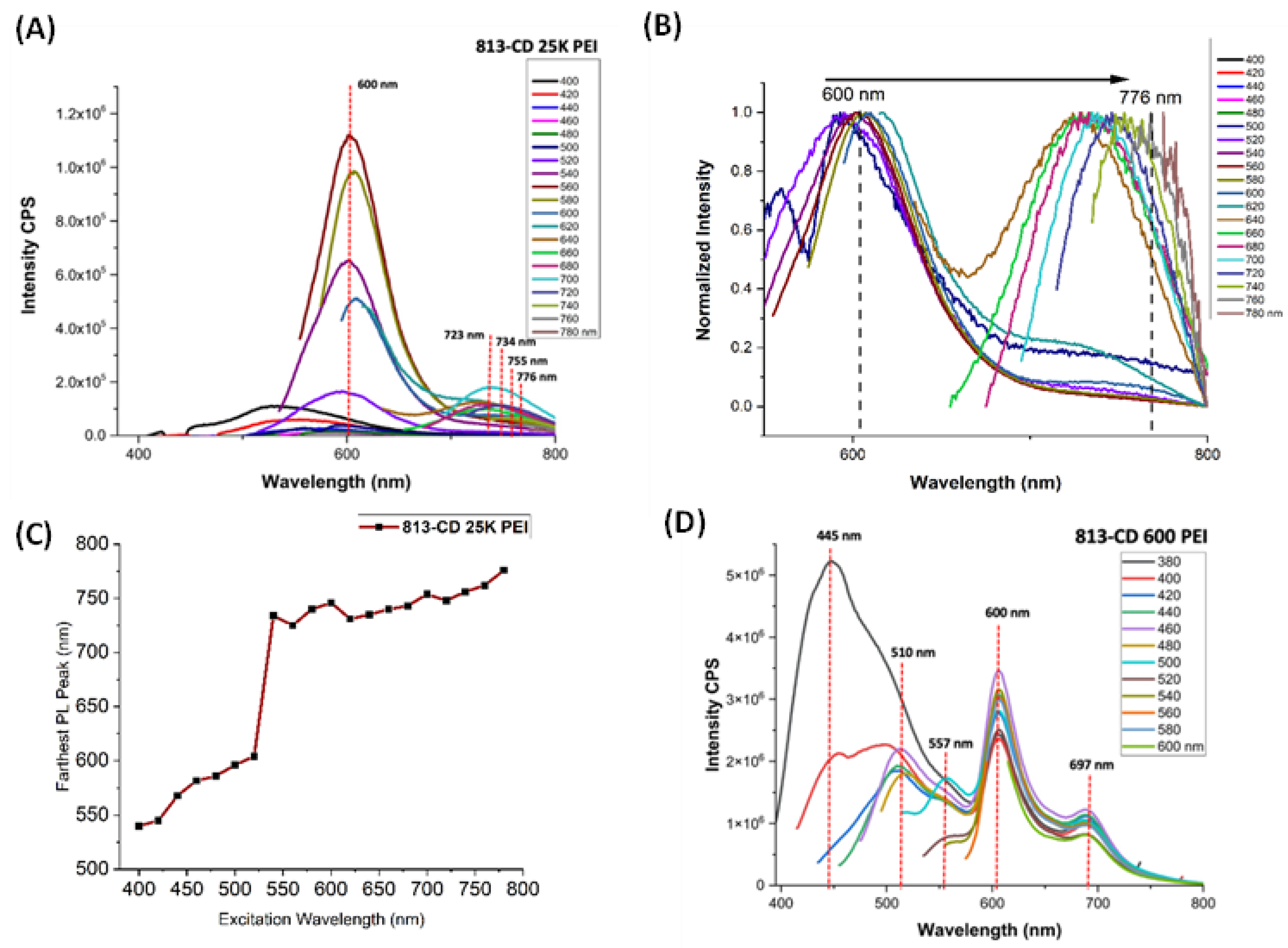
3.2. Solvatochromic PL Behavior of 813-CD
3.3. Optical Manipulation of 813CDs: 600 PEI vs. 25K PEI
3.4. Synthesis of 813-CD/PVP Nanocomposite Film
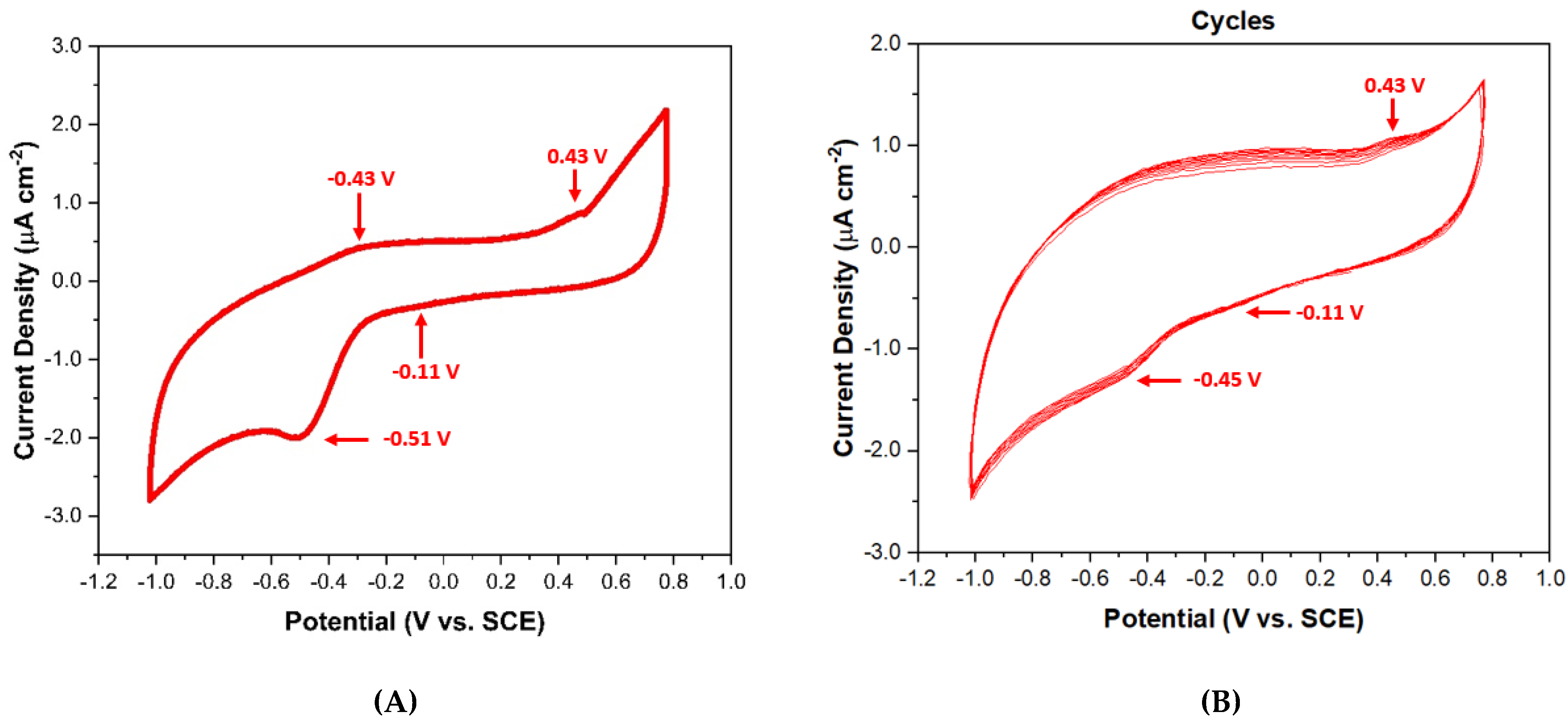
3.5. Cyclic Voltammetry Assessment of 813-CD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, Y.; Zhao, J.; Wang, S.; Lin, T.; Ye, F.; Zhao, S. Carbon Dots with Absorption Red-Shifting for Two-Photon Fluorescence Imaging of Tumor Tissue pH and Synergistic Phototherapy. ACS Appl. Mater. Interfaces 2021, 13, 35365–35375. [Google Scholar] [CrossRef]
- Wen, Y.; Jia, Q.; Nan, F.; Zheng, X.; Liu, W.; Wu, J.; Ren, H.; Ge, J.; Wang, P. Pheophytin Derived Near-Infrared-Light Responsive Carbon Dot Assembly as a New Phototheranotic Agent for Bioimaging and Photodynamic Therapy. Chem. Asian J. 2019, 14, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chen, J.; Jiang, K.; Tang, Z.; Wang, Y.; Li, Z.; Liu, C.; Wu, A.; Lin, H. Ce6-Modified Carbon Dots for Multimodal-Imaging-Guided and Single-NIR-Laser-Triggered Photothermal/Photodynamic Synergistic Cancer Therapy by Reduced Irradiation Power. ACS Appl. Mater. Interfaces 2019, 11, 5791–5803. [Google Scholar] [CrossRef]
- Du, J.; Xu, N.; Fan, J.; Sun, W.; Peng, X. Carbon Dots for In Vivo Bioimaging and Theranostics. Small 2019, 15, e1805087. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.; Huang, C.; Liu, G.; Leung, K.C.-F.; Wáng, Y.X.J. High Performance Photoluminescent Carbon Dots for In Vitro and In Vivo Bioimaging: Effect of Nitrogen Doping Ratios. Langmuir 2015, 31, 8063–8073. [Google Scholar] [CrossRef]
- Yang, S.-T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.-P. Carbon Dots for Optical Imaging In Vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lin, X.; Mei, Y.; Luo, Y.; Yang, M.; Kuang, Y.; Yi, X.; Zeng, W.; Huang, Q.; Zhong, B. Recent Advances in Carbon Dots for In Vitro/Vivo Fluorescent Bioimaging: A Mini-Review. Front. Chem. 2022, 10, 905475. [Google Scholar] [CrossRef]
- Ji, C.; Zhou, Y.; Leblanc, R.M.; Peng, Z. Recent Developments of Carbon Dots in Biosensing: A Review. ACS Sensors 2020, 5, 2724–2741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Mintz, K.J.; Sharma, S.K.; Leblanc, R.M. Carbon Dots: Diverse Preparation, Application, and Perspective in Surface Chemistry. Langmuir 2019, 35, 9115–9132. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, G.; Jia, Y.; Ji, C.; Wang, Y.; Zhou, Y.; Leblanc, R.M.; Peng, Z. Carbon dot composites for bioapplications: A review. J. Mater. Chem. B 2022, 10, 843–869. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Miyanji, E.H.; Zhou, Y.; Pardo, J.; Hettiarachchi, S.D.; Li, S.; Blackwelder, P.L.; Skromne, I.; Leblanc, R.M. Carbon dots: Promising biomaterials for bone-specific imaging and drug delivery. Nanoscale 2017, 9, 17533–17543. [Google Scholar] [CrossRef]
- Gavalas, S.; Kelarakis, A. Towards Red Emissive Systems Based on Carbon Dots. Nanomaterials 2021, 11, 2089. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, Y.-S.; Xu, W.-N.; Zhao, Y.-F.; Xie, H.-Y.; Li, H.-J.; Chen, X.-F.; Zhao, R.-S.; Guo, D.-S. Far-red to near-infrared fluorescent probes based on silicon-substituted xanthene dyes for sensing and imaging. TrAC Trends Anal. Chem. 2020, 122, 115704. [Google Scholar] [CrossRef]
- Jun, Y.W.; Kim, H.R.; Reo, Y.J.; Dai, M.; Ahn, K.H. Addressing the autofluorescence issue in deep tissue imaging by two-photon microscopy: The significance of far-red emitting dyes. Chem. Sci. 2017, 8, 7696–7704. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, Y.; Wang, L. Synthesis and Applications of Red-Emissive Carbon Dots. Chem. Rec. 2019, 19, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Jia, Q.; Liu, W.; Guo, L.; Liu, Q.; Lan, M.; Zhang, H.; Meng, X.; Wang, P. Red-Emissive Carbon Dots for Fluorescent, Photoacoustic, and Thermal Theranostics in Living Mice. Adv. Mater. 2015, 27, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, L.; Jiang, K.; Wu, A.; Lin, H. Toward High-Efficient Red Emissive Carbon Dots: Facile Preparation, Unique Properties, and Applications as Multifunctional Theranostic Agents. Chem. Mater. 2016, 28, 8659–8668. [Google Scholar] [CrossRef]
- Gao, D.; Zhao, H.; Chen, X.; Fan, H. Recent advance in red-emissive carbon dots and their photoluminescent mechanisms. Mater. Today Chem. 2018, 9, 103–113. [Google Scholar] [CrossRef]
- Hallaji, Z.; Bagheri, Z.; Kalji, S.-O.; Ermis, E.; Ranjbar, B. Recent advances in the rational synthesis of red-emissive carbon dots for nanomedicine applications: A review. Flatchem 2021, 29, 100271. [Google Scholar] [CrossRef]
- Yang, X.; Ai, L.; Yu, J.; Waterhouse, G.I.N.; Sui, L.; Ding, J.; Zhang, B.; Yong, X.; Lu, S. Photoluminescence mechanisms of red-emissive carbon dots derived from non-conjugated molecules. Sci. Bull. 2022, 67, 1450–1457. [Google Scholar] [CrossRef]
- Apter, B.; Lapshina, N.; Barhom, H.; Fainberg, B.; Handelman, A.; Accardo, A.; Diaferia, C.; Ginzburg, P.; Morelli, G.; Rosenman, G. Fluorescence Phenomena in Amyloid and Amyloidogenic Bionanostructures. Crystals 2020, 10, 668. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Ren, J.; Malfatti, L.; Enzo, S.; Carbonaro, C.M.; Calvillo, L.; Granozzi, G.; Innocenzi, P. Boron oxynitride two-colour fluorescent dots and their incorporation in a hybrid organic-inorganic film. J. Colloid Interface Sci. 2020, 560, 398–406. [Google Scholar] [CrossRef]
- Kundelev, E.V.; Tepliakov, N.V.; Leonov, M.Y.; Maslov, V.G.; Baranov, A.V.; Fedorov, A.V.; Rukhlenko, I.D.; Rogach, A.L. Towards Bright Red-Emissive Carbon Dots through Controlling Interaction among Surface Emission Centers. J. Phys. Chem. Lett. 2020, 11, 8121–8127. [Google Scholar] [CrossRef]
- Reckmeier, C.J.; Wang, Y.; Zboril, R.; Rogach, A.L. Influence of Doping and Temperature on Solvatochromic Shifts in Optical Spectra of Carbon Dots. J. Phys. Chem. C 2016, 120, 10591–10604. [Google Scholar] [CrossRef]
- Alaş, M.; Genç, R. Solvatochromic Surface-Passivated Carbon Dots for Fluorometric Moisture Sensing in Organic Solvents. ACS Appl. Nano Mater. 2021, 4, 7974–7987. [Google Scholar] [CrossRef]
- Basu, N.; Mandal, D. Solvatochromic Response of Carbon Dots: Evidence of Solvent Interaction with Different Types of Emission Centers. J. Phys. Chem. C 2018, 122, 18732–18741. [Google Scholar] [CrossRef]
- Wang, H.; Haydel, P.; Sui, N.; Wang, L.; Liang, Y.; Yu, W.W. Wide emission shifts and high quantum yields of solvatochromic carbon dots with rich pyrrolic nitrogen. Nano Res. 2020, 13, 2492–2499. [Google Scholar] [CrossRef]
- Vijeata, A.; Chaudhary, G.R.; Umar, A.; Chaudhary, S. Distinctive Solvatochromic Response of Fluorescent Carbon Dots Derived from Different Components of Aegle Marmelos Plant. Eng. Sci. 2021, 15, 197–209. [Google Scholar] [CrossRef]
- Kundu, A.; Park, B.; Oh, J.; Sankar, K.V.; Ray, C.; Kim, W.S.; Jun, S.C. Multicolor emissive carbon dot with solvatochromic behavior across the entire visible spectrum. Carbon 2020, 156, 110–118. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Zhu, Y.; Jiang, H.; Tan, D.; Xu, Z.; Mei, T.; Li, J.; Xue, L.; Wang, X. Green emitting N,S-co-doped carbon dots for sensitive fluorometric determination of Fe(III) and Ag(I) ions, and as a solvatochromic probe. Microchim. Acta 2018, 185, 510. [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Pal, A.; Rahman, M.A.; Ali, M.; Khan, J.A.; Sk, M.P. Insights on the solvatochromic effects in N-doped yellow-orange emissive carbon dots. New J. Chem. 2018, 42, 19837–19843. [Google Scholar] [CrossRef]
- Ishchenko, A.A.; Kulinich, A.V.; Bondarev, S.L.; Knyukshto, V.N. Electronic structure and fluorescent properties of malononitrile-based merocyanines with positive and negative solvatochromism. Opt. Spectrosc. 2008, 104, 57–68. [Google Scholar] [CrossRef]
- Domínguez, M.; Rezende, M.C. Towards a unified view of the solvatochromism of phenolate betaine dyes. J. Phys. Org. Chem. 2010, 23, 156–170. [Google Scholar] [CrossRef]
- Ren, J.; Sun, J.; Sun, X.; Song, R.; Xie, Z.; Zhou, S. Precisely Controlled Up/Down-Conversion Liquid and Solid State Photoluminescence of Carbon Dots. Adv. Opt. Mater. 2018, 6, 1800115. [Google Scholar] [CrossRef]
- Yoshinaga, T.; Shinoda, M.; Iso, Y.; Isobe, T.; Ogura, A.; Takao, K.-I. Glycothermally Synthesized Carbon Dots with Narrow-Bandwidth and Color-Tunable Solvatochromic Fluorescence for Wide-Color-Gamut Displays. ACS Omega 2021, 6, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A. Enhancement of the optical properties of PVP using Zn1-xSnxS for UV-region optical applications. Appl. Phys. A 2021, 127, 1–9. [Google Scholar] [CrossRef]
- Agool, I.R.; Kadhim, K.J.; Hashim, A. Synthesis of (PVA–PEG–PVP–ZrO2) nanocomposites for energy release and gamma shielding applications. Int. J. Plast. Technol. 2017, 21, 444–453. [Google Scholar] [CrossRef]
- Carotenuto, G.; Pepe, G.P.; Nicolais, L. Preparation and characterization of nano-sized Ag/PVP composites for optical applications. Eur. Phys. J. B 2000, 16, 11–17. [Google Scholar] [CrossRef]
- Husain, M.S.B.; Gupta, A.; Alashwal, B.Y.; Sharma, S. Synthesis of PVA/PVP based hydrogel for biomedical applications: A review. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 40, 2388–2393. [Google Scholar] [CrossRef]
- Rao, C.V.S.; Ravi, M.; Raja, V.; Bhargav, P.B.; Sharma, A.K.; Rao, V.V.R.N. Preparation and characterization of PVP-based polymer electrolytes for solid-state battery applications. Iran. Polym. J. 2012, 21, 531–536. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, R.Y. The Role of Polymers in Cosmetics: Recent Trends. In Cosmetic Nanotechnology; ACS Symposium Series; American Chemical Society: Hattiesburg, MS, USA, 2007; Volume 961, pp. 3–56. [Google Scholar]
- Feng, T.; Zhu, S.; Zeng, Q.; Lu, S.; Tao, S.; Liu, J.; Yang, B. Supramolecular Cross-Link-Regulated Emission and Related Applications in Polymer Carbon Dots. ACS Appl. Mater. Interfaces 2018, 10, 12262–12277. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, H.; Xu, J.; Wu, Y.; Zang, Y.; Sun, J. Color Emission Carbon Dots with Quench-ResixAstant Solid-State Fluorescence for Light-Emitting Diodes. ACS Sustain. Chem. Eng. 2021, 9, 3901–3908. [Google Scholar] [CrossRef]
- Ren, J.; Stagi, L.; Innocenzi, P. Fluorescent carbon dots in solid-state: From nanostructures to functional devices. Prog. Solid State Chem. 2021, 62, 100295. [Google Scholar] [CrossRef]
- Javanbakht, S.; Namazi, H. Solid state photoluminescence thermoplastic starch film containing graphene quantum dots. Carbohydr. Polym. 2017, 176, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Pal, A.; Sk, M.P. Review—Aggregation-Induced Emission in Carbon Dots for Potential Applications. ECS J. Solid State Sci. Technol. 2021, 10, 021001. [Google Scholar] [CrossRef]
- Li, H.; Su, D.; Gao, H.; Yan, X.; Kong, D.; Jin, R.; Liu, X.; Wang, C.; Lu, G. Design of Red Emissive Carbon Dots: Robust Performance for Analytical Applications in Pesticide Monitoring. Anal. Chem. 2020, 92, 3198–3205. [Google Scholar] [CrossRef] [PubMed]
- Havrdova, M.; Hola, K.; Skopalik, J.; Tománková, K.; Petr, M.; Cepe, K.; Polakova, K.; Tucek, J.; Bourlinos, A.B.; Zboril, R. Toxicity of carbon dots-Effect of surface functionalization on the cell viability, reactive oxygen species generation and cell cycle. Carbon 2016, 99, 238–248. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A.C. A two-stage poly(ethylenimine)-mediated cytotoxicity: Implications for gene transfer/therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef]
- Iida, T.; Mori, T.; Katayama, Y.; Niidome, T. Overall interaction of cytosolic proteins with the PEI/DNA complex. J. Control. Release 2007, 118, 364–369. [Google Scholar] [CrossRef]
- Kircheis, R.; Wightman, L.; Wagner, E. Design and gene delivery activity of modified polyethylenimines. Adv. Drug Deliv. Rev. 2001, 53, 341–358. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Zhang, C. Polyethyleneimine-Functionalized Fluorescent Carbon Dots: Water Stability, pH Sensing, and Cellular Imaging. Chemnanomat 2015, 1, 122–127. [Google Scholar] [CrossRef]
- Yin, J.-Y.; Liu, H.-J.; Jiang, S.; Chen, Y.; Yao, Y. Hyperbranched Polymer Functionalized Carbon Dots with Multistimuli-Responsive Property. ACS Macro Lett. 2013, 2, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Ashcraft, A.; Liu, K.; Mukhopadhyay, A.; Paulino, V.; Liu, C.; Bernard, B.; Husainy, D.; Phan, T.; Olivier, J.-H.H. A Molecular Strategy to Lock-in the Conformation of a Perylene Bisimide-Derived Supramolecular Polymer. Angew. Chem. Int. Ed. 2020, 59, 7487–7493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zahran, E.M.; Quiroga, B.A.; Perez, J.; Mintz, K.J.; Peng, Z.; Liyanage, P.Y.; Pandey, R.R.; Chusuei, C.C.; Leblanc, R.M. Size-dependent photocatalytic activity of carbon dots with surface-state determined photoluminescence. Appl. Catal. B Environ. 2019, 248, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Li, D.; Zhou, X.; Zhou, G.; Xiao, H.; Zhou, D.; Tian, P.; Guo, R.; Qu, S. Highly Emissive Carbon Dots in Solid State and Their Applications in Light-Emitting Devices and Visible Light Communication. ACS Sustain. Chem. Eng. 2019, 7, 9301–9308. [Google Scholar] [CrossRef]
- Xu, X.; Cai, L.; Hu, G.; Mo, L.; Zheng, Y.; Hu, C.; Lei, B.; Zhang, X.; Liu, Y.; Zhuang, J. Red-emissive carbon dots from spinach: Characterization and application in visual detection of time. J. Lumin. 2020, 227, 117534. [Google Scholar] [CrossRef]
- Hu, Y.; Al Awak, M.M.; Yang, F.; Yan, S.; Xiong, Q.; Wang, P.; Tang, Y.; Yang, L.; LeCroy, G.E.; Hou, X.; et al. Photoexcited state properties of carbon dots from thermally induced functionalization of carbon nanoparticles. J. Mater. Chem. C 2016, 4, 10554–10561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Wei, F.; Wu, X.; Zhou, P.; Chen, Q.; Cen, Y.; Xu, G.; Cheng, X.; Zhang, A.; Hu, Q. PEI modified orange emissive carbon dots with excitation-independent fluorescence emission for cellular imaging and siRNA delivery. Carbon 2021, 177, 403–411. [Google Scholar] [CrossRef]
- Ru, Y.; Waterhouse, G.I.N.; Lu, S. Aggregation in carbon dots. Aggregate 2022, 3, e296. [Google Scholar] [CrossRef]
- Nigam, S.; Rutan, S. Principles and Applications of Solvatochromism. Appl. Spectrosc. 2001, 55, 362A–370A. [Google Scholar] [CrossRef]
- de Melo, C.E.A.; Nandi, L.G.; Domínguez, M.; Rezende, M.C.; Machado, V.G. Solvatochromic behavior of dyes with dimethylamino electron-donor and nitro electron-acceptor groups in their molecular structure. J. Phys. Org. Chem. 2015, 28, 250–260. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Xu, T.; She, M.; Chen, J.; Jia, X.; Liu, P.; Liu, X.; Li, J. Red emissive carbon dots as a fluorescent sensor for fast specific monitoring and imaging of polarity in living cells. J. Mater. Chem. A 2023, 11, 2679–2689. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Microchim. Acta 2019, 186, 583. [Google Scholar] [CrossRef] [PubMed]
- Kosheleva, I.M.; Gembitskii, P.A.; Chmarin, A.I.; Kolesova, L.M.; Zhuk, D.S.; Kargin, V.A. Some properties and the structure of high-molecular polyethylenimine. Russ. Chem. Bull. 1971, 20, 1536–1541. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Portney, N.G.; Cui, D.; Budak, G.; Ozbay, E.; Ozkan, M.; Ozkan, C.S. Zeta potential: A surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed. Microdevices 2008, 10, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhuo, P.; Yin, H.; Fan, Y.; Zhang, J.; Liu, X.; Chen, Z. Solid-State Fluorescent Carbon Dots with Aggregation-Induced Yellow Emission for White Light-Emitting Diodes with High Luminous Efficiencies. ACS Appl. Mater. Interfaces 2019, 11, 24395–24403. [Google Scholar] [CrossRef]
- Wei, S.; Li, Z.; Lu, W.; Liu, H.; Zhang, J.; Chen, T.; Tang, B.Z. Multicolor Fluorescent Polymeric Hydrogels. Angew. Chem. Int. Ed. 2021, 60, 8608–8624. [Google Scholar] [CrossRef]
- Feng, Z.; Adolfsson, K.H.; Xu, Y.; Fang, H.; Hakkarainen, M.; Wu, M. Carbon dot/polymer nanocomposites: From green synthesis to energy, environmental and biomedical applications. Sustain. Mater. Technol. 2021, 29, e00304. [Google Scholar] [CrossRef]
- Gan, Q.; Qin, N.; Zhu, Y.; Huang, Z.; Zhang, F.; Gu, S.; Xie, J.; Zhang, K.; Lu, L.; Lu, Z. Polyvinylpyrrolidone-Induced Uniform Surface-Conductive Polymer Coating Endows Ni-Rich LiNi0.8Co0.1Mn0.1O2 with Enhanced Cyclability for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 12594–12604. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Zhang, X.; Wang, L.; Yang, J.; Fan, E.; Wu, F.; Chen, R.; Li, L. Improved Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Cathode Materials Induced by a Facile Polymer Coating for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 6205–6213. [Google Scholar] [CrossRef]
- Kaur, G.; Gates, B.D. Review—Surface Coatings for Cathodes in Lithium Ion Batteries: From Crystal Structures to Electrochemical Performance. J. Electrochem. Soc. 2022, 169, 043504. [Google Scholar] [CrossRef]
- Lee, J.-E.; Kim, M.-C.; Moon, S.-H.; Kim, E.-S.; Shin, Y.-K.; Choi, S.; Kwon, S.-H.; Kim, S.-J.; Kwon, H.-J.; Park, K.-W. Role of polyvinylpyrrolidone in the electrochemical performance of Li2MnO3 cathode for lithium-ion batteries. RSC Adv. 2019, 9, 10297–10304. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-Doped Graphene Quantum Dots with Oxygen-Rich Functional Groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef]
- Hu, S.; Huang, Q.; Lin, Y.; Wei, C.; Zhang, H.; Zhang, W.; Guo, Z.; Bao, X.; Shi, J.; Hao, A. Reduced graphene oxide-carbon dots composite as an enhanced material for electrochemical determination of dopamine. Electrochim. Acta 2014, 130, 805–809. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Wang, L.; Bu, F.; Wei, J.; Pan, D.; Wu, M. Hierarchical 3D All-Carbon Composite Structure Modified with N-Doped Graphene Quantum Dots for High-Performance Flexible Supercapacitors. Small 2018, 14, 1801498. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xie, Y. Phosphomolybdic acid cluster bridging carbon dots and polyaniline nanofibers for effective electrochemical energy storage. J. Mater. Sci. 2019, 54, 4842–4858. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Q.; Chen, Q.; Li, C.M.; Chen, J. Synergistically boosting the electrochemical performance of polypyrrole-coated activated carbon derived from carbon dots for a high-performance supercapacitor. Chem. Commun. 2021, 57, 9264–9267. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhou, M.; Wang, G.; Wang, Q.; Yan, M.; Bi, H. Morphology-dependent electrochemical performance of nitrogen-doped carbon dots@polyaniline hybrids for supercapacitors. Int. J. Energy Res. 2019, 43, 7529–7540. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Gu, J.; Zhang, X.; Bartoli, M.; Domena, J.B.; Zhou, Y.; Zhang, W.; Paulino, V.; Ferreira, B.C.L.B.; et al. Cancer cells inhibition by cationic carbon dots targeting the cellular nucleus. J. Colloid Interface Sci. 2023, 637, 193–206. [Google Scholar] [CrossRef] [PubMed]


| OPTICAL ANALYSIS OF 813 CDS IN VARIOUS SOLVENTS OF DECREASING POLARITY | |||
|---|---|---|---|
| SOLVENT | Polarity Index (P) | Farthest PL (nm) | PL Intensity (CPS) |
| WATER | 1.00 | 698 | |
| ETHANOL | 0.654 | 650 | |
| ACETIC ACID | 0.648 | 655 | |
| DMSO | 0.444 | 610 | |
| DMF | 0.386 | 655 | |
| METHYLENE CHLORIDE | 0.309 | 655 | |
| CHLOROFORM | 0.259 | 653 | |
| ETHYL ACETATE | 0.228 | 650 | |
| ETHER | 0.117 | 650 | |
| TOLUENE | 0.099 | 650 | |
| HEXANE | 0.009 | 590 | |
| Carbon Dots | Zeta Potential (mV) |
|---|---|
| 813-CD-600 PEI | −5.11 mV |
| 813-CD-25K PEI | +18.8 mV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domena, J.B.; Celebic, E.; Ferreira, B.C.L.B.; Zhou, Y.; Zhang, W.; Chen, J.; Bartoli, M.; Tagliaferro, A.; Johnson, Q.; Chauhan, B.P.S.; et al. Investigation into Red Emission and Its Applications: Solvatochromic N-Doped Red Emissive Carbon Dots with Solvent Polarity Sensing and Solid-State Fluorescent Nanocomposite Thin Films. Molecules 2023, 28, 1755. https://doi.org/10.3390/molecules28041755
Domena JB, Celebic E, Ferreira BCLB, Zhou Y, Zhang W, Chen J, Bartoli M, Tagliaferro A, Johnson Q, Chauhan BPS, et al. Investigation into Red Emission and Its Applications: Solvatochromic N-Doped Red Emissive Carbon Dots with Solvent Polarity Sensing and Solid-State Fluorescent Nanocomposite Thin Films. Molecules. 2023; 28(4):1755. https://doi.org/10.3390/molecules28041755
Chicago/Turabian StyleDomena, Justin B., Ermin Celebic, Braulio C. L. B. Ferreira, Yiqun Zhou, Wei Zhang, Jiuyan Chen, M. Bartoli, A. Tagliaferro, Qiaxian Johnson, Bhanu P. S. Chauhan, and et al. 2023. "Investigation into Red Emission and Its Applications: Solvatochromic N-Doped Red Emissive Carbon Dots with Solvent Polarity Sensing and Solid-State Fluorescent Nanocomposite Thin Films" Molecules 28, no. 4: 1755. https://doi.org/10.3390/molecules28041755
APA StyleDomena, J. B., Celebic, E., Ferreira, B. C. L. B., Zhou, Y., Zhang, W., Chen, J., Bartoli, M., Tagliaferro, A., Johnson, Q., Chauhan, B. P. S., Paulino, V., Olivier, J.-H., & Leblanc, R. M. (2023). Investigation into Red Emission and Its Applications: Solvatochromic N-Doped Red Emissive Carbon Dots with Solvent Polarity Sensing and Solid-State Fluorescent Nanocomposite Thin Films. Molecules, 28(4), 1755. https://doi.org/10.3390/molecules28041755