Carbon-Based Nanomaterials for Catalytic Wastewater Treatment: A Review
Abstract
:1. Introduction
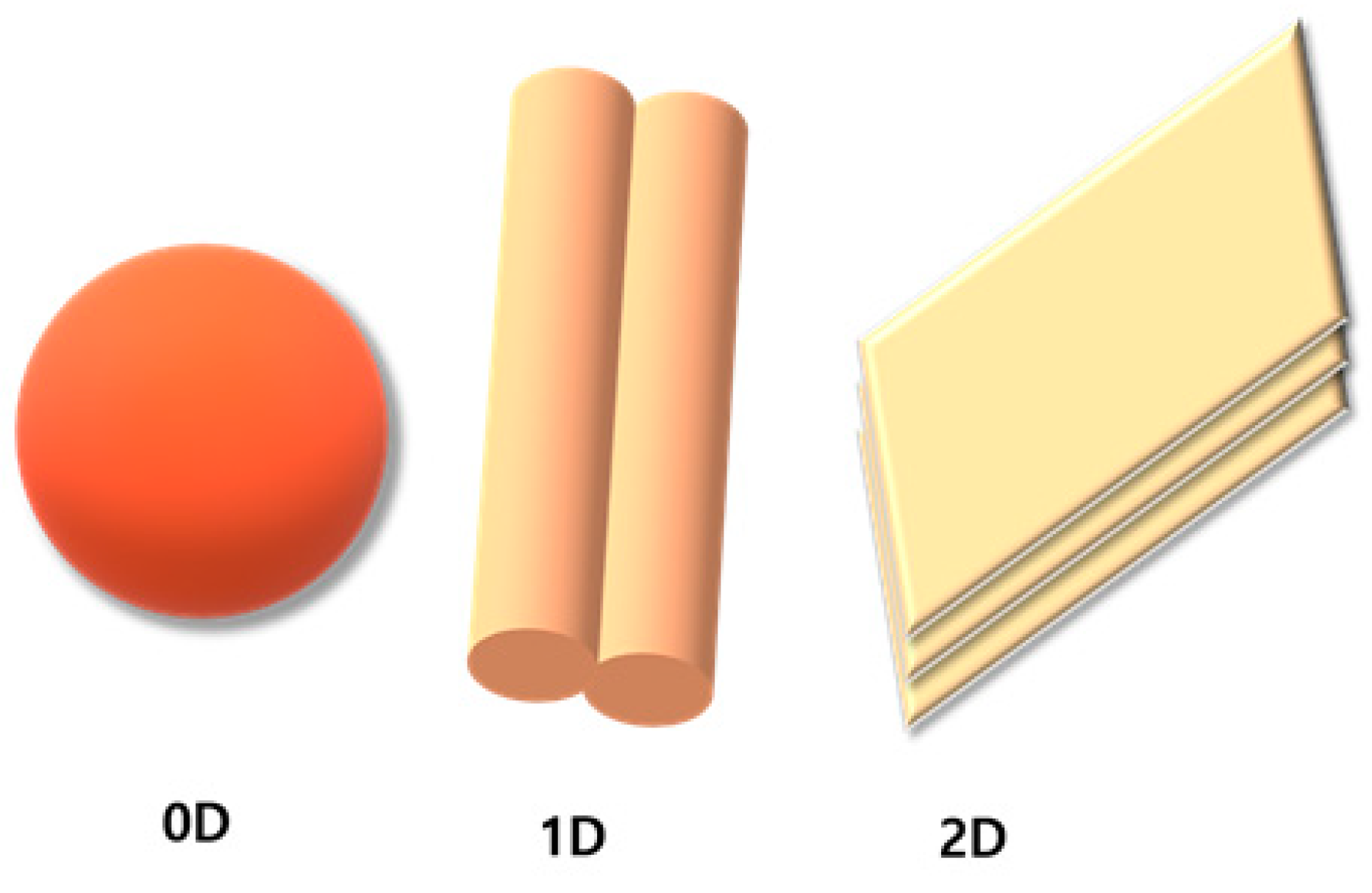
2. Properties of Different CBMs
2.1. 0D CBMs
2.2. 1D CBMs
2.3. 2D CBMs
3. CBM as Fenton-like Catalysts for Wastewater Treatment
- (i)
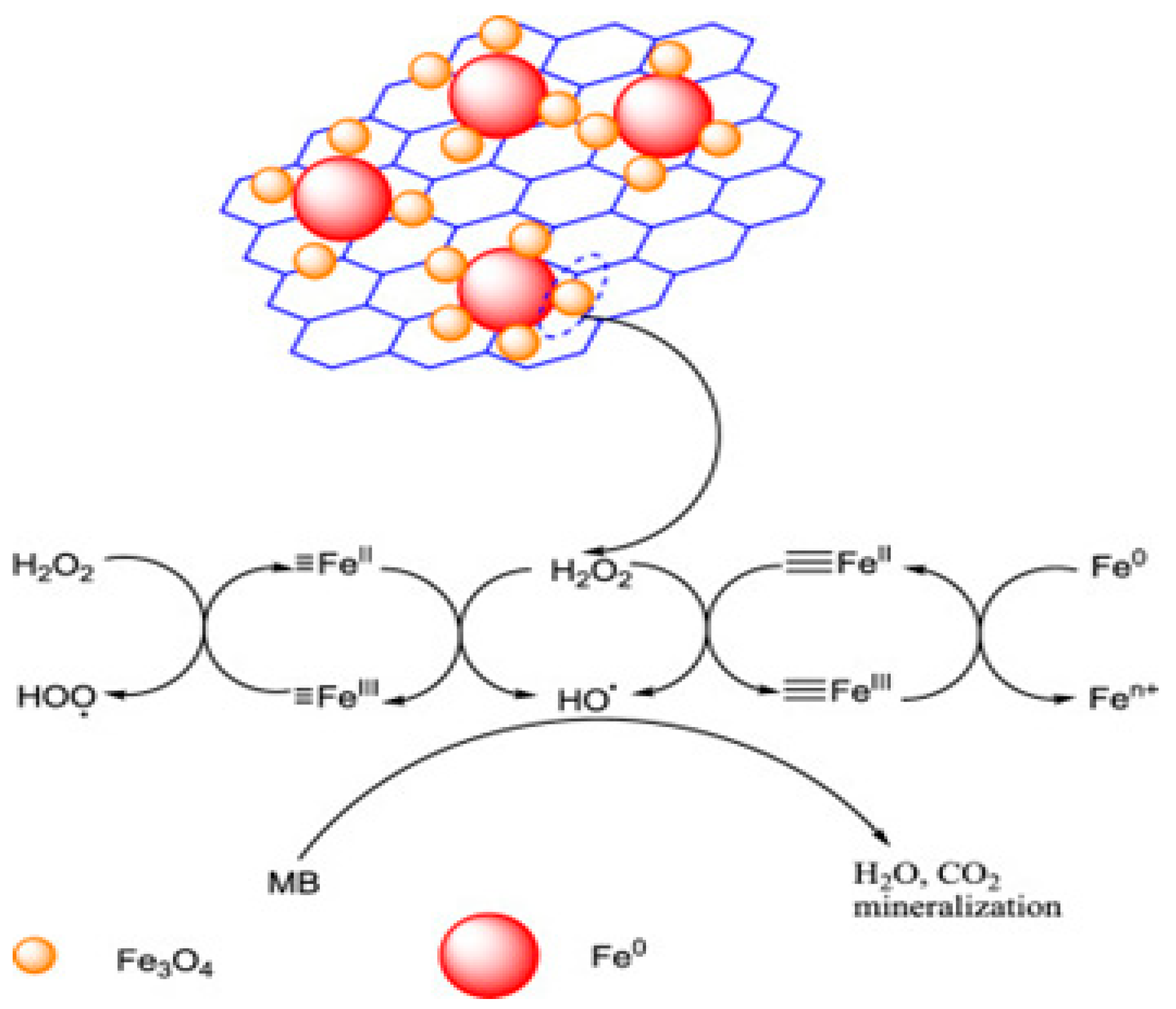
- Traditional Fenton reagents: The conventional system consists of H2O2 and Fe2+, which react with organic molecules through •OH produced by the catalytic decomposition of H2O2 (Equations (1) and (2)). As the •OH has a high oxidation potential, it is quickly oxidized, leading to a very fast reaction. However, many researchers have confirmed that the consumption rate of H2O2 is low, making it relatively difficult to apply directly to drinking water treatment.Fe2+ + H2O2 → Fe3+ + ·OH + OH−Fe3+ + H2O2 → Fe2+ + HO2· + H+
- (ii)
- Fenton-like reactions: Many researchers have worked to improve the traditional Fenton oxidation method and a large number of improved Fenton reagents such as H2O2/Fe3+, H2O2/O3, light-Fenton reagents, and electro-Fenton reagents have appeared. This method is similar to the Fenton reaction, although Fenton-like reagents were used [39].
- (iii)
- Light-Fenton reaction: This reaction introduces light sources (UV/visible light) for the Fenton reaction. The UV and Fenton catalysts can have a synergistic effect on the catalytic decomposition of H2O2, which greatly improves the oxidation of Fenton reagents. However, the amount of UV light incident at the surface of the earth is relatively low (approximately 4%), and visible light is 43%; thus, the Fenton system in the presence of light is of great significance and enhances the degree of mineralization. In this case, the Fenton-like catalyst first absorbs photons from the light source and oxidizes water molecules to produce hydroxyl radicals. Again, the electrons of the iron atoms experience charge transfer with oxygen atoms, and Fe(II) is oxidized to Fe(III) by the dissolved oxygen (O2) in the solution, so that HOO· and ·OH are generated during the reactions [49].
- (iv)
- Electro-Fenton method: This method is an electrochemical advanced oxidation process that can produce H2O2 when it reacts with Fe2+, produced by the oxidation of the Fe anode, generating ·OH and Fe3+. This makes use of the strong oxidizing power of ·OH to catalyze the degradation of organic matter.
- (v)
- Ultrasound-Fenton method: The ultrasound-Fenton method can be used for the pyrolysis of pollutants owing to the local high temperature and pressure generated by the ultrasonic treatment. Moreover, the strong oxidation potential of the hydroxyl radicals generated in high-temperature and high-pressure environments has an oxidizing effect on pollutants.
- (vi)
- Microwave-Fenton method: This method is similar to the ultrasound method, where the only difference is the use of microwave radiation instead of ultrasound, which promotes the decomposition of H2O2 to produce ·OH and helps to destroy the organic pollutants in wastewater.
3.1. Role of CBM in the Fenton Reaction
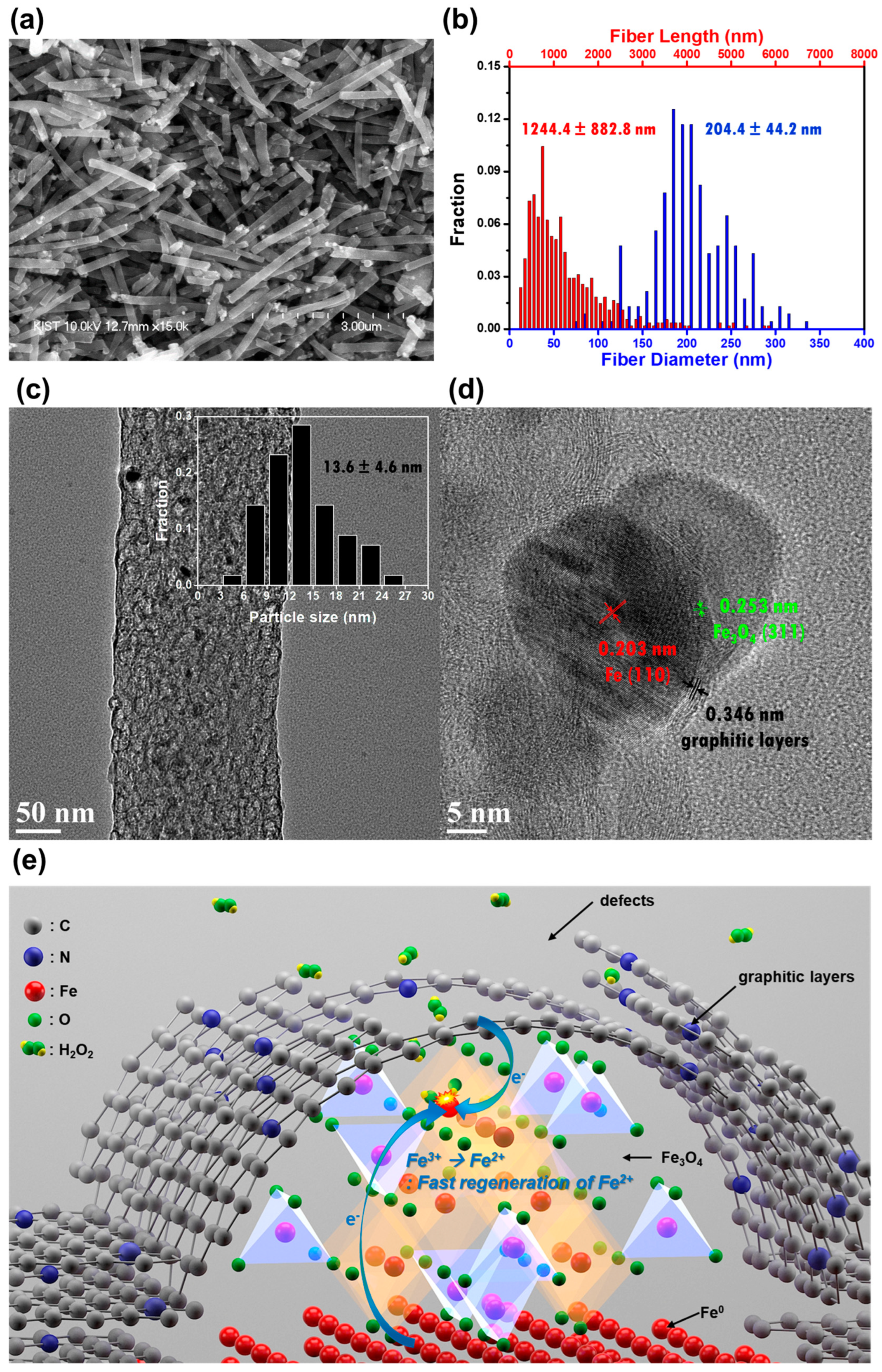
3.1.1. 0D CBM
D CBM for Fenton Reaction
D CBM for Fenton Reaction
4. Carbon-Based Photocatalysts
4.1. OD CBM Photocatalysts
4.2. 1D CBM Photocatalysts
4.3. 2D CBM Photocatalysts
4.3.1. Graphene-Derived Materials for Photocatalytic Wastewater Treatment
4.3.2. Graphdiyne Based Materials for Photocatalytic Waste Water Treatment
4.3.3. g-C3N4 Derived Materials for Photocatalytic Waste Water Treatment
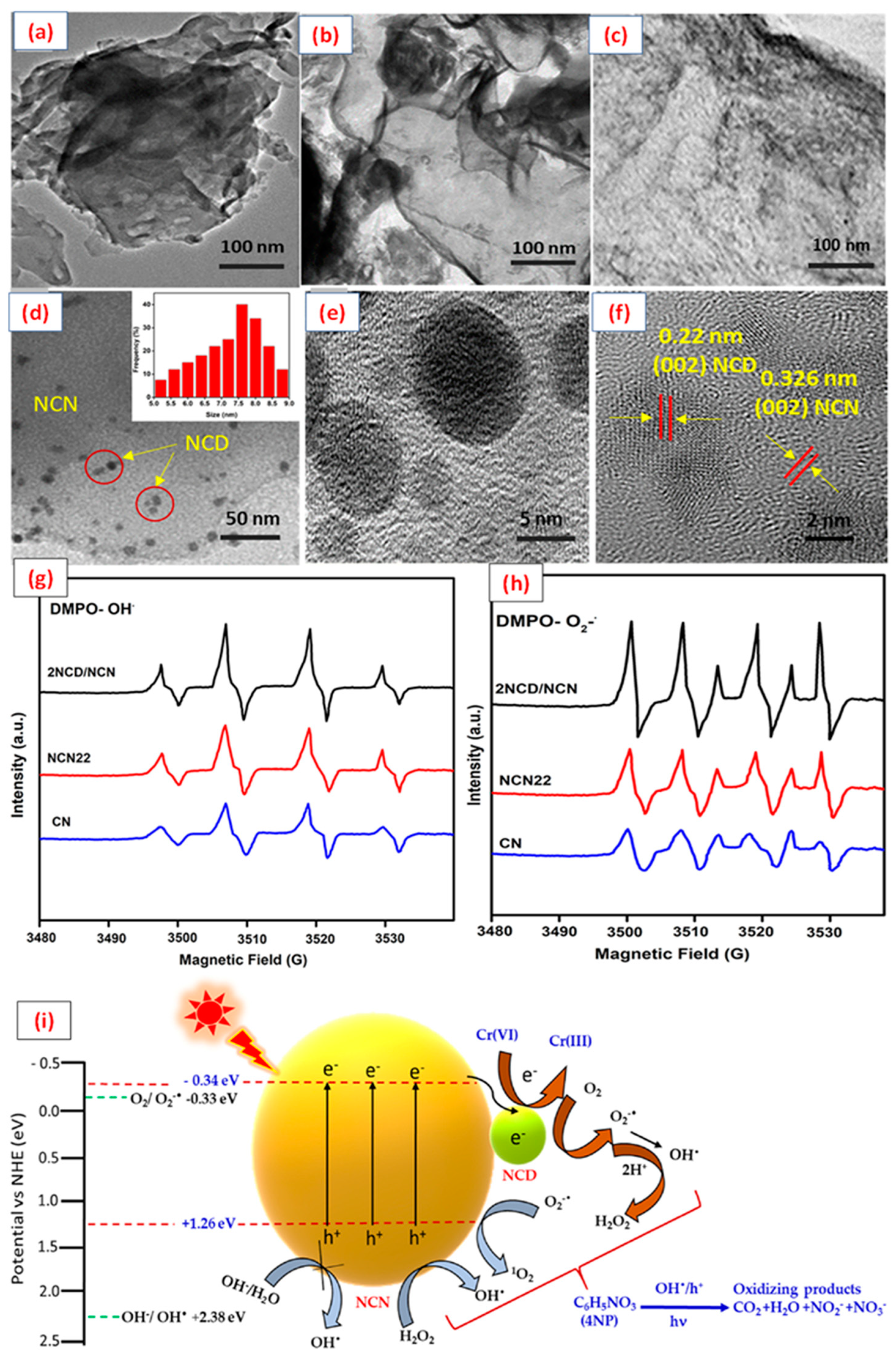
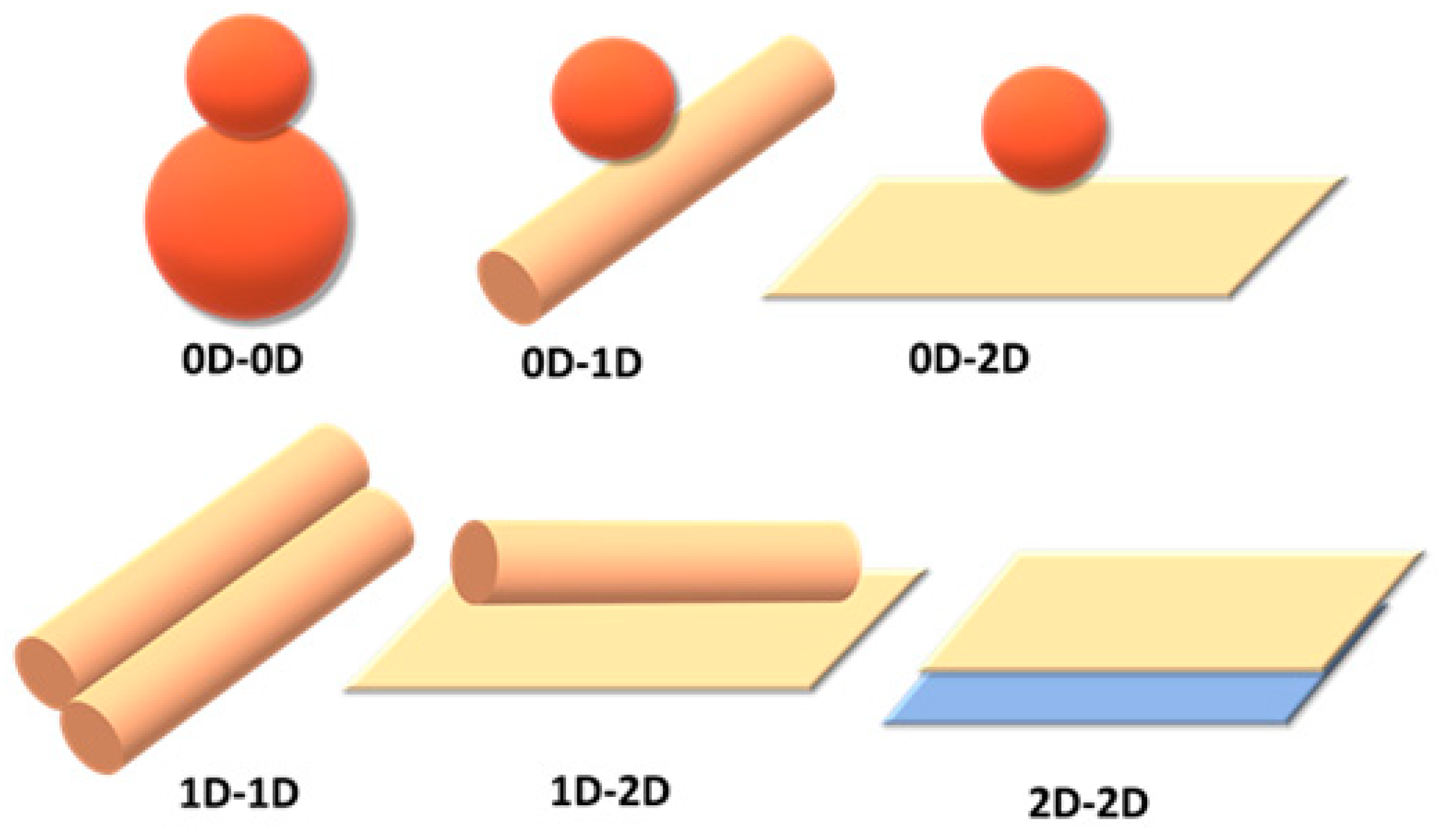
4.3.4. Multidimensional Hybrid CBM-Based Photocatalysts
5. Conclusions and Prospects
- Fenton-like reactions are currently in use for the treatment of aqueous organic pollutants; however, further investigation of the reaction mechanism and its efficient application is required. Moreover, novel and inexpensive catalysts that do not cause secondary pollution and that behave in an environmentally friendly manner should be developed.
- In addition, current research is mainly focused on the multi-component hybridization of 0D/1D/2D CBM and other CBMs for photocatalytic applications to improve photocatalytic performance. However, the development of 3D CBMs with other carbonaceous materials is still lacking. Therefore, future research can attempt to couple 3D CBMs with carbonaceous materials of various dimensions, which can be expected to bring new prospects and stronger photocatalytic performance.
- Compared to traditional metal-based modifiers, carbonaceous materials are relatively simple and cost-effective. However, it is worth noting that the cost of some carbon materials, calculated on a laboratory scale, such as 0D-CD are still far from being viable for large-scale applications. Therefore, the development of low-dimensional low-cost carbon materials for hybrid CBMs is not only the exploration of new synthetic CBMs but also the source of raw materials selected in the future.
- As photocatalytic applications involve many steps, such as photon absorption, charge formation, charge carrier separation and recombination, surface reactions for radical generation, and targeted reactions, the final photo efficiency is accurately controlled by each step and should be integrated on an experimental and theoretical basis.
- Despite the substantial advances in the synthesis and catalytic activity of green catalysts, future research should focus on reducing the cost of the synthesis of CBM-based materials and improving their catalytic efficiency.
- Biowaste-derived CBMs should be used in wastewater treatment processes.
- Magnetic CBMs should be considered for the synthesis of materials, in order to increase their activity and potential for reuse.
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Al-Tohamy, R.; Ali, S.; Li, F.; Okasha, K.; Mahmoud, Y.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approach for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, L.; Parida, K. A review of solar and visible light active oxo-bridged materials for energy and environment. Catal. Sci. Technol. 2017, 7, 2153–2164. [Google Scholar] [CrossRef]
- Cao, H.-L.; Cai, F.-Y.; Yu, K.; Zhang, Y.-Q.; Lü, J.; Cao, R. Photocatalytic Degradation of Tetracycline Antibiotics over CdS/Nitrogen-Doped–Carbon Composites Derived from in Situ Carbonization of Metal–Organic Frameworks. ACS Sustain. Chem. Eng. 2019, 7, 10847–10854. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.; Alexandre-Franco, M.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef]
- Saibu, S.; Adebusoye, S.; Oyetibo, G. Aerobic bacterial transformation and biodegradation of dioxins: A review. Bioresour. Bioprocess. 2020, 7, 7. [Google Scholar] [CrossRef]
- Saleh, I.; Zouari, N.; Al-Ghouti, M. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, M.; Oturan, N.; Li, Y.; Su, P.; Oturan, M. Enhanced activation of hydrogen peroxide using nitrogen doped graphene for effective removal of herbicide 2,4-D from water by iron-free electrochemical advanced oxidation. Electrochim. Acta 2019, 297, 582–592. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Rehman, M.N.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Dual Z-scheme core-shell PANI-CeO2-Fe2O3-NiO heterostructured nanocomposite for dyes remediation under sunlight and bacterial disinfection. Environ. Res. 2022, 215, 114140. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Rehman, M.N.; Mahmood, K.; Batool, S.; Hasan, M.; Rehman, K.; Iqbal, F. Enhancement in carrier separation of ZnO-Ho2O3-Sm2O3 heterostructured nanocomposite with rGO and PANI supported direct dual Z-scheme for antimicrobial inactivation and sunlight driven photocatalysis. Adv. Powder Technol. 2021, 32, 3770–3787. [Google Scholar] [CrossRef]
- Munawar, T.; Nadeem, M.S.; Mukhtar, F.; Rehman, M.N.; Riaz, M.; Batool, S.; Hasan, M.; Iqbal, F. Transition metal-doped SnO2 and graphene oxide (GO) supported nanocomposites as efficient photocatalysts and antibacterial agents. Environ. Sci. Pollut. Res. 2022, 29, 90995–91016. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Enhanced sunlight-absorption of Fe2O3 covered by PANI for the photodegradation of organic pollutants and antimicrobial inactivation. Adv. Powder Technol. 2022, 33, 103708. [Google Scholar] [CrossRef]
- Parida, K.; Mohapatra, L. Carbonate intercalated Zn/Fe layered double hydroxide: A novel photocatalyst for the enhanced photo degradation of azo dyes. Chem. Eng. J. 2012, 179, 131–139. [Google Scholar] [CrossRef]
- Rohilla, S.; Gupta, A.; Kumar, V.; Kumari, S.; Petru, M.; Amor, N.; Noman, M.T.; Dalal, J. Excellent UV-Light Triggered Photocatalytic Performance of ZnO.SiO2 Nanocomposite for Water Pollutant Compound Methyl Orange Dye. Nanomaterials 2021, 11, 2548. [Google Scholar] [CrossRef]
- Shimi, A.K.; Parvathiraj, C.; Kumari, S.; Dalal, J.; Kumar, V.; Wabaidurd, S.M.; Alothman, Z.A. Green synthesis of SrO nanoparticles using leaf extract of Albizia julibrissin and its recyclable photocatalytic activity: An eco-friendly approach for treatment of industrial wastewater. Environ. Sci. Adv. 2022, 1, 849–861. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.; Kumar, P.; Rangasamy, G.; Hemavathy, R.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef]
- Fu, T.; Zhang, B.; Gao, X.; Cui, S.; Guan, C.-Y.; Zhang, Y.; Zhang, B.; Peng, Y. Recent progresses, challenges, and opportunities of carbon-based materials applied in heavy metal polluted soil remediation. Sci. Total Environ. 2023, 856, 158810. [Google Scholar] [CrossRef]
- Quan, C.; Zhou, Y.; Wang, J.; Wu, C.; Gao, N. Biomass-based carbon materials for CO2 capture: A review. J. CO2 Util. 2023, 68, 102373. [Google Scholar] [CrossRef]
- Dutta, V.; Verma, R.; Yuan, M.-H.; Batoo, K.M.; Jayavel, R.; Chauhan, A.; Lin, K.-Y.A.; Balasubramani, R.; Ghotekar, S. Bio-Inspired Synthesis of Carbon-Based Nanomaterials and Their Potential Environmental Applications: A State-of-the-Art Review. Inorganics 2022, 10, 169. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental Applications of Carbon-Based Nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Photocatalytic water splitting hydrogen production via environmental benign carbon-based nanomaterials. Int. J. Hydrogen Energy 2021, 46, 33696–33717. [Google Scholar] [CrossRef]
- Liu, Z.; Ling, Q.; Cai, Y.; Xu, L.; Su, J.; Yu, K.; Wu, X.; Xu, J.; Hu, B.; Wang, X. Synthesis of carbon-based nanomaterials and their application in pollution management. Nanoscale Adv. 2022, 4, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Biglova, Y.; Mustafin, A. Nucleophilic cyclopropanation of [60]fullerene by the addition–elimination mechanism. RSC Adv. 2019, 9, 22428–22498. [Google Scholar] [CrossRef]
- Javey, A. The 2008 Kavli Prize in Nanoscience: Carbon Nanotubes. ACS Nano 2008, 2, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.; Araujo, P. Perspectives on the 2010 Nobel Prize in Physics for Graphene. ACS Nano 2010, 4, 6297–6302. [Google Scholar] [CrossRef]
- Fei, J.; Peng, X.; Jiang, L.; Yuan, X.; Chen, X.; Zhao, Y.; Zhang, W. Recent advances in graphitic carbon nitride as a catalyst for heterogeneous Fenton-like reactions. Dalton Trans. 2021, 50, 16887–16908. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ye, X.; Qi, M.; Chen, M.; Zhang, L.; Zhang, J. Zero to Three Dimension Structure Evolution from Carbon Allotropes to Phosphorus Allotropes. Adv. Mater. Interfaces 2022, 2201941. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Vorobiev, A.; Gazizov, R.; Borschevskii, A.; Markov, V.; Ioutsi, V.; Brotsman, V.; Sidorov, L. Fullerene as Photocatalyst: Visible-Light Induced Reaction of Perfluorinated α,ω-Diiodoalkanes with C60. J. Phys. Chem. A 2017, 121, 113–121. [Google Scholar] [CrossRef]
- Jozeliūnaitė, A.; Valčeckas, D.; Orentas, E. Fullerene soot and a fullerene nanodispersion as recyclable heterogeneous off-the-shelf photocatalysts. RSC Adv. 2021, 11, 4104–4111. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-D.; Dey, N.; Seo, H.; Kim, Y.; Lim, D.; Lee, M. Photocatalytic decomposition of toluene by nanodiamond-supported TiO2 prepared using atomic layer deposition. Appl. Catal. A Gen. 2011, 408, 148–155. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.; Zainal, Z.; Yusof, N. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Julkapli, N.M. Nano-diamond based photocatalysis for solar hydrogen production. Int. J. Hydrogen Energy 2020, 45, 31538–31554. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Central Science 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Song, W.; Zhao, B.; Yang, B. Spectroscopic studies of the optical properties of carbon dots: Recent advances and future prospects. Mater. Chem. Front. 2020, 4, 472–488. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Wang, D.; Zhang, J. Graphdiyne: Synthesis, properties, and applications. Chem. Soc. Rev. 2019, 48, 908–936. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.; Lau, S. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Rabeya, R.; Mahalingam, S.; Manap, A.; Satgunam, M.; Akhtaruzzaman, M.; Chia, C. Structural defects in graphene quantum dots: A review. Int. J. Quantum Chem. 2022, 122, e26900. [Google Scholar] [CrossRef]
- Jegannathan, P.; Yousefi, A.T.; Karim, M.; Kadri, N. Enhancement of graphene quantum dots based applications via optimum physical chemistry: A review. Biocybern. Biomed. Eng. 2018, 38, 481–497. [Google Scholar] [CrossRef]
- Sebastian, F.; Zorn, N.; Settele, S.; Lindenthal, S.; Berger, F.; Bendel, C.; Li, H.; Flavel, B.; Zaumseil, J. Absolute Quantification of sp3 Defects in Semiconducting Single-Wall Carbon Nanotubes by Raman Spectroscopy. J. Phys. Chem. Lett. 2022, 13, 3542–3548. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.H.; Eckhardt, H.; Kertesz, M. Structure-property predictions for new planar forms of carbon: Layered phases containing sp2 and sp atoms. J. Chem. Phys. 1987, 87, 6687–6699. [Google Scholar] [CrossRef]
- Zheng, J.-J.; Zhao, X.; Zhao, Y.; Gao, X. Two-Dimensional Carbon Compounds Derived from Graphyne with Chemical Properties Superior to Those of Graphene. Sci. Rep. 2013, 3, 1271. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Xie, K.; Fang, J.; Li, L.; Deng, J.; Chen, F. Progress of graphite carbon nitride with different dimensions in the photocatalytic degradation of dyes: A review. J. Alloy. Compd. 2022, 901, 163589. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.-K. In Situ Construction of g-C3N4/g-C3N4 Metal-Free Heterojunction for Enhanced Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Li, S.; Qiu, Y.; Feng, Y.; Liu, Y.; Ding, D.; Xu, L.; Ma, X.; Sun, W.; Chen, H. Carbonized paramagnetic complexes of Mn (II) as contrast agents for precise magnetic resonance imaging of sub-millimeter-sized orthotopic tumors. Nat. Commun. 2022, 13, 1938. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Shahzad, A.; Tahir, K.; Kim, J.; Moztahida, M.; Jang, J.; Alam, M.; Lee, S.-H.; Jung, H.-Y.; Lee, D. Photo-Fenton reaction for the degradation of sulfamethoxazole using a multi-walled carbon nanotube-NiFe2O4 composite. Chem. Eng. J. 2020, 382, 123053. [Google Scholar] [CrossRef]
- Zou, C.-y.; Meng, Z.-d.; Ji, W.-c.; Liu, S.-q.; Shen, Z.; Zhang, Y.; Jiang, N.-s. Preparation of a fullerene [60]-iron oxide complex for the photo-fenton degradation of organic contaminants under visible-light irradiation. Chin. J. Catal. 2018, 39, 1051–1059. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, R.; Liu, J.; Zhou, Q.; Zhu, J.; Liang, X.; Xi, Y.; He, H. Fullerol modification ferrihydrite for the degradation of acid red 18 under simulated sunlight irradiation. J. Mol. Catal. A Chem. 2016, 424, 393–401. [Google Scholar] [CrossRef]
- Nekoeinia, M.; Salehriahi, F.; Moradlou, O.; Yousefinejad, H.K.S. Enhanced Fenton-like catalytic performance of N-doped graphene quantum dot incorporated CuCo2O4. New J. Chem. 2018, 42, 9209–9220. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, P.; Jin, M.; Gao, G.; Xi, Q.; Zhou, H.; Xu, G.; Xu, J. Promoting the Photo-Fenton catalytic activity with carbon dots: Broadening light absorption, higher applicable pH and better reuse performance. Mol. Catal. 2020, 481, 110254. [Google Scholar] [CrossRef]
- Ago, H.; Kugler, T.; Cacialli, F.; Salaneck, W.; Shaffer, M.; Windle, A.; Friend, R. Work Functions and Surface Functional Groups of Multiwall Carbon Nanotubes. J. Phys. Chem. B 1999, 103, 8116–8121. [Google Scholar] [CrossRef]
- Seo, J.; Lee, H.-J.; Lee, H.; Kim, H.-E.; Lee, J.-Y.; Kim, H.; Lee, C. Enhanced production of reactive oxidants by Fenton-like reactions in the presence of carbon materials. Chem. Eng. J. 2015, 273, 502–508. [Google Scholar] [CrossRef]
- Peng, J.; Xue, J.; Li, J.; Du, Z.; Wang, Z.; Gao, S. Catalytic effect of low concentration carboxylated multi-walled carbon nanotubes on the oxidation of disinfectants with Cl-substituted structure by a Fenton-like system. Chem. Eng. J. 2017, 321, 325–334. [Google Scholar] [CrossRef]
- Yoo, S.; Jang, D.; Joh, H.-I.; Lee, S. Iron oxide/porous carbon as a heterogeneous Fenton catalyst for fast decomposition of hydrogen peroxide and efficient removal of methylene blue. J. Mater. Chem. A 2017, 5, 748–755. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, Y.; Xian, H.; Yan, L.; Fu, H.; Zhu, G.; Xi, Y.; Zhu, J.; He, H. CNTs/ferrihydrite as a highly efficient heterogeneous Fenton catalyst for the degradation of bisphenol A: The important role of CNTs in accelerating Fe(III)/Fe(II) cycling. Appl. Catal. B Environ. 2020, 270, 118891. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Qian, J. Graphene aerogel-based catalysts in Fenton-like reactions for water decontamination: A short review. Chem. Eng. J. Adv. 2021, 8, 100171. [Google Scholar] [CrossRef]
- Shi, H.; Chen, X.; Liu, K.; Ding, X.; Liu, W.; Xu, M. Heterogeneous Fenton ferroferric oxide-reduced graphene oxide-based composite microjets for efficient organic dye degradation. J. Colloid Interface Sci. 2020, 572, 39–47. [Google Scholar] [CrossRef]
- Sadegh, F.; Politakos, N.; de San Roman, E.; Sanz, O.; Modarresi-Alam, A.; Tomovska, R. Toward enhanced catalytic activity of magnetic nanoparticles integrated into 3D reduced graphene oxide for heterogeneous Fenton organic dye degradation. Sci. Rep. 2021, 11, 18343. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Tian, Z.; Zhang, L.; Guo, Y.; Yan, S. Enhanced heterogeneous Fenton degradation of Methylene Blue by nanoscale zero valent iron (nZVI) assembled on magnetic Fe3O4/reduced graphene oxide. J. Water Process Eng. 2015, 5, 101–111. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J. Degradation of sulfamethazine using Fe3O4-Mn3O4/reduced graphene oxide hybrid as Fenton-like catalyst. J. Hazard. Mater. 2017, 324, 653–664. [Google Scholar] [CrossRef]
- Yang, B.; Tian, Z.; Wang, B.; Sun, Z.; Zhang, L.; Guo, Y.; Li, H.; Yan, S. Facile synthesis of Fe3O4/hierarchical-Mn3O4/graphene oxide as a synergistic catalyst for activation of peroxymonosulfate for degradation of organic pollutants. RSC Adv. 2015, 5, 20674–20683. [Google Scholar] [CrossRef]
- Wan, Z.; Hu, J.; Wang, J. Removal of sulfamethazine antibiotics using CeFe-graphene nanocomposite as catalyst by Fenton-like process. J. Environ. Manag. 2016, 182, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Zhanga, Z.; Muhammad, Y.; Chenc, Y.; Shaha, S.J.; Peng, Y.; Shao, S.; Wang, R.; Li, X.; Liu, H.; Zhao, Z. Construction of ultra-stable and Z-scheme Fe-Graphdiyne/MIL-100(Fe) photo-Fenton catalyst with C=C-FeIO interface for the highly enhanced catalytic degradation of Dinotefuran. Chem. Eng. J. 2021, 426, 131621. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: Preparation, modification and environmental application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kanazawa, S.; Kofuji, Y.; Sakamoto, H.; Ichikawa, S.; Tanaka, S.; Hirai, T. Sunlight-Driven Hydrogen Peroxide Production from Water and Molecular Oxygen by Metal-Free Photocatalysts. Angew. Chem. Int. Ed. 2014, 53, 13454–13459. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Sampaio, M.; Teixo, J.; Silva, C.; Faria, J.; Silva, A. Photo-Fenton degradation assisted by in situ generation of hydrogen peroxide using a carbon nitride photocatalyst. J. Water Process Eng. 2020, 37, 101467. [Google Scholar] [CrossRef]
- Bicalho, H.; Lopez, J.; Binatti, I.; Batista, P.; Ardisson, J.; Resende, R.; Lorençon, E. Facile synthesis of highly dispersed Fe(II)-doped g-C3N4 and its application in Fenton-like catalysis. Mol. Catal. 2017, 435, 156–165. [Google Scholar] [CrossRef]
- Ma, J.; Wang, K.; Wang, C.; Chen, X.; Zhu, W.; Zhu, G.; Yao, W.; Zhu, Y. Photocatalysis-self-Fenton system with high-fluent degradation and high mineralization ability. Appl. Catal. B Environ. 2020, 276, 119150. [Google Scholar] [CrossRef]
- Sun, B.; Li, H.; Li, X.; Liu, X.; Zhang, C.; Xu, H.; Zhao, X. Degradation of Organic Dyes over Fenton-Like Cu2O–Cu/C Catalysts. Ind. Eng. Chem. Res. 2018, 57, 14011–14021. [Google Scholar] [CrossRef]
- Lam, F.; Yip, A.; Hu, X. Copper/MCM-41 as a Highly Stable and pH-insensitive Heterogeneous Photo-Fenton-like Catalytic Material for the Abatement of Organic Wastewater. Ind. Eng. Chem. Res. 2007, 46, 3328–3333. [Google Scholar] [CrossRef]
- Jiang, N.; Lyu, L.; Yu, G.; Zhang, L.; Hu, C. A dual-reaction-center Fenton-like process on –C≡N–Cu linkage between copper oxides and defect-containing g-C3N4 for efficient removal of organic pollutants. J. Mater. Chem. A 2018, 6, 17819–17828. [Google Scholar] [CrossRef]
- Das, G.; Shim, J.; Bhatnagar, A.; Tripathi, K.; Kim, T. Biomass-derived Carbon Quantum Dots for Visible-Light-Induced Photocatalysis and Label-Free Detection of Fe(III) and Ascorbic acid. Sci. Rep. 2019, 9, 15084. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wang, C.; Xu, K.; He, H.; Li, Q.; Yang, C.; Gao, X. N-CQDs Act as Electronic Warehouse in N-CQDs/CdS Regulate Adsorption Energy to Promote Photocatalytic Selective Oxidation of Aromatic Alcohols. Colloids Surf. A Physicochem. Eng. Aspects 2022, 641, 128559. [Google Scholar] [CrossRef]
- Athulya, M.; Bony, K.J.; Chacko, A.R.; Mohan, C.; Mathew, B. Review on Carbon Quantum Dot Based Semiconductor Photocatalysts for the Abatement of Refractory Pollutants. ChemPhysChem 2022, 23, 1–17. [Google Scholar]
- Qu, D.; Zheng, M.; Du, P.; Zhou, Y.; Zhang, L.; Li, D.; Tan, H.; Zhao, Z.; Xie, Z.; Sun, Z. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 2013, 5, 12272–12277. [Google Scholar] [CrossRef]
- Cai, A.; Wang, X.; Qi, Y.; Ma, Z. Hierarchical ZnO/S,N:GQD composites: Biotemplated synthesis and enhanced visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2017, 391, 484–490. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Kobylański, M.P.; Gołąbiewska, A.; Nadolna, J.; Zaleska-Medynska, A.; Malankowska, A. Quantum dot-decorated semiconductor micro- and nanoparticles: A review of their synthesis, characterization and application in photocatalysis. Adv. Colloid Interface Sci. 2018, 256, 352–372. [Google Scholar] [CrossRef]
- Serp, P.; Corrias, M.; Kalck, P. Carbon nanotubes and nanofibers in catalysis. Appl. Catal. A Gen. 2003, 253, 337–358. [Google Scholar] [CrossRef]
- Kokubo, K.; Matsubayashi, K.; Tategaki, H.; Takada, H.; Oshima, T. Facile synthesis of highly water-soluble fullerenes more than half-covered by hydroxyl groups. ACS Nano 2008, 2, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Miao, J.; Yang, Z.; Xiao, F.-X.; Yang, H.; Liu, B.; Yang, Y. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dai, M.; Zhang, S.; Dai, M.; Zhang, P.; Wang, S.; He, Z. Recent Progress on Carbon-Nanotube-Based Materials for Photocatalytic Applications: A Review. Solar RRL 2022, 6, 2200243. [Google Scholar] [CrossRef]
- Sarkodie, B.; Amesimeku, J.; Frimpong, C.; Howard, E.; Quan, F.; Zhenzhen, X. Photocatalytic degradation of dyes by novel electrospun nanofibers: A. review. Chemosphere 2022, 313, 137654. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qian, J.; Wang, N.; Xing, J.; Liu, L. In-situ synthesis of CNT/TiO2 heterojunction nanocomposite and its efficient photocatalytic degradation of Rhodamine B dye. Inorg. Chem. Commun. 2020, 119, 108071. [Google Scholar] [CrossRef]
- Azzam, E.; Fathy, N.; El-Khouly, S.; Sami, R. Enhancement the photocatalytic degradation of methylene blue dye using fabricated CNTs/TiO2/AgNPs/Surfactant nanocomposites. J. Water Process Eng. 2019, 28, 311–321. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.; Esvandi, Z.; Khatooni, H.; Ramavandi, B. Evaluation of two cationic dyes removal from aqueous environments using CNT/MgO/CuFe2O4 magnetic composite powder: A comparative study. J. Environ. Chem. Eng. 2021, 9, 104752. [Google Scholar] [CrossRef]
- Mohamed, A.; Yousef, S.; Abdelnaby, M.A.; Osman, T.; Hamawandi, B.; Toprak, M.; Muhammed, M.; Uheida, A. Photocatalytic degradation of organic dyes and enhanced mechanical properties of PAN/CNTs composite nanofibers. Sep. Purif. Technol. 2017, 182, 219–223. [Google Scholar] [CrossRef]
- Koo, Y.; Littlejohn, G.; Collins, B.; Yun, Y.; Shanov, V.; Schulz, M.; Pai, D.; Sankar, J. Synthesis and characterization of Ag–TiO2–CNT nanoparticle composites with high photocatalytic activity under artificial light. Compos. Part B Eng. 2014, 57, 105–111. [Google Scholar] [CrossRef]
- Chen, W.; Fan, Z.; Zhang, B.; Ma, G.; Takanabe, K.; Zhang, X.; Lai, Z. Enhanced Visible-Light Activity of Titania via Confinement inside Carbon Nanotubes. J. Am. Chem. Soc. 2011, 133, 14896–14899. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers, Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Dutta, V.; Singh, P.; Shandilya, P.; Sharma, S.; Raizada, P.; Saini, A.; Gupta, V.; Hosseini-Bandegharaei, A.; Agarwal, S.; Rahmani-Sani, A. Review on advances in photocatalytic water disinfection utilizing graphene and graphene derivatives-based nanocomposites. J. Environ. Chem. Eng. 2019, 7, 103132. [Google Scholar] [CrossRef]
- Karim, A.; Selvaraj, A. Graphene composites in photocatalytic oxidation of aqueous organic contaminants—A state of art. Process Saf. Environ. Prot. 2021, 146, 136–160. [Google Scholar] [CrossRef]
- Upadhyay, R.; Soin, N.; Roy, S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Xuad, Q.; Zhu, B.; Cheng, B.; Yua, J.; Zhou, M.; Ho, W. Photocatalytic H2 evolution on graphdiyne/g-C3N4 hybrid nanocomposites. Appl. Catal. B Environ. 2019, 255, 117770. [Google Scholar]
- Liang, S.; Deng, H.; Zhou, Z.; Wong, W.-Y. Fabrication of graphdiyne and its analogues for photocatalytic application. EcoMat. 2022, e12297. [Google Scholar] [CrossRef]
- Wang, S.; Yi, L.; Halpert, J.E.; Lai, X.; Liu, Y.; Cao, H.; Yu, R.; Wang, D.; Li, Y. A Novel and Highly Efficient Photocatalyst Based on P25–Graphdiyne Nanocomposite. Small 2012, 8, 265. [Google Scholar] [CrossRef]
- Hou, H.; Shao, G.; Yang, W. Recent advances in g-C3N4-based photocatalysts incorporated by MXenes and their derivatives. J. Mater. Chem. A 2021, 9, 13722–13745. [Google Scholar] [CrossRef]
- Patnaik, S.; Martha, S.; Parida, K. An overview of the structural, textural and morphological modulations of g-C3N4 towards photocatalytic hydrogen production. RSC Adv. 2016, 6, 46929–46951. [Google Scholar] [CrossRef]
- Liu, W.; Wang, B.; Zhang, M. Effect of Process Parameters on the Microstructure and Performance of TiO2-Loaded Activated Carbon. ACS Omega 2021, 50, 35076–35092. [Google Scholar] [CrossRef] [PubMed]
- Tay, Q.; Kanhere, P.; Ng, C.; Chen, S.; Chakraborty, S.; Huan, A.; Sum, T.; Ahuja, R.; Chen, Z. Defect Engineered g-C3N4 for Efficient Visible Light Photocatalytic Hydrogen Production. Chem. Mater. 2015, 27, 4930–4933. [Google Scholar] [CrossRef]
- Lan, H.; Li, L.; An, X.; Liu, F.; Chen, C.; Liu, H.; Qu, J. Microstructure of carbon nitride affecting synergetic photocatalytic activity: Hydrogen bonds vs. structural defects. Appl. Catal. B Environ. 2017, 204, 49–57. [Google Scholar] [CrossRef]
- Jin, X.; Wu, Y.; Zhang, Q.; Wang, F.; Chen, P.; Liu, H.; Huang, S.; Wu, J.; Tu, N.; Lv, W.; et al. Defect-modified reduced graphitic carbon nitride (RCN) enhanced oxidation performance for photocatalytic degradation of diclofenac. Chemosphere 2020, 258, 127343. [Google Scholar] [CrossRef]
- Chen, M.; Li, M.; Lee, S.; Zhao, X.; Lin, S. Constructing novel graphitic carbon nitride-based nanocomposites–From the perspective of material dimensions and interfacial characteristics. Chemosphere 2022, 302, 134889. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Yan, L.; Jing, C. Impact of doped metals on urea-derived g-C3N4 for photocatalytic degradation of antibiotics: Structure, photoactivity and degradation mechanisms. Appl. Catal. B Environ. 2019, 244, 475–485. [Google Scholar] [CrossRef]
- Ma, X.; Lv, Y.; Xu, J.; Liu, Y.; Zhang, R.; Zhu, Y. A Strategy of Enhancing the Photoactivity of g-C3N4 via Doping of Nonmetal Elements: A First-Principles Study. J. Phys. Chem. C 2012, 116, 23485–23493. [Google Scholar] [CrossRef]
- Akhundi, A.; Moshfegh, A.Z.; Habibi-Yangjeh, A.; Sillanpää, M. Simultaneous Dual-Functional Photocatalysis by g-C3N4-Based Nanostructures. ACS EST Eng. 2022, 2, 564–585. [Google Scholar] [CrossRef]
- Guo, R.-t.; Wang, J.; Bi, Z.-x.; Chen, X.; Hu, X.; Pan, W.-g. Recent advances and perspectives of g–C3N4–based materials for photocatalytic dyes degradation. Chemosphere 2022, 295, 133834. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, Q.; Li, K.; Zhang, Y.; Liu, E.; Li, X. Recent advances on g–C3N4–based Z-scheme photocatalysts: Structural design and photocatalytic applications. Int. J. Hydrogen Energy 2023, 48, 196–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, J.; Ding, Y.; Liu, B.; Zhao, L.; Zhang, S. Research progress on g–C3N4–based photocatalysts for organic pollutants degradation in wastewater: From exciton and carrier perspectives. Ceram. Int. 2021, 47, 31005–31030. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, L.; Su, R.; Hu, B.; Wang, Z.; Jin, Y.; Wang, Y.; Besenbacher, F.; Dong, M. Nanostructured heterogeneous photo-catalysts for hydrogen production and water splitting: A comprehensive insight. Appl. Mater. Today 2019, 17, 159–182. [Google Scholar] [CrossRef]
- Patial, S.; Sonu; Sudhaik, A.; Chandel, N.; Ahamad, T.; Raizada, P.; Singh, P.; Chaukura, N.; Selvasembian, R. A Review on Carbon Quantum Dots Modified g-C3N4-Based Photocatalysts and Potential Application in Wastewater Treatment. Appl. Sci. 2022, 12, 11286. [Google Scholar] [CrossRef]
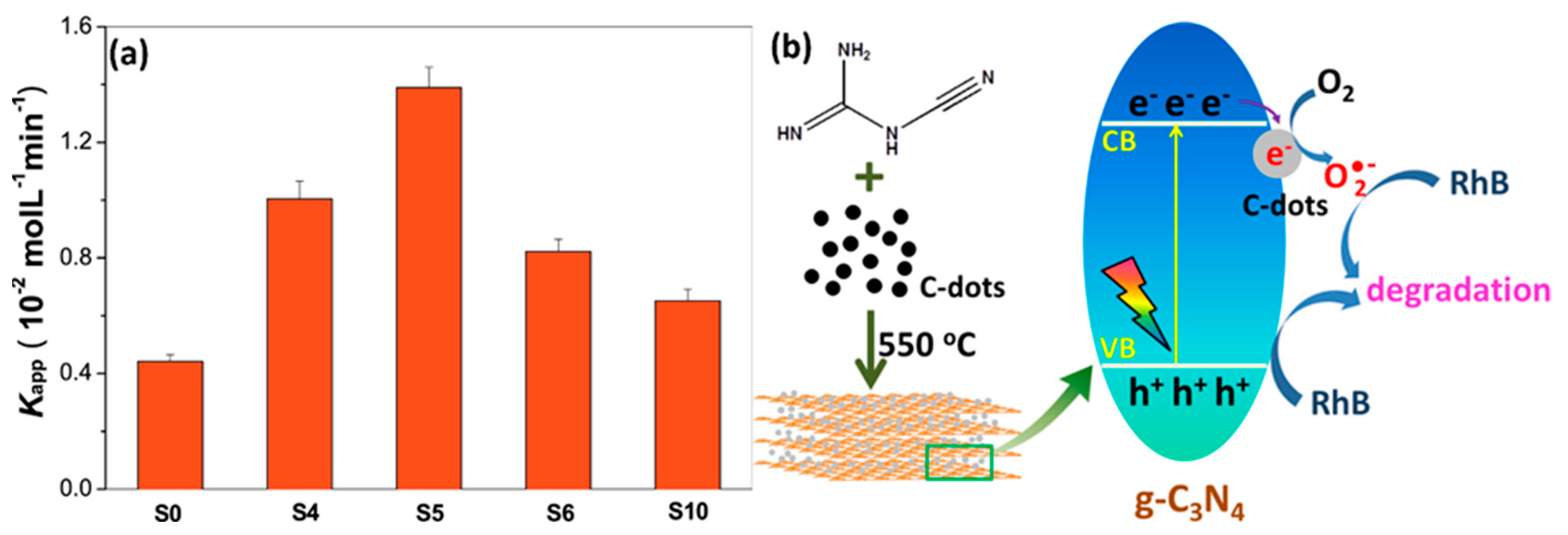
- Fang, S.; Xia, Y.; Lv, K.; Li, Q.; Sun, J.; Li, M. Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4. Appl. Catal. B Environ. 2016, 185, 225–232. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Geng, F.; Guo, L.-H.; Wan, B.; Yang, Y. Carbon dots decorated graphitic carbon nitride as an efficient metal-free photocatalyst for phenol degradation. Appl. Catal. B Environ. 2016, 180, 656–662. [Google Scholar] [CrossRef]
- Liu, H.; Liang, J.; Fu, S.; Li, L.; Cui, J.; Gao, P.; Zhao, F.; Zhou, J. N doped carbon quantum dots modified defect-rich g-C3N4 for enhanced photocatalytic combined pollutions degradation and hydrogen evolution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124552. [Google Scholar] [CrossRef]
- Li, H.; Huang, G.; Xu, H.; Yang, Z.; Xu, X.; Li, J.; Qu, A.; Chen, Y. Enhancing photodegradation activity of g-C3N4 via decorating with S-doped carbon nitride quantum dots by in situ polymerization. J. Solid State Chem. 2020, 292, 121705. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Liu, F.; Jiang, W.; Zhang, D.; Liang, J. Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation. Water Res. 2019, 151, 8–19. [Google Scholar] [CrossRef]
- Mohapatra, L.; Patra, D.; Zaidi, S.; Yoo, S. Heterointerface engineered and highly dual-functional N-doped carbon dot /N-rich g-C3N4 hybrid photocatalysts. Mater. Today Chem. 2022, 26, 101081. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, H.; Wang, L.; Yan, J.; Li, H.; Song, Y.; Huang, L.; Cai, G. The CNT modified white C3N4 composite photocatalyst with enhanced visible-light response photoactivity. Dalton Trans. 2013, 42, 7604–7613. [Google Scholar] [CrossRef]
- Sun, L.; Du, T.; Hu, C.; Chen, J.; Lu, J.; Lu, Z.; Han, H. Antibacterial Activity of Graphene Oxide/g-C3N4 Composite through Photocatalytic Disinfection under Visible Light. ACS Sustain. Chem. Eng. 2017, 5, 8693–8701. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Guo, X.; Chen, Z.; Zhang, W.; Wang, Y.; Tang, X.; Zhang, Y.; Zhao, Y. Sulfur-doped g-C3N4/rGO porous nanosheets for highly efficient photocatalytic degradation of refractory contaminants. J. Mater. Sci. Technol. 2020, 41, 117–126. [Google Scholar] [CrossRef]
- He, B.; Feng, M.; Chen, X.; Sun, J. Multidimensional (0D-3D) functional nanocarbon: Promising material to strengthen the photocatalytic activity of graphitic carbon nitride. Green Energy Environ. 2021, 6, 823–845. [Google Scholar] [CrossRef]
- Li, F.; Tang, M.; Li, T.; Zhang, L.; Hu, C. Two-dimensional graphene/g-C3N4 in-plane hybrid heterostructure for enhanced photocatalytic activity with surface-adsorbed pollutants assistant. Appl. Catal. B Environ. 2020, 268, 118397. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohapatra, L.; Cheon, D.; Yoo, S.H. Carbon-Based Nanomaterials for Catalytic Wastewater Treatment: A Review. Molecules 2023, 28, 1805. https://doi.org/10.3390/molecules28041805
Mohapatra L, Cheon D, Yoo SH. Carbon-Based Nanomaterials for Catalytic Wastewater Treatment: A Review. Molecules. 2023; 28(4):1805. https://doi.org/10.3390/molecules28041805
Chicago/Turabian StyleMohapatra, Lagnamayee, Dabin Cheon, and Seung Hwa Yoo. 2023. "Carbon-Based Nanomaterials for Catalytic Wastewater Treatment: A Review" Molecules 28, no. 4: 1805. https://doi.org/10.3390/molecules28041805
APA StyleMohapatra, L., Cheon, D., & Yoo, S. H. (2023). Carbon-Based Nanomaterials for Catalytic Wastewater Treatment: A Review. Molecules, 28(4), 1805. https://doi.org/10.3390/molecules28041805





