Phenolic Compound Profile and Antioxidant Capacity of Flax (Linum usitatissimum L.) Harvested at Different Growth Stages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yield and Total Phenolic Content
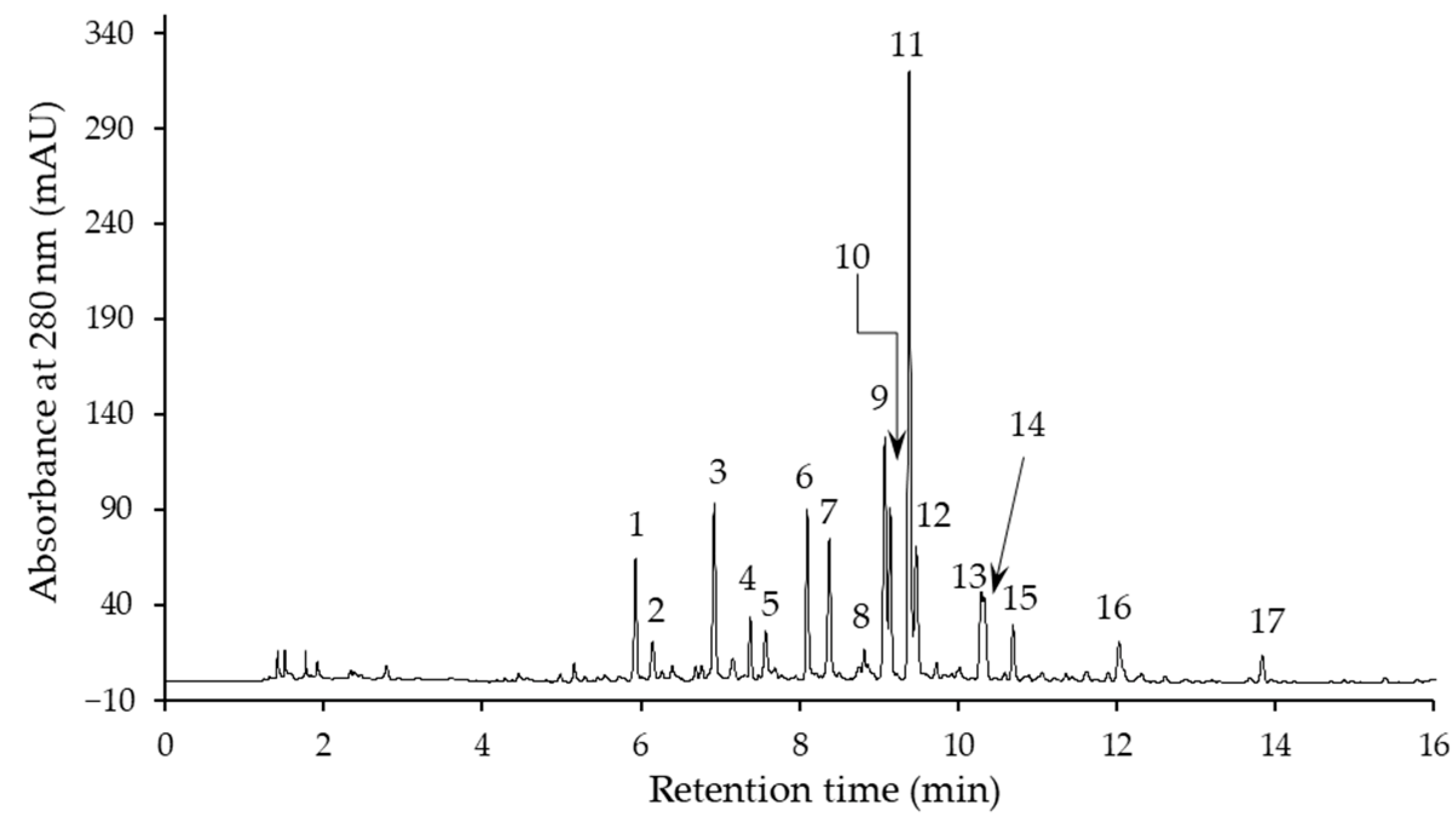
2.2. Phenolic Compound Profile
2.3. Antioxidant Capacity
3. Materials and Methods
3.1. Plant Material and Cultivation Conditions
3.2. Chemicals
3.3. Preparation of the Extracts
3.4. Determination of the Total Phenolic Content
3.5. Phenolic Compound Profile Analysis
3.6. Trolox Equivalent Antioxidant Capacity Determination
3.7. Determination of the Ferric-Reducing Antioxidant Power
3.8. Determination of the DPPH Radical Scavenging Activity
3.9. β-Carotene-Linoleic Acid Emulsion Oxidation
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zuk, M.; Richter, D.; Matuła, J.; Szopa, J. Linseed, the multipurpose plant. Ind. Crops Prod. 2015, 75, 165–177. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Van Remoortel, V.; Danthine, S.; Paquot, M.; Blecker, C. Flaxseed proteins: Food uses and health benefits. Int. J. Food Sci. Technol. 2011, 46, 221–228. [Google Scholar] [CrossRef]
- Wu, S.; Wang, X.; Qi, W.; Guo, Q. Bioactive protein/peptides of flaxseed: A review. Trends Food Sci. Technol. 2019, 92, 184–193. [Google Scholar] [CrossRef]
- Karamać, M.; Kosińska-Cagnazzo, A.; Kulczyk, A. Use of Different Proteases to Obtain Flaxseed Protein Hydrolysates with Antioxidant Activity. Int. J. Mol. Sci. 2016, 17, 1027. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Lim, S. A Review of Extraction Techniques and Food Applications of Flaxseed Mucilage. Foods 2022, 11, 1677. [Google Scholar] [CrossRef] [PubMed]
- Mysiukiewicz, O.; Barczewski, M. Utilization of linseed cake as a postagricultural functional filler for poly(lactic acid) green composites. J. Appl. Polym. Sci. 2019, 136, 47152. [Google Scholar] [CrossRef]
- Garros, L.; Drouet, S.; Corbin, C.; Decourtil, C.; Fidel, T.; de Lacour, J.L.; Leclerc, E.A.; Renouard, S.; Tungmunnithum, D.; Doussot, J.; et al. Insight into the Influence of Cultivar Type, Cultivation Year, and Site on the Lignans and Related Phenolic Profiles, and the Health-Promoting Antioxidant Potential of Flax (Linum usitatissimum L.) Seeds. Molecules 2018, 23, 2636. [Google Scholar] [CrossRef]
- Czemplik, M.; Mierziak, J.; Szopa, J.; Kulma, A. Flavonoid C-glucosides Derived from Flax Straw Extracts Reduce Human Breast Cancer Cell Growth In Vitro and Induce Apoptosis. Front. Pharmacol. 2016, 7, 282. [Google Scholar] [CrossRef]
- Tchoumtchoua, J.; Mathiron, D.; Pontarin, N.; Gagneul, D.; van Bohemen, A.-I.; N’Nang, E.O.; Mesnard, F.; Petit, E.; Fontaine, J.-X.; Molinié, R.; et al. Phenolic Profiling of Flax Highlights Contrasting Patterns in Winter and Spring Varieties. Molecules 2019, 24, 4303. [Google Scholar] [CrossRef]
- Touré, A.; Xueming, X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Sicilia, T.; Niemeyer, H.B.; Honig, D.M.; Metzler, M. Identification and Stereochemical Characterization of Lignans in Flaxseed and Pumpkin Seeds. J. Agric. Food Chem. 2003, 51, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Adolphe, J.L.; Whiting, S.J.; Juurlink, B.H.J.; Thorpe, L.U.; Alcorn, J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br. J. Nutr. 2010, 103, 929–938. [Google Scholar] [CrossRef]
- Kyselka, J.; Rabiej, D.; Dragoun, M.; Kreps, F.; Burčová, Z.; Němečková, I.; Smolová, J.; Bjelková, M.; Szydłowska-Czerniak, A.; Schmidt, Š.; et al. Antioxidant and antimicrobial activity of linseed lignans and phenolic acids. Eur. Food Res. Technol. 2017, 243, 1633–1644. [Google Scholar] [CrossRef]
- Prasad, K. Antioxidant Activity of Secoisolariciresinol Diglucoside-derived Metabolites, Secoisolariciresinol, Enterodiol, and Enterolactone. Int. J. Angiol. 2000, 9, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, A.; Fliniaux, O.; Quéro, A.; Molinié, R.; Demailly, H.; Hano, C.; Paetz, C.; Roscher, A.; Grand, E.; Kovensky, J.; et al. Kinetics of the incorporation of the main phenolic compounds into the lignan macromolecule during flaxseed development. Food Chem. 2017, 217, 1–8. [Google Scholar] [CrossRef]
- Struijs, K.; Vincken, J.-P.; Doeswijk, T.G.; Voragen, A.G.J.; Gruppen, H. The chain length of lignan macromolecule from flaxseed hulls is determined by the incorporation of coumaric acid glucosides and ferulic acid glucosides. Phytochemistry 2009, 70, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Herchi, W.; Bahashwan, S.; Trabelsi, H.; Sebei, K.; Boukhchina, S. Effect of seed maturation stages on physical properties and antioxidant activity in flaxseed (Linum usitatissimum L.). Food Sci. Technol. 2015, 35, 598–604. [Google Scholar] [CrossRef]
- Dubois, J.; Mabry, T.J. The C-glycosylflavonoids of flax, Linum usitatissimum. Phytochemistry 1971, 10, 2839–2840. [Google Scholar] [CrossRef]
- Mierziak, J.; Wojtasik, W.; Kostyn, K.; Czuj, T.; Szopa, J.; Kulma, A. Crossbreeding of transgenic flax plants overproducing flavonoids and glucosyltransferase results in progeny with improved antifungal and antioxidative properties. Mol. Breed. 2014, 34, 1917–1932. [Google Scholar] [CrossRef]
- Elboutachfaiti, R.; Molinié, R.; Mathiron, D.; Maillot, Y.; Fontaine, J.-X.; Pilard, S.; Quéro, A.; Brasselet, C.; Dols-Lafargue, M.; Delattre, C.; et al. Secondary Metabolism Rearrangements in Linum usitatissimum L. after Biostimulation of Roots with COS Oligosaccharides from Fungal Cell Wall. Molecules 2022, 27, 2372. [Google Scholar] [CrossRef]
- Pontarin, N.; Molinié, R.; Mathiron, D.; Tchoumtchoua, J.; Bassard, S.; Gagneul, D.; Thiombiano, B.; Demailly, H.; Fontaine, J.-X.; Guillot, X.; et al. Age-Dependent Metabolic Profiles Unravel the Metabolic Relationships within and between Flax Leaves (Linum usitatissimum). Metabolites 2020, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, S29–S45. [Google Scholar] [CrossRef]
- Flax Council of Canada. Growing Flax: Production, Management & Diagnostic Guide, 5th ed.; Flax Council of Canada: Winnipeg, Canada, 2020; Available online: https://www.flaxcouncil.ca/quadrant/media/files/pdfs/150101_FCOC-growers-guide-v11.pdf (accessed on 13 February 2020).
- Gai, F.; Karamać, M.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Sunflower (Helianthus annuus L.) Plants at Various Growth Stages Subjected to Extraction—Comparison of the Antioxidant Activity and Phenolic Profile. Antioxidants 2020, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Karamać, M.; Gai, F.; Longato, E.; Meineri, G.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Antioxidant Activity and Phenolic Composition of Amaranth (Amaranthus caudatus) during Plant Growth. Antioxidants 2019, 8, 173. [Google Scholar] [CrossRef]
- Karamać, M.; Gai, F.; Peiretti, P.G. Effect of the Growth Stage of False Flax (Camelina sativa L.) on the Phenolic Compound Content and Antioxidant Potential of the Aerial Part of the Plant. Pol. J. Food Nutr. Sci. 2020, 70, 189–198. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Karamać, M.; Janiak, M.; Longato, E.; Meineri, G.; Amarowicz, R.; Gai, F. Phenolic Composition and Antioxidant Activities of Soybean (Glycine max (L.) Merr.) Plant during Growth Cycle. Agronomy 2019, 9, 153. [Google Scholar] [CrossRef]
- McCall, A.C.; Fordyce, J.A. Can optimal defence theory be used to predict the distribution of plant chemical defences? J. Ecol. 2010, 98, 985–992. [Google Scholar] [CrossRef]
- Drabińska, N.; Jeż, M.; Nogueira, M. Variation in the Accumulation of Phytochemicals and Their Bioactive Properties among the Aerial Parts of Cauliflower. Antioxidants 2021, 10, 1597. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, J.; Mitić, S.; Mitić, M.; Kocić, G.; Pavlović, A.; Tošić, S. Variation in the Phenolic Compounds Profile and Antioxidant Activity in Different Parts of Hawthorn (Crataegus pentagyna Willd.) during Harvest Periods. Pol. J. Food Nutr. Sci. 2019, 69, 367–378. [Google Scholar] [CrossRef]
- Kozłowska, M.; Ścibisz, I.; Przybył, J.L.; Ziarno, M.; Żbikowska, A.; Majewska, E. Phenolic Contents and Antioxidant Activity of Extracts of Selected Fresh and Dried Herbal Materials. Pol. J. Food Nutr. Sci. 2021, 71, 269–278. [Google Scholar] [CrossRef]
- Hemmati, S.; von Heimendahl, C.B.I.; Klaes, M.; Alfermann, A.W.; Schmidt, T.J.; Fuss, E. Pinoresinol-Lariciresinol Reductases with Opposite Enantiospecificity Determine the Enantiomeric Composition of Lignans in the Different Organs of Linum usitatissimum L. Planta Medica 2010, 76, 928–934. [Google Scholar] [CrossRef]
- Geng, P.; Sun, J.; Zhang, M.; Li, X.; Harnly, J.M.; Chen, P. Comprehensive characterization of C-glycosyl flavones in wheat (Triticum aestivum L.) germ using UPLC-PDA-ESI/HRMSn and mass defect filtering. J. Mass Spectrom. 2016, 51, 914–930. [Google Scholar] [CrossRef]
- Ferreres, F.; Silva, B.M.; Andrade, P.B.; Seabra, R.M.; Ferreira, M.A. Approach to the study of C-glycosyl flavones by ion trap HPLC-PAD-ESI/MS/MS: Application to seeds of quince (Cydonia oblonga). Phytochem. Anal. 2003, 14, 352–359. [Google Scholar] [CrossRef]
- Benayad, Z.; Gómez-Cordovés, C.; Es-Safi, N.E. Characterization of Flavonoid Glycosides from Fenugreek (Trigonella foenum-graecum) Crude Seeds by HPLC–DAD–ESI/MS Analysis. Int. J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar] [CrossRef] [PubMed]
- Materska, M. Flavone C-glycosides from Capsicum annuum L.: Relationships between antioxidant activity and lipophilicity. Eur. Food Res. Technol. 2015, 240, 549–557. [Google Scholar] [CrossRef]
- Mekky, R.H.; Abdel-Sattar, E.; Segura-Carretero, A.; Contreras, M.D.M. A comparative study on the metabolites profiling of linseed cakes from Egyptian cultivars and antioxidant activity applying mass spectrometry-based analysis and chemometrics. Food Chem. 2022, 395, 133524. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Beta, T. Qualitative and quantitative analysis of the major phenolic compounds as antioxidants in barley and flaxseed hulls using HPLC/MS/MS. J. Sci. Food Agric. 2012, 92, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Brigante, F.I.; Mas, A.L.; Pigni, N.B.; Wunderlin, D.A.; Baroni, M.V. Targeted metabolomics to assess the authenticity of bakery products containing chia, sesame and flax seeds. Food Chem. 2020, 312, 126059. [Google Scholar] [CrossRef] [PubMed]
- Quartieri, A.; García-Villalba, R.; Amaretti, A.; Raimondi, S.; Leonardi, A.; Rossi, M.; Tomàs-Barberàn, F. Detection of novel metabolites of flaxseed lignans in vitro and in vivo. Mol. Nutr. Food Res. 2016, 60, 1590–1601. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Guo, X.; Brennan, C.S.; Li, T.; Fu, X.; Chen, G.; Liu, R.H. Effect of germination on lignan biosynthesis, and antioxidant and antiproliferative activities in flaxseed (Linum usitatissimum L.). Food Chem. 2016, 205, 170–177. [Google Scholar] [CrossRef]
- Corbin, C.; Drouet, S.; Mateljak, I.; Markulin, L.; Decourtil, C.; Renouard, S.; Lopez, T.; Doussot, J.; Lamblin, F.; Auguin, D.; et al. Functional characterization of the pinoresinol–lariciresinol reductase-2 gene reveals its roles in yatein biosynthesis and flax defense response. Planta 2017, 246, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Courts, F.L.; Williamson, G. The Occurrence, Fate and Biological Activities of C-glycosyl Flavonoids in the Human Diet. Crit. Rev. Food Sci. Nutr. 2015, 55, 1352–1367. [Google Scholar] [CrossRef]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of Flavonoid O-Glycoside, C-Glycoside and Their Aglycones on Antioxidant Capacity and Metabolism during In Vitro Digestion and In Vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef]
- Kosińska, A.; Penkacik, K.; Wiczkowski, W.; Amarowicz, R. Presence of Caffeic Acid in Flaxseed Lignan Macromolecule. Plant Foods Hum. Nutr. 2011, 66, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Waszkowiak, K.; Gliszczyńska-Świgło, A.; Barthet, V.; Skręty, J. Effect of Extraction Method on the Phenolic and Cyanogenic Glucoside Profile of Flaxseed Extracts and Their Antioxidant Capacity. J. Am. Oil Chem. Soc. 2015, 92, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Orak, H.H.; Karamać, M.; Amarowicz, R.; Orak, A.; Janiak, M.A.; Tenikecier, H.S. Variations of genotypes of Vicia species as influenced by seed phenolic compounds and antioxidant activity. Zemdirb. Agric. 2022, 109, 35–42. [Google Scholar] [CrossRef]
- Herman, M.; Janiak, M.A.; Sadlik, J.K.; Piekoszewski, W.; Amarowicz, A. Iron, Zinc, Copper, Manganese and Chromium in Green Teas, Their Transfer to Extracts and Correlations between Contents of Elements and Bioactive Compounds. Pol. J. Food Nutr. Sci. 2022, 72, 421–429. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Karamać, M.; Orak, H.H.; Amarowicz, R.; Orak, A.; Piekoszewski, W. Phenolic contents and antioxidant capacities of wild and cultivated white lupin (Lupinus albus L.) seeds. Food Chem. 2018, 258, 1–7. [Google Scholar] [CrossRef] [PubMed]

| Growth Stage | Days after Sowing | Extraction Yield (%) | Total Phenolic Content | |
|---|---|---|---|---|
| mg GAE/g Extract | mg GAE/g FM | |||
| Stem extension | 45 | 20.4 ± 0.8 a | 33.6 ± 2.9 a | 1.06 ± 0.17 b,c |
| Visible bud | 51 | 17.8 ± 1.2 b | 27.9 ± 4.8 a | 1.01 ± 0.13 b,c |
| Flowering | 58 | 16.9 ± 1.3 b,c | 29.2 ± 1.7 a | 1.33 ± 0.20 a |
| Brown capsule | 65 | 14.4 ± 0.1 d | 33.4 ± 2.1 a | 1.22 ± 0.09 a,b |
| Seed ripening | 72 | 16.0 ± 0.7 c | 32.8 ± 2.6 a | 1.43 ± 0.12 a |
| Mature seed | 99 | 10.2 ± 0.4 e | 33.1 ± 2.7 a | 0.88 ± 0.05 c |
| Compound No. 1 | λmax (nm) | [M−H]− (m/z) | MS2 Ions (m/z) | Identified Compound |
|---|---|---|---|---|
| 1 | 259 | 341 | 179, 161 | Coniferin (coniferyl alcohol β-d-glucoside) |
| 2 | 298 sh, 329 | 341 | 179, 161, 131 | Caffeic acid hexoside I |
| 3 | 299 sh, 326 | 341 | 179, 161 | Caffeic acid hexoside II |
| 4 | 256 sh, 271, 347 | 609 | 591, 519, 489, 399, 369 | Lucenin-2 (luteolin 6,8-di-C-glucoside) |
| 5 | 286, 314 | 191 | p-Coumaric acid ethyl ester | |
| 6 2 | 271, 336 | 593 | 575, 503, 473, 383, 353 | Vicenin-2 (apigenin 6,8-di-C-β-d-glucoside) |
| 7 | 256 sh, 270, 348 | 579 | 561, 519, 489, 459, 399, 369 | Luteolin 6,8-C-hexoside-C-pentoside |
| 8 | 271, 333 | 593 | 575, 503, 473, 383, 353 | Apigenin 6,8-di-C-hexoside |
| 9 | 271, 335 | 563 | 545, 503, 473, 443, 383, 353 | Apigenin 8-C-hexoside 6-C-pentoside |
| 10 2 | 271, 336 | 563 | 545, 503, 473, 443, 383, 353 | Schaftoside (apigenin 8-C-α-l-arabinoside 6-C-β-d-glucoside) |
| 11 2 | 256 sh, 269, 349 | 447 | 429, 357, 327, 297 | Isoorientin (luteolin 6-C-β-d-glucoside) |
| 12 2 | 257 sh, 270, 349 | 447 | 429, 357, 327, 297 | Orientin (luteolin 8-C-β-d-glucoside) |
| 13 2 | 269, 337 | 431 | 341, 323, 311, 283, 269 | Vitexin (apigenin 8-C-glucoside) |
| 14 | 272 | 565 | 519, 357, 339, 327 | Dehydrodiconiferyl alcohol-4-O-glucoside (DCG) |
| 15 2 | 270, 338 | 431 | 341, 323, 311, 283, 269 | Isovitexin (apigenin 6-C-glucoside) |
| 16 | 299 sh, 330 | 401 | 222 | Caffeic acid derivative |
| 17 | 278 | 685 | 667, 521, 363, 303 | Unknown |
| Compound | Stem Extension | Visible Bud | Flowering | Brown Capsule | Seed Ripening | Mature Seed |
|---|---|---|---|---|---|---|
| Coniferin 1 | 0.642 ± 0.028 c | 0.598 ± 0.086 c | 0.555 ± 0.023 c | 1.07 ± 0.16 b | 1.71 ± 0.27 a | 0.719 ± 0.118 b,c |
| Caffeic acid hexoside I 1 | 0.628 ± 0.045 a | 0.341 ± 0.039 b,c | 0.397 ± 0.037 b | 0.439 ± 0.022 b | 0.291 ± 0.037 c | 0.280 ± 0.044 c |
| Caffeic acid hexoside II 1 | 2.39 ± 0.18 a | 1.37 ± 0.10 b,c | 1.43 ± 0.16 b | 1.29 ± 0.06 b,c | 0.991 ± 0.161 c | 0.421 ± 0.121 d |
| Lucenin-2 2 | 1.46 ± 0.18 a | 1.23 ± 0.06 a,b | 1.05 ± 0.13 b,c | 1.14 ± 0.05 a,b,c | 0.880 ± 0.073 c | 0.907 ± 0.149 c |
| p-Coumaric acid ethyl ester 3 | 0.224 ± 0.015 b | 0.232 ± 0.012 b | 0.233 ± 0.011 a,b | 0.261 ± 0.006 a | 0.216 ± 0.003 b | 0.223 ± 0.009 b |
| Vicenin-2 | 3.43 ± 0.22 a | 2.78 ± 0.21 b | 2.89 ± 0.12 b | 2.87 ± 0.19 b | 2.05 ± 0.13 c | 1.82 ± 0.11 c |
| Luteolin 6,8-C-hexoside-C-pentoside 2 | 3.77 ± 0.30 a | 3.21 ± 0.21 a,b | 3.19 ± 0.26 ab | 3.55 ± 0.14 a,b | 3.08 ± 0.16 b | 3.17 ± 0.26 a,b |
| Apigenin 6,8-di-C-hexoside 4 | 0.445 ± 0.023 a | 0.368 ± 0.027 a | 0.357 ± 0.020 a,b | 0.347 ± 0.016 a,b | 0.250 ± 0.015 b,c | 0.146 ± 0.092 c |
| Apigenin 8-C-hexoside 6-C-pentoside 4 | 3.05 ± 0.38 a | 2.45 ± 0.16 b | 2.35 ± 0.18 b | 2.42 ± 0.21 b | 1.68 ± 0.10 c | 1.41 ± 0.11 c |
| Schaftoside | 3.32 ± 0.10 a | 2.67 ± 0.18 b,c | 2.80 ± 0.034 b,c | 2.97 ± 0.28 a,b | 2.30 ± 0.23 c,d | 2.12 ± 0.18 d |
| Isoorientin | 14.3 ± 1.6 a | 11.3 ± 1.2 b | 11.8 ± 0.6 a,b | 12.8 ± 0.3 a,b | 11.3 ± 1.1 b | 7.90 ± 1.01 c |
| Orientin | 4.80 ± 0.30 a | 4.18 ± 0.41 a | 4.26 ± 0.22 a | 4.49 ± 0.09 a | 3.97 ± 0.43 a | 2.04 ± 0.30 b |
| Vitexin | 1.29 ± 0.06 a,b | 1.20 ± 0.12 ab | 1.30 ± 0.04 a,b | 1.33 ± 0.05 a | 1.09 ± 0.11 b | 0.684 ± 0.068 c |
| DCG 1 | 0.172 ± 0.015 d | 0.470 ± 0.081 c | 0.397 ± 0.017 c,d | 0.577 ± 0.088 c | 0.790 ± 0.094 b | 1.35 ± 0.16 a |
| Isovitexin | 0.943 ± 0.092 a,b,c | 0.828 ± 0.076 b,c | 0.987 ± 0.033 a,b | 1.07 ± 0.02 a | 0.905 ± 0.068 a,b,c | 0.781 ± 0.067 c |
| Caffeic acid derivative 1 | 1.06 ± 0.16 a | 0.558 ± 0.061 c | 0.854 ± 0.074 a,b | 1.10 ± 0.06 a | 0.878 ± 0.122 a,b | 0.741 ± 0.124 b,c |
| Unknown 1 | 0.063 ± 0.004 c | 0.212 ± 0.009 a,b | 0.251 ± 0.009 a | 0.207 ± 0.018 a,b | 0.278 ± 0.067 a | 0.159 ± 0.016 b |
| ∑ Phenolic compounds | 42.0 ± 3.5 a | 34.0 ± 2.7 b | 35. 1 ± 1.9 b | 38.0 ± 1.0 a,b | 32.7 ± 2.2 b | 24.9 ± 2.3 c |
| ∑ Flavone C-glycosides | 36.8 ± 3.1 a | 30.3 ± 2.5 b | 31.0 ± 1.6 a,b | 33.0 ± 1.1 a,b | 27.6 ± 2.2 b | 21.0 ± 1.8 c |
| Compound | Stem Extension | Visible Bud | Flowering | Brown Capsule | Seed Ripening | Mature Seed |
|---|---|---|---|---|---|---|
| Coniferin 1 | 20.0 ± 0.5 b,c | 22.0 ± 4.9 b,c | 25.3 ± 3.2 b,c | 40.0 ± 7.8 b | 75.4 ± 15 a | 19.1 ± 3.2 c |
| Caffeic acid hexoside I 1 | 19.6 ± 2.0 a | 12.5 ± 2.2 b,c | 18.1 ± 3.1 a,b | 16.4 ± 1.7 a,b | 12.8 ± 1.9 b,c | 7.45 ± 1.10 c |
| Caffeic acid hexoside II 1 | 74.4 ± 7.4 a | 49.9 ± 3.2 b,c | 65.3 ± 12.8 a,b | 48.1 ± 3.2 b,c | 43.5 ± 8.2 c | 11.2 ± 3.1 d |
| Lucenin-2 2 | 45.4 ± 6.6 a | 45.1 ± 5.1 a | 47.9 ± 9.9 a | 42.7 ± 3.0 a | 38.5 ± 3.5 a,b | 24.1 ± 3.7 b |
| p-Coumaric acid ethyl ester 3 | 6.96 ± 0.12 c,d | 8.48 ± 1.01 b,c | 10.6 ± 1.4 a | 9.76 ± 0.50 a,b | 9.49 ± 0.63 a,b | 5.93 ± 0.22 d |
| Vicenin-2 | 107 ± 11 a,b | 102 ± 10 b | 132 ± 17 a | 107 ± 2 a,b | 89.9 ± 5.27 b,c | 48.3 ± 2.5 d |
| Luteolin 6,8-C-hexoside-C-pentoside 2 | 117 ± 12 a,b | 117 ± 12 a,b | 146 ± 23 a | 133 ± 4 a | 135 ± 8 a | 84.4 ± 5.3 b |
| Apigenin 6,8-di-C-hexoside 4 | 13.8 ± 0.9 a,b | 13.5 ± 1.4 a,b | 16.3 ± 2.3 a | 13.0 ± 0.4 a,b | 11.0 ± 0.7 b | 3.85 ± 2.37 c |
| Apigenin 8-C-hexoside 6-C-pentoside 4 | 95.3 ± 15.0 a,b | 89.4 ± 8.8 a,b | 107 ± 17 a | 90.1 ± 5.6 a,b | 73.6 ± 2.1 b | 37.4 ± 2.1 c |
| Schaftoside | 104 ± 9 b | 97.3 ± 5.5 b | 128 ± 11 a | 111 ± 6 a,b | 101 ± 11 b | 56.3 ± 5.0 c |
| Isoorientin | 446 ± 64 a | 414 ± 50 a | 538 ± 73 a | 480 ± 26 a | 498 ± 60 a | 210 ± 23 b |
| Orientin | 150 ± 13 a | 152 ± 15 a | 194 ± 25 a | 168 ± 7 a | 174 ± 24 a | 54.0 ± 6.9 b |
| Vitexin | 40.1 ± 2.1 b | 43.7 ± 3.6 b | 59.3 ± 6.9 a | 49.7 ± 1.13 a,b | 47.8 ± 5.6 b | 18.2 ± 1.4 c |
| DCG 1 | 5.35 ± 0.38 c | 17.3 ± 4.2 b | 18.0 ± 0.9 b | 21.7 ± 4.3 b | 34.7 ± 5.4 a | 35.7 ± 3.7 a |
| Isovitexin | 29.4 ± 3.3 b,c | 30.2 ± 3.4 b | 45.0 ± 5.3 a | 39.8 ± 1.9 a | 39.7 ± 4.0 a | 20.7 ± 1.3 c |
| Caffeic acid derivative 1 | 33.1 ± 5.7 a,b | 20.4 ± 3.1 b | 39.1 ± 6.5 a | 41.0 ± 4.3 a | 38.6 ± 7.1 a | 19.7 ± 3.0 b |
| Unknown 1 | 1.98 ± 0.24 d | 7.77± 0.84 b,c | 11.4 ± 0.8 a,b | 7.75 ± 1.09 b,c | 12.2 ± 3.1 a | 4.23 ± 0.36 c,d |
| ∑ Phenolic compounds | 1309 ± 148 a | 1243 ± 123 a | 1601 ± 219 a | 1418 ± 62 a | 1436 ± 142 a | 660 ± 48 b |
| ∑ Flavone C-glycosides | 1147 ± 133 a | 1105 ± 110 a | 1413 ± 190 a | 1233 ± 39 a | 1209 ± 123 a | 557 ± 37 b |
| Growth Stage | TEAC | FRAP | EC50 (mg/mL) | Non-Oxidized β-Carotene (%) 1 | ||
|---|---|---|---|---|---|---|
| mmol TE/g Extract | µmol TE/g FM | mmol Fe2+/g Extract | µmol Fe2+/g FM | |||
| Stem extension | 0.184 ± 0.058 a | 5.78 ± 1.98 b,c | 0.763 ± 0.032 a,b | 23.8 ± 1.7 c | 0.167 ± 0.041 b,c | 68.6 ± 1.6 c,d |
| Visible bud | 0.150 ± 0.054 a | 5.46 ± 1.92 c | 0.715 ± 0.017 b,c | 26.1 ± 1.3 b,c | 0.201 ± 0.040 a,b | 68.0 ± 2.0 d |
| Flowering | 0.199 ± 0.011 a | 9.08 ± 1.23 a | 0.728 ± 0.024 a,b,c | 33.2 ± 3.6 a | 0.164 ± 0.015 b,c | 69.6 ± 2.2 c,d |
| Brown capsule | 0.222 ± 0.017 a | 8.11 ± 0.59 a,b | 0.783 ± 0.025 a | 28.7 ± 0.6 b | 0.152 ± 0.002 c | 73.7 ± 0.7 a,b |
| Seed ripening | 0.226 ± 0.022 a | 9.93 ± 1.39 a | 0.738 ± 0.028 a,b | 32.3 ± 1.9 a | 0.174 ± 0.014 b,c | 71.1 ± 1.4 b,c |
| Mature seed | 0.200 ± 0.018 a | 5.32 ± 0.39 c | 0.669 ± 0.060 c | 17.8 ± 1.3 d | 0.228 ± 0.014 a | 75.6 ± 1.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gai, F.; Janiak, M.A.; Sulewska, K.; Peiretti, P.G.; Karamać, M. Phenolic Compound Profile and Antioxidant Capacity of Flax (Linum usitatissimum L.) Harvested at Different Growth Stages. Molecules 2023, 28, 1807. https://doi.org/10.3390/molecules28041807
Gai F, Janiak MA, Sulewska K, Peiretti PG, Karamać M. Phenolic Compound Profile and Antioxidant Capacity of Flax (Linum usitatissimum L.) Harvested at Different Growth Stages. Molecules. 2023; 28(4):1807. https://doi.org/10.3390/molecules28041807
Chicago/Turabian StyleGai, Francesco, Michał A. Janiak, Katarzyna Sulewska, Pier Giorgio Peiretti, and Magdalena Karamać. 2023. "Phenolic Compound Profile and Antioxidant Capacity of Flax (Linum usitatissimum L.) Harvested at Different Growth Stages" Molecules 28, no. 4: 1807. https://doi.org/10.3390/molecules28041807
APA StyleGai, F., Janiak, M. A., Sulewska, K., Peiretti, P. G., & Karamać, M. (2023). Phenolic Compound Profile and Antioxidant Capacity of Flax (Linum usitatissimum L.) Harvested at Different Growth Stages. Molecules, 28(4), 1807. https://doi.org/10.3390/molecules28041807







