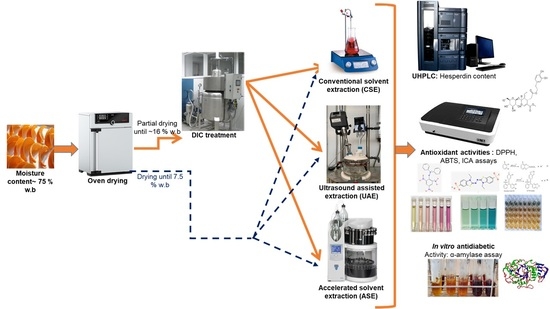
Intensifying Effect of Instant Controlled Pressure Drop (DIC) Pre-Treatment on Hesperidin Recovery from Orange Byproducts: In Vitro Antioxidant and Antidiabetic Activities of the Extracts
Abstract
1. Introduction
2. Results
2.1. Total Phenols, Flavonoids, and Hesperidin Contents of Extracts
2.2. Antioxidant and Antidiabetic Activities of Extracts
2.2.1. DPPH, ABTS Radical Scavenging Activities and Iron Chelating Activity
2.2.2. In Vitro Antidiabetic Activity
2.3. Principal Components Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sample Preparation
4.2. Oven Drying of Orange Byproducts
4.3. Pretreatment by DIC Dehydration of Orange Byproducts
4.4. Extraction Methods
4.4.1. Conventional Solvent Extraction (CSE)
4.4.2. Ultrasound-Assisted Extraction (UAE)
4.4.3. Accelerated Solvent Extraction (ASE)
4.4.4. Intensifying Extraction Using DIC Pretreatment
4.5. Analytical Methods
4.5.1. Determination of Total Phenol and Flavonoid Contents
4.5.2. Analysis of Hesperidin by UHPLC
4.5.3. Determination of Radical Scavenging Activity by DPPH Assay
4.5.4. Determination of Radical Scavenging Activity by ABTS Assay
4.5.5. Iron Chelating Activity
4.5.6. In Vitro α-Amylase Inhibition Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Citrus World Statistics. Edition 2022. New Citrus World Statistics Publication. Available online: https://fruittoday.com/en/new-citrus-world-statistics-publication/ (accessed on 21 November 2022).
- M’hiri, N.; Ioannou, I.; Mihoubi Boudhrioua, N.; Ghoul, M. Extraction methods of citrus peel phenolic compounds: A review. Food Rev. Int. 2014, 30, 265–290. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Mihoubi-Boudhrioua, N. Phytochemical characteristics of Citrus peel and effect of conventional and nonconventional processing on phenolic compounds: A review. Food Rev. Int. 2017, 33, 587–619. [Google Scholar] [CrossRef]
- Chaudhari, S.Y.; Ruknuddin, G.; Prajapati, P. Ethno medicinal values of Citrus genus: A review. Med. J. DY Patil Univ. 2016, 9, 560. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Hypoglycaemic and anti-diabetic activity of selected African medicinal plants. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 224–237. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6971502/ (accessed on 15 December 2019). [PubMed]
- Singh, B.; Pal Singh, J.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Stanisic, D.; Costa, A.F.; Fávaro, W.J.; Tasic, L.; Seabra, A.B. Anticancer Activities of Hesperidin and Hesperetin In vivo and their Potentiality against Bladder Cancer. J. Nanomed. Nanotechnol. 2018, 9, 515. [Google Scholar] [CrossRef]
- Tocmo, R.; Pena-Fronteras, J.; Caalumba, F.K.; Mendoza, M.; Johnson, J.J. Valorization of pomelo (Citrus grandis Osbeck) peel: A review of current utilization, phytochemistry, bioactivities, and mechanisms of action. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1969–2012. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K. Therapeutic and Nutraceutical Potential of Bioactive Compounds Extracted from Fruit Residues. Crit. Rev. Food Sci. Nutr. 2014, 55, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Benayad, O.; Bouhrim, M.; Tiji, S.; Kharchoufa, L.; Addi, M.; Drouet, S.; Hano, C.; Lorenzo, J.M.; Bendaha, H.; Bnouham, M.; et al. Phytochemical Profile, α-Glucosidase, and α-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus aurantium (L.) Peel, a Valuable By-Product from Northeastern Morocco. Biomolecules. 2021, 11, 1555. [Google Scholar] [CrossRef]
- Asabi, O.A.; Oisemuzeimen, J.O.; Abiodun, O.O.; Blessing, K. Antioxidant and In-vitro Antidiabetic Activities of Fermented Peels of Citrus x Sinensis (L.) Osbeck (Rutaceae). Prog. Chem. Biochem. Res. 2021, 4, 414–425. [Google Scholar] [CrossRef]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavonone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, F.; Haidari, F.; Hedayati, M.; Zakerkish, M.; Ahmadi, K. Blood pressure lowering and anti-inflammatory effects of hesperidin in type 2 diabetes; a randomized double-blind controlled clinical trial. Phytother Res. 2018, 32, 1073–1079. [Google Scholar] [CrossRef]
- Homayouni, F.; Haidari, F.; Hedayati, M.; Zakerkish, M.; Ahmadi, K. Hesperidin Supplementation Alleviates Oxidative DNA Damage and Lipid Peroxidation in Type 2 Diabetes: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Phytother Res. 2017, 31, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Pharmacological Significance of Hesperidin and Hesperetin, Two Citrus Flavonoids, as Promising Antiviral Compounds for Prophylaxis Against and Combating COVID-19. Nat. Prod. Commun. 2021, 16, 1–15. [Google Scholar] [CrossRef]
- Padilla de la Rosa, D.; Ruiz-Palomino, P.; Guadalupe, M.; García-Fajardo, J.; Sandoval-Fabián, G.; Arriola-Guevara, E. A Green Process for the Extraction and Purification of Hesperidin from Mexican Lime Peel (Citrus aurantifolia Swingle) that is Extendible to the Citrus Genus. Processes 2018, 6, 266. [Google Scholar] [CrossRef]
- Cheigh, C.I.; Chung, E.Y.; Chung, M.S. Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. J. Food Eng. 2012, 110, 472–477. [Google Scholar] [CrossRef]
- Khadhraoui, B.; Ummat, V.; Tiwari, B.K.; Fabiano-Tixier, A.S.; Chemat, F. Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason Sonochem. 2021, 76, 105625. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, H.M. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef]
- Anticona, M.; Blesa, J.; Frigola, A.; Esteve, M.J. High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods. Foods 2020, 9, 811. [Google Scholar] [CrossRef]
- Inoue, T.; Tsubaki, S.; Ogawa, K.; Onishi, K.; Azuma, J.I. Isolation of hesperidin from peels of thinned Citrus unshiu fruits by microwave-assisted extraction. Food Chem. 2010, 123, 542–547. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Mihoubi Boudhrioua, N. Effect of different operating conditions on the extraction of phenolic compounds in orange peel. Food Bioprod. Process. 2015, 96, 161–170. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Mihoubi Boudhrioua, N.; Ghoul, M. Antioxidants of Maltease orange peel: Comparative investigation of the efficiency of four extraction methods. J. Appl. Pharm. Sci. 2017, 96, 161–170. [Google Scholar] [CrossRef]
- Wang, Z.; Mei, X.; Chen, X.; Rao, S.; Ju, T.; Li, J.; Yang, Z. Extraction and recovery of bioactive soluble phenolic compounds from brocade orange (Citrus sinensis) peels: Effect of different extraction methods thereon. LWT 2023, 173, 114337. [Google Scholar] [CrossRef]
- Chadni, M.; Isidore, E.; Diemer, E.; Ouguir, O.; Brunois, F.; Catteau, R.; Cassan, L.; Ioannou, I. Optimization of Extraction Conditions to Improve Chlorogenic Acid Content and Antioxidant Activity of Extracts from Forced Witloof Chicory Roots. Foods 2022, 11, 1217. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef]
- Shehata, M.G.; Abd El Aziz, N.M.; Youssef, M.M.; El-Sohaimy, S.A. Optimization conditions of ultrasound-assisted extraction of phenolic compounds from orange peels using response surface methodology. J. Food Process. Preserv. 2021, 27, 2268. [Google Scholar] [CrossRef]
- Ghasempour, N.; Elhami, A.H.R.; Javanmard, M.; Azarpazhouh, E.; Armin, M. Optimization of conditions of ultrasound-assisted extraction of phenolic compounds from orange pomace (Citrus sinensis). Int. J. Biol. Chem. 2019, 12, 2. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Barrales, F.M.; Silveira, P.; Barbosa, P.d.P.M.; Ruviaro, A.R.; Paulino, B.N.; Pastore, G.M.; Martinez, J. Recovery of phenolic compounds from citrus by-products using pressurized liquids–an application to orange peel. Food Bioprod. Process. 2018, 112, 9–21. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Chen, X.; Huang, P.; Chen, K.; Ma, Y.; Agarry, E.I.; Kan, J. Optimization and comparison of nonconventional extraction techniques for soluble phenolic compounds from brocade orange (Citrus sinensis) peels. Food Chem. 2022, 87, 4917–4929. [Google Scholar] [CrossRef]
- Repaji, M.; Cegledi, E.; Kruk, V.; Pedisi, S.; Çınar, F.; Kovacevi, B.D.; Žuti, I.; Dragovi´-Uzelac, V. Accelerated Solvent Extraction as a Green Tool for the Recovery of Polyphenols and Pigments from Wild Nettle Leaves. Processes 2020, 8, 803. [Google Scholar] [CrossRef]
- El Kantar, S.; Rajha, H.N.; Maroun, R.G.; Louka, N. Intensification of polyphenols extraction from orange peels using infrared as a novel and energy saving pretreatment. J. Food Sci. 2020, 85, 414–420. [Google Scholar] [CrossRef]
- Allaf, T.; Mounir, S.; Tomao, V.; Chemat, F. Instant Controlled Pressure Drop Combined to Ultrasounds as Innovative Extraction Process Combination: Fundamental Aspects. Procedia Eng. 2012, 42, 1061–1078. [Google Scholar] [CrossRef]
- Allaf, T.; Besombes, C.; Tomao, V.; Chemat, F.; Allaf, K. Coupling DIC and Ultrasound in Solvent Extraction Processes. In Instant Controlled Pressure Drop (D.I.C.); Springer: New York, NY, USA, 2014; Chapter Part III; pp. 151–161. [Google Scholar] [CrossRef]
- Allaf, T.; Tomao, V.; Ruiz, K.; Chemat, F. Instant controlled pressure drop technology and ultrasound assisted extraction for sequential extraction of essential oil and antioxidants. Ultrason Sonochem. 2013, 20, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Louati, I.; Bahloul, N.; Besombes, C.; Allaf, K.; Kechaou, N. Instant Controlled Pressure-Drop as Texturing Pretreatment for Intensifying both Final Drying Stage and Extraction of Phenolic Compounds to valorize Orange Industry By-products (Citrus Sinensis L.). Food Bioprod. Process. 2018, 114, 85–94. [Google Scholar] [CrossRef]
- Mounir, S.; Téllez-Pérez, C.; Sunooj, K.V.; Allaf, K. Texture and Color Characteristics of Swell Dried Ready-to-Eat Zaghloul Date Snacks: Effect of Operative Parameters of Instant Controlled Pressure Drop Process. J. Texture Stud. 2019, 51, 276–289. [Google Scholar] [CrossRef]
- Namir, M.; Elzahar, K.; Ramadan, M.F.; Allaf, K. Cactus pear peel snacks prepared by instant pressure drop texturing: Effect of process variables on bioactive compounds and functional properties. J. Food Meas. Charact. 2016, 11, 388–400. [Google Scholar] [CrossRef]
- Peng, J.; Bi, J.; Yi, J.; Wu, X.; Zhou, M.; Lyu, J.; Liu, J. Engineering Texture Properties of Instant Controlled Pressure Drop (DIC) Dried Carrot Chips via Modulating Osmotic Conditions. Food Bioproc. Tech. 2018, 11, 1674–1685. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, D.; Tan, C.; Hu, Y.; Sundararajan, B.; Zhou, Z. Profiling of flavonoid and antioxidant activity of fruit tissues from 27 Chinese local citrus cultivars. Plants 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Some methodical problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Diemer, E.; Chadni, M.; Grimi, N.; Ioannou, I. Optimization of the Accelerated Solvent Extraction of Caffeoylquinic Acids from Forced Chicory Roots and Antioxidant Activity of the Resulting Extracts. Foods 2022, 11, 3214. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O. Characterization of the antioxidant properties of phenolic extracts from some citrus peels. J. Food Sci. Technol. 2011, 49, 729–736. [Google Scholar] [CrossRef]






| Variables | DPPH-RSA | ABTS-RSA | ICA | Hesperidin | TPC | TFC |
|---|---|---|---|---|---|---|
| DPPH-RSA | 1 | 0.413 | 0.177 | 0.306 | 0.361 | 0.367 |
| ABTS-RSA | - | 1 | 0.787 | 0.944 | 0.945 | 0.913 |
| ICA | - | - | 1 | 0.776 | 0.658 | 0.720 |
| Hesperidin | - | - | - | 1 | 0.970 | 0.991 |
| TPC | - | - | - | - | 1 | 0.962 |
| TFC | - | - | - | - | - | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Abdallah, M.; Chadni, M.; M’hiri, N.; Brunissen, F.; Rokbeni, N.; Allaf, K.; Besombes, C.; Ioannou, I.; Boudhrioua, N. Intensifying Effect of Instant Controlled Pressure Drop (DIC) Pre-Treatment on Hesperidin Recovery from Orange Byproducts: In Vitro Antioxidant and Antidiabetic Activities of the Extracts. Molecules 2023, 28, 1858. https://doi.org/10.3390/molecules28041858
Ben Abdallah M, Chadni M, M’hiri N, Brunissen F, Rokbeni N, Allaf K, Besombes C, Ioannou I, Boudhrioua N. Intensifying Effect of Instant Controlled Pressure Drop (DIC) Pre-Treatment on Hesperidin Recovery from Orange Byproducts: In Vitro Antioxidant and Antidiabetic Activities of the Extracts. Molecules. 2023; 28(4):1858. https://doi.org/10.3390/molecules28041858
Chicago/Turabian StyleBen Abdallah, Mariem, Morad Chadni, Nouha M’hiri, Fanny Brunissen, Nesrine Rokbeni, Karim Allaf, Colette Besombes, Irina Ioannou, and Nourhene Boudhrioua. 2023. "Intensifying Effect of Instant Controlled Pressure Drop (DIC) Pre-Treatment on Hesperidin Recovery from Orange Byproducts: In Vitro Antioxidant and Antidiabetic Activities of the Extracts" Molecules 28, no. 4: 1858. https://doi.org/10.3390/molecules28041858
APA StyleBen Abdallah, M., Chadni, M., M’hiri, N., Brunissen, F., Rokbeni, N., Allaf, K., Besombes, C., Ioannou, I., & Boudhrioua, N. (2023). Intensifying Effect of Instant Controlled Pressure Drop (DIC) Pre-Treatment on Hesperidin Recovery from Orange Byproducts: In Vitro Antioxidant and Antidiabetic Activities of the Extracts. Molecules, 28(4), 1858. https://doi.org/10.3390/molecules28041858