Hybrid Composite of Sn(IV)-Porphyrin and Mesoporous Structure for Enhanced Visible Light Photocatalytic Degradation of Organic Dyes
Abstract
1. Introduction
2. Results and Discussion
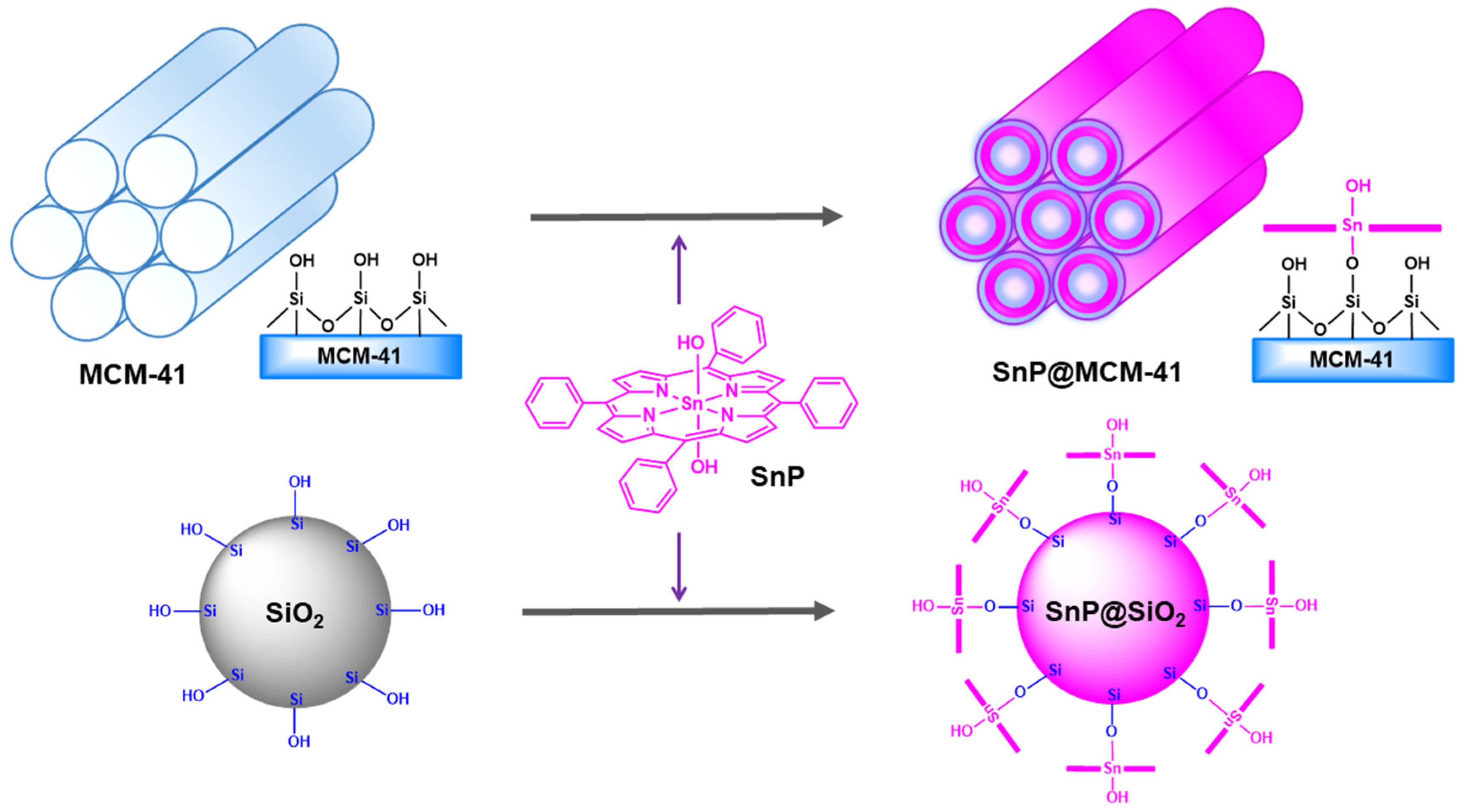
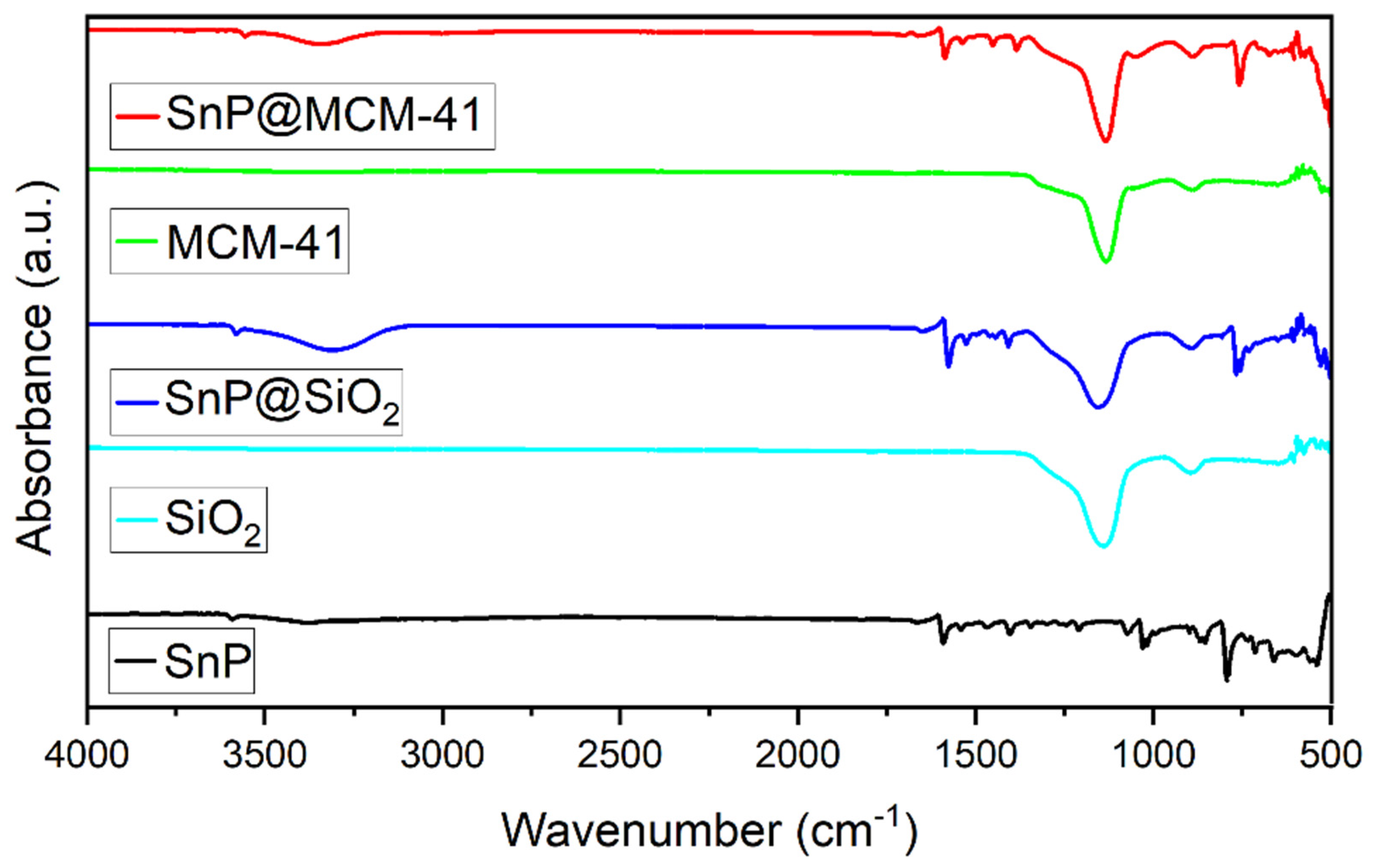
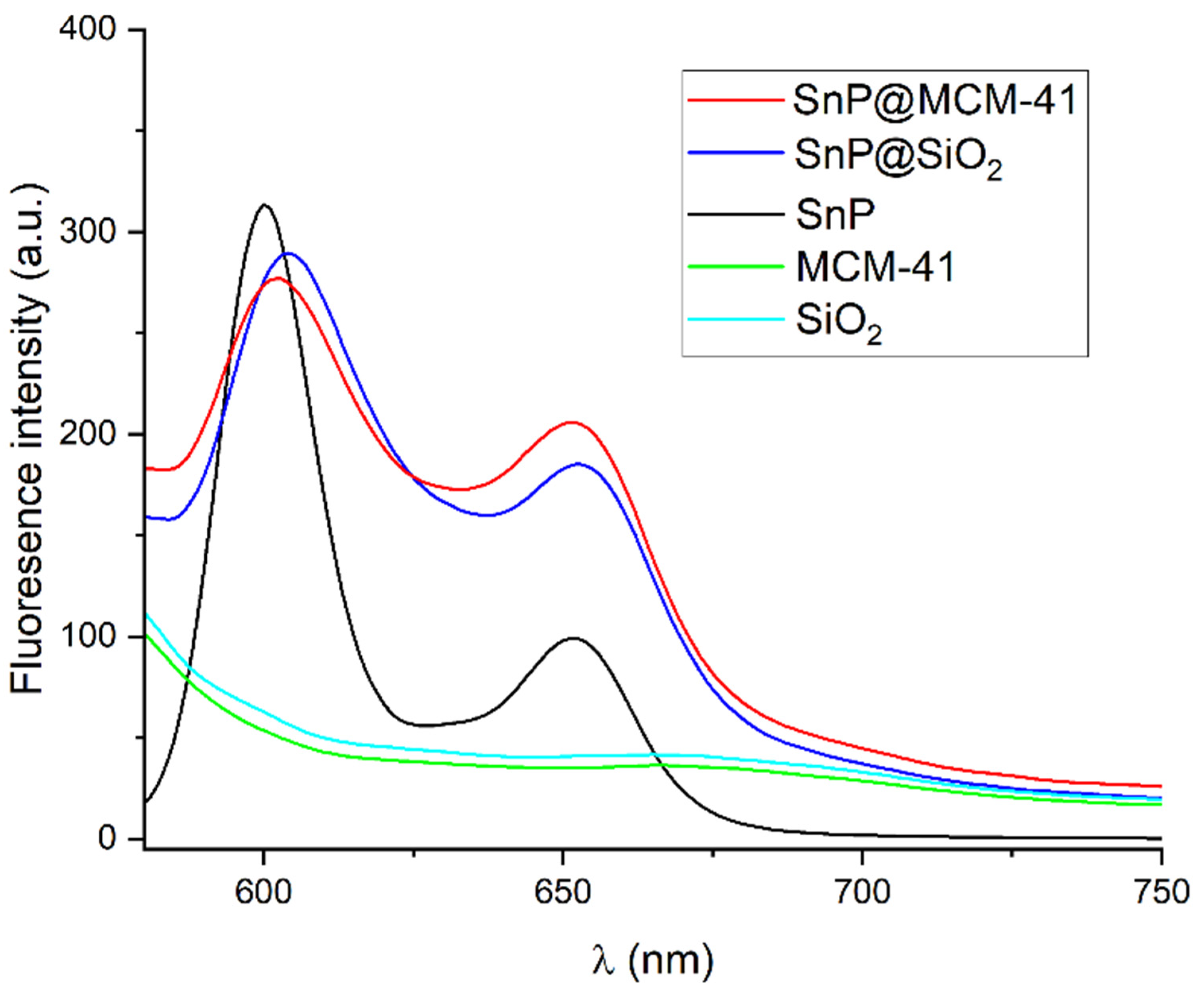
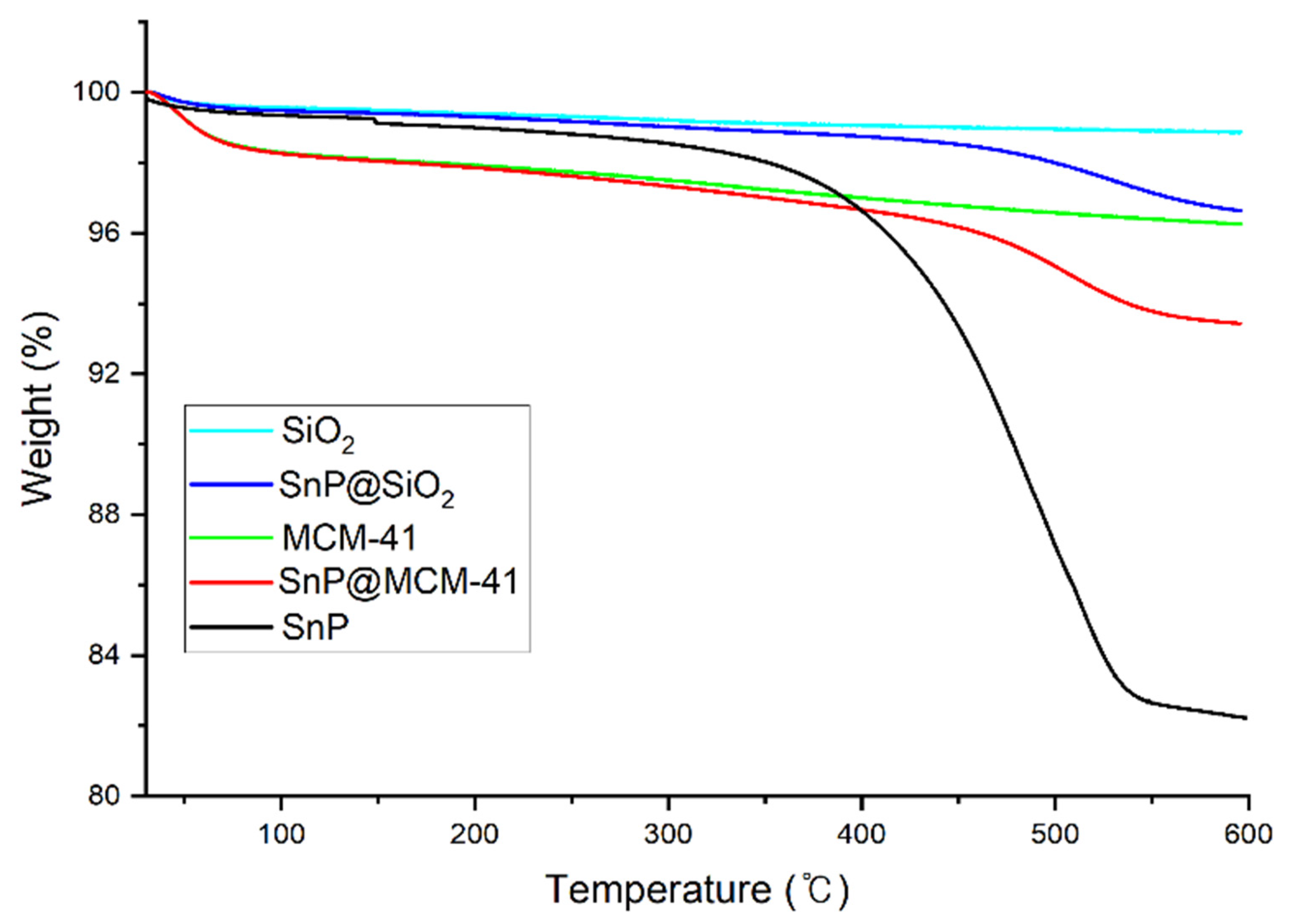
2.1. Fabrication and Characterization
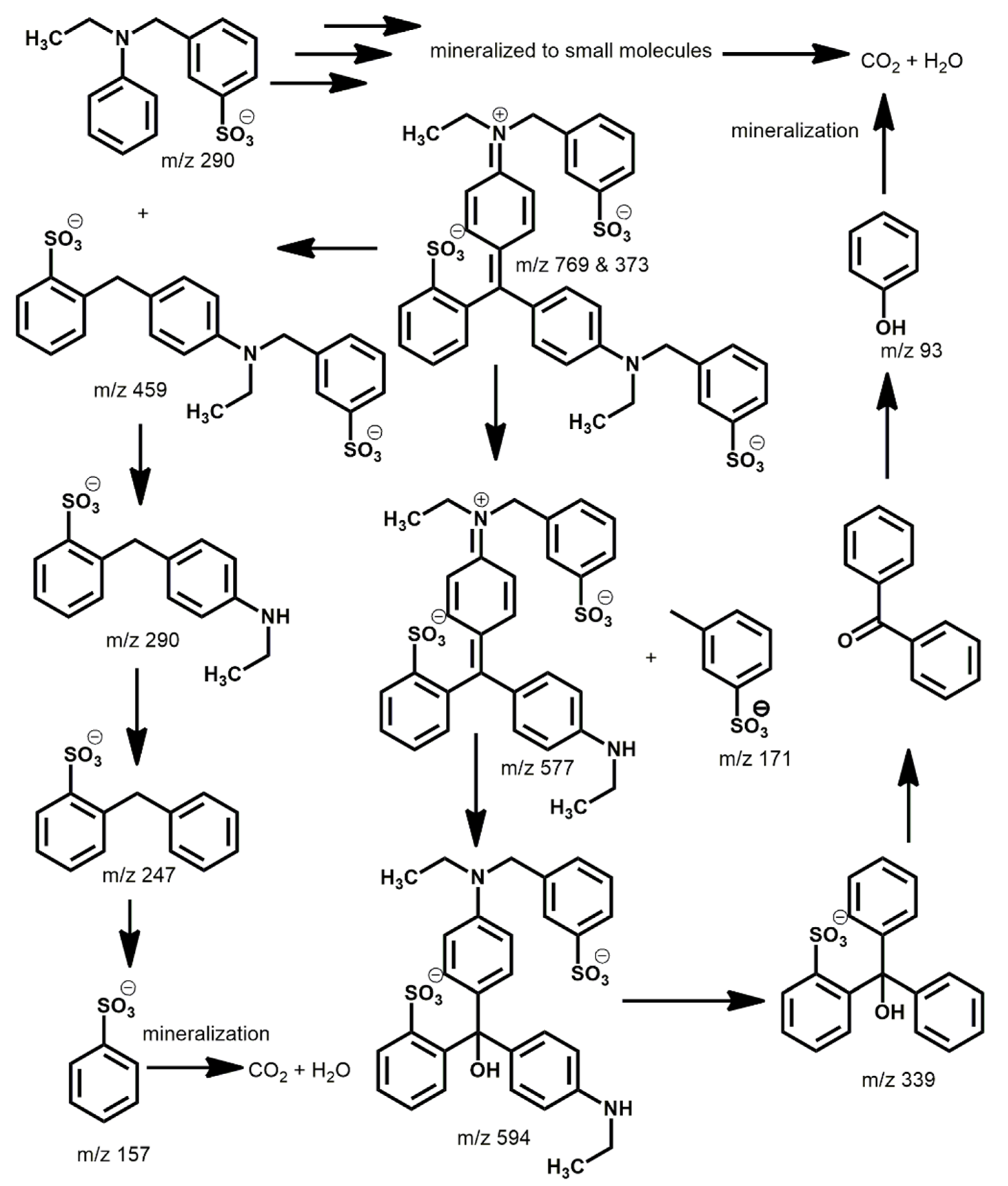
2.2. Photocatalytic Degradation of Organic Dyes
3. Materials and Methods
3.1. Fabrication of SnP@MCM−41
3.2. Fabrication of SnP@SiO2
3.3. Photocatalytic Degradation Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.; Von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Kumar Singh, B.; Paul Nathanail, C.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical Pollution: A Growing Peril and Potential Catastrophic Risk to Humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, H.; Yu, X.; Jia, Y.; Chang, Y.; Gao, S. Morphology regulated Bi2WO6 nanoparticles on TiO2 nanotubes by solvothermal Sb3+ doping as effective photocatalysts for wastewater treatment. Electrochim. Acta 2020, 330, 135167. [Google Scholar] [CrossRef]
- Parvulescu, V.I.; Epron, F.; Garcia, H.; Granger, P. Recent Progress and Prospects in Catalytic Water Treatment. Chem. Rev. 2022, 122, 2981–3121. [Google Scholar] [CrossRef] [PubMed]
- Ain, Q.U.; Rasheed, U.; Yaseen, M.; Zhang, H.; Tong, Z. Superior dye degradation and adsorption capability of polydopamine modified Fe3O4-pillared bentonite composite. J. Hazard. Mater. 2020, 397, 122758. [Google Scholar] [CrossRef] [PubMed]
- Glugoski, L.P.; de Jesus Cubas, P.; Fujiwara, S.T. Reactive Black 5 dye degradation using filters of smuggled cigarette modified with Fe3+. Environ. Sci. Pollut. Res. 2017, 24, 6143–6150. [Google Scholar] [CrossRef]
- Balcha, A.; Yadav, O.P.; Dey, T. Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res. 2016, 23, 25485–25493. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef]
- O’Neill, J.S.; Kearney, L.; Brandon, M.P.; Pryce, M.T. Design Components of Porphyrin-Based Photocatalytic Hydrogen Evolution Systems: A Review. Coord. Chem. Rev. 2022, 467, 214599. [Google Scholar] [CrossRef]
- Mota, H.P.; Quadrado, R.F.N.; Iglesias, B.A.; Fajardo, A.R. Enhanced Photocatalytic Degradation of Organic Pollutants Mediated by Zn(II)-Porphyrin/Poly(Acrylic Acid) Hybrid Microparticles. Appl. Catal. B Environ. 2020, 277, 119208. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Gao, Y.; Yang, W.; Li, Y.; Guo, C. Porous metalloporphyrinic nanospheres constructed from metal 5,10,15,20-tetraksi(4′-ethynylphenyl)porphyrin for efficient catalytic degradation of organic dyes. RSC Adv. 2018, 8, 7330–7339. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Jeon, K.-S.; Oh, S.; Kim, H.-J.; Asahi, T.; Masuhara, H.; Yoon, M. Synthesis of Sn-Porphyrin-Intercalated Trititanate Nanofibers: Optoelectronic Properties and Photocatalytic Activities. Chem. Mater. 2007, 19, 1984–1991. [Google Scholar] [CrossRef]
- Kim, W.; Park, J.; Jo, H.J.; Kim, H.-J.; Choi, W. Visible Light Photocatalysts Based on Homogeneous and Heterogenized Tin Porphyrins. J. Phys. Chem. C 2008, 112, 491–499. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.-Y.; Shen, D.-H.; Song, Y.-Y.; Wang, X.; Liu, Z.-G. Immobilization of cobalt porphyrin on CeO2@SiO2 core-shell nanoparticles as a novel catalyst for selective oxidation of diphenylmethane. J. Mol. Catal. A Chem. 2013, 367, 7–11. [Google Scholar] [CrossRef]
- Yoo, H.-Y.; Yan, S.; Ra, J.W.; Jeon, D.; Goh, B.; Kim, T.-Y.; Mackeyev, Y.; Ahn, Y.-Y.; Kim, H.-J.; Wilson, L.J.; et al. Tin porphyrin immobilization significantly enhances visible-light-photosensitized degradation of Microcystins: Mechanistic implications. Appl. Catal. B Environ. 2016, 199, 33–44. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Y.; Wang, X.; Yang, W.; Bai, F.; Zhang, B.; Alarid, L.; Bian, K.; Fan, H. pH-Dependent Assembly of Porphyrin-Silica Nanocomposites and Their Application in Targeted Photodynamic Therapy. Nano Lett. 2017, 17, 6916–6921. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Martínez-Edo, G.; Balmori, A.; Pontón, I.; Martí del Rio, A.; Sánchez-García, D. Functionalized Ordered Mesoporous Silicas (MCM-41): Synthesis and Applications in Catalysis. Catalysts 2018, 8, 617. [Google Scholar] [CrossRef]
- Costa, J.A.S.; Jesus, R.A.; Santos, D.; Manoa, J.F.; Romao, L.P.C.; Paranhos, C.M. Recent progresses in the adsorption of organic, inorganic, and gas compounds by MCM-41-based mesoporous materials. Microporous Mesoporous Mater. 2020, 291, 109698. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, K.-M.; Ahn, T.K.; Kim, S.K.; Kim, K.S.; Kim, D.; Kim, H.-J. Novel fullerene–porphyrin–fullerene triad linked by metal axial coordination: Synthesis, X-ray crystal structure, and spectroscopic characterizations of trans-bis([60]fullerenoacetato)tin(iv) porphyrin. Chem. Commun. 2004, 2594–2595. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeon, W.S.; Lim, J.H.; Hong, C.S.; Kim, H.-J. Synthesis, X-ray crystal structure, and electrochemistry of trans-bis(ferrocenecarboxylato)(tetraphenylporphyrinato)tin(IV). Polyhedron 2007, 26, 2517–2522. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, J.H.; Choi, H.; Lee, T.; Ko, J.; Yoon, M.; Kim, H.-J. Photoregulated Fluorescence Switching in Axially Coordinated Tin(IV) Porphyrinic Dithienylethene. Inorg. Chem. 2008, 47, 2411–2415. [Google Scholar] [CrossRef]
- Kim, M.K.; Shee, N.K.; Lee, J.; Yoon, M.; Kim, H.-J. Photoinduced Electron Transfer upon Supramolecular Complexation of (Porphyrinato) Sn-Viologen with Cucurbit[7]uril. Photochem. Photobiol. Sci. 2019, 18, 1996–2002. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Fluorescent chemosensing for aromatic compounds by supramolecular complex composed of tin(IV) porphyrin, viologen, and cucurbit[8]uril. Chem. Commun. 2019, 55, 10575–10578. [Google Scholar] [CrossRef]
- Kim, H.-J.; Bampos, N.; Sanders, J.K.M. Assembly of Dynamic Heterometallic Oligoporphyrins Using Cooperative Zinc-Nitrogen, Ruthenium-Nitrogen, and Tin-Oxygen Coordination. J. Am. Chem. Soc. 1999, 121, 8120–8121. [Google Scholar] [CrossRef]
- Indelli, M.T.; Chiorboli, C.; Ghirotti, M.; Orlandi, M.; Scandola, F.; Kim, H.J.; Kim, H.-J. Photoinduced Electron Transfer in Ruthenium(II)/Tin(IV) Multiporphyrin Arrays. J. Phys. Chem. B 2010, 114, 14273–14282. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Shee, N.K.; Park, K.-M.; Kim, H.-J. Assembly and X-ray crystal structures of heterometallic multiporphyrins with complementary coordination between ruthenium (II) and tin (IV) porphyrins. Inorg. Chim. Acta 2019, 488, 1–7. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jo, H.J.; Kim, J.; Kim, S.-Y.; Kim, D.; Kim, K. Supramolecular self-assembly of tin(IV) porphyrin channels stabilizing single-file chains of water molecules. CrystEngComm 2005, 7, 417–420. [Google Scholar] [CrossRef]
- Li, C.; Park, K.-M.; Kim, H.-J. Ionic assembled hybrid nanoparticle consisting of tin(IV) porphyrin cations and polyoxomolybdate anions, and photocatalytic hydrogen production by its visible light sensitization. Inorg. Chem. Commun. 2015, 60, 8–11. [Google Scholar] [CrossRef]
- Shee, N.K.; Lee, C.-J.; Kim, H.-J. Octahedral Tin(IV) Porphyrin-Based Porous Square-Grid Coordination Networks Exhibiting Selective Uptake of CO2 over N2. Bull. Korean Chem. Soc. 2022, 43, 103–109. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, H.-J. Photocatalytic Hydrogen Production by the Sensitization of Sn(IV)-Porphyrin Embedded in a Nafion Matrix Coated on TiO2. Molecules 2022, 27, 3770. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Supramolecular Porphyrin Nanostructures Based on Coordination-Driven Self-Assembly and Their Visible Light Catalytic Degradation of Methylene Blue Dye. Nanomaterials 2020, 10, 2314. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, H.-J. Self-Assembled Nanomaterials Based on Complementary Sn(IV) and Zn(II)-Porphyrins, and Their Photocatalytic Degradation for Rhodamine B Dye. Molecules 2021, 26, 3598. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Three Isomeric Zn(II)-Sn(IV)-Zn(II) Porphyrin-Triad-Based Supramolecular Nanoarchitectures for the Morphology-Dependent Photocatalytic Degradation of Methyl Orange. ACS Omega 2022, 7, 9775–9784. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Morphology-controlled self-assembled nanostructures of complementary metalloporphyrin triads through intermolecular coordination tuning and their photocatalytic degradation for Orange II. Inorg. Chem. Front. 2022, 9, 5538–5548. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Coordination framework materials fabricated by the self-assembly of Sn(IV) porphyrins with Ag(I) ions for the photocatalytic degradation of organic dyes in wastewater. Inorg. Chem. Front. 2022, 9, 1270–1280. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Sn(IV) Porphyrin-Based Ionic Self-Assembled Nanostructures and Their Application in Visible Light Photo-Degradation of Malachite Green. Catalysts 2022, 12, 799. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Sn(IV)-Porphyrin-Based Nanostructures Featuring Pd(II)-Mediated Supramolecular Arrays and Their Photocatalytic Degradation of Acid Orange 7 Dye. Int. J. Mol. Sci. 2022, 23, 13702. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Supramolecular squares of Sn(IV)porphyrins with Re(I)-corners for the fabrication of self-assembled nanostructures performing photocatalytic degradation of Eriochrome Black T dye. Inorg. Chem. Front. 2022, 10, 174–183. [Google Scholar] [CrossRef]
- Jank, M.; Köser, H.; Lücking, F.; Martienssen, M.; Wittchen, S. Decolorization and Degradation of Erioglaucine (Acid Blue 9) Dye in Wastewater. Environ. Technol. 1998, 19, 741–747. [Google Scholar] [CrossRef]
- Larowska, D.; O’Brien, J.M.; Senge, M.O.; Burdzinski, G.; Marciniak, B.; Lewandowska-Andralojc, A. Graphene Oxide Functionalized with Cationic Porphyrins as Materials for the Photodegradation of Rhodamine B. J. Phys. Chem. C 2020, 124, 15769–15780. [Google Scholar] [CrossRef] [PubMed]
- Khezrianjoo, S.; Revanasiddappa, H.D. Effect of operational parameters and kinetic study on the photocatalytic degradation of m-cresol purple using irradiated ZnO in aqueous medium. Water Qual. Res. J. 2015, 51, 69–78. [Google Scholar] [CrossRef]
- Liu, D.; Song, J.; Chung, J.S.; Hur, S.H.; Choi, W.M. ZnO/Boron Nitride Quantum Dots Nanocomposites for the Enhanced Photocatalytic Degradation of Methylene Blue and Methyl Orange. Molecules 2022, 27, 6833. [Google Scholar] [CrossRef] [PubMed]
- Rojas-García, E.; García-Martínez, D.C.; López-Medina, R.; Rubio-Marcos, F.; Castañeda-Ramírez, A.A.; Maubert-Franco, A.M. Photocatalytic Degradation of Dyes Using Titania Nanoparticles Supported in Metal-Organic Materials Based on Iron. Molecules 2022, 27, 7078. [Google Scholar] [CrossRef] [PubMed]
- Borhade, A.V.; Tope, D.R.; Dabhade, G.B. Removal of erioglaucine dye from aqueous medium using ecofriendly synthesized ZnMnO3 photocatalyst. J. Surf. Sci. Nanotech. 2017, 15, 74–80. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, L.; Chen, J.; Ji, L.; Zhou, Y.; Wang, Y.; Cai, L.; Guo, J.; Song, W. Fine Characterization of Natural SiO2-Doped Catalyst Derived from Mussel Shell with Potential Photocatalytic Performance for Organic Dyes. Catalysts 2020, 10, 1130. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Niaei, A.; Khataee, A. Photocatalytic degradation of the herbicide erioglaucine in the presence of nanosized titanium dioxide: Comparison and modeling of reaction kinetics. J. Environ. Sci. Health Part B 2006, 41, 1273–1290. [Google Scholar] [CrossRef]
- Azar, F.; Yang, H.-B.; Venault, L.; Faure, S. Degradation of erioglaucine dye under γ-irradiation. Procedia Chem. 2012, 7, 647–653. [Google Scholar] [CrossRef]
- Jain, R.; Sikarwar, S. Photodestruction and COD removal of toxic dye erioglaucine by TiO2-UV process: Influence of operational parameters. Int. J. Phys. Sci. 2008, 3, 299–305. [Google Scholar]
- Shenoy, S.; Jang, E.; Park, T.J.; Gopinath, C.S.; Sridharan, K. Cadmium sulfide nanostructures: Influence of morphology on the photocatalytic degradation of erioglaucine and hydrogen generation. Appl. Surf. Sci. 2019, 483, 696–705. [Google Scholar] [CrossRef]
- Khezrianjoo, S.; Revanasiddappa, H.D. Electrochemical oxidation of m-cresol purple dye in aqueous media. Water Qual. Res. J. Can. 2015, 50, 305–313. [Google Scholar] [CrossRef]
- Chatterjee, S.; Tyagi, A.K.; Ayyub, P. Efficient Photocatalytic Degradation of Rhodamine B Dye by Aligned Arrays of Self Assembled Hydrogen Titanate Nanotubes. J. Nanomater. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Sundararajan, M.; Sailaja, V.; Kennedy, L.J.; Vijaya, J.J. Photocatalytic degradation of Rhodamine B under visible light using nanostructured zinc doped cobalt ferrite: Kinetics and mechanism. Ceram. Int. 2017, 43, 540–548. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, J.; Li, C.; Yu, J.; Jiang, X.; Zheng, Y.; Hu, W.; Jiao, F. Enhanced photocatalytic degradation of rhodamine B, methylene blue and 4-nitrophenol under visible light irradiation using TiO2/MgZnAl layered double hydroxide. J. Mater. Sci. Mater. Electron. 2018, 29, 7002–7014. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, Z.; He, X.; Yang, X.; Chen, X.; Gao, Z. Constructing a Z-scheme Heterojunction of Egg-Like Core@shell CdS@TiO2 Photocatalyst via a Facile Reflux Method for Enhanced Photocatalytic Performance. Nanomaterials 2019, 9, 222. [Google Scholar] [CrossRef]
- Luna-Flores, A.; Morales, M.A.; Agustín-Serrano, R.; Portillo, R.; Luna-López, J.A.; Pérez-Sánchez, G.F.; Luz, A.D.H.-d.l.; Tepale, N. Improvement of the Photocatalytic Activity of ZnO/Burkeite Heterostructure Prepared by Combustion Method. Catalysts 2019, 9, 817. [Google Scholar] [CrossRef]
- Liu, Z.; Song, Y.; Wang, Q.; Jia, Y.; Tan, X.; Du, X.; Gao, S. Solvothermal fabrication and construction of highly photoelectrocatalytic TiO2 NTs/Bi2MoO6 heterojunction based on titanium mesh. J. Colloid Interface Sci. 2019, 556, 92–101. [Google Scholar] [CrossRef]
- Jo, H.J.; Jung, S.H.; Kim, H.-J. Synthesis and Hydrogen-Bonded Supramolecular Assembly of Trans-Dihydroxotin (IV) Tetrapyridylporphyrin Complexes. Bull. Korean Chem. Soc. 2004, 25, 1869–1873. [Google Scholar]



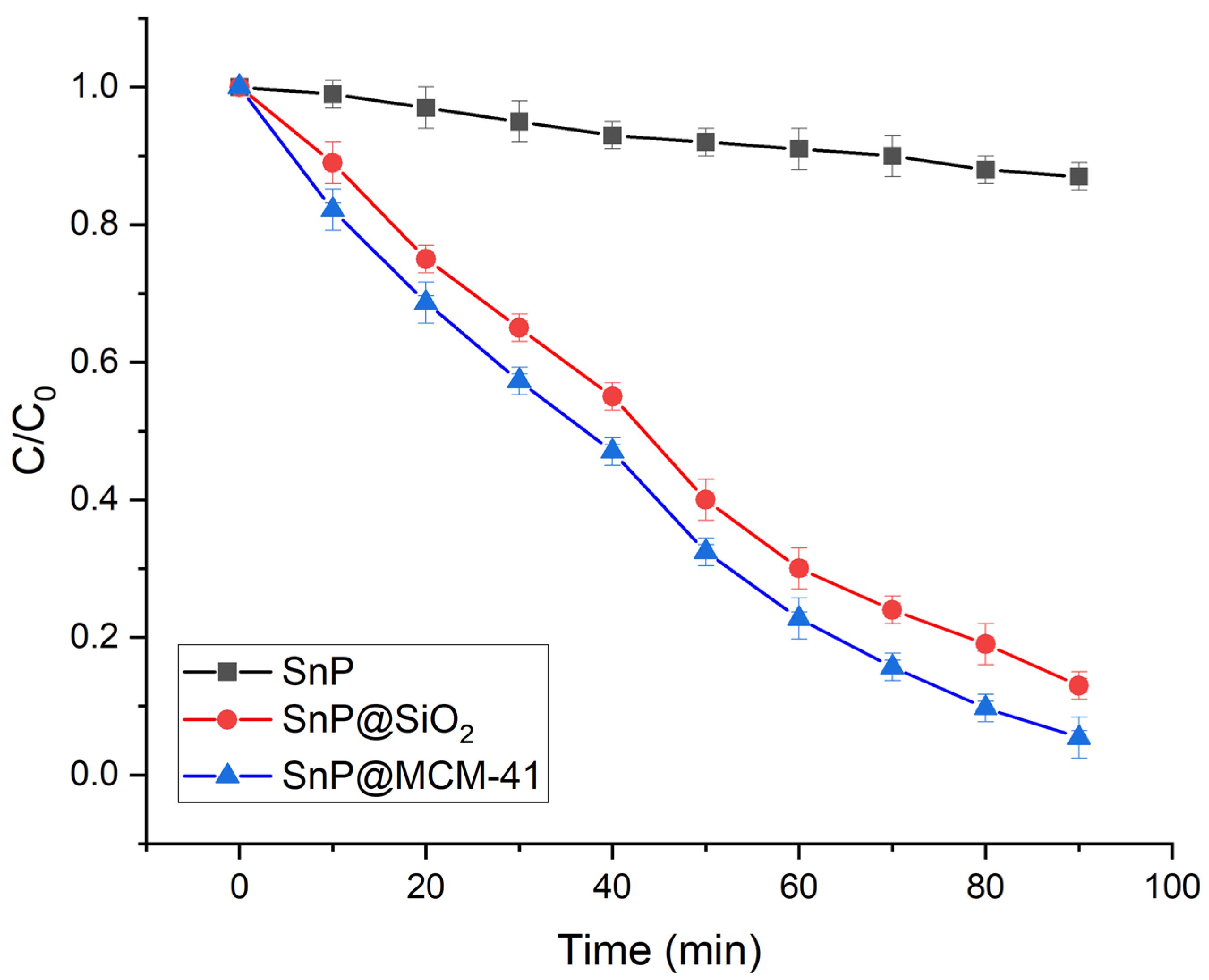
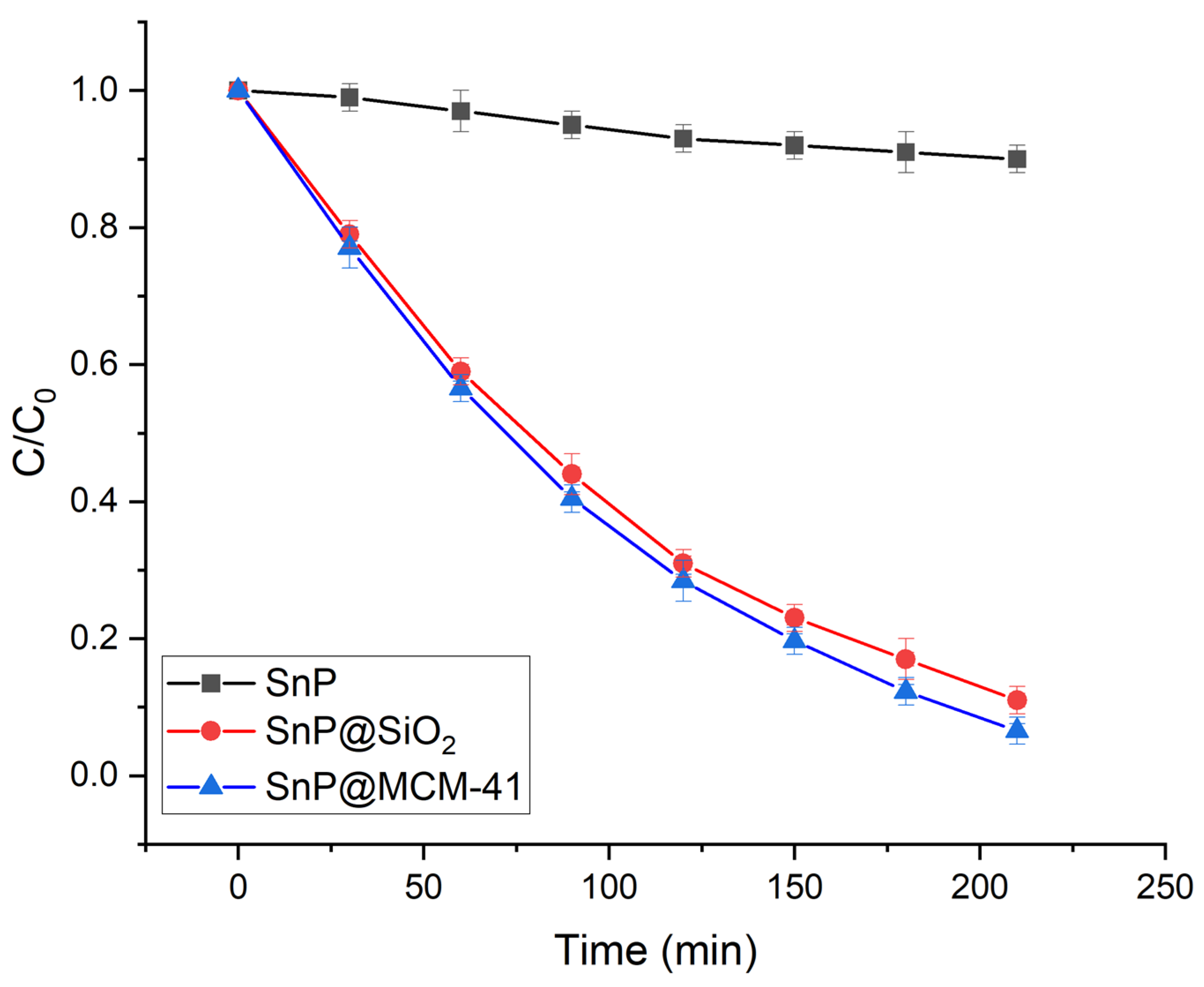
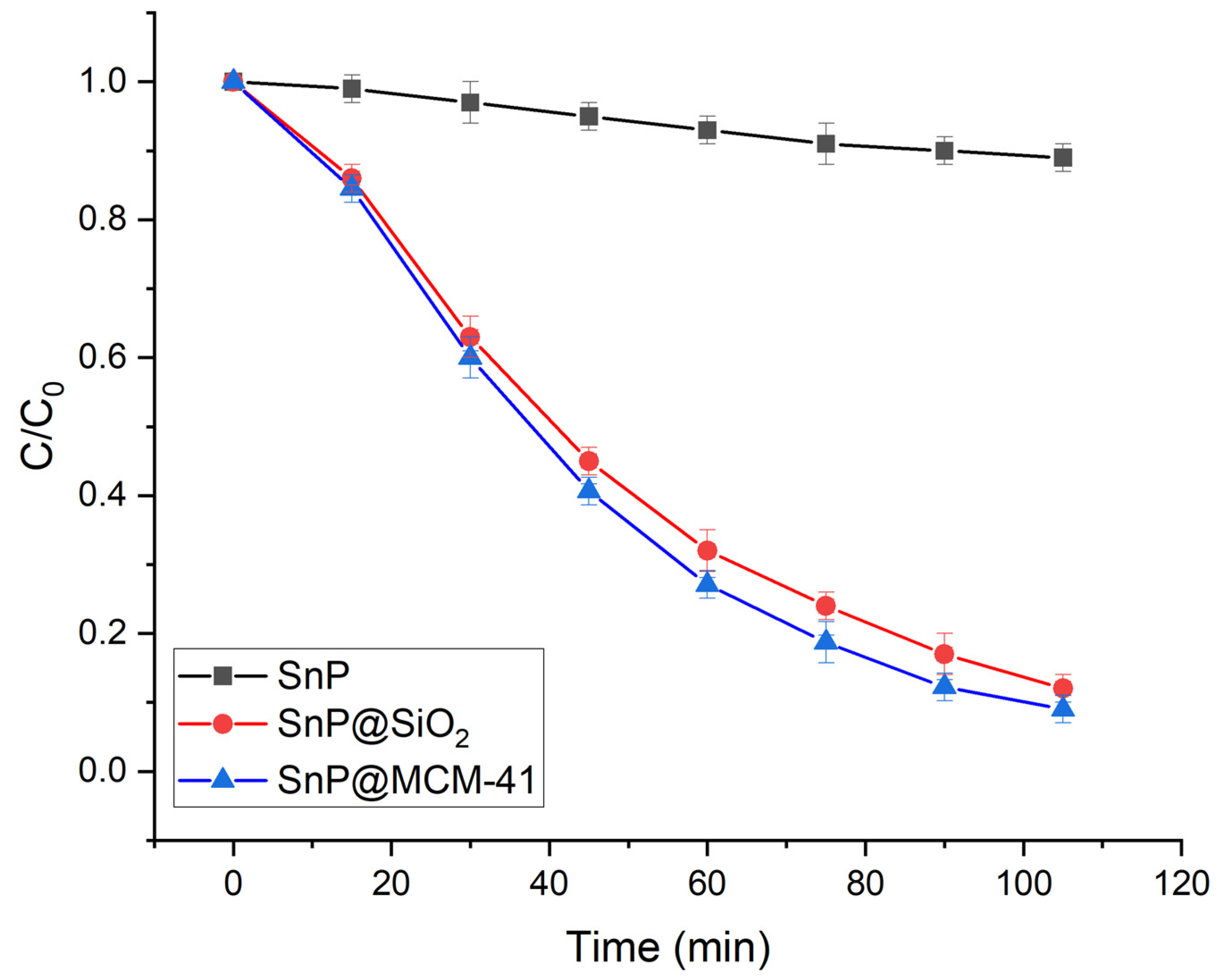
| Photocatalyst | Dye | Rate Constant (min−1) | References |
|---|---|---|---|
| TiO2 (P−25) | EG | 0.0473 | [48] |
| γ−Irradiation | EG | 0.0018 | [49] |
| TiO2 (UV) | EG | 0.025 | [50] |
| CdS (one-dimensional) | EG | 0.008 | [51] |
| SnP | EG | 0.002 | This study |
| SnP@SiO2 | EG | 0.022 | This study |
| SnP@MCM−41 | EG | 0.029 | This study |
| Electrochemical-oxidation | MCP | 0.32 | [52] |
| ZnO | MCP | 0.022 | [53] |
| SnP | MCP | 0.001 | This study |
| SnP@SiO2 | MCP | 0.021 | This study |
| SnP@MCM−41 | MCP | 0.024 | This study |
| TiO2 (P−25) | RhB | 0.001 | [53] |
| Co0.6Zn0.4Fe2O4 | RhB | 0.015 | [54] |
| TiO2/MgZnAl | RhB | 0.005 | [55] |
| CdS | RhB | 0.003 | [56] |
| ZnO | RhB | 0.009 | [57] |
| TiO2 NTs/Bi2MoO6 | RhB | 0.0077 | [58] |
| SnP | RhB | 0.0006 | This study |
| SnP@SiO2 | RhB | 0.011 | This study |
| SnP@MCM−41 | RhB | 0.013 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shee, N.K.; Park, B.-H.; Kim, H.-J. Hybrid Composite of Sn(IV)-Porphyrin and Mesoporous Structure for Enhanced Visible Light Photocatalytic Degradation of Organic Dyes. Molecules 2023, 28, 1886. https://doi.org/10.3390/molecules28041886
Shee NK, Park B-H, Kim H-J. Hybrid Composite of Sn(IV)-Porphyrin and Mesoporous Structure for Enhanced Visible Light Photocatalytic Degradation of Organic Dyes. Molecules. 2023; 28(4):1886. https://doi.org/10.3390/molecules28041886
Chicago/Turabian StyleShee, Nirmal Kumar, Beom-Hyeok Park, and Hee-Joon Kim. 2023. "Hybrid Composite of Sn(IV)-Porphyrin and Mesoporous Structure for Enhanced Visible Light Photocatalytic Degradation of Organic Dyes" Molecules 28, no. 4: 1886. https://doi.org/10.3390/molecules28041886
APA StyleShee, N. K., Park, B.-H., & Kim, H.-J. (2023). Hybrid Composite of Sn(IV)-Porphyrin and Mesoporous Structure for Enhanced Visible Light Photocatalytic Degradation of Organic Dyes. Molecules, 28(4), 1886. https://doi.org/10.3390/molecules28041886







