Erucic Acid—Both Sides of the Story: A Concise Review on Its Beneficial and Toxic Properties
Abstract
:1. Introduction
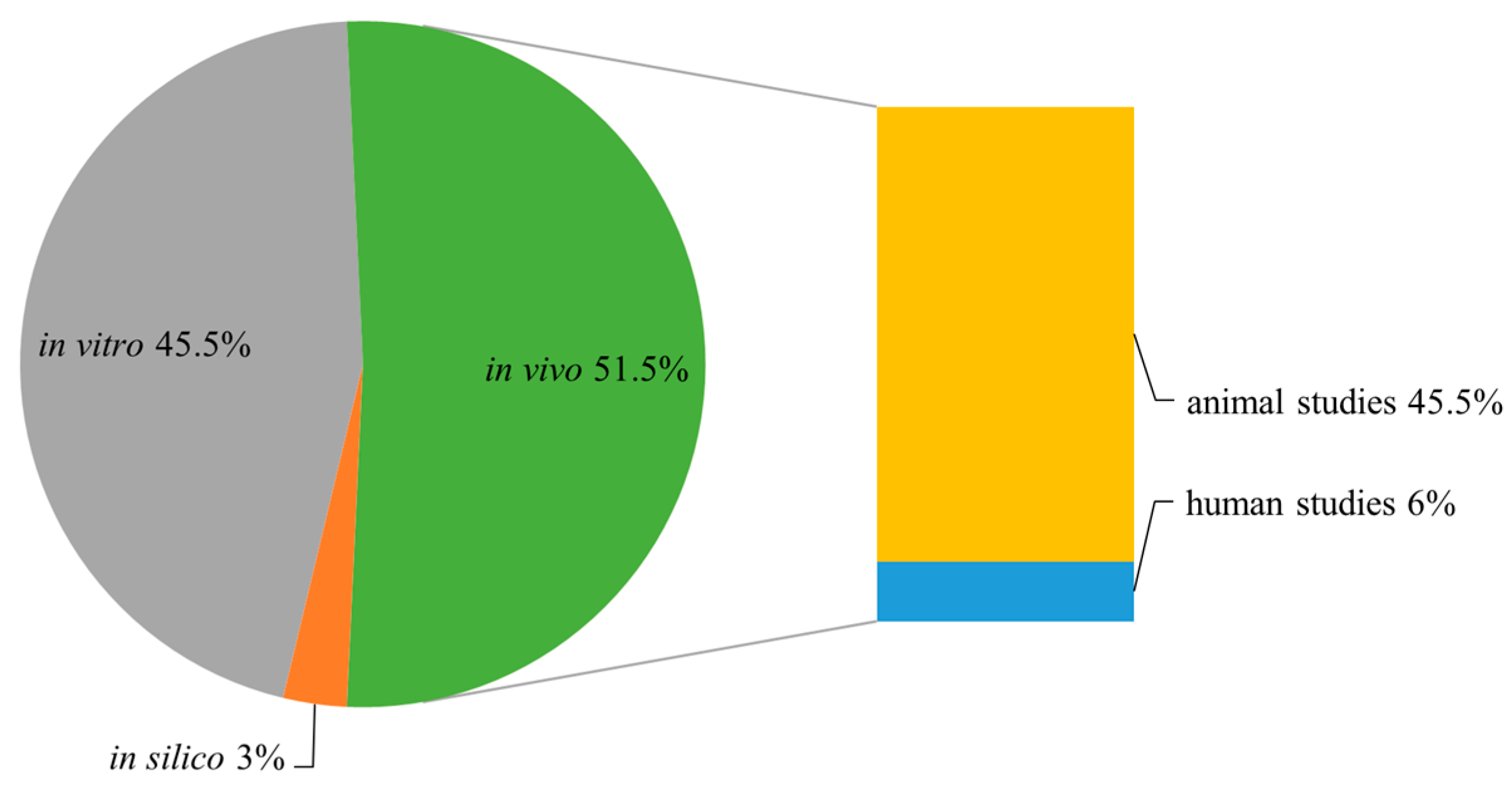
2. Results
2.1. EA Toxic Effects
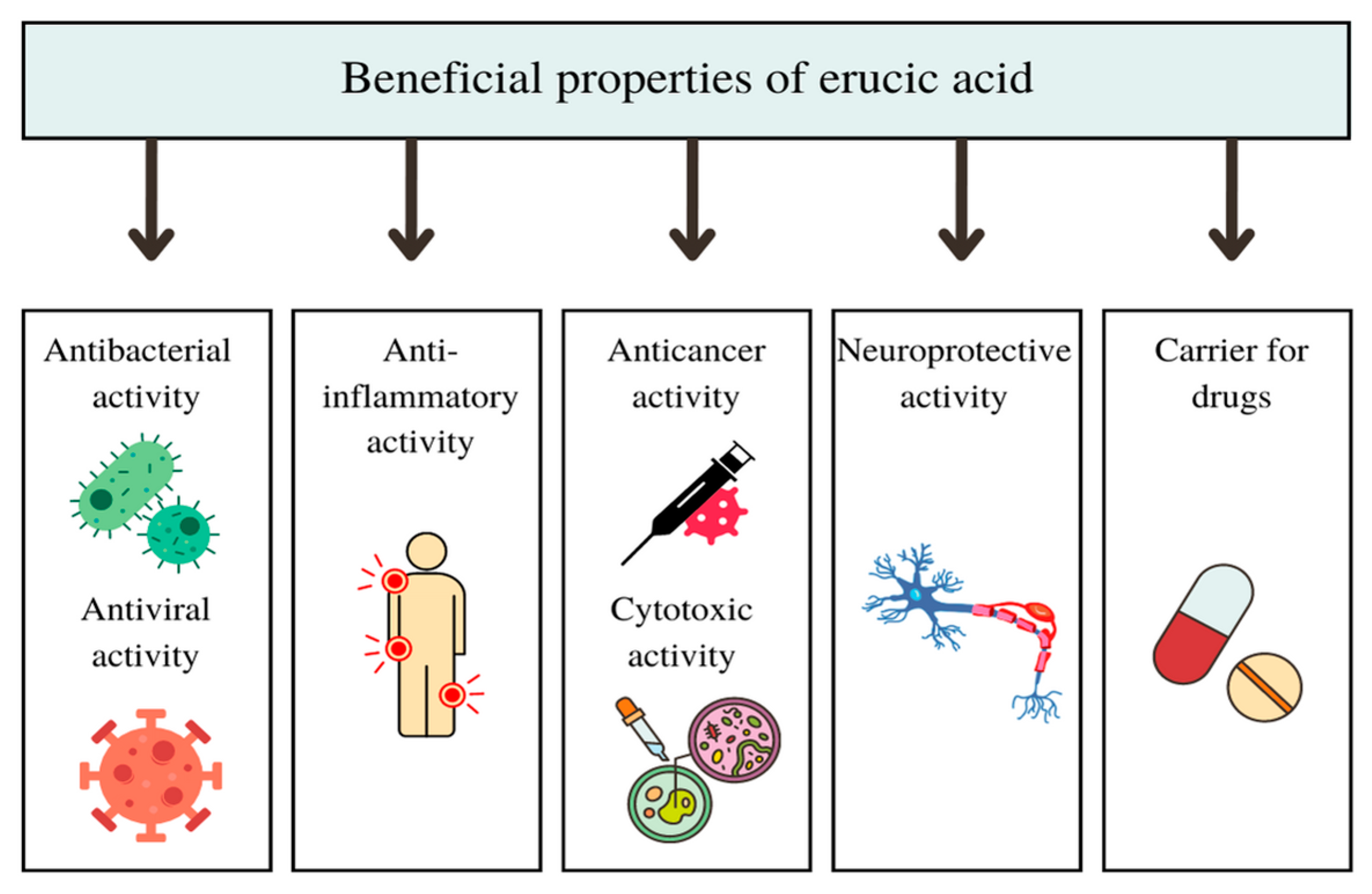
2.2. EA Beneficial Effects
2.2.1. Antibacterial and Antiviral Activity of EA and Its Derivatives In Vitro and In Vivo
2.2.2. Anti-Inflammatory Activity of EA In Vitro and In Vivo
2.2.3. Cytotoxic and Anticancer Activity of EA and Its Derivatives In Vitro and In Vivo
2.2.4. Neuroprotective Activity of EA In Vivo
2.2.5. Use of EA as a Carrier for Other Drugs
3. Materials and Methods
4. Conclusions and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B. Erucic acid in feed and food. EFSA J. 2016, 14, e04593. [Google Scholar]
- Heijkenskjöld, L.; Ernster, L. Studies of the mode of action of erucic acid on heart metabolism. Acta Med. Scand. 1975, 198, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, H. Erucic acid and the Spanish oil epidemic. Lancet 1981, 318, 1293. [Google Scholar] [CrossRef] [PubMed]
- Kilbourne, E.M. Toxic-oil syndrome: Epidemic with an elusive etiology. Chest 1985, 88, 324–325. [Google Scholar] [CrossRef]
- Terracini, B. Toxic Oil Syndrome: Ten Years of Progress, 1st ed.; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2004. [Google Scholar]
- Fatouh, M. Is Lorenzo’s oil effective for the treatment of children with X-linked adrenoleukodystrophy? A systematic review of the literature. Arch. Dis. Child. 2022, 107, A199–A200. [Google Scholar]
- Hulan, H.W.; Argentieri, M.P.; Mahadevan, S.; Sauer, F.D.; Corner, A.H. Relationship between erucic acid and myocardial changes in male rats. Lipids 1976, 11, 9–15. [Google Scholar] [CrossRef]
- Ishinaga, M.; Sato, J.; Kitagawa, Y.; Sugimoto, E.; Kito, M. Perturbation of phospholipid metabolism by erucic acid in male Sprague-Dawley rat heart. J. Biochem. 1982, 92, 253–263. [Google Scholar] [CrossRef]
- Murphy, C.C.; Murphy, E.J.; Golovko, M.Y. Erucic acid is differentially taken up and metabolized in rat liver and heart. Lipids 2008, 43, 391–400. [Google Scholar] [CrossRef]
- De Wildt, D.J.; Speijers, G.J.A. Influence of dietary rapeseed oil and erucic acid upon myocardial performance and hemodynamics in rats. Toxicol. Appl. Pharmacol. 1984, 74, 99–108. [Google Scholar] [CrossRef]
- Bozcali, E.; Süzer, Ö.; Gürsoy, H.N.; Atukeren, P.; Gümüstas, K.M. Effects of erucic acid supplemented feeding on chronic doxorubucin toxicity in rats. Int. J. Clin. Exp. 2009, 2, 337. [Google Scholar]
- Altinoz, M.A.; Bilir, A.; Elmaci, İ. Erucic acid, a component of Lorenzo’s oil and PPAR-δ ligand modifies C6 glioma growth and toxicity of doxorubicin. Experimental data and a comprehensive literature analysis. Chem. Biol. Interact. 2018, 294, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Bierenbaum, M.L.; Chen, Y.; Lei, H.; Watkins, T. Relationship between dietary fatty acid, selenium, and degenerative cardiomyopathy. Med. Hypotheses 1992, 39, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Lemaitre, R.N.; King, I.B.; Song, X.; Steffen, L.M.; Folsom, A.R.; Mozaffarian, D. Long-chain monounsaturated Fatty acids and incidence of congestive heart failure in 2 prospective cohorts. Circulation 2013, 127, 1512–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kageyama, K.; Nagasawa, T.; Kimura, S.; Kobayashi, T.; Kinoshita, Y. Cytotoxic activity of unsaturated fatty acids to lymphocytes. Can. J. Biochem. 1980, 58, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, E.N.; Gray, T.J.; Lake, B.G. Effect of very long chain fatty acids on peroxisomal β-oxidation in primary rat hepatocyte cultures. Lipids 1985, 20, 929–932. [Google Scholar] [CrossRef]
- Kawano, H.; Nishizawa-Tanaka, Y.; Yasuda, S.; Sakai, D.; Miyake, M.; Mayumi, T.; Hama, T. Induction of peroxisomal β-oxidation and fatty acid content in primary cultures of rat hepatocytes. J. Clin. Biochem. Nutr. 1997, 23, 145–153. [Google Scholar] [CrossRef]
- El Bioukar, B.; Straehli, F.; Ng, K.H.; Rolland, M.O.; Hashimoto, T.; Carreau, J.P.; Deschatrette, J. Resistance to erucic acid as a selectable marker for peroxisomal activity: Isolation of revertants of an infantile Refsum disease cell line. J. Inherit. Metab. Dis. 1994, 17, 41–59. [Google Scholar] [CrossRef]
- Takahashi, A.; Dohi, H.; Egashira, Y.; Hirai, S. Erucic acid derived from rosemary regulates differentiation of mesenchymal stem cells into osteoblasts/adipocytes via suppression of peroxisome proliferator-activated receptor γ transcriptional activity. Phytother. Res. 2020, 34, 1358–1366. [Google Scholar] [CrossRef]
- Plötz, T.; von Hanstein, A.S.; Krümmel, B.; Laporte, A.; Mehmeti, I.; Lenzen, S. Structure-toxicity relationships of saturated and unsaturated free fatty acids for elucidating the lipotoxic effects in human EndoC-βH1 beta-cells. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165525. [Google Scholar] [CrossRef]
- Goc, A.; Niedzwiecki, A.; Rath, M. Anti-borreliae efficacy of selected organic oils and fatty acids. BMC Complement Altern. Med. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowska, A.; Roszkowski, P.; Bielenica, A.; Olejarz, W.; Stępień, K.; Struga, M. Anticancer and antimicrobial effects of novel ciprofloxacin fatty acids conjugates. Eur. J. Med. Chem. 2020, 185, 111810. [Google Scholar] [CrossRef]
- Liang, X.; Huang, Y.; Pan, X.; Hao, Y.; Chen, X.; Jiang, H.; Yang, Z. Erucic acid from Isatis indigotica Fort. suppresses influenza A virus replication and inflammation in vitro and in vivo through modulation of NF-κB and p38 MAPK pathway. J. Pharm. Anal. 2020, 10, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Henry, G.E.; Momin, R.A.; Nair, M.G.; Dewitt, D.L. Antioxidant and cyclooxygenase activities of fatty acids found in food. J. Agric. Food Chem. 2002, 50, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Melzig, M.F.; Henke, K. Inhibition of thrombin activity by selected natural products in comparison to neutrophil elastase. Planta Med. 2005, 71, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Aparna, V.; Dileep, K.V.; Sadasivan, C.; Haridas, M. Computational and thermodynamic analyses of the phospholipase A2 inhibition by erucic acid and linoleic acid. Med. Chem. Res. 2013, 22, 1102–1106. [Google Scholar] [CrossRef]
- Gan, L.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Zhou, X.Q. Erucic acid impairs intestinal immune function of on-growing grass carp (Ctenopharyngodon idella). Aquaculture 2020, 519, 734916. [Google Scholar] [CrossRef]
- Hawkins, R.A.; Sangster, K.; Arends, M.J. The apoptosis-inducing effects of polyunsaturated fatty acids (PUFAs) on benign and malignant breast cells in vitro. Breast 1999, 8, 16–20. [Google Scholar] [CrossRef]
- Erdlenbruch, B.; Jendrossek, V.; Gerriets, A.; Vetterlein, F.; Eibl, H.; Lakomek, M. Erucylphosphocholine: Pharmacokinetics, biodistribution and CNS-accumulation in the rat after intravenous administration. Cancer Chemother. Pharmacol. 1999, 44, 484–490. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Struga, M.; Roszkowski, P.; Koliński, M.; Kmiecik, S.; Jałbrzykowska, K.; Bielenica, A. The effect of conjugation of ciprofloxacin and moxifloxacin with fatty acids on their antibacterial and anticancer activity. Int. J. Mol. Sci. 2022, 23, 6261. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Olejarz, W.; Kubiak-Tomaszewska, G.; Ciechanowicz, A.K.; Struga, M. The Effect of Fatty Acids on Ciprofloxacin Cytotoxic Activity in Prostate Cancer Cell Lines—Does Lipid Component Enhance Anticancer Ciprofloxacin Potential? Cancers 2022, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Ko, H.J.; Jeon, S.J.; Lee, S.; Lee, H.E.; Kim, H.N.; Ryu, J.H. The memory-enhancing effect of erucic acid on scopolamine-induced cognitive impairment in mice. Pharmacol. Biochem. Behav. 2016, 142, 85–90. [Google Scholar] [CrossRef]
- Ünal, İ.; Cansız, D.; Sürmen, M.G.; Sürmen, S.; Sezer, Z.; Beler, M.; Üstündag, Ü.V.; Güzel, E.; Alturfan, A.A.; Emekli-Alturfan, E. Identification of molecular network of gut-brain axis associated with neuroprotective effects of PPARδ-ligand erucic acid in rotenone-induced Parkinson’s disease model in zebrafish. Eur. J. Neurosci. 2022, 57, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Judy, K.D.; Olivi, A.; Buahin, K.G.; Domb, A.; Epstein, J.I.; Colvin, O.M.; Brem, H. Effectiveness of controlled release of a cyclophosphamide derivative with polymers against rat gliomas. J. Neurosurg. 1995, 82, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Olivi, A.; Ewend, M.G.; Utsuki, T.; Tyler, B.; Domb, A.J.; Brat, D.J.; Brem, H. Interstitial delivery of carboplatin via biodegradable polymers is effective against experimental glioma in the rat. Cancer Chemother. Pharmacol. 1996, 39, 90–96. [Google Scholar]
- Golenser, J.; Domb, A.; Teomim, D.; Tsafack, A.; Nisim, O.; Ponka, P.; Cabantchik, Z.I. The treatment of animal models of malaria with iron chelators by use of a novel polymeric device for slow drug release. J. Pharmacol. Exp. Ther. 1997, 281, 1127–1135. [Google Scholar]

| Treatment | Effect | Ref. |
|---|---|---|
| Animal Studies | ||
| 1.4 or 2.6 g EA/100 g diet |
| [3] |
| 5.4% EA in diet |
| [8] |
| 5% EA in diet |
| [10] |
| 8.9% EA in diet |
| [11] |
| 0.5 or 5% EA in diet |
| [12] |
| EA and DOX (5 mg/kg + 100 mg/kg) |
| [13] |
| Human Studies | ||
| 500 mL mustard oil/month |
| [14] |
| prospective study |
| [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galanty, A.; Grudzińska, M.; Paździora, W.; Paśko, P. Erucic Acid—Both Sides of the Story: A Concise Review on Its Beneficial and Toxic Properties. Molecules 2023, 28, 1924. https://doi.org/10.3390/molecules28041924
Galanty A, Grudzińska M, Paździora W, Paśko P. Erucic Acid—Both Sides of the Story: A Concise Review on Its Beneficial and Toxic Properties. Molecules. 2023; 28(4):1924. https://doi.org/10.3390/molecules28041924
Chicago/Turabian StyleGalanty, Agnieszka, Marta Grudzińska, Wojciech Paździora, and Paweł Paśko. 2023. "Erucic Acid—Both Sides of the Story: A Concise Review on Its Beneficial and Toxic Properties" Molecules 28, no. 4: 1924. https://doi.org/10.3390/molecules28041924
APA StyleGalanty, A., Grudzińska, M., Paździora, W., & Paśko, P. (2023). Erucic Acid—Both Sides of the Story: A Concise Review on Its Beneficial and Toxic Properties. Molecules, 28(4), 1924. https://doi.org/10.3390/molecules28041924






