Design, Synthesis, and Pharmacology of New Triazole-Containing Quinolinones as CNS Active Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
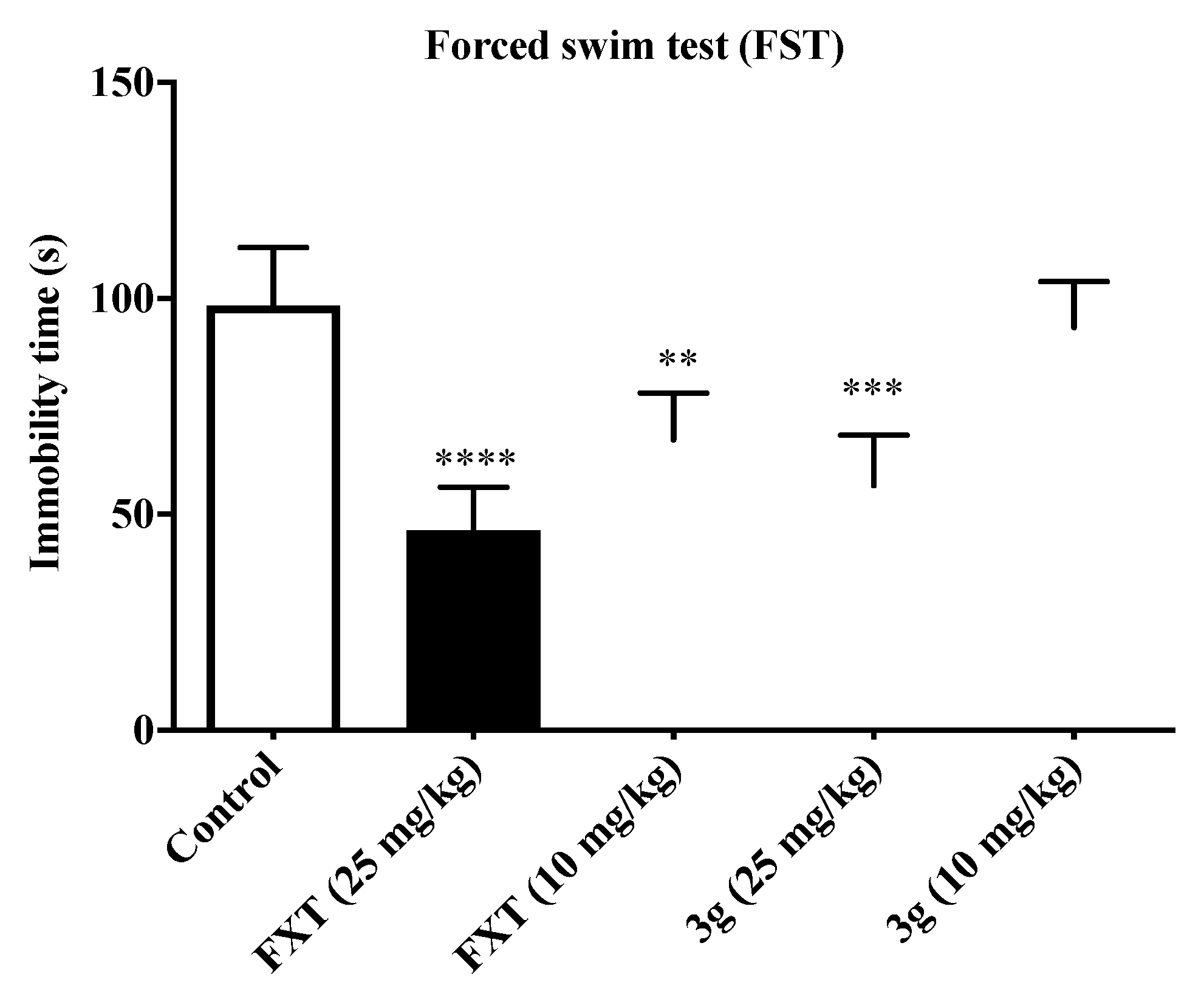
2.2. Antidepressant Activities
2.3. Antiseizure Activity and Neurotoxicity
2.4. Effects of Compound 3g on the Level of Neurotransmitters GABA and 5-HT in Mouse Brain
2.5. Molecular Docking, Drug-Like Properties, and Pharmacokinetic Properties Prediction
3. Materials and Methods
3.1. Chemiscal Part
3.1.1. Synthesis Procedure of 6-Acetyl-3,4-dihydroquinolin-2(1H)-one (1)
3.1.2. Synthesis Procedure of N-substituted-6-acetyl-3,4-dihydro-2(1H)-quinolinone (2a–l)
3.1.3. Synthesis Procedure of Target Compounds (3a–3l)
3.2. In Vivo Pharmacology
3.3. Molecular Docking, Drug-Like Properties, and Pharmacokinetic Properties Prediction
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- World Health Organisation (WHO). Depression and Other Common Mental Disorders Global Health Estimates; WHO: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf (accessed on 1 September 2020).
- Singh, G.; Sander, J.W. The global burden of epilepsy report: Implications for low- and middle-income countries. Epilepsy Behav. 2020, 5, 106949. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, B.; Czuczwar, S.J. Epilepsy coexisting with depression. Pharmacol. Rep. 2016, 68, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- De Silva, S.; Isuru, A.; Rodrigo, A.; Kuruppuarachchi, L. Prevalence and correlates of depression in patients with epilepsy in Sri Lanka. Ceylon Med. J. 2021, 66, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Y.; Hu, Z.; Jiang, J.; Ye, J.; Zhou, Y.; Yu, Z.; Tang, H. Effectiveness of acupuncture for anxiety and depression in irritable bowel syndrome: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e24958. [Google Scholar] [CrossRef] [PubMed]
- Valente, K.D.; Busatto, F.G. Depression and temporal lobe epilepsy represent an epiphenomenon sharing similar neural networks: Clinical and brain structural evidences. Arq. Neuropsiquiatr. 2013, 71, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Butler, T.; Harvey, P.; Cardozo, L.; Zhu, Y.S.; Mosa, A.; Tanzi, E.; Pervez, F. Epilepsy, depression, and growth hormone. Epilepsy Behav. 2019, 94, 297–300. [Google Scholar] [CrossRef]
- Wei, Z.; Ren, L.; Wang, X.; Liu, C.; Cao, M.; Hu, M.; Jiang, Z.; Hui, B.; Xia, F.; Yang, Q.; et al. Network of depression and anxiety symptoms in patients with epilepsy. Epilepsy Res. 2021, 175, 106696. [Google Scholar] [CrossRef]
- Borgmann, M.; Holtkamp, M.; Adli, M.; Behr, J. Depression und Epilepsie: Zwei Krankheitsbilder mit gemeinsamen Ursachen? [Depression and epilepsy: Two clinical pictures with common causes?]. Nervenarzt 2016, 87, 724–730. [Google Scholar] [CrossRef]
- Kanner, A.M. The treatment of depressive disorders in epilepsy: What all neurologists should know. Epilepsia 2013, 54, 3–12. [Google Scholar] [CrossRef]
- Kanner, A.M. Depression in Epilepsy: A Neurobiologic Perspective. Epilepsy Curr. 2005, 5, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Ojemann, L.M.; Friel, P.N.; Trejo, W.J.; Dudley, D.L. Effect of doxepin on seizure frequency in depressed epileptic patients. Neurology 1982, 33, 646–648. [Google Scholar] [CrossRef]
- Rosenstein, D.; Nelson, J.; Jacobs, S. Seizures associated with antidepressants: A review. J. Clin. Psychol. 1993, 54, 289–299. [Google Scholar]
- Liu, B.; Anderson, G.; Mittmann, N.; To, T.; Axcell, T.; Shear, N. Use of selective serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet 1998, 351, 1303–1307. [Google Scholar] [CrossRef]
- Warden, S.J.; Robling, A.G.; Sanders, M.S.; Bliziotes, M.M.; Turner, C.H. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology 2005, 146, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Diem, S.J.; Blackwell, T.L.; Stone, K.L.; Yaffe, K.; Haney, E.M.; Bliziotes, M.M.; Ensrud, K.E. Use of antidepressants and rates of hip bone loss in older women: The study of osteoporotic fractures. Arch. Intern. Med. 2007, 167, 1240–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, T.; Coupland, C.; Morriss, R.; Arthur, A.; Moore, M.; Hippisley-Cox, J. Antidepressant use and risk of epilepsy and seizures in people aged 20 to 64 years: Cohort study using a primary care database. BMC Psychiatry 2015, 15, 315. [Google Scholar] [CrossRef] [Green Version]
- Maguire, M.J.; Marson, A.G.; Nevitt, S.J. Antidepressants for people with epilepsy and depression. Cochrane Database Syst. Rev. 2021, 4, CD010682. [Google Scholar]
- Górska, N.; Słupski, J.; Cubała, W.J.; Wiglusz, M.S.; Gałuszko-Węgielnik, M. Antidepressants in epilepsy. Neurol. Neurochir. Pol. 2018, 52, 657–661. [Google Scholar] [CrossRef]
- Steinert, T.; Fröscher, W. Epileptic Seizures Under Antidepressive Drug Treatment: Systematic Review. Pharmacopsychiatry 2018, 51, 121–135. [Google Scholar] [CrossRef]
- Guan, L.P.; Quan, Z.S. 3,4-DHQLO and Triazole and Its Related Analogues with Anticonvulsant Effects. Mini Rev. Med. Chem. 2016, 16, 323–342. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, X.; Wang, T.; Xiao, J. Quinolone hybrids and their anti-cancer activities: An overview. Eur. J. Med. Chem. 2019, 165, 59–79. [Google Scholar] [CrossRef]
- Kalkhambkar, R.G.; Aridoss, G.; Kulkarni, G.M. Synthesis and biological studies of some new acrylic acid ethyl esters of quinolinone. Monatsh. Chem. 2012, 143, 1075–1086. [Google Scholar] [CrossRef]
- Kumar, S.; Bawa, S.; Drabu, S.; Gupta, H.; Machwal, L.; Kumar, R. Synthesis, antidepressant and antifungal evaluation of novel 2-chloro-8-methylquinoline amine derivatives. Eur. J. Med. Chem. 2011, 46, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, Y.; Sakurai, Y.; Sato, S. 3,4-dihydro-2(1H)-quinolinone as a novel anti- depressant drug: Synthesis and pharmacology of 1-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-3,4-dihydro-5-methoxy-2(1H)-quinolinone and its derivatives. J. Med. Chem. 2000, 43, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Bauman, J.N.; Frederick, K.S.; Sawant, A.; Walsky, R.L.; Cox, L.M.; Obach, R.S.; Kalgutkar, A.S. Comparison of the bioactivation potential of the antidepressant and hepatotoxin nefazodone with aripiprazole, a structural analog and marketed drug. Drug Metab. Dispos. 2008, 36, 1016–1029. [Google Scholar] [CrossRef]
- Weber, J.; Lyseng-Williamson, K.A.; Scott, L.J. Aripiprazole: In major depressive disorder. CNS Drugs 2008, 22, 807–813. [Google Scholar] [CrossRef]
- Furukawa, Y.; Hamza, T.; Cipriani, A.; Furukawa, T.A.; Salanti, G.; Ostinelli, E.G. Optimal dose of aripiprazole for augmentation therapy of antidepressant-refractory depression: Preliminary findings based on a systematic review and dose-effect meta-analysis. Br. J. Psychiatry 2022, 221, 440–447. [Google Scholar] [CrossRef]
- Deng, X.Q.; Song, M.X.; Zheng, Y.; Quan, Z.S. Design, synthesis and evaluation of the antidepressant and anticonvulsant activities of triazole-containing quinolinones. Eur. J. Med. Chem. 2014, 73, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.R.; Walker, K.A.; Hirschfeld, D.R.; Bruno, J.J.; Yang, D.S.; Maloney, P.J. 3,4-Dihydroquinolin-2(1H)-ones as combined inhibitors of thromboxane A2 synthase and cAMP phosphodiesterase. J. Med. Chem. 1992, 35, 620–628. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]
- Cryan, J.F.; Valentino, R.J.; Lucki, I. Assessing substrates underlying the. behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 2005, 29, 547–569. [Google Scholar] [CrossRef]
- Bourin, M.; Chenu, F.; Ripoll, N.; David, D.J. A proposal of decision tree to screen putative antidepressants using forced swim and tail suspension tests. Behav. Brain. Res. 2005, 164, 266–269. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 97, 52587. [Google Scholar]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [PubMed]
- Yuen, E.S.; Trocóniz, I.F. Can pentylenetetrazole and maximal electroshock rodent seizure models quantitatively predict antiepileptic efficacy in humans? Seizure 2015, 24, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sills, G.J.; Rogawski, M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef] [PubMed]
- Song, M.X.; Rao, B.-Q.; Cheng, B.B.; Wu, Y.; Zeng, H.; Luo, Y.; Deng, X.Q. Design, Synthesis and Evaluation of the Antidepressant and Anticonvulsant Activities of Triazole-Containing Benzo[d]oxazoles. CNS Neurol. Disord. Drug Targets 2017, 16, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Song, M.X.; Huang, Y.S.; Zhou, Q.G.; Deng, X.Q.; Yao, X.D. Synthesis of ring-opened derivatives of triazole-containing quinolinones and their antidepressant and anticonvulsant activities. Bioorg. Chem. 2021, 106, 104505. [Google Scholar] [CrossRef]
- Kendell, S.F.; Krystal, J.H.; Sanacora, G. GABA and glutamate systems as therapeutic targets in depression and mood disorders. Expert Opin. Ther. Targets. 2005, 9, 153–168. [Google Scholar] [CrossRef]
- Croarkin, P.E.; Levinson, A.J.; Daskalakis, Z.J. Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci. Biobehav. Rev. 2011, 35, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Henning, A.; Grimm, S.; Schulte, R.F.; Beck, J.; Dydak, U.; Schnepf, B.; Boeker, H.; Boesiger, P.; Northoff, G. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch. Gen. Psychiatry 2009, 66, 478–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.J.; Wang, S.B.; Quan, Z.S. Synthesis and antidepressant activities of 4-(substituted-phenyl)tetrazolo[1,5-a]quinazolin-5(4H)-ones and their derivatives. Mol. Divers. 2015, 19, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Keppel Hesselink, J.M. Phenytoin: A step by step insight into its multiple mechanisms of action—80 years of mechanistic studies in neuropharmacology. J. Neurol. 2017, 264, 2043–2047. [Google Scholar] [CrossRef]
- Collins, G.S. Effect of aminooxyacetic acid, thiosemicarbazide and haloperidol on the metabolism and half-lives of glutamate and GABA in rat brain. Biochem. Pharmacol. 1973, 22, 101–111. [Google Scholar] [CrossRef]










| Compd. | -R | Maximal Electroshock Test | Pentylenetetrazole Test | Rotarod Test | |||
|---|---|---|---|---|---|---|---|
| 0.5 h | 4 h | 0.5 h | 4 h | 0.5 h | 4 h | ||
| 3a | -C3H7 | 300 | - | - | - | - | - |
| 3b | -C4H9 | 300 | - | - | - | - | - |
| 3c | -C5H11 | 100 | 300 | - | - | 300 | - |
| 3d | -C6H13 | 300 | 300 | - | - | 300 | - |
| 3e | -C7H15 | 300 | - | - | - | - | - |
| 3f | -CH2C6H5 | 100 | 300 | - | - | 300 | - |
| 3g | -CH2C6H4(2-F) | 100 | - | - | - | - | - |
| 3h | -CH2C6H4(3-F) | 300 | - | - | - | - | - |
| 3i | -CH2C6H4(4-F) | 300 | - | - | - | - | - |
| 3j | -CH2C6H4(2-Cl) | 300 | - | - | - | - | - |
| 3k | -CH2C6H4(3-CI) | - | - | - | - | - | - |
| 3l | -CH2C6H4(4-CI) | 300 | 300 | - | - | 300 | - |
| Carbamazepine | - | 30 | 100 | - | - | 100 | 100 |
| Valproate | - | 300 | - | 300 | - | - | - |
| Compounds | ED50 a | TD50 b | PI c |
|---|---|---|---|
| 3c | 63.4 (58.0–69.3) | 264.1 (240.1–290.5) | 4.2 |
| 3f | 78.9 (71.7–86.8) | 253.5 (230.5–278.8) | 3.2 |
| 3g | 84.9 (75.16–95.83) | 439.9 (394.45–490.70) | 5.2 |
| Carbamazepine | 8.7 (8.3–10.2) | 41.5 (38.1–46.3) | 4.8 |
| Valproate | 288 (257–329) | 432 (370–492) | 1.5 |
| Compound | MW | CLogP | HBD | HBA | n-ROTB | Lipinski’s Violation |
|---|---|---|---|---|---|---|
| Rule | 500 | ≤5 | ≤5 | <10 | ≤10 | ≤1 |
| 3a | 297.355 | 1.549 | 0 | 4 | 4 | 0 |
| 3b | 311.382 | 2.005 | 0 | 4 | 5 | 0 |
| 3c | 325.408 | 2.462 | 0 | 4 | 6 | 0 |
| 3d | 339.435 | 2.918 | 0 | 4 | 7 | 0 |
| 3e | 353.461 | 3.374 | 0 | 4 | 8 | 0 |
| 3f | 345.398 | 2.26 | 0 | 4 | 4 | 0 |
| 3g | 363.388 | 2.466 | 0 | 4 | 4 | 0 |
| 3h | 363.388 | 2.466 | 0 | 4 | 4 | 0 |
| 3i | 363.388 | 2.466 | 0 | 4 | 4 | 0 |
| 3j | 379.843 | 2.925 | 0 | 4 | 4 | 0 |
| 3k | 379.843 | 2.925 | 0 | 4 | 4 | 0 |
| 3l | 379.843 | 2.925 | 0 | 4 | 4 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Song, M.; Hua, Y.; Zhu, Y.; Liu, W.; Xia, Q.; Deng, X.; Huang, Y. Design, Synthesis, and Pharmacology of New Triazole-Containing Quinolinones as CNS Active Agents. Molecules 2023, 28, 1987. https://doi.org/10.3390/molecules28041987
Zhao W, Song M, Hua Y, Zhu Y, Liu W, Xia Q, Deng X, Huang Y. Design, Synthesis, and Pharmacology of New Triazole-Containing Quinolinones as CNS Active Agents. Molecules. 2023; 28(4):1987. https://doi.org/10.3390/molecules28041987
Chicago/Turabian StyleZhao, Wennan, Mingxia Song, Yi Hua, Yangnv Zhu, Wenli Liu, Qishan Xia, Xianqing Deng, and Yushan Huang. 2023. "Design, Synthesis, and Pharmacology of New Triazole-Containing Quinolinones as CNS Active Agents" Molecules 28, no. 4: 1987. https://doi.org/10.3390/molecules28041987
APA StyleZhao, W., Song, M., Hua, Y., Zhu, Y., Liu, W., Xia, Q., Deng, X., & Huang, Y. (2023). Design, Synthesis, and Pharmacology of New Triazole-Containing Quinolinones as CNS Active Agents. Molecules, 28(4), 1987. https://doi.org/10.3390/molecules28041987





