Recent Developments in Chemical Derivatization of Microcrystalline Cellulose (MCC): Pre-Treatments, Functionalization, and Applications
Abstract
1. Introduction
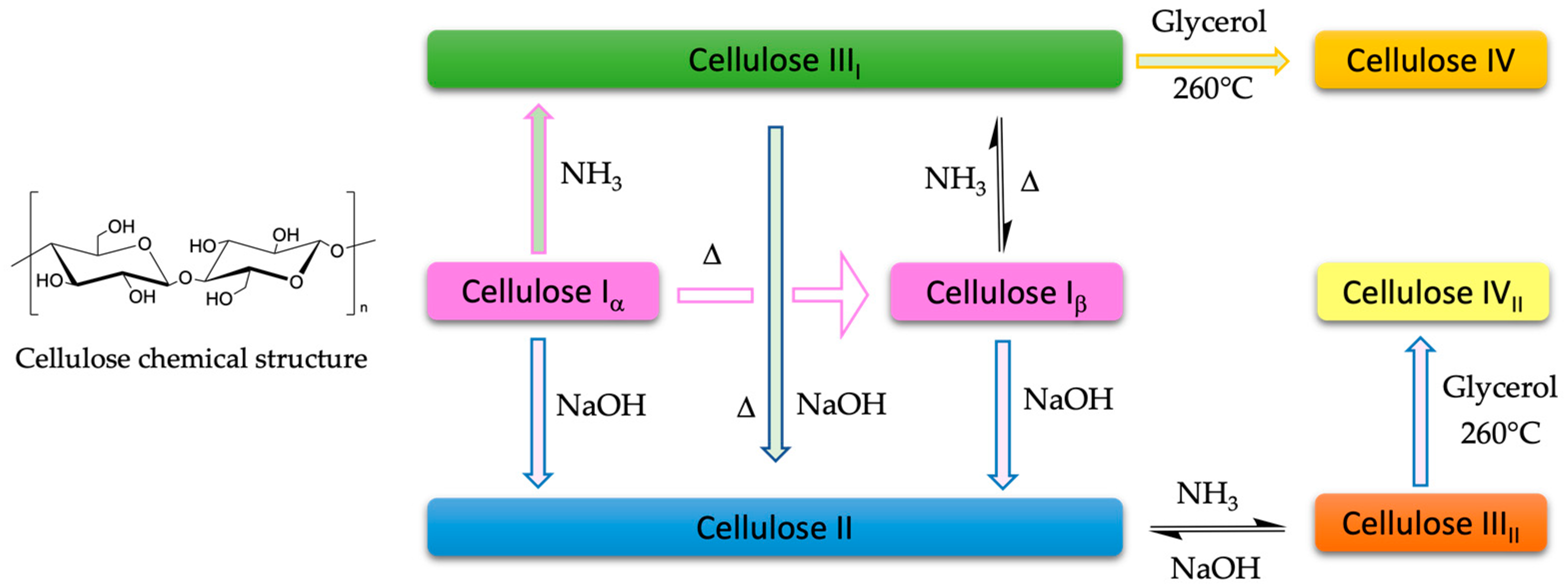
Microcrystalline Cellulose
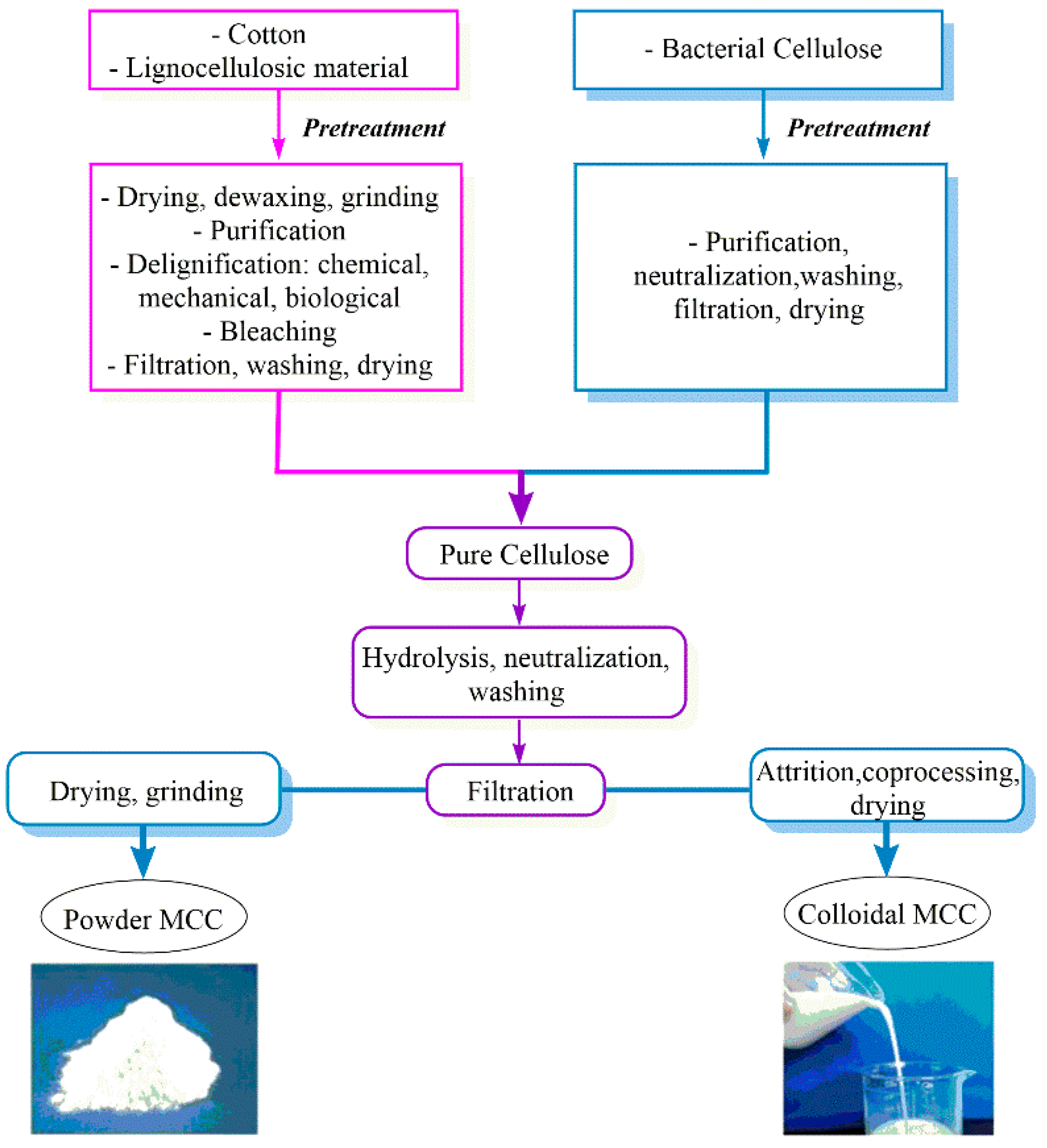
2. MCC Pre-Treatment
2.1. Physical Pre-Treatment
2.2. Biological Pre-Treatment
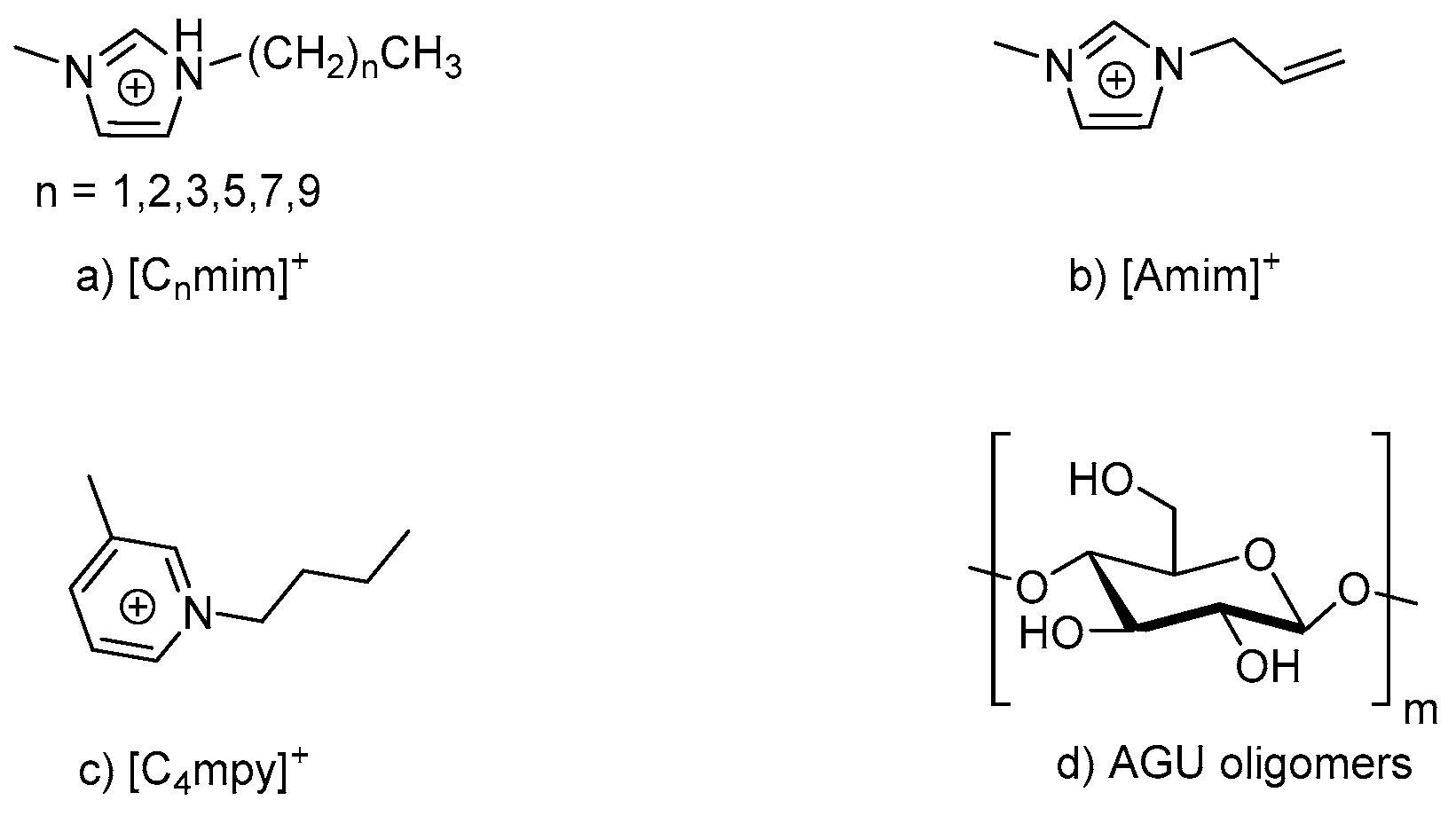
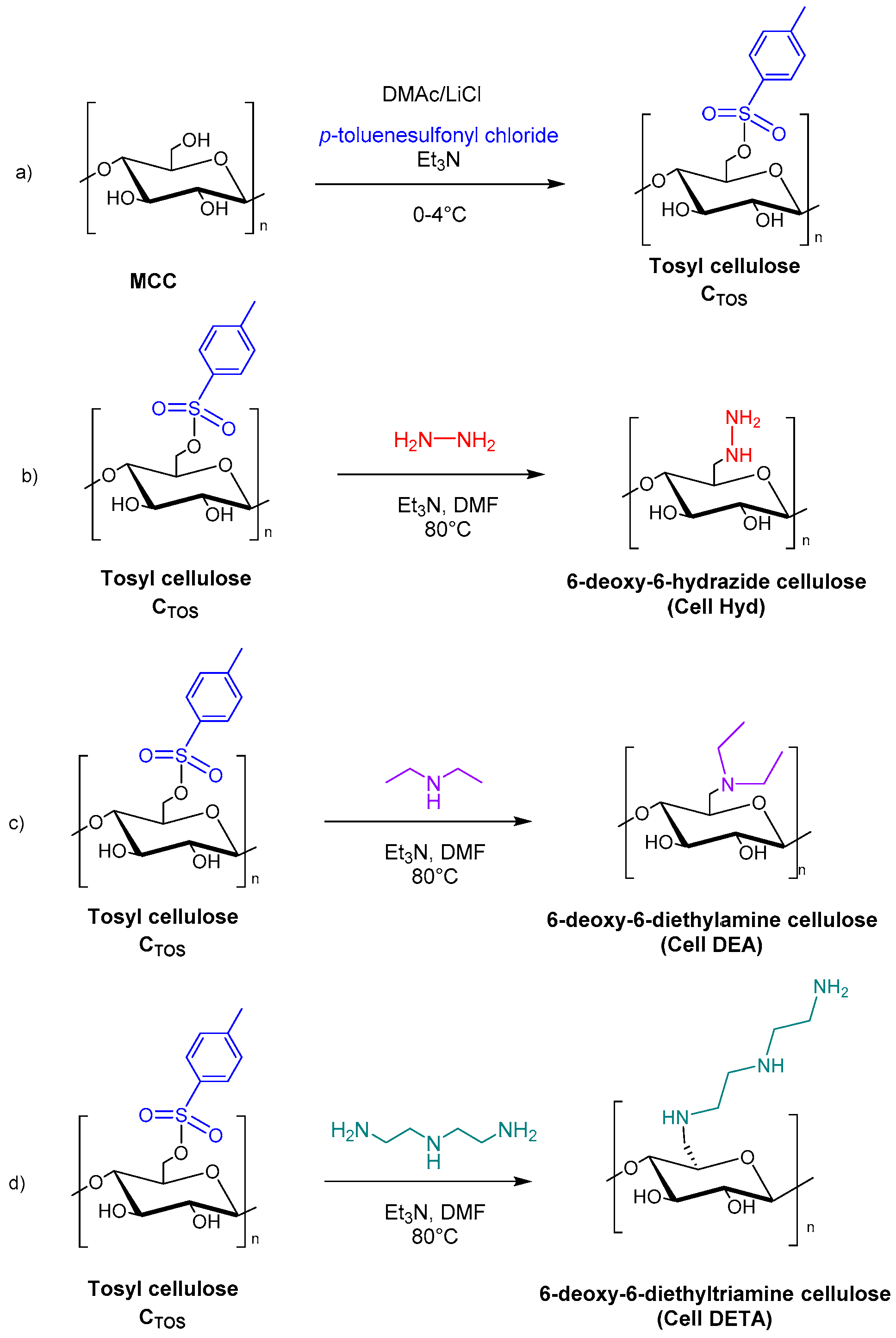
2.3. Chemical Pre-Treatment
3. MCC Functionalization and Applications
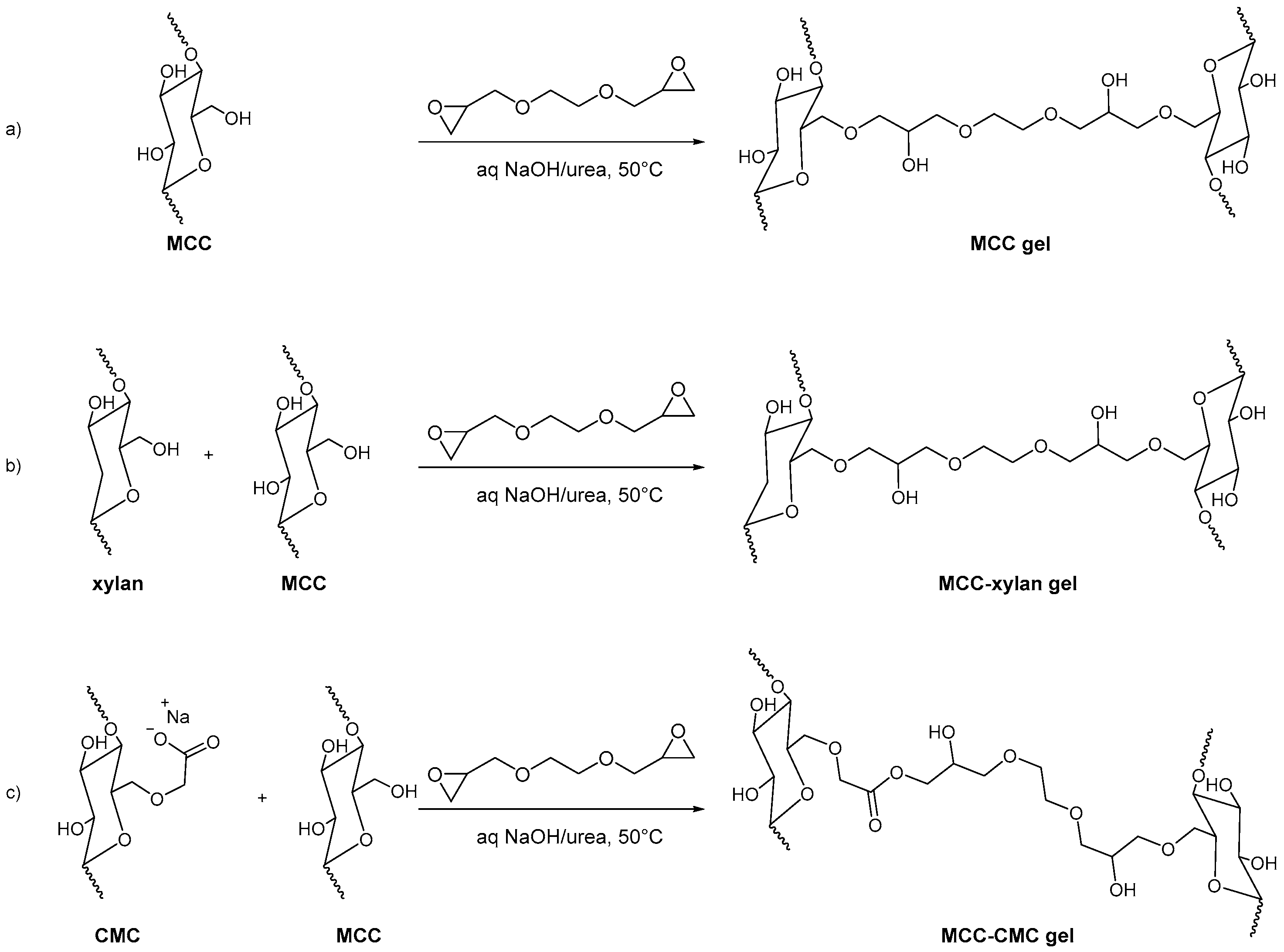
3.1. Adsorbents
3.2. Flame Retardants
3.3. Reinforcing Agent
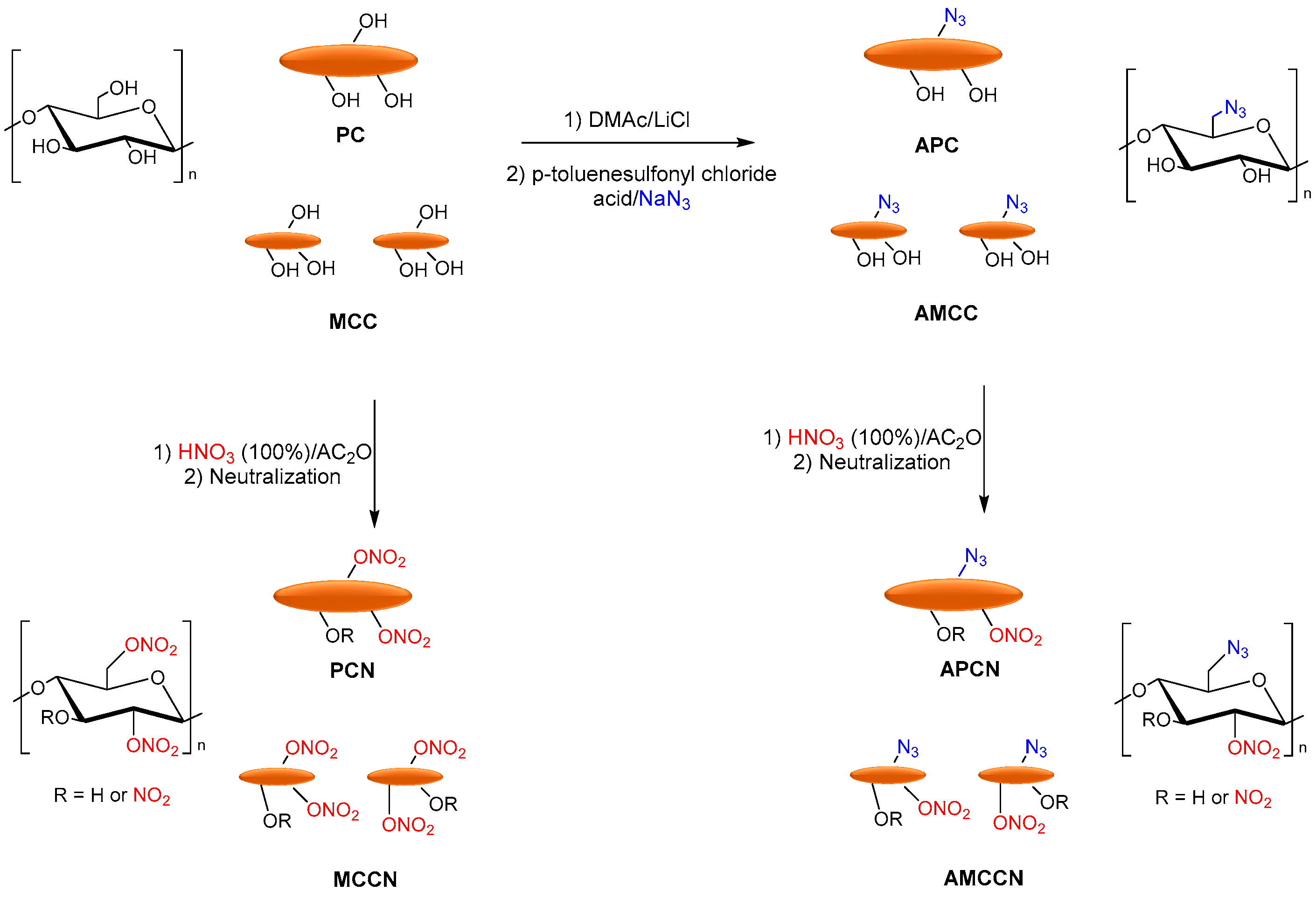
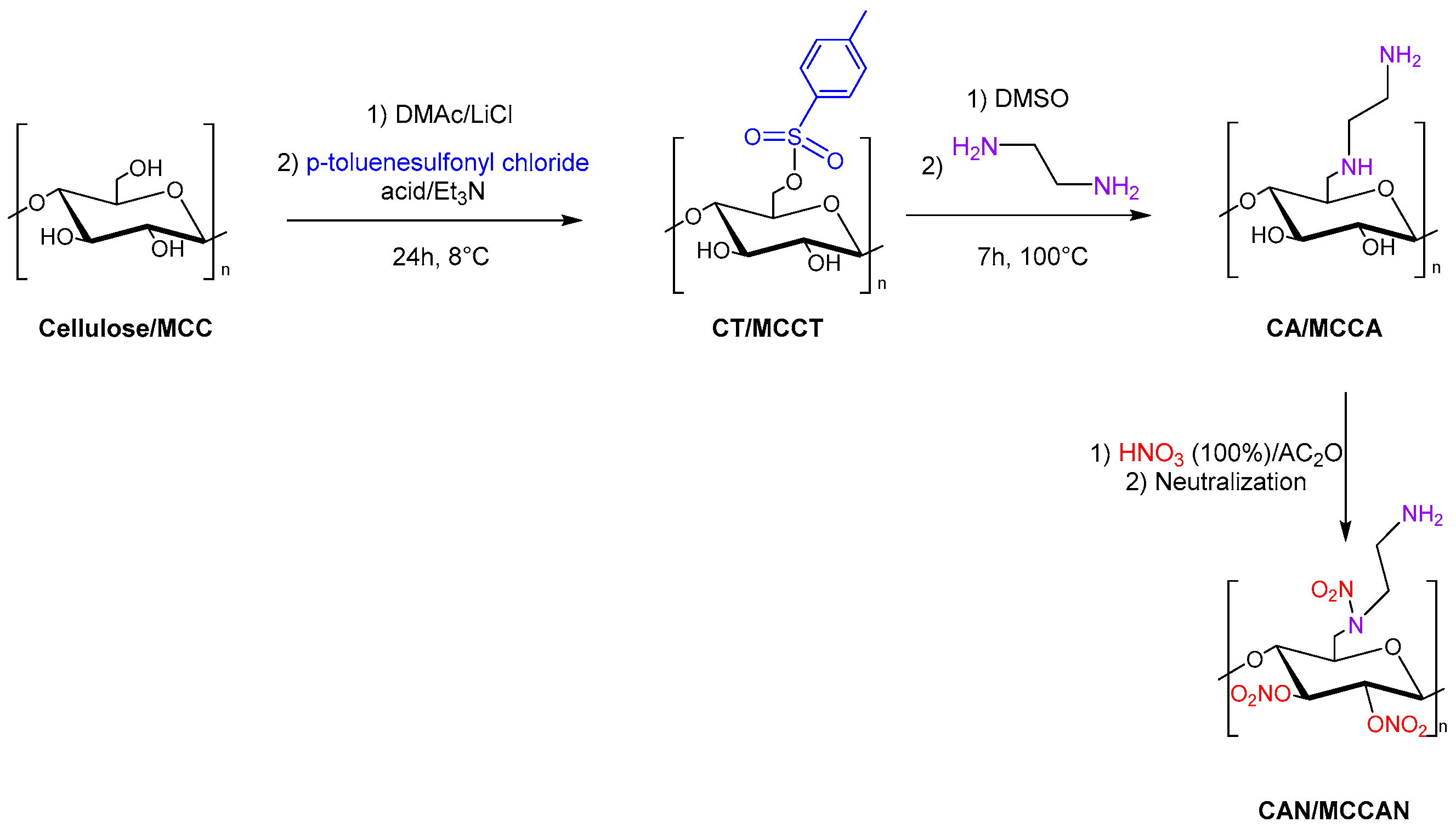
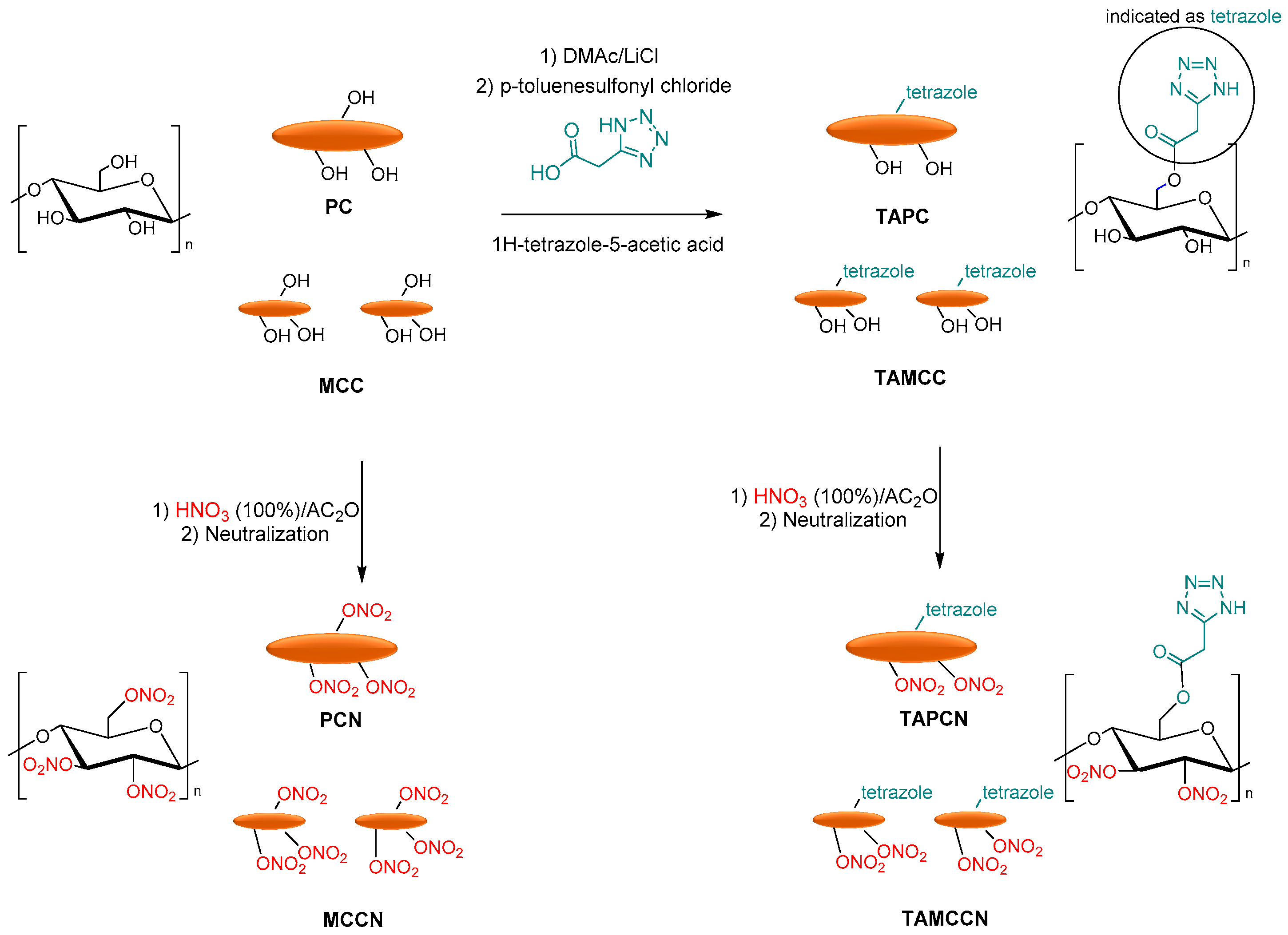
3.4. Energetic Materials
4. Biomedical Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Processing and characterization of natural cellulose fibers/thermoset polymer composites. Carbohydr. Polym. 2014, 109, 102–117. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Manuspiya, H. A Critical Review on Cellulose: From Fundamental to an Approach on Sensor Technology. Renew. Sustain. Energy Rev. 2012, 90, 402–412. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated Cellulose—Its Barrier Properties and Applications in Cellulosic Materials: A Review. J. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Londström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Ohad, D.; Danon, M.D.; Hestrin, S. Synthesis of cellulose by Acetobacter xylinum. V. Ultrastructure of polymer. J. Cell. Biol. 1962, 12, 31–46. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Hui Chuin, C.T.; Sabar, S.; Fazita, M.R.N.; Taiwo, O.F.A.; Hassan, T.M.; Haafiz, M.K.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Horii, F.; Hirai, A.; Kitamaru, R. CP/MAS carbon-13 NMR spectra of the crystalline components of native celluloses. Macromolecules 1987, 20, 2117. [Google Scholar] [CrossRef]
- Sugiyama, J.; Persson, J.; Chanzy, H. Combined infrared and electron diffraction study of the polymorphism of native celluloses. Macromolecules 1991, 24, 2461. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. Crystal Structure and Hydrogen Bonding System in Cellulose Iα from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef]
- Gautam, S.P.; Bundela, P.S.; Pandey, A.K.; Jamaluddin, J.; Awasthi, M.K.; Sarsaiya, S. A review on systematic study of cellulose. J. Appl. Nat. Sci. 2010, 2, 330–343. [Google Scholar] [CrossRef]
- Naomi, R.; Bt Hj Idrus, R.; Fauzi, M.B. Plant-vs. Bacterial-derived cellulose for wound healing: A review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, M.; Liu, R.; Wang, D.; Liu, X.; Liu, W.; Ma, L.; Li, T.; Huang, Y. Modified native cellulose fibers—A novel efficient adsorbent for both fluoride and arsenic. J. Hazard. Mater. 2011, 185, 93–100. [Google Scholar] [CrossRef]
- Silva, L.S.; Lima, L.C.B.; Silva, F.C.; Matos, J.M.E.; Santos, M.R.M.C.; Junior, L.S.S.; Sousa, K.S.; da Silva Filho, E.C. Dye anionic sorption in aqueous solution onto a cellulose surface chemically modified with aminoethanethiol. Chem. Eng. J. 2013, 218, 89–98. [Google Scholar] [CrossRef]
- Bhasney, S.M.; Mondal, K.; Kumar, A.; Katyiar, V. Effect of microcrystalline cellulose [MCC] fibres on the morphological and crystalline behaviour of high-density polyethylene [HDPE]/polylactic acid [PLA] blends. Compos. Sci. Technol. 2020, 187, 107941. [Google Scholar] [CrossRef]
- Imeson, A. Food Stabilisers, Thickeners and Gelling Agents, 1st ed.; Wiley online Library: London, UK, 2009; pp. 218–236. [Google Scholar]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef]
- Lin, Q.; Zheng, Y.; Wang, G.; Shi, X.; Zhang, T.; Yu, J.; Sun, J. Protein adsorption behaviors of carboxymethylated bacterial cellulose membranes. Int. J. Biol. Macromol. 2015, 73, 264–269. [Google Scholar] [CrossRef]
- Hu, T.; Hu, X.; Tang, C.; Liu, D. Adsorbent grafted on cellulose by in situ synthesis of EDTA-like groups and its properties of metal ion adsorption from aqueous solution. Cellulose 2022, 29, 941–952. [Google Scholar] [CrossRef]
- Astrini, N.; Anah, L.; Haryadi, H.R. Adsorption of heavy metal ion from aqueous solution by using cellulose based hydrogel composite. Macromol. Symp. 2015, 353, 191–197. [Google Scholar] [CrossRef]
- Bothra, S.; Upadhyay, Y.; Kumar, R.; Kumar, S.A.; Sahoo, S.K.J. Chemically modified cellulose strips with pyridoxal conjugated red fluorescent gold nanoclusters for nanomolar detection of mercuric ions. Biosens. Bioelectron. 2017, 90, 329–335. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, B.; Wang, T.; Yang, L.; Xu, X.; Chen, C.; Wei, F.; Lv, W.; Zhang, L.; Sun, D. Synthesis of cellulose–silica nanocomposites by in situ biomineralization during fermentation. Cellulose 2019, 27, 703–712. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, T.-J.; Zhang, D.-W.; Li, H.-Y.; Ma, Y.-R.; Qi, L.-M.; Zhou, Y.-L.; Zhang, X.-X. Amperometric hydrogen peroxide biosensor based on the immobilization of heme proteins on gold nanoparticles–bacteria cellulose nanofibers nanocomposite. Talanta 2011, 84, 71–77. [Google Scholar] [CrossRef]
- Li, G.; Sun, K.; Li, D.; Lv, P.; Wang, Q.; Huang, F.; Wei, Q. Biosensor based on bacterial cellulose-Au nanoparticles electrode modified with laccase for hydroquinone detection. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 408–414. [Google Scholar] [CrossRef]
- Schlesinder, M.; Giese, M.; Blusch, L.K.; Hamad, W.Y.; MacLachlan, M.J. Chiral nematic cellulose–gold nanoparticle composites from mesoporous photonic cellulose. Chem. Commun. 2014, 51, 530–533. [Google Scholar] [CrossRef]
- Schlesinger, M.; Hamad, W.Y.; MacLachlan, M.J. Optically tunable chiral nematic mesoporous cellulose films. Soft Matter 2015, 11, 4686–4694. [Google Scholar] [CrossRef]
- Chen, H.; Yan, X.; Feng, Q.; Zhao, P.; Xu, X.; Ng, D.H.L.; Bian, L. Citric acid/Cysteine Modified Cellulose-Based Materials: Green Preparation and its Applications in AntiCounterfeiting, Chemical Sensing, and UV Shielding. ACS Sustain. Chem. Eng. 2017, 5, 11387–11394. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, C.; Zhang, W.; Gu, J. Green Synthesis of Cellulose Nanofibrils Decorated with Ag Nanoparticles and Their Application in Colorimetric Detection of l-Cysteine. ACS Sustain. Chem. Eng. 2020, 8, 12713–12721. [Google Scholar] [CrossRef]
- Rajnish, K.N.; Samuel, M.S.; Datta, S.; Chandrasekar, N.; Balaji, R.; Jose, S.; Selvarajan, E. Immobilization of cellulase enzymes on nano and micro-materials for breakdown of cellulose for biofuel production—A narrative review. Int. J. Biol. Macromol. 2021, 182, 1793–1802. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Cheng, L.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Kian, L.K.; Saba, N.; Jawaid, M.; Fouad, H. Characterization of microcrystalline cellulose extracted from olive fiber. Int. J. Biol. Macromol. 2020, 156, 347–353. [Google Scholar] [CrossRef]
- Taye, M.; Chaudhary, B.U.; Kale, R.D. Extraction and Analysis of Microcrystalline Cellulose from Delignified Serte Leaf Fiber Wastes. J. Nat. Fibers 2019, 18, 1–13. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Alothman, O.Y. Isolation and characterization of microcrystalline cellulose from roselle fibers. Int. J. Biol. Macromol. 2017, 103, 931–940. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M. Microcrystalline cellulose from Posidonia oceanica brown algae: Extraction and characterization. Int. J. Biol. Macromol. 2019, 138, 837–845. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Derradji, M.; Bessa, W. Ecofriendly isolation and characterization of microcrystalline cellulose from giant reed using various acidic media. Cellulose 2019, 26, 7635–7651. [Google Scholar] [CrossRef]
- Alotabi, M.D.; Alshammari, B.A.; Saba, N.; Alothman, O.Y.; Kian, L.K.; Khan, A.; Jawaid, M. Microcrystalline cellulose from fruit bunch stalk of date palm: Isolation and characterization. J. Polym. Environ. 2020, 28, 1766–1775. [Google Scholar] [CrossRef]
- Rasheed, M.; Jawaid, M.; Karim, Z.; Abdullah, L.C. Morphological, physiochemical and thermal properties of microcrystalline cellulose (MCC) extracted from bamboo fiber. Molecules 2020, 25, 2824. [Google Scholar] [CrossRef]
- Yulina, R.; Gustiani, R.S.; Kasipah, C.; Sukardan, M.D. Preparation of microcrystalline cellulose from cotton yarn spinning mills wastes: Effect of pretratment and hydrolysis reaction condition on the product characteristics. E3S Web Conf. 2020, 48, 02004. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sudakova, I.G.; Yatsenkova, O.V.; Garyntseva, N.V.; Rataboul, F.; Djakovitch, L. Optimizing single-stage processes of microcrystalline cellulose production via the peroxide delignification of wood in the presence of a titania catalyst. Catal. Ind. 2018, 10, 360–367. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, M.; Ling, C.; Hou, W.; Yan, Z. Extraction and characterization of microcrystalline cellulose from waste cotton fabrics via hydrothermal method. Waste Manag. 2018, 82, 139–146. [Google Scholar] [CrossRef]
- Benaniba, S.; Djendel, M.; Bonbaaya, R.; Raouache, E.; Kessal, O.; Driss, Z. Experimental investigation on thermomechanical properties of bio-composites reinforced with two lengths of the date palm fibers. J. Nat. Fibers 2022, 19, 10193–10209. [Google Scholar] [CrossRef]
- Karim, M.Z.; Chowdhury, Z.Z.; Abd Hamid, S.B.; Ali, M.E. Statistical optimization for acid hydrolysis of microcrystalline cellulose and its physiochemical characterization by using metal ion catalyst. Materials 2014, 7, 6982–6999. [Google Scholar] [CrossRef]
- Yavorov, N.; Valchev, I.; Radeva, G.; Todorova, D. Kinetic investigation of dilute and hydrolysis of hardwood pulp for microcrystalline cellulose production. Carbohyd. Res. 2020, 488, 107910. [Google Scholar] [CrossRef]
- Trusovs, S. Microcrystalline Cellulose. U.S. Patent 6,392,034 B1, 28 November 2002. [Google Scholar]
- DeLong, E.A. Method of Producing Level off DP Microcrystalline Cellulose and Glucose from Lignocellulosic Material. U.S. Patent 4,645,541, 24 February 1987. [Google Scholar]
- Ha, E.Y.; Landi, C.D. Method for Producing Microcrystalline Cellulose. U.S. Patent 5,769,934, 23 June 1998. [Google Scholar]
- Merci, A.; Urbano, A.; Grossmann, M.V.E.; Tischer, C.A.; Mali, S. Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion. Food Res. Int. 2015, 73, 38–43. [Google Scholar] [CrossRef]
- Chundawat, S.P.S.; Sousa, L.D.; Roy, S.; Yang, Z.; Gupta, S.; Pal, R.; Pingali, S.V. Ammonia-salt solvent promotes cellulosic biomass deconstruction under ambient pretreatment conditions to enable rapid soluble sugar production at ultra-low enzyme loadings. Green Chem. 2020, 22, 204–218. [Google Scholar] [CrossRef]
- Lan, L.; Chen, H.; Lee, D.; Xu, S.; Skillen, N.; Tedstone, A.; Robertson, P.; Garforth, A.; Daly, H.; Hardacre, C.; et al. Effect of Ball-Milling Pretreatment of Cellulose on Its Photoreforming for H2 Production. ACS Sustain. Chem. Eng. 2022, 10, 4862–4871. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, H. Effect of ball milling on the hydrolysis of microcrystalline cellulose in hot-compressed water. AlChE J. 2011, 57, 793–800. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Órfao, J.J.M.; Pereira, M.F.R. Enhanced direct production of sorbitol by cellulose ball-milling. Green Chem. 2015, 17, 2973–2980. [Google Scholar] [CrossRef]
- Meine, N.; Rinaldi, R.; Schuth, F. Solvent-free catalytic depolymerization of cellulose to water-soluble oligosaccharides. ChemSusChem 2012, 5, 1449–1454. [Google Scholar] [CrossRef]
- Hick, S.M.; Griebel, C.; Restrepo, D.T.; Truitt, J.H.; Buker, E.J.; Bylda, C.; Blair, R.G. Mechanocatalysis for biomass-derived chemicals and fuels. Green Chem. 2010, 12, 468–474. [Google Scholar] [CrossRef]
- Rechulski, M.D.K.; Käldström, M.; Richter, U.; Schüth, F.; Rinaldi, R. Mechanocatalytic depolymerization of Lignocellulose Performed on Hectogram and Kilogram Scales. Ind. Eng. Chem. Res. 2015, 54, 4581–4592. [Google Scholar] [CrossRef]
- Zhu, X.; Jin, Q. Comparison of Three Emerging Dross Recovery Processes in China’s Aluminum Industry from the Perspective of Life Cycle Assessment. ACS Sustain. Chem. Eng. 2021, 9, 6776–6787. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Hernandez, J.G. Sustainability Assessment of Mechanochemistry by using the twelve principles of green chemistry. ChemSusChem 2021, 14, 2145–2162. [Google Scholar] [CrossRef]
- Xiu, H.; Cheng, R.; Li, J.; Ma, F.; Song, T.; Yang, X.; Feng, P.; Zhang, X.; Kozliak, E.; Ji, Y. Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose. Green Process Synth. 2019, 8, 348–354. [Google Scholar] [CrossRef]
- Barakat, A.; Mayer-Laigle, C.; Solhy, A.; Arancon, R.A.D.; DeVries, H.; Luque, R. Mechanical pretreatments of lignocellulosic biomass: Towards facile and environmentally sound technologies for biofuels production. RSC Adv. 2014, 4, 48109–48127. [Google Scholar] [CrossRef]
- Benoit, M.; Rodrigues, A.; de Oliveria Vigier, K.; Fourré, E.; Barrault, J.; Tatibouët, J.-M.; Jérôme, F. Combination of ball-milling and non-thermail atmospheric plasma as physical treatments for the saccharification of microcrystalline cellulose. Green. Chem. 2012, 14, 2212–2215. [Google Scholar] [CrossRef]
- Huang, F.; Long, Z.; Liu, S.; Qin, Z. Dielectric barrier discharge plasma pretreatment on hydrolysis of microcrystalline cellulose. Plasma Sci. Technol. 2017, 19, 045504. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. Power ultrasound in organic synthesis: Moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006, 35, 180–196. [Google Scholar] [CrossRef]
- Tischer, P.C.S.F.; Sierakowski, M.R.; Westfahl, H.; Tischer, C.A. Nanostructural Reorganization of Bacterial Cellulose by Ultrasonic Treatment. Biomacromolecules 2010, 11, 1217–1224. [Google Scholar] [CrossRef]
- Velmurugan, R.; Muthukumar, K. Utilization of sugarcane bagasse for bioethanol production: Sono-assisted acid hydrolysis approach. Bioresour. Technol. 2011, 102, 7119–7123. [Google Scholar] [CrossRef]
- Mikkola, J.; Kirilin, A.; Tuuf, J.; Pranovich, A.; Holmbom, V.; Kustov, L.M.; Murzin, D.Y.; Salmi, T. Ultrasound enhancement of cellulose processing in ionic liquids: From dissolution towards functionalization. Green Chem. 2007, 9, 1229–1237. [Google Scholar] [CrossRef]
- Vizireanu, S.; Panaitescu, D.M.; Nicolae, C.; Frone, A.N.; Chiulan, I.; Ionita, M.D.; Satulu, V.; Carpen, L.G.; Petrescu, S.; Birjega, R.; et al. Cellulose defbrillation and functionalization by plasma in liquid treatment. Sci. Rep. 2018, 8, 15473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Benoit, M.; de Oliveira Vigier, K.; Barrault, J.; Jégou, G.; Philippe, M.; Jérôme, F. Pretreatment of microcrystalline cellulose by ultrasound: Effect of particle size in the heterogeneously-catalyzed hydrolysis of cellulose to glucose. Green Chem. 2013, 15, 963. [Google Scholar] [CrossRef]
- Zhang, M.-F.; Qin, Y.-H.; Ma, J.-Y.; Yang, L.; Wu, Z.-K.; Wang, T.-L.; Wang, W.-G.; Wang, C.-W. Depolymerization of microcrystalline cellulose by the combination of ultrasound and Fenton reagent. Ultrason. Sonochem. 2016, 31, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Allen, S.; Taylor, G. Design and operation of an integrated membrane reactor for enzymatic cellulose hydrolysis. Biochem. Eng. J. 2002, 12, 223–229. [Google Scholar] [CrossRef]
- Chen, Y.; Stipanovic, A.J.; Winter, W.T.; Wilson, D.B.; Kim, Y.-J. Effect of digestion by pure cellulases on crystallinity and average chain length for bacterial and microcrystalline celluloses. Cellulose 2007, 14, 283–293. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, S.; Zhang, T. Homogeneous alkalization of cellulose in N-methylmorpholine-N-oxide/water solution. Cellulose 2017, 24, 1235–1245. [Google Scholar] [CrossRef]
- Carrillo-Varela, I.; Vidal, C.; Vidaurre, S.; Parra, C.; Machuca, A.; Briones, R.; Mendonca, R.T. Alkalization of kraft pulps from pine and eucalyptus and its effect on enzymatic saccharification and viscosity control of cellulose. Polymers 2022, 14, 3127. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, H.; Luque, R. Advances on biomass pretreatment using ionic liquids: An overview. Energy Environ. Sci. 2011, 4, 3913–3929. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Wang, J.; Zhang, S. Effect of cationic structure on cellulose dissolution in ionic liquids: A molecular dynamics study. Chem. Phys. Chem. 2012, 13, 3126–3133. [Google Scholar] [CrossRef]
- Isik, M.; Sardom, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef]
- Liu, L.; Chen, H. Enzymatic hydrolysis of cellulose materials treated with ionic liquid [BMIM] Cl. Chin. Sci. Bull. 2006, 51, 2432–2436. [Google Scholar] [CrossRef]
- Berga, L.; Bruce, I.; Nicol, T.W.J.; Holding, A.J.; Isobe, N.; Shimizu, S.; Walker, A.J.; Reid, J.E.S.J. Cellulose dissolution and regeneration using a non-aqueous, non-stoichiometric protic ionic liquid system. Cellulose 2020, 27, 9593–9603. [Google Scholar] [CrossRef]
- Turner, M.B.; Spear, S.K.; Huddleston, J.G.; Holbrey, J.D.; Rogers, R.D. Ionic liquid salt-induced inactivation and unfolding of cellulase from Trichoderma reesei. Green Chem. 2003, 5, 443–447. [Google Scholar] [CrossRef]
- Zhao, H.; Jones, C.I.L.; Baker, G.A.; Xia, S.; Olubajo, O.; Person, V.N. Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J. Biotechnol. 2009, 139, 47–54. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Li, W.; Zhang, B.; Liu, Y. Effect of solvent pre-treatment on the structures and dissolution of microcrystalline cellulose in lithium chloride/dimethylacetamide. Cellulose 2019, 26, 3095–3109. [Google Scholar] [CrossRef]
- Jin, H.; Zha, C.; Gu, L. Direct dissolution of cellulose in NaOH/thiourea/urea aqueous solution. Carbohydr. Res. 2007, 342, 851–858. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, G.; Liu, C.; Wu, M.; Tang, Y.; Li, B.; Peng, H. Ultrafast improvement of cellulose accessibility via non-dissolving pretreatment with LiBr⋅3H2O under room temperature. Carbohydr. Polym. 2022, 284, 119180. [Google Scholar] [CrossRef]
- Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martin, J.M.; Fierro, J.L.G. High enhancement of the hydrolysis rate of cellulose after pretreatment with inorganic salt hydrates. Green Chem. 2020, 22, 3860–3866. [Google Scholar] [CrossRef]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrad, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment. A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef]
- Huang, T.; Nie, J.; Yang, J.-H.; Qi, X.-D.; Zhou, Z.-W.; Wang, Y. Bio-inspired functionalization of microcrystalline cellulose aerogel with high adsorption performance toward dyes. Carbohydr. Polym. 2018, 198, 546–555. [Google Scholar]
- Hu, D.; Wang, P.; Li, J.; Wang, L. Functionalization of Microcrystalline Cellulose with N,N-dimethyldodecylamine for the Removal of Congo Red Dye from an Aqueous Solution. Bioresources 2014, 9, 5951–5962. [Google Scholar] [CrossRef]
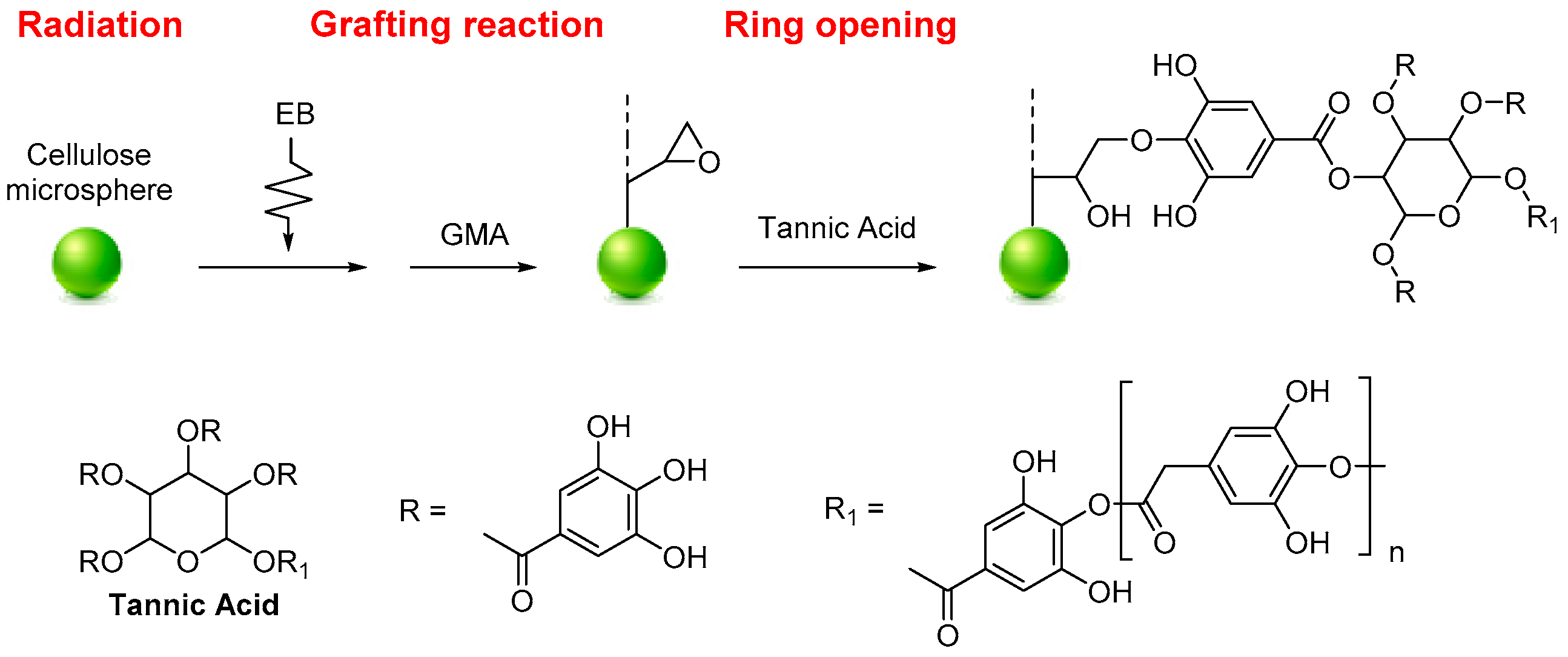
- Du, J.; Zhang, M.; Dong, Z.; Yang, X.; Zhao, L. Facile fabrication of tannic acid functionalized microcrystalline cellulose for selective recovery of Ga(III) and In(III) from potential leaching solution. Sep. Purif. Technol. 2022, 286, 120442. [Google Scholar] [CrossRef]
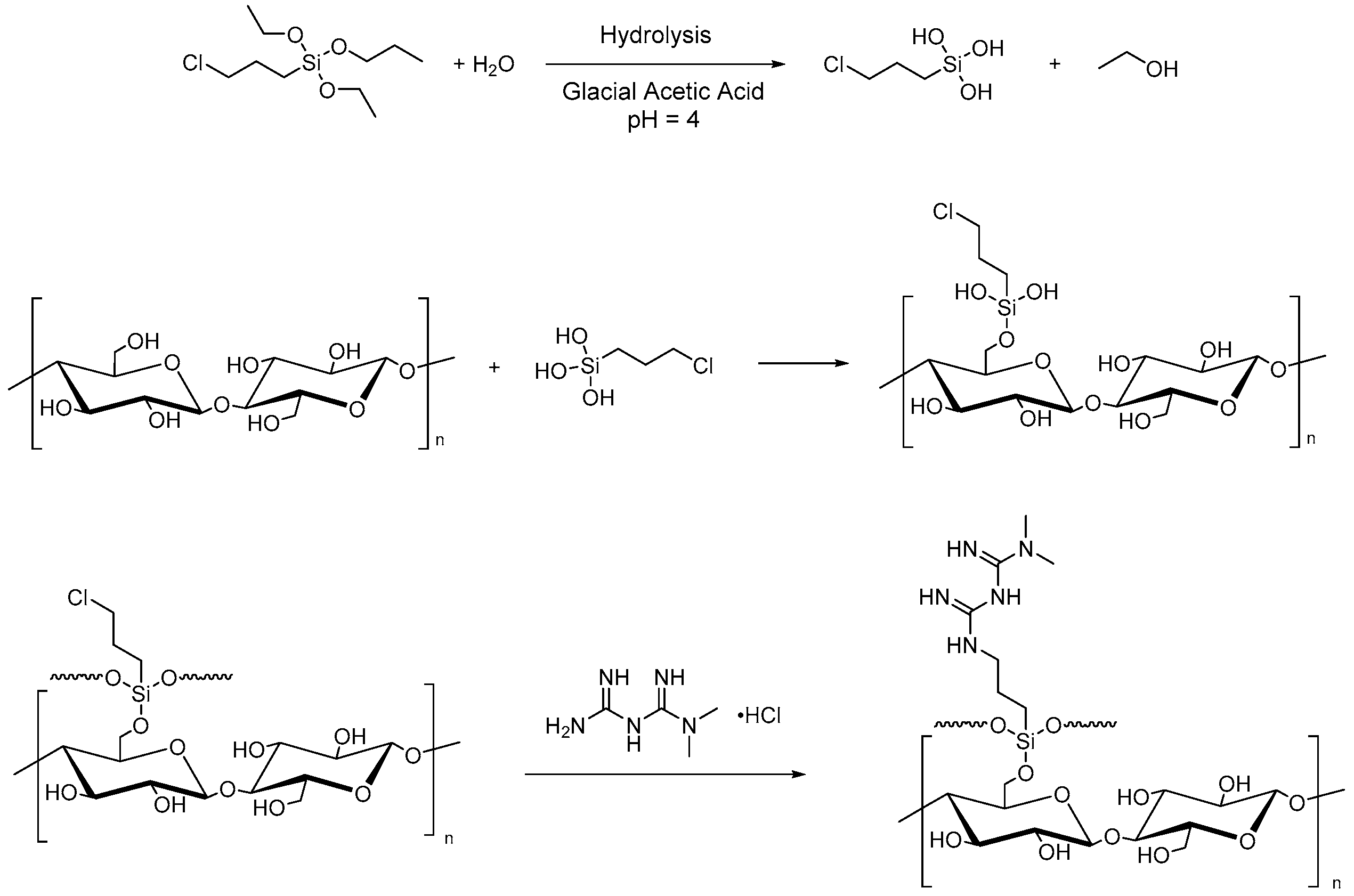
- Rafieian, F.; Mousavi, M.; Yu, Q.; Jonoobi, M. Amine functionalization of microcrystalline cellulose assisted by (3-chloropropyl)triethoxysilane. Int. J. Biol. Macromol. 2019, 130, 280–287. [Google Scholar] [CrossRef] [PubMed]
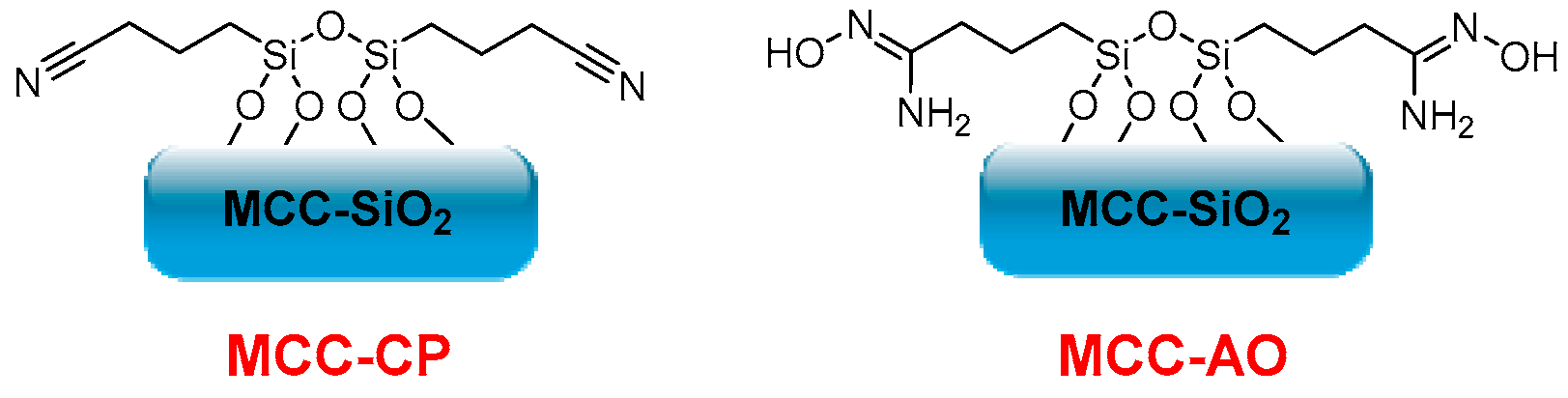
- Gunathilake, C.; Dassanayake, R.S.; Abidi, N.; Jaroniec, M. Amidoxime-Functionalized Microcrystalline Cellulose-Mesoporous Silica Composites for Carbon Dioxide Sorption at Elevated Temperatures. J. Mater. Chem. A 2016, 4, 4808. [Google Scholar] [CrossRef]
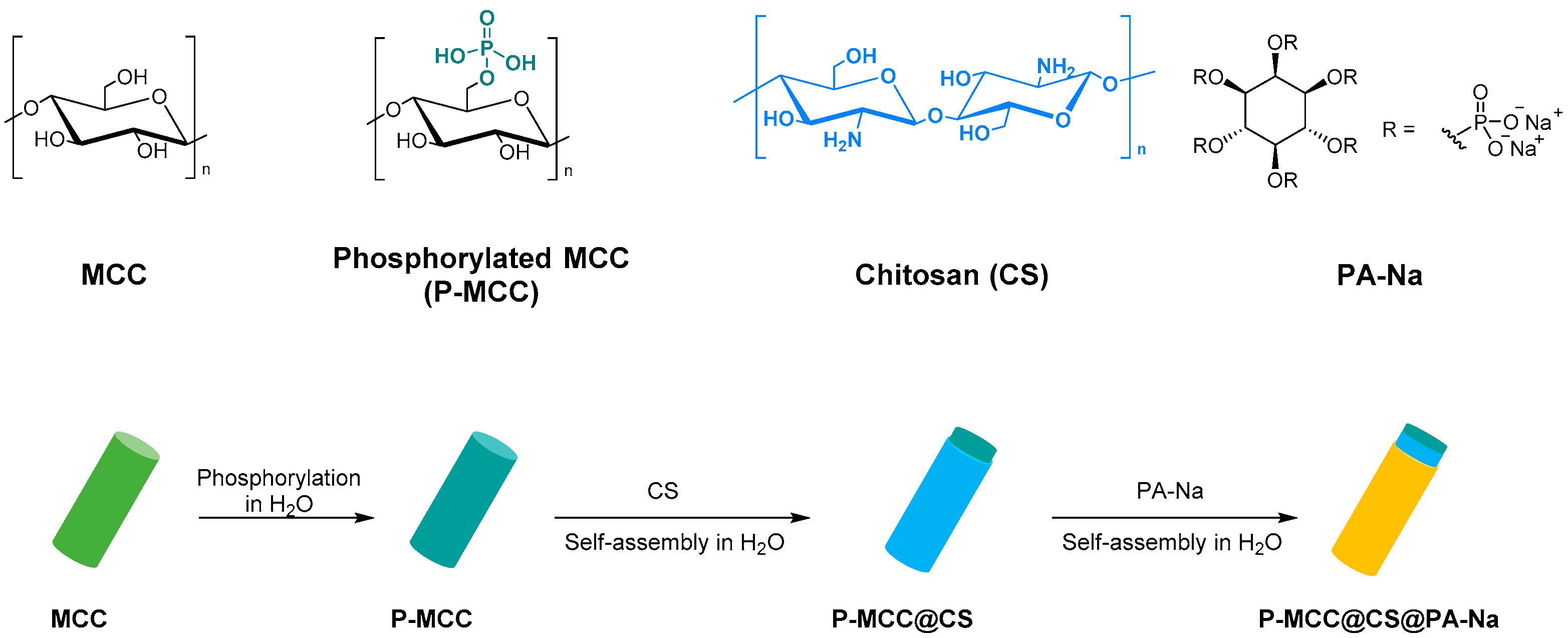
- Yuan, H.-B.; Tang, R.-C.; Yu, C.-B. Flame Retardant Functionalization of Microcrystalline Cellulose by Phosphorylation Reaction with Phytic Acid. Int. J. Mol. Sci. 2021, 22, 9631. [Google Scholar] [CrossRef]
- Lou, G.; Ma, Z.; Dai, J.; Bai, Z.; Fu, S.; Huo, S.; Qian, L.; Song, P. Fully Biobased Surface-Functionalized Microcrystalline Cellulose via Green Self-Assembly toward Fire-Retardant, Strong, and Tough Epoxy Biocomposites. ACS Sustain. Chem. Eng. 2021, 9, 13595–13605. [Google Scholar] [CrossRef]
- Ashori, A.; Nourbakhsh, A. Performance properties of microcrystalline cellulose as a reinforcing agent in wood plastic composites. Compos. B. Eng. 2010, 41, 578–581. [Google Scholar] [CrossRef]
- Ratnakumar, A.; Rathnayake, W.S.M.; Karunanayake, L.; Samarasekara, A.M.P.B.; Amarasinghe, D.A.S. Microcrystalline Cellulose as Reinforcing Agents for Polypropylene Composites. Trop. Agric. Res. 2020, 31, 106–114. [Google Scholar] [CrossRef]
- Jankauskaite, V.; Abzalbekuly, B.; Lisauskaite, A.; Procycevas, I.; Fataraite, E.; Vitkauskiene, A.; Janakhmetov, U. Silicone Rubber and Microcrystalline Cellulose Composites with Antimicrobial Properties. Mater. Sci. 2014, 20, 42–49. [Google Scholar] [CrossRef]
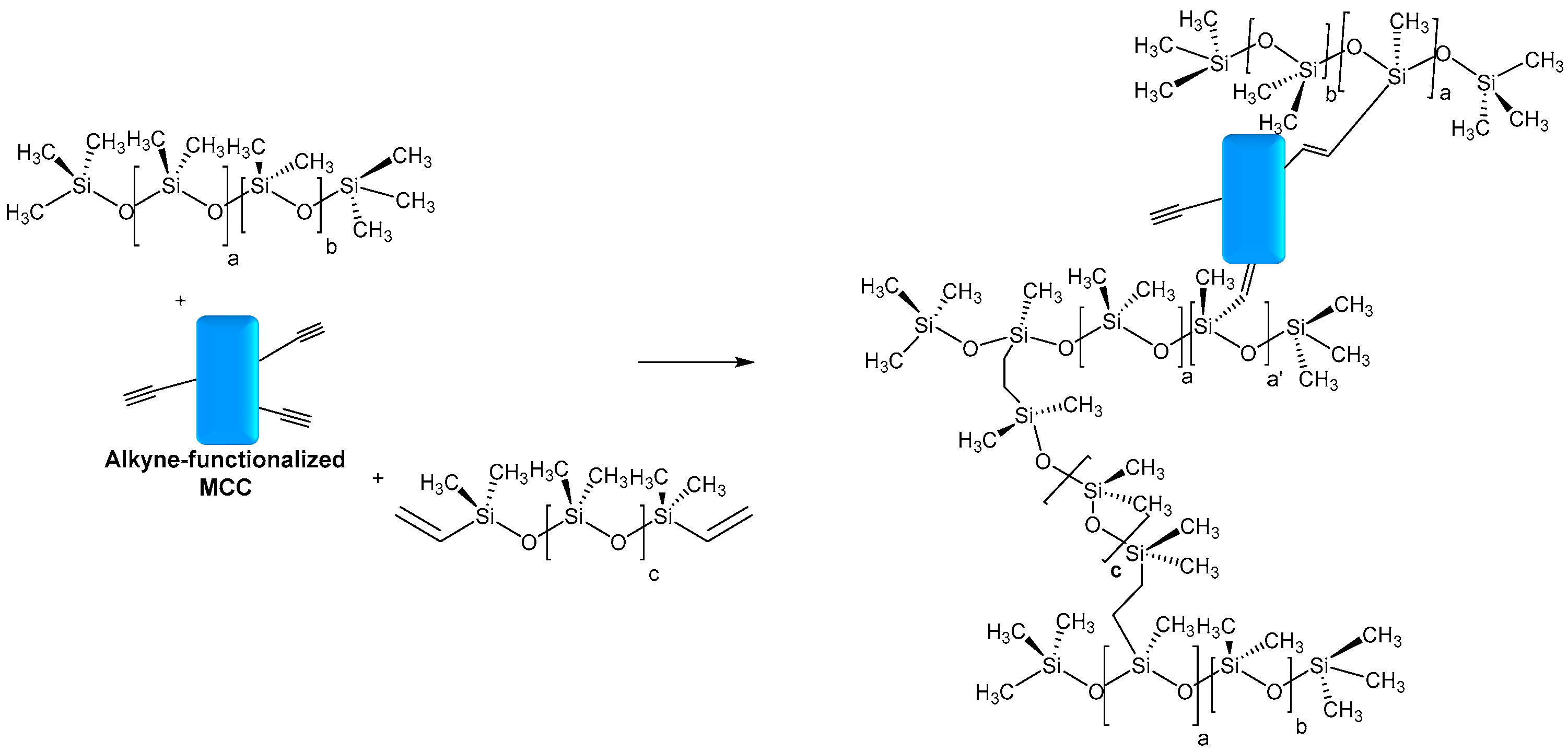
- Deng, S.; Binauld, S.; Mangiante, G.; Frances, J.M.; Charlot, A.; Bernard, J.; Zhoub, X.; Fleurya, E. Microcrystalline cellulose as reinforcing agent in silicone elastomers. Carbohydr. Polym. 2016, 151, 899–906. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Krumm, B.; Khimeche, K.; Mezrou, A. A promising energetic biopolymer based on azide-functionalized microcrystalline cellulose: Synthesis and characterization. Carbohydr. Polym. 2020, 249, 116820. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Krumm, B. New insensitive nitrogen-rich energetic polymers based on amino-functionalized cellulose and microcrystalline cellulose: Synthesis and characterization. Fuel 2020, 277, 118258. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Khimeche, K. Tetrazole-functionalized microcrystalline cellulose: A promising biopolymer for advanced energetic materials. J. Chem. Eng. 2020, 400, 125960. [Google Scholar] [CrossRef]
- Kollár, P.; Závalová, V.; Hošek, J.; Havelka, P.; Sopuch, T.; Karpisek, M.; Třetinová, D.; Suchý, P. Cytotoxicity and effects on inflammatory response of modified types of cellulose in macrophage-like THP-1 cells. Int. Immunopharmacol. 2011, 11, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Kalita, R.D.; Nath, Y.; Ochubiojo, M.E.; Buragohain, A.K. Extraction and characterization of microcrystalline cellulose from fodder grass; Setaria glauca (L) P. Beauv, and its potential as a drug delivery vehicle for isoniazid, a first line antituberculosis drug. Colloids Surf. B Biointerfaces 2013, 108, 85–89. [Google Scholar] [CrossRef]
- Kundu, D.; Banerjee, T. Development of microcrystalline cellulose based hydrogels for the in vitro delivery of Cephalexin. Heliyon 2020, 6, e03027. [Google Scholar] [CrossRef] [PubMed]
- Nazir, F.; Iqbal, M. Synthesis, Characterization and Cytotoxicity Studies of Aminated Microcrystalline Cellulose Derivatives against Melanoma and Breast Cancer Cell Lines. Polymers 2020, 12, 2634. [Google Scholar] [CrossRef]
- Pavliňák, D.; Švachová, V.; Vojtek, L.; Zarzycká, J.; Hyršl, P.; Alberti, M.; Vojtová, L. Cytotoxicity Studies of Aminated Microcrystalline Cellulose Derivatives against Melanoma and Breast Cancer Cell Lines. Open Chem. 2015, 13, 229–235. [Google Scholar]
- Garba, Z.N.; Lawan, I.; Zhou, W.; Zhang, M.; Wang, L.; Yuan, Z. Microcrystalline cellulose (MCC) based materials as emerging adsorbents for the removal of dyes and heavy metals—A review. Sci. Total Environ. 2020, 717, 135070. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupidi, G.; Pastore, G.; Marcantoni, E.; Gabrielli, S. Recent Developments in Chemical Derivatization of Microcrystalline Cellulose (MCC): Pre-Treatments, Functionalization, and Applications. Molecules 2023, 28, 2009. https://doi.org/10.3390/molecules28052009
Lupidi G, Pastore G, Marcantoni E, Gabrielli S. Recent Developments in Chemical Derivatization of Microcrystalline Cellulose (MCC): Pre-Treatments, Functionalization, and Applications. Molecules. 2023; 28(5):2009. https://doi.org/10.3390/molecules28052009
Chicago/Turabian StyleLupidi, Gabriele, Genny Pastore, Enrico Marcantoni, and Serena Gabrielli. 2023. "Recent Developments in Chemical Derivatization of Microcrystalline Cellulose (MCC): Pre-Treatments, Functionalization, and Applications" Molecules 28, no. 5: 2009. https://doi.org/10.3390/molecules28052009
APA StyleLupidi, G., Pastore, G., Marcantoni, E., & Gabrielli, S. (2023). Recent Developments in Chemical Derivatization of Microcrystalline Cellulose (MCC): Pre-Treatments, Functionalization, and Applications. Molecules, 28(5), 2009. https://doi.org/10.3390/molecules28052009





