CO2 Capture Membrane for Long-Cycle Lithium-Air Battery
Abstract
1. Introduction
2. Results and Discussion
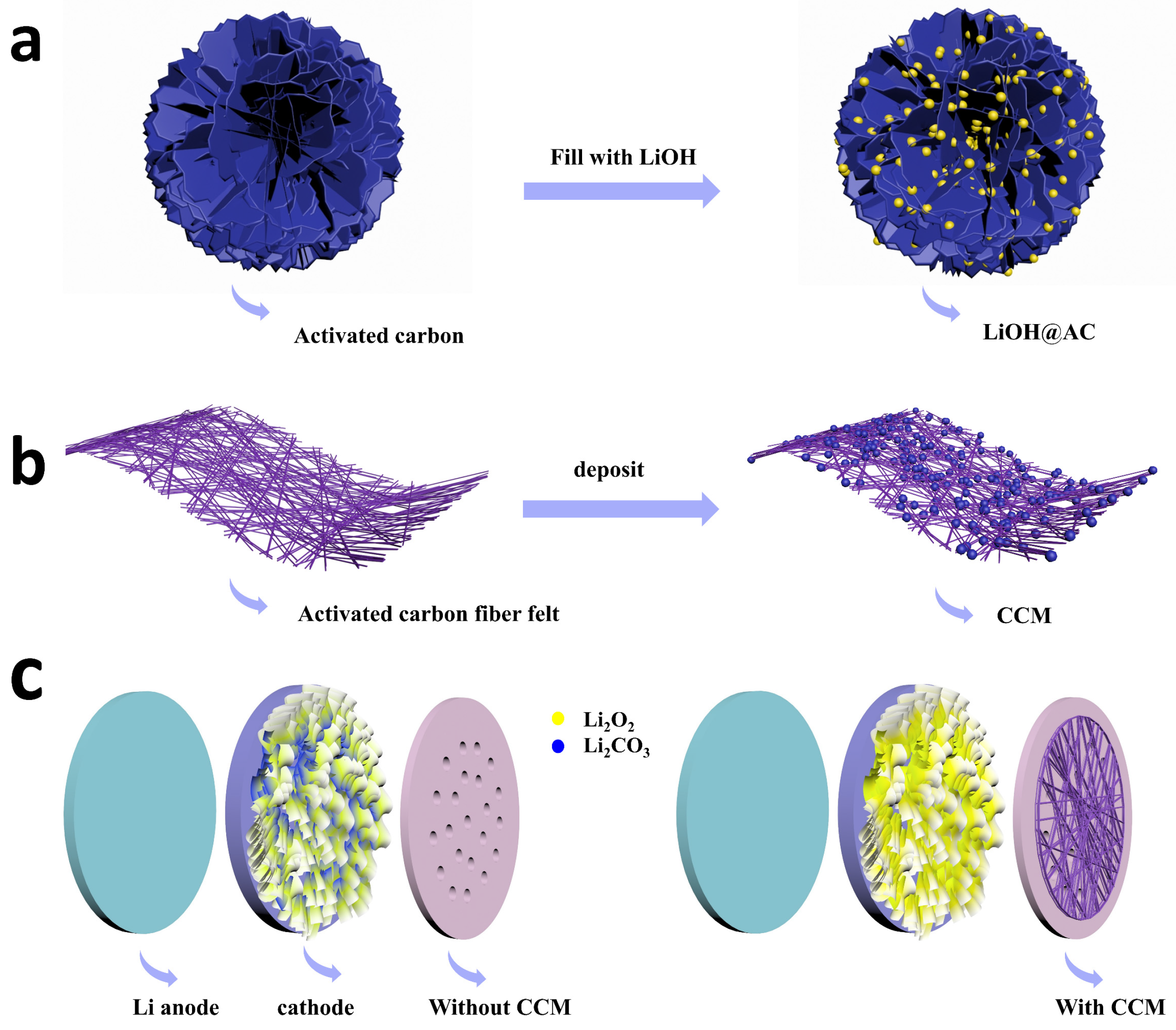
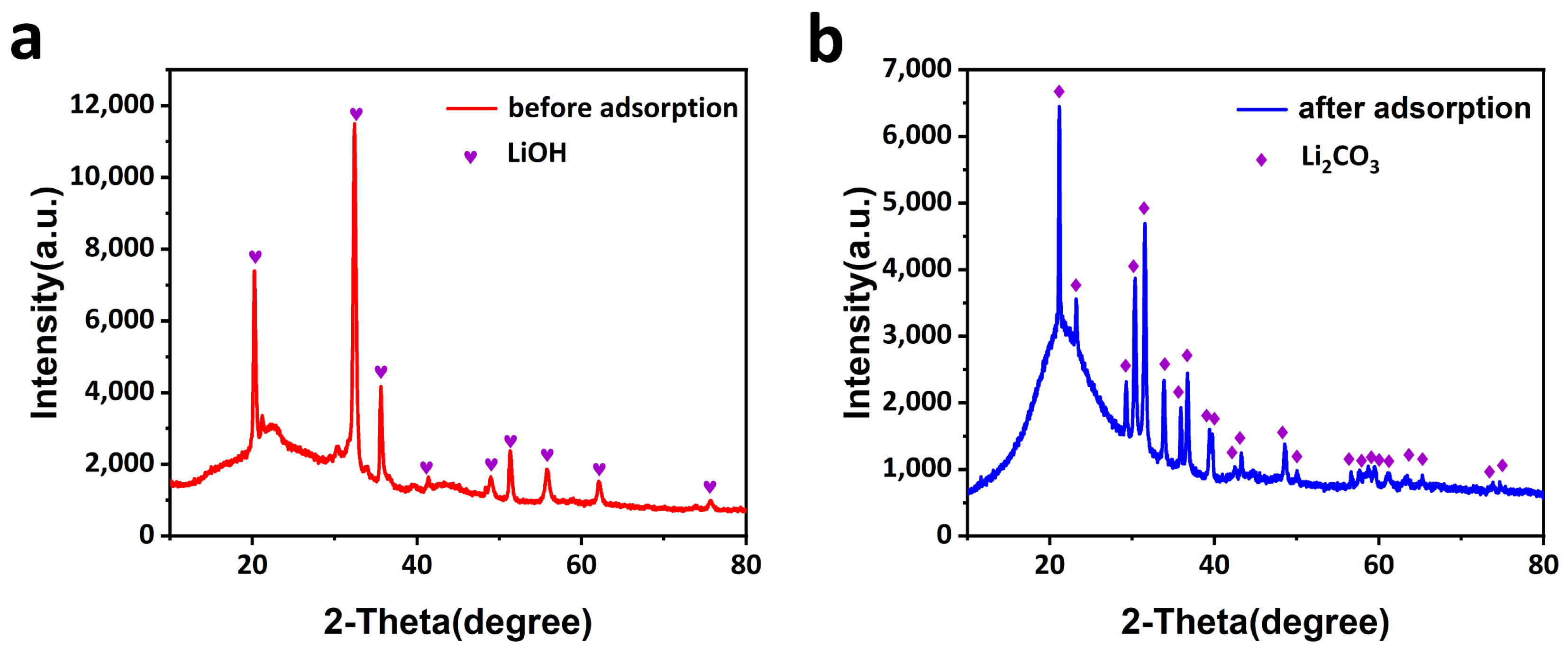
2.1. Design and Preparation of CCM
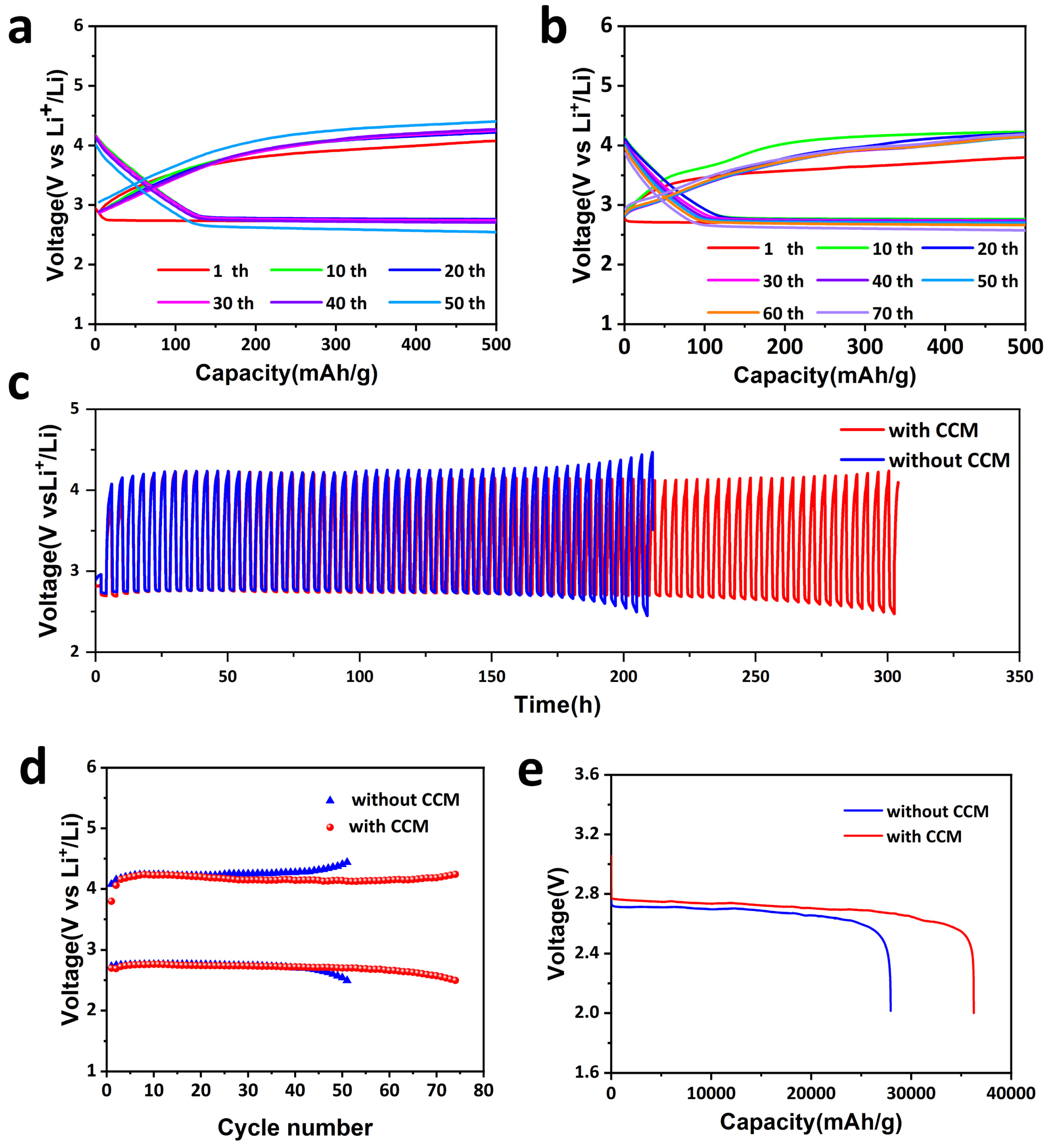
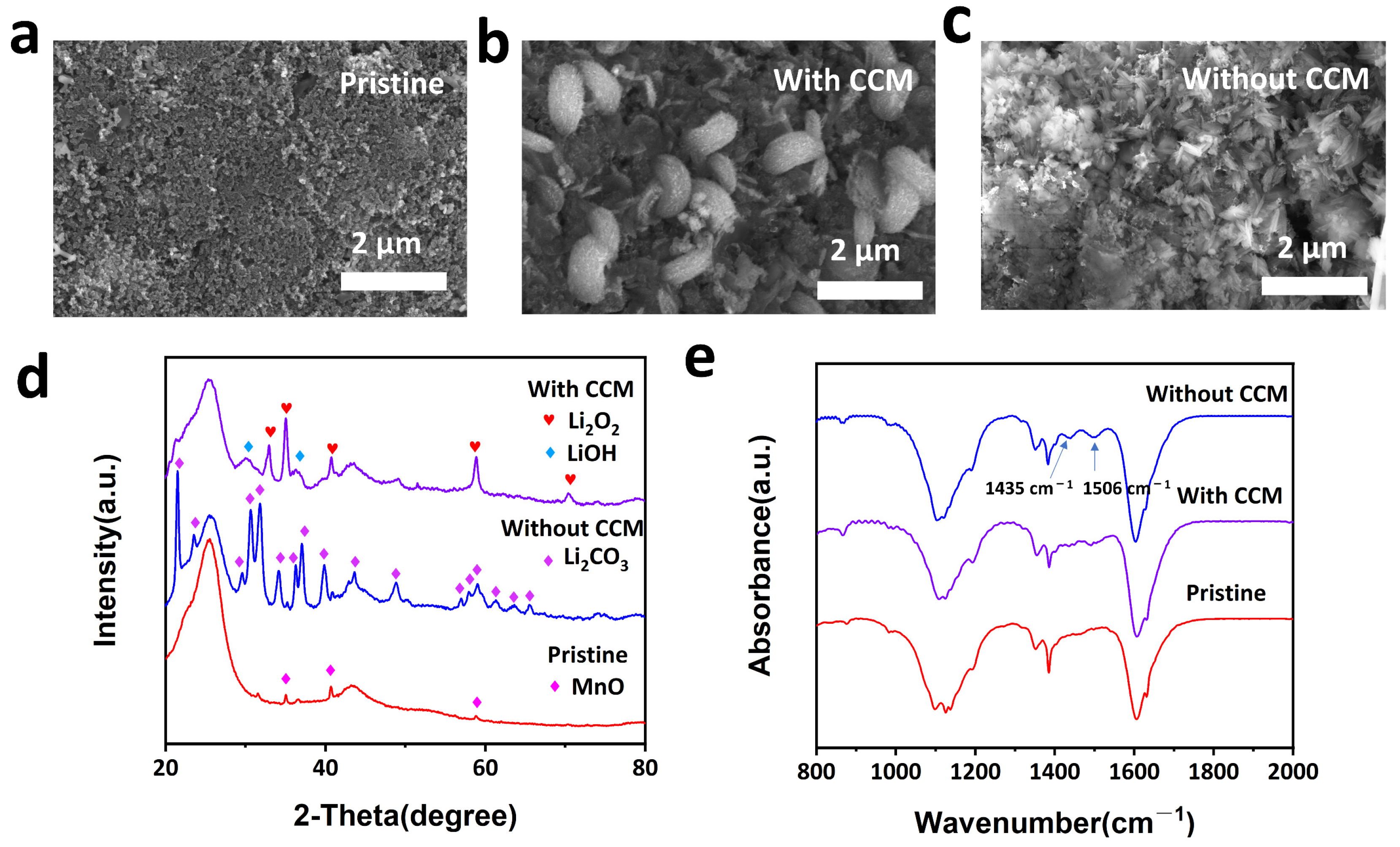
2.2. Performance of LABs with CCM
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Preparation of Activated Carbon Filled with Lithium Hydroxide (LiOH@AC)
3.4. Preparation of CCM
3.5. Breakthrough Experiments
3.6. Assembly of Lithium-Air Battery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, J.; Wang, Z.; Xu, D.; Meng, F.; Zhang, X. 3D ordered macroporous LaFeO3 as efficient electrocatalyst for Li-O2 batteries with enhanced rate capability and cyclic performance. Energy Environ. Sci. 2014, 7, 2213–2219. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, D.; Xu, J.; Zhang, X. Oxygen electrocatalysts in metal-air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Freunberger, S.A.; Chen, Y.; Bruce, P.G. A reversible and higher-rate Li-O2 battery. Science 2012, 337, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Feng, X.; Jin, X.; Shao, M.; Su, Y.; Zhang, Y.; Zhang, X. Protecting the lithium metal anode for a safe flexible lithium-air battery in ambient air. Angew. Chem. Int. Ed. 2019, 58, 18240–18245. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Li, M.; Di, J.; Bai, P.; Song, L.; Wang, X.; Li, F.; Liang, S.; Xu, J.; Yu, J. A highly stable and flexible zeolite electrolyte solid-state Li-air battery. Nature 2021, 592, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, H.; Wang, J.; Zhang, T. Oxygen-free cell formation process obtaining LiF protected electrodes for improved stability in lithium-oxygen batteries. Energy Storage Mater. 2019, 23, 670–677. [Google Scholar] [CrossRef]
- Ma, J.; Meng, F.; Yu, Y.; Liu, D.; Yan, J.; Zhang, Y.; Zhang, X.; Jiang, Q. Prevention of dendrite growth and volume expansion to give high-performance aprotic bimetallic Li-Na alloy-O2 batteries. Nat. Chem. 2019, 11, 64–70. [Google Scholar] [CrossRef]
- Zhu, Z.; Ni, Y.; Lv, Q.; Geng, J.; Xie, W.; Li, F.; Chen, J. Surface plasmon mediates the visible light–responsive lithium–oxygen battery with Au nanoparticles on defective carbon nitride. Proc. Natl. Acad. Sci. USA 2021, 118, e2024619118. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C. A review of high energy density lithium–air battery technology. J. Appl. Electrochem. 2014, 44, 5–22. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Liu, T.; Meng, B.; Li, X.; Liang, G.; Hu, X.; Wang, Q.J. Broadband high photoresponse from pure monolayer graphene photodetector. Nat. Commun. 2013, 4, 1811. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Araez, N.; Novák, P. Critical aspects in the development of lithium–air batteries. J. Solid State Electrochem. 2013, 17, 1793–1807. [Google Scholar] [CrossRef]
- Li, F.; Zhang, T.; Zhou, H. Challenges of non-aqueous Li-O2 batteries: Electrolytes, catalysts, and anodes. Energy Environ. Sci. 2013, 6, 1125–1141. [Google Scholar] [CrossRef]
- Lim, H.; Lim, H.; Park, K.; Seo, D.; Gwon, H.; Hong, J.; Goddard, W.A.; Kim, H.; Kang, K. Toward a Lithium–“Air” Battery: The Effect of CO2 on the Chemistry of a Lithium–Oxygen Cell. J. Am. Chem. Soc. 2013, 135, 9733–9742. [Google Scholar] [CrossRef]
- Abraham, K.M. A brief history of non-aqueous metal-air batteries. ECS Trans. 2008, 3, 67–71. [Google Scholar] [CrossRef]
- Laoire, C.O.; Mukerjee, S.; Abraham, K.M.; Plichta, E.J.; Hendrickson, M.A. Elucidating the mechanism of oxygen reduction for lithium-air battery applications. J. Phys. Chem. C 2009, 113, 20127–20134. [Google Scholar] [CrossRef]
- Laoire, C.O.; Mukerjee, S.; Abraham, K.M.; Plichta, E.J.; Hendrickson, M.A. Influence of nonaqueous solvents on the electrochemistry of oxygen in the rechargeable lithium−air battery. J. Phys. Chem. C 2010, 114, 9178–9186. [Google Scholar] [CrossRef]
- Zhang, X.; Mu, X.; Yang, S.; Wang, P.; Guo, S.; Han, M.; He, P.; Zhou, H. Research progress for the development of Li-air batteries: Addressing parasitic reactions arising from air composition. Energy Environ. Mater. 2018, 1, 61–74. [Google Scholar] [CrossRef]
- Chen, K.; Huang, G.; Ma, J.; Wang, J.; Yang, D.; Yang, X.; Yu, Y.; Zhang, X. The Stabilization Effect of CO2 in Lithium–Oxygen/CO2 Batteries. Angew. Chem. Int. Ed. 2020, 59, 16661–16667. [Google Scholar] [CrossRef]
- Zou, X.; Lu, Q.; Liao, K.; Shao, Z. Towards practically accessible aprotic Li-air batteries: Progress and challenges related to oxygen-permeable membranes and cathodes. Energy Storage Mater. 2022, 45, 869–902. [Google Scholar] [CrossRef]
- Guo, Z.; Dong, X.; Yuan, S.; Wang, Y.; Xia, Y. Humidity effect on electrochemical performance of Li-O2 batteries. J. Power Sources 2014, 264, 1–7. [Google Scholar] [CrossRef]
- Bhatt, M.D.; Geaney, H.; Nolan, M.; O’Dwyer, C. Key scientific challenges in current rechargeable non-aqueous Li-O2 batteries: Experiment and theory. Phys. Chem. Chem. Phys. 2014, 16, 12093–12130. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.D.; Black, R.; Williams, Z.; Fernandes, R.; Cuisinier, M.; Berg, E.J.; Novak, P.; Murphy, G.K.; Nazar, L.F. Towards a stable organic electrolyte for the lithium oxygen battery. Adv. Energy Mater. 2015, 5, 1400867. [Google Scholar] [CrossRef]
- Shao, Y.; Ding, F.; Xiao, J.; Zhang, J.; Xu, W.; Park, S.; Zhang, J.; Wang, Y.; Liu, J. Making Li-air batteries rechargeable: Material challenges. Adv. Funct. Mater. 2013, 23, 987–1004. [Google Scholar] [CrossRef]
- Crowther, O.; Salomon, M. Oxygen selective membranes for Li-Air (O2) batteries. Membranes 2012, 2, 216–227. [Google Scholar] [CrossRef]
- Ruan, Y.; Sun, J.; Song, S.; Yu, L.; Chen, B.; Li, W.; Qin, X. A perfluorocarbon–silicone oil oxygen–selective membrane for ambient operation of aprotic Li-air batteries. Electrochem. Commun. 2018, 96, 93–97. [Google Scholar] [CrossRef]
- Jaradat, A.; Zhang, C.; Singh, S.K.; Ahmed, J.; Ahmadiparidari, A.; Majidi, L.; Rastegar, S.; Hemmat, Z.; Wang, S.; Ngo, A.T.; et al. High performance air breathing flexible lithium–air battery. Small 2021, 17, 2102072. [Google Scholar] [CrossRef]
- Li, C.; Wei, J.; Qiu, K.; Wang, Y. Li–air battery with a superhydrophobic Li-protective layer. ACS Appl. Mater. Interfaces 2020, 12, 23010–23016. [Google Scholar] [CrossRef]
- Zou, X.; Liao, K.; Wang, D.; Lu, Q.; Zhou, C.; He, P.; Ran, R.; Zhou, W.; Jin, W.; Shao, Z. Water-proof, electrolyte-nonvolatile, and flexible Li-air batteries via O2-permeable silica-aerogel-reinforced polydimethylsiloxane external membranes. Energy Storage Mater. 2020, 27, 297–306. [Google Scholar] [CrossRef]
- Huang, C.; Wang, P.; Li, Y. Fabrication of electrospun CO2 adsorption membrane for zinc-air battery application. Chem. Eng. J. 2020, 395, 125031. [Google Scholar] [CrossRef]
- Ouyang, L.; Xiao, J.; Jiang, H.; Yuan, S. Nitrogen-doped porous carbon materials derived from graphene oxide/melamine resin composites for CO2 adsorption. Molecules 2021, 26, 5293. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, J.; Wang, X.; Liu, M.; Liu, Y. Synthesis, characterization and application of amine-functionalized hierarchically micro-mesoporous silicon composites for CO2 capture in flue gas. Molecules 2022, 27, 3429. [Google Scholar] [CrossRef] [PubMed]
- Satyapal, S.; Filburn, T.; Trela, J.; Strange, J. Performance and properties of a solid amine sorbent for carbon dioxide removal in space life support applications. Energy Fuels 2001, 15, 250–255. [Google Scholar] [CrossRef]
- Dawkins, R.P.; Gehrhardt, H.M. Efficient removal of carbon dioxide from an undersea habitat using adsorption techniques. Ocean. Eng. 1970, 2, 27–31. [Google Scholar] [CrossRef]
- Costagliola, M.A.; Prati, M.V.; Perretta, G. Post combustion CO2 capture with calcium and lithium hydroxide. Sci. Rep. 2022, 12, 10518. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Lee, J.; Bokare, A.D.; Kwon, S.; Park, D.; Jung, W.; Choi, J.; Yang, Y.; Lee, J.; Choi, W. LiOH-embedded zeolite for carbon dioxide capture under ambient conditions. J. Ind. Eng. Chem. 2015, 22, 350–356. [Google Scholar] [CrossRef]
- Tovar, T.M.L.M. Supported lithium hydroxide for carbon dioxide adsorption in water-saturated environments. Adsorption 2017, 23, 51–56. [Google Scholar] [CrossRef]
- Miller, D.D.W.A. Effect of water vapor on the LiOH-CO2 reaction. Ind. Eng. Chem. Fundam. 1970, 9, 454–457. [Google Scholar]
- Maas, D.A.B.A. Factors influencing rate of carbon dioxide reaction with lithium hydroxide. Ind. Eng. Chem. Process Des. Dev. 1971, 10, 489–494. [Google Scholar]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Liu, P.; Wang, Y.; Yang, J.; Li, J.; Li, L. CO2 capture from high-humidity flue gas using a stable metal-organic framework. Molecules 2022, 27, 5608. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Grimaud, A.; Lepoivre, F.; Yang, C.; Tarascon, J.M. Chemical vs electrochemical formation of Li2CO3 as a discharge product in Li-O2/CO2 batteries by controlling the superoxide intermediate. J. Phys. Chem. Lett. 2017, 8, 214–222. [Google Scholar] [CrossRef] [PubMed]


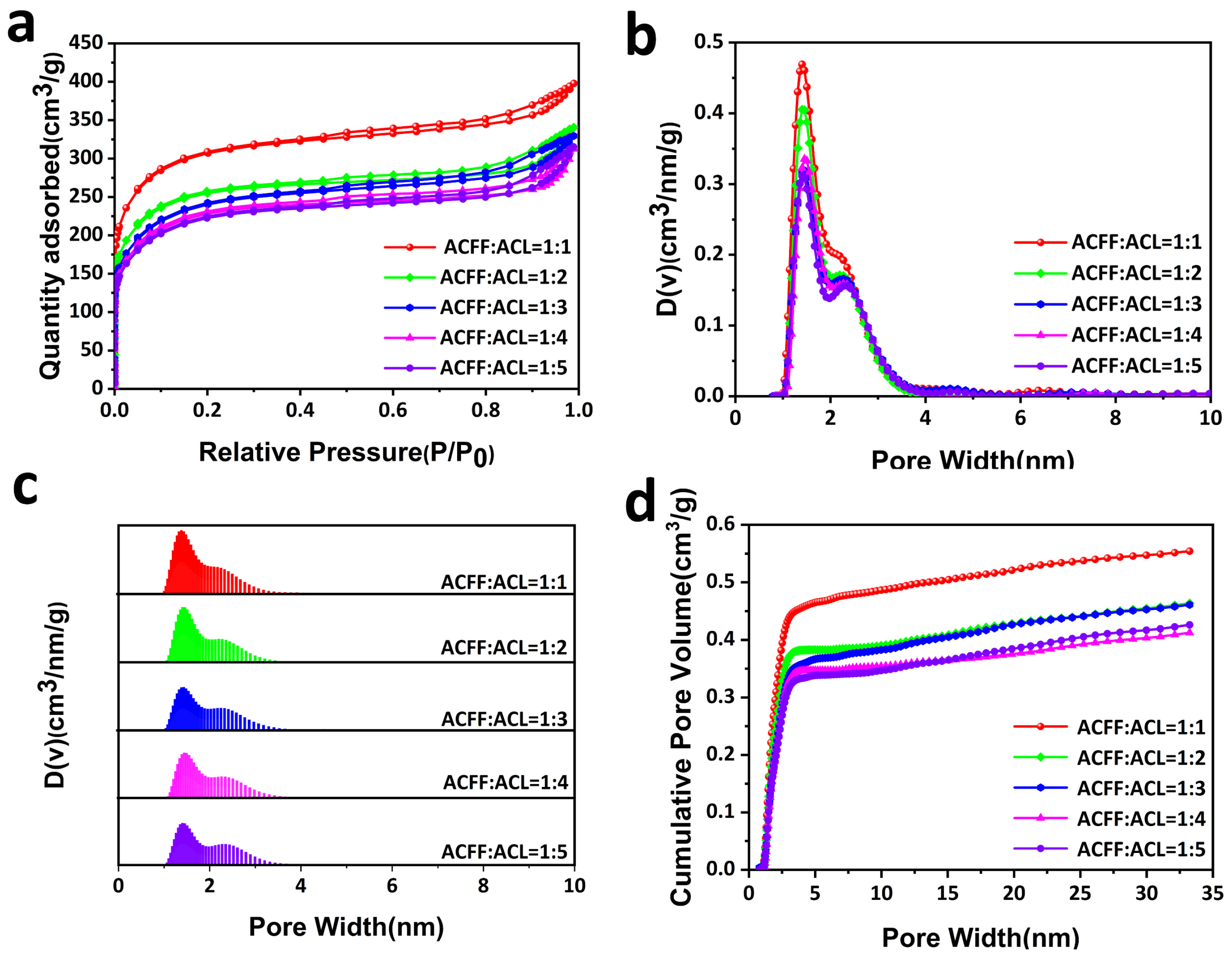



| ACFF:AC (LiOH) | Volume (cm3 g−1) | BET Surface Area (m2 g−1) |
|---|---|---|
| AC | 0.769 | 1548 |
| 1:1 | 0.554 | 1137 |
| 1:2 | 0.464 | 943 |
| 1:3 | 0.461 | 875 |
| 1:4 | 0.413 | 833 |
| 1:5 | 0.426 | 811 |
| Support | LiOH Loading (wt%) | Temperature (°C) | CO2 Content | Adsorption Capacity (mmol of CO2/g of Sorbent |
|---|---|---|---|---|
| LEZ-13X [36] | 75% | 25 | 7000 ppm | 4.51 |
| LEZ-5A [36] | 75% | 25 | 7000 ppm | 4.43 |
| Norit SX Ultra [37] | 30% | 25 | 1% CO2 99% He | 3.4 |
| Commercial activated carbon (this work) | 50% | 25 | Pure CO2 | 8.5 (208 cm3 g−1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Chen, Y.; Zhao, Y.; Yao, C.; Liu, Y.; Liu, X. CO2 Capture Membrane for Long-Cycle Lithium-Air Battery. Molecules 2023, 28, 2024. https://doi.org/10.3390/molecules28052024
Wang J, Chen Y, Zhao Y, Yao C, Liu Y, Liu X. CO2 Capture Membrane for Long-Cycle Lithium-Air Battery. Molecules. 2023; 28(5):2024. https://doi.org/10.3390/molecules28052024
Chicago/Turabian StyleWang, Jiawei, Yanli Chen, Yunfeng Zhao, Chongyan Yao, Yibo Liu, and Xizheng Liu. 2023. "CO2 Capture Membrane for Long-Cycle Lithium-Air Battery" Molecules 28, no. 5: 2024. https://doi.org/10.3390/molecules28052024
APA StyleWang, J., Chen, Y., Zhao, Y., Yao, C., Liu, Y., & Liu, X. (2023). CO2 Capture Membrane for Long-Cycle Lithium-Air Battery. Molecules, 28(5), 2024. https://doi.org/10.3390/molecules28052024






