New Type of Tannins Identified from the Seeds of Cornus officinalis Sieb. et Zucc. by HPLC-ESI-MS/MS
Abstract
:1. Introduction
2. Results and Discussion
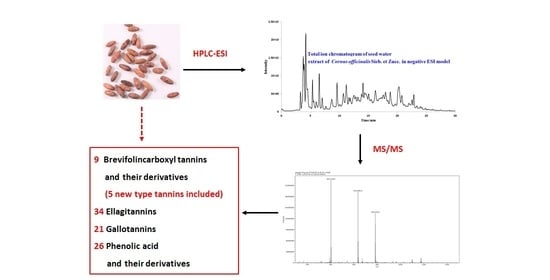
2.1. General
2.2. Brevifolincarboxylic Tannins and Their Derivatives
2.3. Ellagitannins
2.4. Gallotannins
2.5. Phenolic Acid and Their Derivatives
2.5.1. Phenolic Acids
2.5.2. Hydroxycinnamic Acids and Their Derivatives
2.5.3. Hydroxybenzoic Acids and Their Derivatives
2.6. Non-Phenolic Compounds
2.7. Total Phenolic Content (TPC)
3. Materials and Methods
3.1. Solvents and Reagents
3.2. Plant Source
3.3. Sample Preparation
3.4. LC-MS Analysis
3.5. Total Phenolic Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, K.J.; Cheng, C.S.; Yan, G.Q.; Lu, W.L.; Ge, J.F.; Cheng, Y.X.; Li, N. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J. Ethnopharmacol. 2014, 153, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Feng, Z.L.; Chen, H.B.; Wang, F.S.; Lu, J.H. Corni Fructus: A review of chemical constituents and pharmacological activities. Chin. Med. 2018, 13, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czerwinska, M.E.; Melzig, M.F. Cornus mas and Cornus Officinalis-Analogies and Differences of Two Medicinal Plants Traditionally Used. Front. Pharmacol. 2018, 9, 894. [Google Scholar] [CrossRef]
- He, K.; Song, S.; Zou, Z.; Feng, M.; Wang, D.; Wang, Y.; Li, X.; Ye, X. The hypoglycemic and synergistic effect of loganin, morroniside, and ursolic acid isolated from the fruits of Cornus officinalis. Phytother. Res. 2015, 30, 283–291. [Google Scholar] [CrossRef]
- Mau, J.; Chen, C.; Hsieh, P. Antimicrobial effect of extracts from Chinese chive, cinnamon, and corni fructus. J. Agric. Food Chem. 2001, 49, 183–188. [Google Scholar] [CrossRef]
- Park, C.H.; Cho, E.J.; Yokozawa, T. Protection against hypercholesterolemia by Corni fructus extract and its related protective mechanism. J. Med. Food 2009, 12, 973–981. [Google Scholar] [CrossRef]
- Cao, G.; Cai, H.; Cai, B.; Tu, S. Effect of 5-hydroxymethylfurfural derived from processed Cornus officinalis on the prevention of high glucose-induced oxidative stress in human umbilical vein endothelial cells and its mechanism. Food Chem. 2013, 140, 273–279. [Google Scholar] [CrossRef]
- Telang, N.T.; Li, G.; Sepkovic, D.W.; Bradlow, H.L.; Wong, G.Y. Anti-proliferative effects of Chinese herb Cornus officinalis in a cell culture model for estrogen receptor-positive clinical breast cancer. Mol. Med. Rep. 2012, 5, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhakta, H.K.; Park, C.H.; Yokozawa, T.; Tanaka, T.; Jung, H.A.; Choi, J.S. Potential anticholinesterase and β-site amyloid precursor protein cleaving enzyme 1 inhibitory activities of cornuside and gallotannins from Cornus officinalis fruits. Arch. Pharm. Res. 2017, 40, 836–853. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Noh, J.S.; Tanaka, T.; Roh, S.S.; Lee, J.C.; Yokozawa, T. Polyphenol isolated from Corni Fructus, 7-O-galloyl-d-sedoheptulose, modulates advanced glycation endproduct-related pathway in type 2 diabetic db/db mice. Arch. Pharm. Res. 2015, 38, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Li, Z.; Lam, S.; Peng, W.; He, Y. Reuse of Cornus officinalis nutlet for bioenergy. BioResources 2022, 17, 6411–6444. [Google Scholar] [CrossRef]
- Przybylska, D.; Kucharska, A.Z.; Cybulska, I.; Sozánski, T.; Piórecki, N.; Fecka, I. Cornus mas L. Stones: A Valuable By-Product as an Ellagitannin Source with High Antioxidant Potential. Molecules 2020, 25, 4646. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, R.; Piwnicki, K. Pestki owoców jako cenny surowiec wtórny przemysłu spożywczego. Postep. Tech. Przetwórstwa Spożywczego 2007, 2, 62–66. [Google Scholar]
- Mendu, V.; Harman-Ware, A.E.; Crocker, M.; Jae, J.; Stork, J.; Morton, S.; Placido, A.; Huber, G.; DeBolt, S. Identification and thermochemical analysis of high-lignin feed stocks for biofuel and biochemical production. Biotechnol. Biofuels 2011, 4, 43. [Google Scholar] [CrossRef] [Green Version]
- Kostíc, M.D.; Velickovíc, A.V.; Jokovic, N.; Stamenkovíc, O.S.; Veljkovíc, V.B. Optimization and kinetic modeling of esterification of the oil obtained from waste plum stones as a pretreatment step in biodiesel production. Waste Manag. 2016, 48, 619–629. [Google Scholar] [CrossRef]
- Anwar, M.; Rasul, M.; Ashwath, N. Optimization of biodiesel production from stone fruit kernel oil. Energy Procedia 2019, 160, 268–276. [Google Scholar] [CrossRef]
- Lee, J.; Jang, D.S.; Kim, N.H.; Lee, Y.M.; Kim, J.; Kim, J.S. Galloyl Glucoses from the Seeds of Cornus officinalis with Inhibitory Activity against Protein Glycation, Aldose Reductase, and Cataractogenesis ex Vivo. Biol. Pharm. Bull. 2011, 343, 443–446. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Li, J.; Jiang, H. Cornus officinalis Sieb. et Zucc. Seeds as a Dietary Source of Ellagic Acid. Nat. Prod. Commun. 2022, 17, 1–5. [Google Scholar] [CrossRef]
- Bubba, M.D.; Checchini, L.; Chiuminatto, U.; Doumett, S.; Fibbi, D.; Giordani, E. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of polyphenolic composition of four cultivars of Fragaria vesca L. berries and their comparative evaluation. J. Mass Spectrom. 2012, 47, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xia, W. Analysis of phenolic compounds in chinese olive (Canarium album L.) fruit by RPHPLC-DAD-ESI-MS. Food Chem. 2007, 105, 1307–1311. [Google Scholar] [CrossRef]
- Tanaka, T.; Nonaka, G.; Nishioka, I. Tannins and related compounds. C. Reaction of dehydrohexahydroxydiphenic acid esters with bases, and its application to the structure determination of pomegranate tannins, granatins A and B. Chem. Pharm. Bull. 1990, 38, 2424–2428. [Google Scholar] [CrossRef] [Green Version]
- Saijo, R.; Nonaka, G.I.; Nishioka, I.; Chen, I.S.; Hwang, T.H. Tannins and Related Compounds. LXXXVIII.: Isolation and Characterization of Hydrolyzable Tannins from Mallotus japonicus (THUNB.) MUELLER-ARG. and M. philippinensis (LAM.) MUELLER-ARG. Chem. Pharm. Bull. 1989, 37, 2940–2947. [Google Scholar] [CrossRef] [Green Version]
- Quideau, S.; Feldman, K.S. Ellagitannin Chemistry. Chem. Rev. 1996, 96, 475–503. [Google Scholar] [CrossRef] [PubMed]
- Akkawi, M.; Abu-Lafi, S.; Abu-Remeleh, Q.; Qutob, M.; Lutgen, P. Phytochemical screening of Pomegranate juice, peels, leaves and membranes water extracts and their effect on β-hematin formation, a comparative study. Pharm. Pharmacol. Int. J. 2019, 7, 193–200. [Google Scholar]
- Lin, J.H.; Nonaka, G.I.; Nishioka, I. Tannins and related compounds. XCIV. Isolation and characterization of seven new hydrolyzable tannins from the leaves of Macaranga tanarius (L.) Muell. et Arg. Chem. Pharm. Bull. 2008, 38, 1218–1223. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T.; Hatano, T.; Yasui, T. Revised structure of isoterchebin, isolated from Cornus officinalis. Heterocycles 1981, 16, 1321–1324. [Google Scholar] [CrossRef]
- Tanaka, T.; Tong, H.; Xu, Y.; Ishimaru, K.; Nonaka, G.; Nishioka, I. Tannins and related compounds CXVII. Isolation and characterization of three new ellagitannins, lagerstannins A, B, C, having a gluconic acid core, from Lagerstroemia speciosa L. pers. Chem. Pharm. Bull. 1992, 40, 2975–2980. [Google Scholar] [CrossRef] [Green Version]
- Tarone, A.; Goupy, P.; Ginies, C.; Marostica, M.; Dufour, C. Advanced characterization of polyphenols from Myrciaria jaboticaba peel and lipid protection in in vitro gastrointestinal digestion. Food Chem. 2021, 359, 129959. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T. Correlation of oxidative transformations of hydrolyzable tannins and plant evolution. Phytochemistry 2000, 55, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Yisimayili, Z.; Abdulla, R.; Tian, Q.; Wang, Y.; Chen, M.; Sun, Z.; Li, Z.; Liu, F.; Aisa, H.A.; Huang, C. A comprehensive study of pomegranate flowers polyphenols and metabolites in rat biological samples by high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2019, 1604, 460472. [Google Scholar] [CrossRef] [PubMed]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A.; Kucharska, A.Z.; Fecka, I. Composition and Antibacterial Activity of Aronia melanocarpa (Michx.) Elliot, Cornus mas L. and Chaenomeles superba Lindl. Leaf Extracts. Molecules 2020, 25, 2011. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Campo, M.; Pinelli, P. HPLC/DAD/ESI-MS analyses and anti-radical activity of hydrolysable tannins from different vegetal species. Food Chem. 2012, 130, 214–221. [Google Scholar] [CrossRef]
- Hanhineva, K.; Rogachev, I.; Kokko, H.; Mintz-Oron, S.; Venger, I.; Kärenlampi, S.; Aharoni, A. Non-targeted analysis of spatial metabolite composition in strawberry (Fragariax ananassa) flowers. Phytochemistry 2008, 69, 2463–2481. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Hatano, T.; Ogawa, N.; Kira, R.; Matsuda, M. Cornusiin A, a dimeric ellagitannin forming four tautomers, and accompanying new tannins in Cornus officinalis. Chem. Pharm. Bull. 1984, 32, 4662–4665. [Google Scholar] [CrossRef]
- Hatano, T.; Ogawa, N.; Kira, R.; Yasuhara, T.; Okuda, T. Tannins of cornaceous plants. I. Cornusiins A, B and C, dimeric monomeric and trimeric hydrolyzable tannins from Cornus officinalis, and orientation of valoneoyl group in related tannins. Chem. Pharm. Bull. 1989, 37, 2083–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatano, T.; Yasuhara, T.; Okuda, T. Tannins of cornaceous plants. II. Cornusiins D, E and F, new dimeric and trimeric hydrolyzable tannins from Cornus officinalis. Chem. Pharm. Bull. 1989, 37, 2665–2669. [Google Scholar] [CrossRef] [Green Version]
- Karvela, E.; Makris, D.; Kefalas, P.; Moutounet, M. Extraction of phenolics in liquid model matrices containing oak chips: Kinetics, liquid chromatography mass spectroscopy characterisation and association with in vitro antiradical activity. Food Chem. 2008, 110, 263272. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Determination of phenolic composition and antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria chiloensis spp. Chiloensis form chiloensis) using HPLC-DAD-ESI-MS and free radical quenching techniques. J. Food Compos. Anal. 2010, 23, 545. [Google Scholar] [CrossRef]
- Seeram, N.P.; Lee, R.; Scheuller, H.S.; Heber, D. Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chem. 2006, 97, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Regazzoni, L.; Arlandini, E.; Garzon, D.; Santagati, N.A.; Beretta, G.; Facino, R.M. A rapid profiling of gallotannins and flavonoids of the aqueous extract of Rhus coriaria L. by flow injection analysis with high-resolution mass spectrometry assisted with database searching. J. Pharm. Biomed. Anal. 2013, 72, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Lu, Y.; Santos, S.A.O.; Silvestre, A.J.D.; Neto, C.P.; Câmara, J.S.; Rocha, S.M. Phenolic profile of Sercial and Tinta Negra Vitis vinifera L. grape skins by HPLC-DAD-ESI-MSn: Novel phenolic compounds in Vitis vinifera L. grape. Food Chem. 2012, 135, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xu, Q.; Jiang, H. Identification and Characterization of Two New Forced Degradation Products of Saikosaponin A under the Stress of Acid Hydrolytic Conditions. Molecules 2016, 21, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Reidah, I.M.; Mohammed, S.A.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC-DAD-ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Magdalena, M.T.; Grazyna, Z. In-depth phytochemical and biological studies on potential AChE inhibitors in red and zigzag clover dry extracts using reversed–phase liquid chromatography (RP-LC) coupled with photodiode array (PDA) and electron spray ionization-quadrupole/time of flight-mass spectrometric (ESI-QToF/MS-MS) detection and thin-layer chromatography-bioautography. Food Chem. 2022, 375, 131846. [Google Scholar]
- Duckstein, S.M.; Stintzing, F.C. Investigation on the phenolic constituents in Hamamelis virginiana leaves by HPLC-DAD and LC-MS/MS. Anal. Bioanal. Chem. 2011, 401, 677–688. [Google Scholar] [CrossRef]
- Ancos, B.D.; González, E.M.; Cano, M.P. Ellagic acid, Vitamin C, and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit. J. Agric. Food Chem. 2000, 48, 4565–4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuncio-Jáuregui, N.; Nowicka, P.; Munera-Picazo, S.; Hernández, F.; Carbonell-Barrachina, A.A.; Wojdyło, A. Identification and quantification of major derivatives of ellagic acid and antioxidant properties of thinning and ripe Spanish pomegranates. J. Funct. Foods 2015, 12, 354–364. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Content of bioactive compounds in the peach kernels and their antioxidant, anti-hyperglycemic, anti-aging properties. Eur. Food Res. Technol. 2019, 245, 1123–1136. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Al-Ghamdi, A.A.; Elshikh, M.S.; Shah, M.H.; Abbasi, A.M. Phytochemical Profiling, Antioxidant and HepG2 Cancer Cells’ Antiproliferation Potential in the Kernels of Apricot Cultivars. Saudi J. Biol. Sci. 2020, 27, 163–172. [Google Scholar] [CrossRef] [PubMed]











| No. | Compound | Assignment | tR /min | MS /(m/z) | MS/MS Fragment Ions /(m/z) | |
|---|---|---|---|---|---|---|
| Brevifolincarboxyl tannins and their derivatives | ||||||
| 1 | A1 | 1 | Brevifolincarboxyl-trigalloyl-hexoside | 19.62 | 909.1014 [M-H]− | 169.0084; 247.0257; 273.0030; 291.0139; 435.0560; 453.0302; 604.7536; 739.0454; 757.0958; 909.1014 |
| 2 | 2 | 21.28 | 909.0992 [M-H]− | 169.0087; 247.0183; 273.0029; 291.0138; 435.0475; 453.057; 757.0576; 909.0732 | ||
| 3 | 3 | 23.80 | 909.1022 [M-H]− | 169.0103; 247.014; 272.9983; 291.0465; 435.0149; 604.9794; 757.0533; 909.0967 | ||
| 4 | A2 | Galloyl- brevifolincarboxyl-hexoside | 18.36 | 605.0013 [M-H]− | 125.0230; 149.0080; 169.0128; 221.0070; 247.0255; 273.0026; 291.0116; 311.0390; 331.0200; 378.02; 383.04; 387.02; 435.03; 453.0401; 465.04; 463.04726; 587.0662; 605.0013 | |
| 5 | A3 | Digalloyl-DHHDP-hexdside | 17.01 | 801.0795 [M-H]− | 125.0068; 169.0126; 191.03401 219.0929; 247.0251; 273.0023; 291.0128; 363.02; 374.07; 378.87; 427.02; 435.055; 445.03; 466.81; 597.02; 621.88; 627.73; 757.0929; 765.0580; 783.0775 | |
| 6 | A4 | DHHDP-trigalloylhexoside | 19.62 | 953.0915 [M-H]− | 169.0084; 247.0257; 273.0031; 291.0140; 435.0559; 587.0396; 605.0547; 757.0971; 909.0996 | |
| 7 | A5 | Monogalloyl-DHHDP-hexoside | 18.36 | 649.1073 [M-H]− | 125.0230; 169.0128; 465.0664; 479.0852; 497.09307; 649.1073 | |
| 8 | A6 | DHHDP-HHDP-galloyl-gluconic acid | 22.55 | 967.1068 [M-H]− | 169.0124; 231.0274; 247.0253; 249.0377; 257.0086; 273.0068; 275.0203; 291.0096; 300.9964; 382.999; 399.01; 427.008; 445.02; 465.047; 581.05473; 597.05606; 749.05748; 765.0391; 917.0799; 935.0724 | |
| 9 | A7 | Peroxide product of DHHDP-trigalloylhexoside | 23.39 | 941.1275 [M-H]− | 247.02572; 291.01381; 435.05605; 453.0698; 587.0662; 605.0627; 739.0645; 757.0970; 843.47; 909.10025 | |
| Ellagitannins | ||||||
| 10 | B1 | 1 | Dimer of valoneoyl-galloyl-hexoside and HHDP-galloyl-hexoside | 9.6 | 708.0711 [M-2H]2−/2 | 169.0131; 249.0417; 275.0207; 300.9971; 450.991; 633.0780; 708.0753; 765.0594; 785.0749; 1114.88 |
| 11 | 2 | 10.09 | 708.0717 [M-2H]2−/2 | 169.0132; 249.0415; 275.0207; 300.9970; 450.9914; 633.0779; 708.0750; 765.0587; 783.0538; 785.0765; 936.2874; 1115.027; 1247.0553 | ||
| 12 | 3 | 10.69 | 708.0712 [M-2H]2−/2 | 125.0222; 169.0109; 249.0379; 275.0164; 300.9970; 450.9913; 633.0474; 708.0393; 765.0600; 783.0393; 785.0564; 1095.3044; 1114.9217; 1159.3596; 1247.0139; 1255.6029; 1334.6029; 1382.7864 | ||
| 12 | B2 | 1 | HHDP-monogalloyl- hexoside | 10.09 | 633.0738 [M-H]− | 125.02; 169.01; 249.03; 275.01; 300.99; 313.05; 331.0601; 450.98; 467.02; 481.05; 633.05 |
| 14 | 2 | 13.46 | 633.5754 [M-H]− | 125.0233; 169.0112; 249.0416; 275.02081; 300.9970; 331.0615; 421.0296; 633.0784; | ||
| 15 | B3 | Bis-HHDP-hexoside | 10.09 | 783.0750 [M-H]− | 249.0376; 275.0208; 300.9970; 450.9977; 481.0508; 765.0378; 783.0750 | |
| 16 | B4 | 1 | Digalloyl-HHDP-hexoside | 11.65 | 785.0776 [M-H]− | 169.0126; 231.0303; 249.0407; 275.0201; 300.9963;419.0536; 466.0678; 483.0679; 568.5199; 743.0063; 785.0776 |
| 17 | 2 | 13.85 | 785.0557 [M-H]− | 125.022; 169.01252; 249.0407; 275.0202; 300.9962; 384.7543; 483.0478; 626.5269; 785.0557 | ||
| 18 | 3 | 16.19 | 785.0776 [M-H]− | 125.0222; 169.0130; 249.0407; 275.0201; 300.9963; 419.0283; 445.0278; 457.5856; 483.1278; 615.0389; 633.0501; 785.0776 | ||
| 19 | B5 | 1 | HHDP-digalloyl-hexoside dimer | 12.51 | 784.0739 [M-2H]2−/2 | 125.02; 169.01324; 249.04253; 275.0206; 300.9970; 450.9906; 597.04; 613.04; 633.06; 699.04; 765.03; 784.0739; 785.0741; 935.06; 1084.85; 1266.88; 1398.82; 1479.9; |
| 20 | 2 | 13.46 | 784.0769 [M-2H]2−/2 | 125.0222; 169.0111; 231.0283; 249.0380; 275.1222; 300.9921; 450.9812; 633.0624; 699.0546; 765.0389; 784.0325; 785.1371; 935.0390; 1182.3044; 1266.8373 | ||
| 21 | 3 | 14.38 | 784.0769 [M-2H]2−/2 | 125.0209; 169.0112; 231.0281; 249.0341; 275.0122; 300.9921; 450.9909; 633.0603; 699.0471; 765.0389; 784.0325; 785.07436; 935.0625; 1266.8373; | ||
| 22 | 4 | 15.51 | 784.0769 [M-2H]2−/2 | 125.0222; 169.0116; 231.0283; 249.0380; 275.0121; 300.9871; 450.9908; 633.0643; 699.0467; 765.0390; 784.0322; 785.07436; 935.0650; 1266.8801; 1464.8974 | ||
| 23 | 5 | 17.95 | 784.0769 [M-2H]2−/2 | 125.0221; 169.0131 231.0283; 249.0378; 275.0163; 300.9920; 450.9910; 633.0602; 699.0458; 765.0387; 784.0320; 785.0743; 935.0391; 1266.8801 | ||
| 24 | 6 | 19.62 | 784.0769 [M-2H]2−/2 | 125.0221; 169.0111 231.0283; 249.0379; 275.0120; 300.9920; 450.9908; 633.0626; 699.0622; 765.0381; 784.0528; 785.0956; 935.0664; 1085.8745; 1348.183 | ||
| 25 | B6 | EA-(HHDP-galloyl)-galloyl-hexoside | 14.38 | 542.0338 [M-H]− | 300.9970; 450.9709; 542.5338; 633.0634; 765.0208; 783.0566; 785.0763 | |
| 26 | B7 | Gallic acid etheric HHDP-digalloyl-hexoside | 15.51 | 953.0909 [M-H]− | 169.0124; 249.0408; 275.0200; 300.9963; 444.97; 462.9903; 597.0273; 615.0253; 765.0391; 783.0339; 785.0754; 909.0972; 953.0909 | |
| 27 | B8 | Trimer of HHDP-galloyl-hexoside and two valoneoyl-digalloyl-hexoside | 15.51 | 1100.0655 [M-2H]2−/2 | 169.0113;275.0207; 249.03796; 300.9969; 450.99503;613.0458; 633.0761; 765.05627;783.0518; 1015.5890; 1100.0655; 1427.1950; 1417.0668; 1567.1799 | |
| 28 | B9 | 1 | Trimer of HHDP-galloyl-hexoside, valoneoyl-digalloyl-hexoside and valoneoyl-trigalloyl-hexoside | 15.51 | 1176.0540 [M-2H]2−/2 | 169.0111; 231.0281; 249.0378; 275.0163; 300.9968; 450.9904; 633.0614; 765.0374; 783.0731; 935.0649; 1091.5994; 1176.0540; 1247.1002; 1417.1671; 1569.1285; 1719.1229; 2050.6591; 2103.4512 |
| 29 | 2 | 17.01 | 1176.0549 [M-2H]2−/2 | 169.0111; 231.0282; 249.0379; 275.0164; 300.9969; 450.9906; 633.0612; 765.0577; 783.0743; 785.0735; 935.0640; 1091.600; 1176.054; 1247.1003; 1417.117; 1567.1327; 1719.1293; 2052.0692 | ||
| 30 | 3 | 17.95 | 1176.0904 [M-2H]2−/2 | 169.0112; 231.0281; 249.0378; 275.0163; 300.9969; 450.9903; 633.0611; 765.0364; 783.0725; 785.0724; 935.0904; 1091.597; 1176.090; 1247.0928; 1417.117; 1567.1253; 1719.0564; | ||
| 31 | 4 | 19.62 | 1176.1170 [M-2H]2−/2 | 169.0112; 231.0249; 249.0379; 275.0164; 300.9970; 450.9906; 633.6614; 765.0375; 783.0783; 785.0733; 935.0643; 1091.6337; 1176.0928; 1247.1001; 1417.1170; 1567.1280; 1719.2001; 2051.6284 | ||
| 32 | 5 | 22.04 | 1176.5870 [M-2H]2−/2 | 169.0090; 231.0282; 249.0378; 275.0164; 300.9969; 450.9906; 633.6618; 765.0379; 783.0949; 785.0731; 935.0652; 1091.5993; 1176.5870; 1247.0970; 14717.0670; 1567.1222; 1719.0538; 2052.0695; 2370.8410 | ||
| 33 | B10 | 1 | HHDP-trigalloyl-hexoside | 17.95 | 468.0396 [M-2H]2−/2 | 125.0231; 169.0108; 249.0377; 275.0168; 300.9920; 392.0275; 468.0396; 614.9811; 767.0531 |
| 34 | 2 | 18.36 | 468.0395 [M-2H]2−/2 | 125.0231; 169.0128; 275.0204; 300.9969; 316.0311; 392.0363; 468.03952; 614.98; 767.0310 | ||
| 35 | B11 | 1 | Dimer of HHDP-digalloyl-hexoside and valoneoyl-digalloyl-hexoside | 17.01 | 860.0783 [M-2H]2−/2 | 125.0222; 169.0111; 231.0280; 249.0378; 275.0164; 300.992; 450.9815; 633.0482; 699.0474; 765.0383; 784.0733; 860.0783; 937.0754; 1087.0227; 1419.839 |
| 36 | 2 | 17.95 | 860.0278 [M-2H]2−/2 | 169.0132; 249.0418; 275.0207; 300.99693; 450.99036; 597.05; 765.05; 775.07; 785.03; 860.0278; 935.06; 937.10097; 937.10; 1087.0970; 1249.10; 1419.1430 | ||
| 37 | 3 | 18.36 | 860.0780 [M-2H]2−/2 | 125.0208; 169.0111; 231.0280; 249.0378; 275.0164; 300.9919; 450.9906; 633.0596; 699.0244; 765.0384; 784.0520; 860.0780; 937.1008; 1087.098; 1267.0512 | ||
| 38 | 4 | 19.62 | 860.0545 [M-2H]2−/2 | 125.0228; 169.0112; 231.0249; 249.0379; 275.0164; 300.992; 450.9911; 633.0595; 699.0457; 765.0577; 784.0731; 860.0545; 937.0749; 1087.098; 1419.194 | ||
| 39 | 5 | 21.28 | 860.0549 [M-2H]2−/2 | 125.0220; 169.0110; 231.0246; 249.0378; 275.0163; 300.992; 450.9906; 633.0466; 699.0358; 765.0386; 784.0525; 860.0549; 937.0749; 1087.063; 1093.38; 1139.656 | ||
| 40 | B12 | 1 | Galloyl-bis-HHDP-hexoside | 20.29 | 935.0833 [M-H]− | 139.01322; 300.997; 581.0539; 597.05; 632.97; 749.05547; 783.01; 917.0757; 935.08338 |
| 41 | 2 | 21.64 | 935.0808 [M-H]− | 169.0110; 231.028; 247.0223; 275.0165; 300.9720; 597.0398; 749.0552; 783.0803; 917.0495; 935.0660 | ||
| 42 | B13 | Ellagic acid pentoside | 17.58 | 433.1349 [M-H]− | 299.9979; 300.9634; 387.1276; 433.0394; 450.8096? | |
| 43 | B14 | Methyl ellagic acid pentoside | 22.55 | 447.0570 [M-H]− | 270.9903; 298.9806; 299.9885; 314.0053; 315.0157; 332.0889; 333.090; 447.05616 | |
| Gallotannins | ||||||
| 44 | C1 | 1 | Mono-galloyl- hexoside | 4.2 | 331.0672 [M-1]− | 125.0230; 169.0128; 211.0240; 331.0694 |
| 45 | 2 | 5.37 | 331.0694 [M-1]− | 125.0230; 169.0128; 211.0239; 331.0694 | ||
| 46 | 3 | 7.05 | 311.0639 [M-1]− | 125.0231; 169.01289; 311.0639 | ||
| 47 | C2 | 1 | Di-galloyl- hexoside | 6.0 | 483.0780 [M-H]− | 125.0230; 169.0128; 311.0690; 483.0778 |
| 48 | 2 | 7.05 | 483.0781 [M-H]− | 125.0230; 169.0128; 311.0690; 483.0778 | ||
| 49 | 3 | 8.69 | 483.0779 [M-H]− | 125.0230;169.01281; 193.0132; 211.0239; 271.0454; 313.0572; | ||
| 50 | 4 | 10.09 | 483.1459 [M-H]− | 125.0231; 169.0128; 193.0130; 211.0239; 241.0342; 271.0451; 313.0572; 331.06935; 483.1459 | ||
| 51 | 5 | 10.69 | 483.0776 [M-H]− | 125.0230; 169.0127; 211.0139; 271.0451; 313.05721; 483.0776 | ||
| 52 | C3 | 1 | Trigalloyl- hexoside | 11.9 | 635.0928 [M-H]− | 125.0229; 169.0124; 313.0568; 465.0655; 635.0928 |
| 53 | 2 | 13.46 | 635.0924 [M-H]− | 125.0231; 169.0128; 211.0239; 271.0454; 313.0569; 465.0657; 483.07735; 635.0924 | ||
| 54 | 3 | 14.39 | 635.0923 [M-H]− | 125.0229; 169.0124; 313.0568; 465.0655; 635.0928 | ||
| 55 | 4 | 15.05 | 635.0930 [M-H]− | 125.0232; 169.0129; 211.024; 271.0452; 313.0574; 465.0648; 483.0775; 635.0930 | ||
| 56 | C4 | 1 | Tetragalloyl- hexoside | 18.36 | 787.1058 [M-H]− | 125.0229; 169.0125; 313.0570; 465.0657; 635.09187; 787.1058 |
| 57 | 2 | 19.62 | 787.1058 [M-H]− | 125.0229; 169.0125; 313.0567; 465.0653; 635.09194; 787.1058 | ||
| 58 | C5 | 1 | Penta-galloyl-hexoside | 20.77 | 939.1118 [M-H]− | 125.02282; 169.01253;313.05; 403.05; 447.04; 465.04; 513.06?; 617.05; 635.05; 787.06; 939.1118 |
| 59 | 2 | 21.28 | 939.1122 [M-H]− | 125.0228; 169.0124; 313.0570; 465.0658; 787.10365; 939.1122 | ||
| 60 | 3 | 22.04 | 939.1120 [M-H]− | 125.0228; 169.0124; 313.0570; 465.0658; 617.0827; 787.1036; 939.1120 | ||
| 61 | C6 | 1 | Hexa-galloyl- hexoside | 23.39 | 545.03 [M-2H]2−/2 | 125.0230; 169.0128; 241.0350; 317.0350; 393.0477; 431.0635; 447.0562; 465.0650; 545.0487; 601.0745; 617.0484; 769.0929 |
| 62 | 2 | 24.00 | 545.03 [M-2H]2−/2 | 125.0231; 169.0219; 241.0345; 317.0403; 393.0465; 431.0635; 447.0562; 465.0662; 545.0591; 601.0754.; 617.0731; 769.0745; 896.8940; 935.1472 | ||
| 63 | C7 | Monogalloyl-heptoside | 5.37 | 361.0796 [M-H]− | 125.0232; 169.0129; 211.0244; 241.0348; 271.04527; 361.0796 | |
| 64 | C8 | Digalloyl-heptoside | 10.69 | 513.0904 [M-H]− | 125.0230; 169.0128; 211.0240; 271.0451; 343.0711; 361.0781; 513.0904 | |
| Phenolic acids and their derivatives | ||||||
| 65 | D1 | Salicylic acid | 0.1 | 136.86152 [M-1]− | 92.91849; 136.86152 | |
| 66 | D2 | Cinnamic acid | 0.1 | 146.93 [M-1]− | 58.85718; 87.92379; 102.94717 | |
| 67 | D3 | 1 | Caffeic acid | 12.84 | 179.03318 [M-1]− | 135.04356; 179.03318 |
| 68 | 2 | Caffeic acid isomer | 15.51 | 179.03323 [M-1]− | 135.0436; 179.03323 | |
| 69 | D4 | p-Coumaric acid | 16.19 | 163.03804 [M-1]− | 119.04825; 163.03804 | |
| 70 | D5 | Syringic acid | 17.01 | 197.04325 [M-1]− | 121.0280; 153.0536; 182.0217; 197.04325 | |
| 71 | D6 | Gallic acid | 6.52 | 169.01284 [M-1]− | 125.0229; 169.01284 | |
| 72 | D7 | Ellagic acid | 21.28 | 300.99706 [M-1]− | 300.99706 | |
| 73 | D8 | Brevifolin carboxylic acid | 13.19 | 291.01377 [M-1]− | 173.022;191.0317; 203; 219; 247.02568; 291.01377 | |
| 74 | D9 | Citric acid derivative 1 | 0.1 | 280.8698 [M-1]− | 102.9471; 118.9424; 130.8875; 146.9368; 162.8384; 174.8779; 190.9262; 218.8685; 236.8784; 262.85823; 280.8698 | |
| 75 | D10 | Citric acid derivative 2 | 0.1 | 336.85 [M-1]− | 102.9472; 146.9371; 190.92668 | |
| 79 | D11 | Caffeoylmalic acid | 3.91 | 295.06706 [M-1]− | 71.01203; 115.0020; 133.0124; 179.05567; 295.06706 | |
| 77 | D12 | Caffeoylmaloyl Fumaric acid | 3.91 | 393.03 [M-1]− | 71.01206; 79.9552; 96.95802; 115.0021; 135.0125; 179.0555; 295.06659 | |
| 78 | D13 | 1 | Caftaric acid | 12.51 | 311.03643 [M-1]− | 87.00687; 135.0421; 149.0081; 179.03326; 311.03643 |
| 79 | 2 | 12.84 | 311.04 [M-1]− | 59.01217; 87.00672; 135.04366; 149.00816; 179.0333 | ||
| 80 | D14 | Digallic acid or digallate | 12.51 | 321.02 [M-1]− | 125.02308; 169.01285 | |
| 81 | D15 | Protocatechuic acid derivative | 3.58 | 333.06135 [M-1]− | 78.95685; 109.8816; 152.9943; 171.004; 241.0099; 333.06135 | |
| 82 | D16 | Feruloyl acid derivative | 12.51 | 373.113 [M-1]− | 193.04875; 149.05987; 373.113 | |
| 83 | D17 | Feruloyl tartaric acid | 17.58 | 325.05 [M-1]− | 59.01226; 87.00679; 103.0017; 134.03563; 149.0081;178.0262; 193.0488; | |
| 84 | D18 | Syringoylmalic acid | 17.01 | 313.05 [M-1]− | 71.0121; 115.0022; 121.02804; 133.0125;153.0536; 182.0219;197.0432 | |
| 85 | D19 | Galloyl malic acid | 8.69 | 285.02 [M-1]− | 71.012; 115.00211; 133.01243; 169.01263; 125.023 | |
| 86 | D20 | Malic acid derivative | 15.51 | 505.15889 [M-1]− | 71.0120; 101.02559; 115.0022; 127.0385; 133.0125; 227.0914; 389.1437; 487.1433; 505.15889 | |
| 87 | D21 | p-Coumaroyltartaric acid | 16.19 | 295.00 [M-1]− | 59.01225; 87.0067; 103.00181; 105.1979;119.04835; 130.9971;163.03806; | |
| 88 | D22 | p-Coumaroyl acid derivative 1 | 22.55 | 331.08094 [M-1]− | 109.0258; 119.0470; 163.0381; 207.0282; 287.0933; 272.0602; 287.0152; 331.08094 | |
| 89 | D23 | p-Coumaroyl acid derivative 2 | 22.04 | 361.0927 [M-1]− | 119.0483;139.0377;163.0381; 207.0282; 287.0471; 302.0470; 317.0244; 361.09278 | |
| 90 | D24 | Valoneic acid bilactone | 15.51 | 468.9910 [M-1]− | 125.0271; 169.0132; 270.9862; 298.9814; 299.9814; 300.9971; 425.01618; 468.9910 | |
| Non-phenolic acids | ||||||
| 91 | E1 | Citric Acid | 0.1 | 190.9274 [M-1]− | 58.9567; 102.94717; 146.93691;190.9274 | |
| 92 | E2 | 1 | Tartaric acid | 3.91 | 149.00 [M-1]− | 59.01211; 72.99147; 87.00666; 103.00173; 105.018; 149.00818 |
| 93 | 2 | 12.84 | 149.00 [M-1]− | 59.01226; 72.9916; 87.00679; 103.00188; 105.016; 149.00811 | ||
| 94 | E3 | 1 | Malic acid | 4.2 | 133.01247 [M-1]− | 71.01199; 115.00212; 133.0124 |
| 95 | 2 | 5.37 | 133.01253 [M-1]− | 71.0121; 115.00218; 133.01253 | ||
| 96 | E4 | 1 | Quinic acid | 4.2 | 191.01939 [M-1]− | 85.02796; 111.0068; 117.0181; 129.0174; 154.9972; 173.0683; 191.0193 |
| 97 | 2 | 5.27 | 85.02792; 111.0068; 191.01934 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, L.; Jiang, H.; Li, M.; Wang, L.; Li, J.-X.; Wang, Y.-Y.; Guo, Q.-X. New Type of Tannins Identified from the Seeds of Cornus officinalis Sieb. et Zucc. by HPLC-ESI-MS/MS. Molecules 2023, 28, 2027. https://doi.org/10.3390/molecules28052027
Li J, Chen L, Jiang H, Li M, Wang L, Li J-X, Wang Y-Y, Guo Q-X. New Type of Tannins Identified from the Seeds of Cornus officinalis Sieb. et Zucc. by HPLC-ESI-MS/MS. Molecules. 2023; 28(5):2027. https://doi.org/10.3390/molecules28052027
Chicago/Turabian StyleLi, Jun, Lin Chen, Hua Jiang, Min Li, Lu Wang, Jia-Xing Li, Yue-Yue Wang, and Qing-Xia Guo. 2023. "New Type of Tannins Identified from the Seeds of Cornus officinalis Sieb. et Zucc. by HPLC-ESI-MS/MS" Molecules 28, no. 5: 2027. https://doi.org/10.3390/molecules28052027
APA StyleLi, J., Chen, L., Jiang, H., Li, M., Wang, L., Li, J. -X., Wang, Y. -Y., & Guo, Q. -X. (2023). New Type of Tannins Identified from the Seeds of Cornus officinalis Sieb. et Zucc. by HPLC-ESI-MS/MS. Molecules, 28(5), 2027. https://doi.org/10.3390/molecules28052027