Evaluation of Antipsychotic Drugs’ Stability in Oral Fluid Samples
Abstract
:1. Introduction
2. Results and Discussion
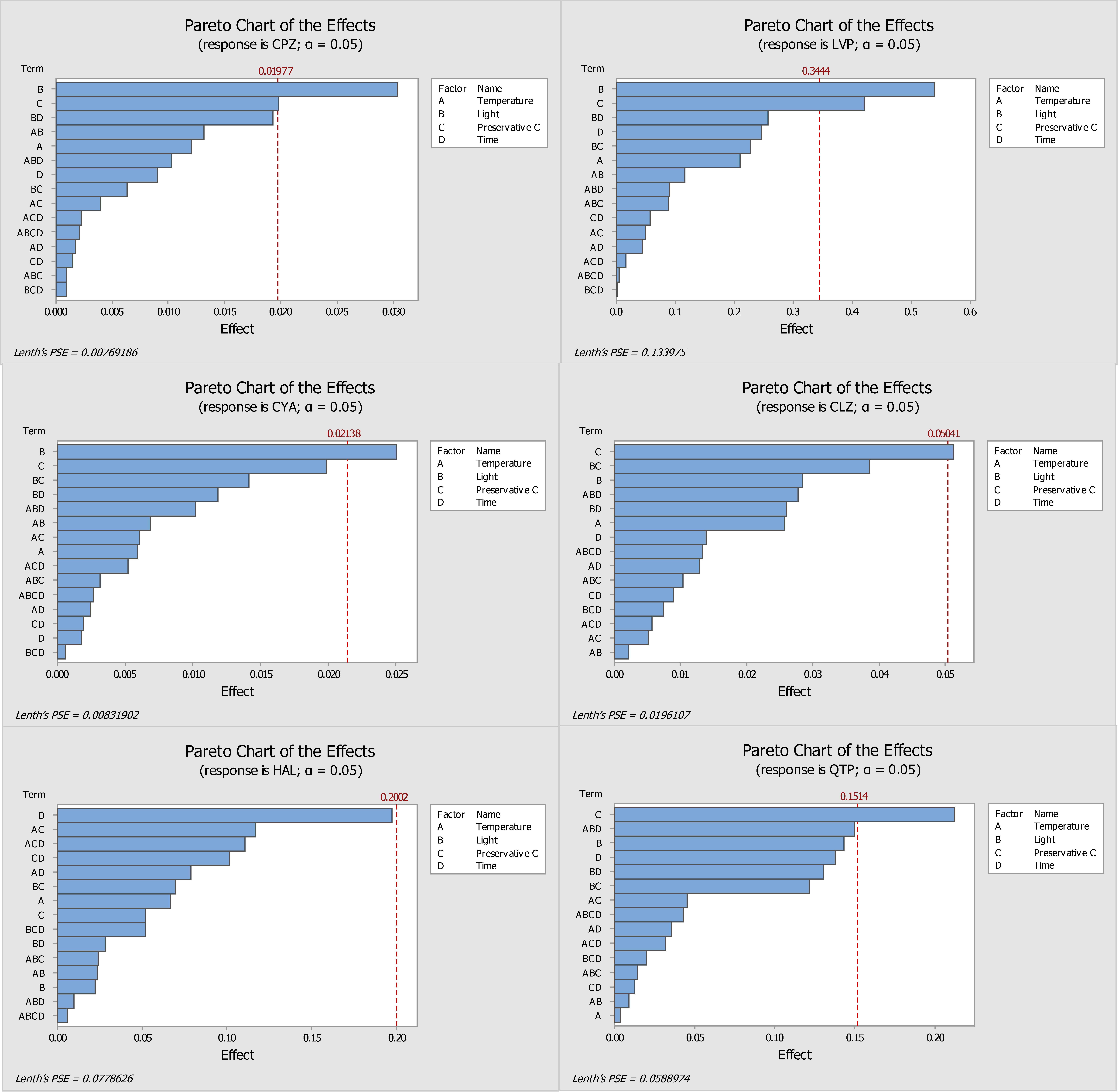
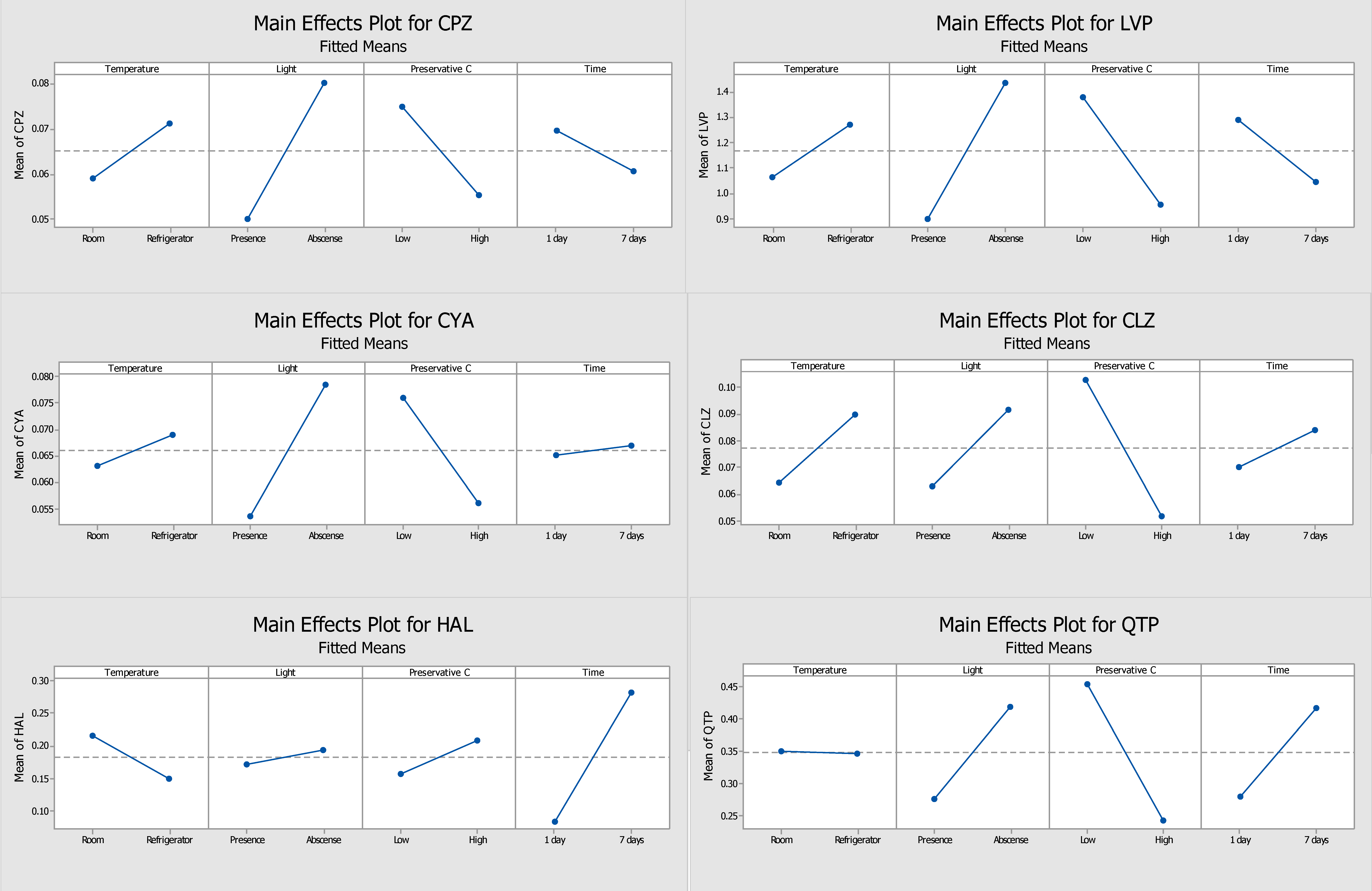
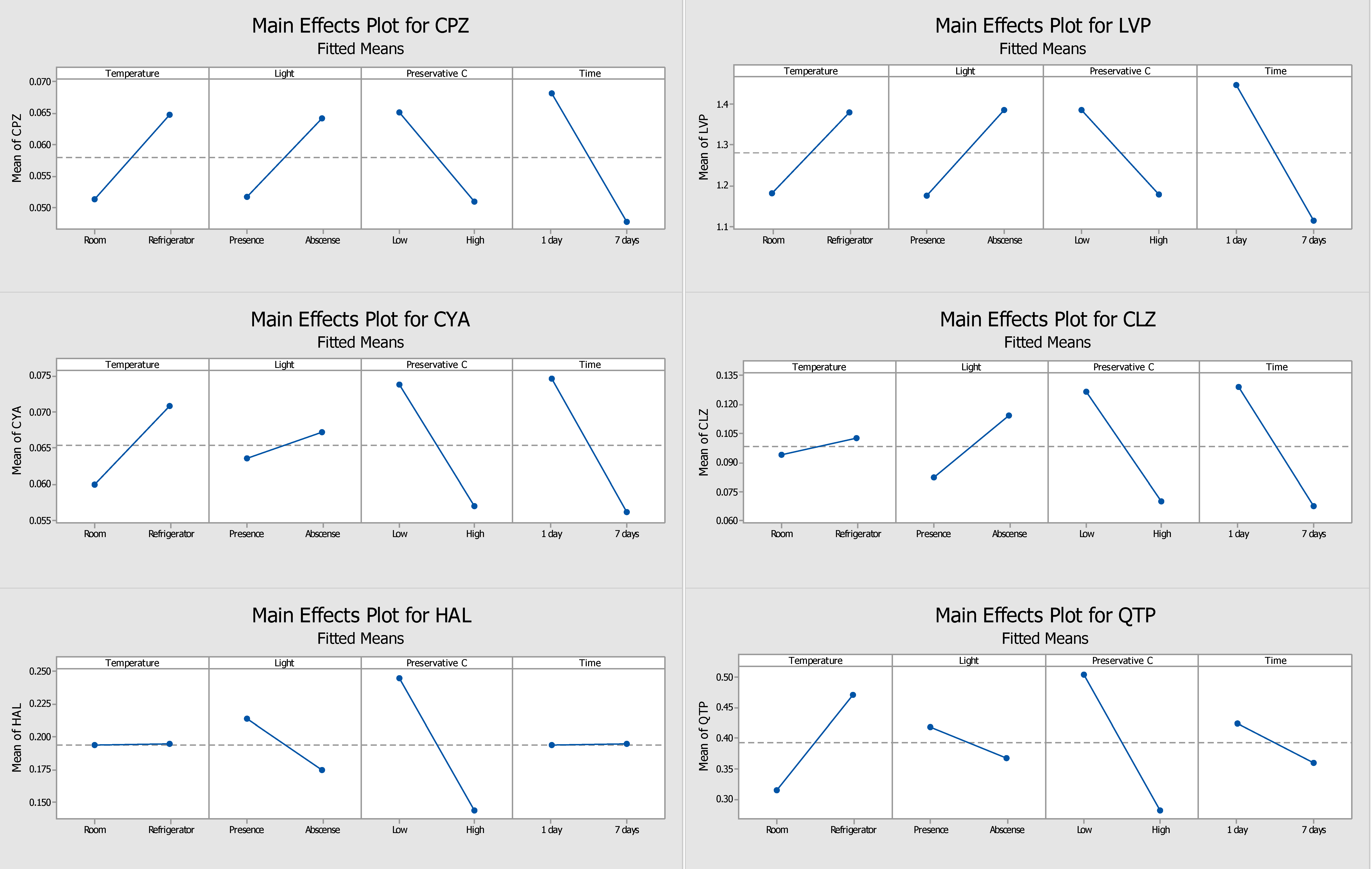
2.1. Optimization of the Stability Protocol
2.2. DOE Performed without Preservative
2.3. Final Conditions for Long-Term Stability Assay
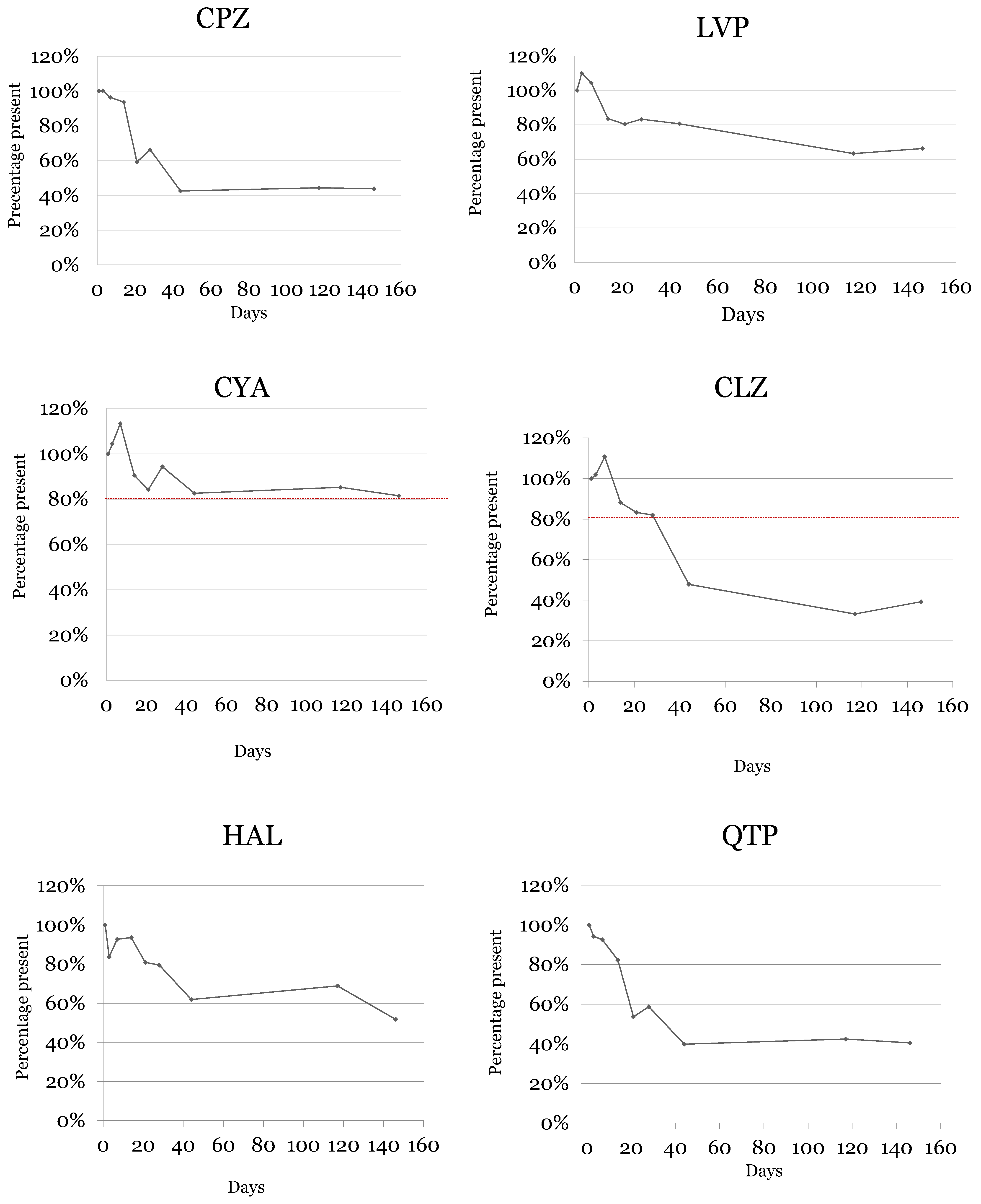
2.4. Samples Stability
3. Materials and Methods
3.1. Reagents and Standards
3.2. Biological Specimens
3.3. Gas Chromatographic and Mass Spectrometric Conditions
3.4. Sample Preparation
3.5. Design of Experiments
3.6. Long-Term Stability Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Caramelo, D.; Rosado, T.; Oliveira, V.; Rodilla, J.M.; Rocha, P.M.M.; Barroso, M.; Gallardo, E. Determination of antipsychotic drugs in oral fluid using dried saliva spots by gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 6141–6153. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Akhondzadeh, S. Schizophrenia: Etiology and pharmacotherapy. IDrugs 2001, 4, 1167–1172. [Google Scholar] [PubMed]
- Cowen, P.J. Psychopharmacology. In Comprehensive Clinical Psychology; Bellack, A.S., Hersen, M., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 135–161. [Google Scholar]
- Nemergut, G.; Sandra, J. Neuroleptics (Typical/Atypical Antipsychotics). In Pain; Abd-Elsayed, A., Ed.; Springer: Cham, Switzerland, 2019; pp. 255–260. [Google Scholar]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadland, S.E.; Levy, S. Objective Testing: Urine and Other Drug Tests. Child Adolesc. Psychiatr. Clin. N. Am. 2016, 25, 549–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmongy, H.; Abdel-Rehim, M. Saliva as an alternative specimen to plasma for drug bioanalysis. A review. TrAC—Trends Anal. Chem. 2016, 83, 70–79. [Google Scholar] [CrossRef]
- Gallardo, E.; Queiroz, J.A. The role of alternative specimens in toxicological analysis. Biomed. Chromatogr. 2008, 22, 795–821. [Google Scholar] [CrossRef]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T.W. Salivary biomarkers: Toward future clinical and diagnostic utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Choo, R.E.; Huestis, M.A. Oral fluid as a diagnostic tool. Clin. Chem. Lab. Med. 2004, 42, 1273–1287. [Google Scholar] [CrossRef]
- Desrosiers, N.A.; Huestis, M.A. Oral Fluid Drug Testing: Analytical Approaches, Issues and Interpretation of Results. J. Anal. Toxicol. 2019, 43, 415–443. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Rehim, A.; Abdel-Rehim, M. Dried saliva spot as a sampling technique for saliva samples. Biomed. Chromatogr. 2014, 28, 875–877. [Google Scholar] [CrossRef]
- Carvalho, J.; Rosado, T.; Barroso, M.; Gallardo, E. Determination of antiepileptic drugs using dried saliva spots. J. Anal. Toxicol. 2019, 43, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Jacques, A.L.B.; dos Santos, M.K.; Limberger, R.P. Development and Validation of a Method Using Dried Oral Fluid Spot to Determine Drugs of Abuse. J. Forensic Sci. 2019, 64, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Marques, H.; Rosado, T.; Barroso, M.; Passarinha, L.; Gallardo, E. Optimization and validation of a procedure using the dried saliva spots approach for the determination of tobacco markers in oral fluid. J. Pharm. Biomed. Anal. 2022, 212, 114648. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Rosado, T.; Barroso, M.; Gallardo, E. New method for the monitoring of antidepressants in oral fluid using dried spot sampling. Pharmaceuticals 2021, 14, 1284. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.-L.; Zhang, M.; Wang, J.; Zeng, S.; Min, J.Z. Potential use of a dried saliva spot (DSS) in therapeutic drug monitoring and disease diagnosis. J. Pharm. Anal. 2021, 12, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Mata, D.C. Stability of 26 sedative hypnotics in six toxicological matrices at different storage conditions. J. Anal. Toxicol. 2016, 40, 663–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, F.T. Stability of analytes in biosamples-an important issue in clinical and forensic toxicology? Anal. Bioanal. Chem. 2007, 388, 1505–1519. [Google Scholar] [CrossRef]
- Saar, E.; Gerostamoulos, D.; Drummer, O.H.; Beyer, J. Assessment of the stability of 30 antipsychotic drugs in stored blood specimens. Forensic Sci. Int. 2012, 215, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.S.; Partridge, S.J.; Handley, S.A.; Flanagan, R.J. Stability of some atypical antipsychotics in human plasma, haemolysed whole blood, oral fluid, human serum and calf serum. Forensic Sci. Int. 2013, 229, 151–156. [Google Scholar] [CrossRef]
- Patteet, L.; Maudens, K.E.; Morrens, M.; Sabbe, B.; Dom, G.; Neels, H. Determination of Common Antipsychotics in Quantisal-Collected Oral Fluid by UHPLC-MS/MS. Ther. Drug Monit. 2016, 38, 87–97. [Google Scholar] [CrossRef]
- Costa, S.; Barroso, M.; Castañera, A.; Dias, M. Design of experiments, a powerful tool for method development in forensic toxicology: Application to the optimization of urinary morphine 3-glucuronide acid hydrolysis. Anal. Bioanal. Chem. 2010, 396, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Barroso, M.; Martinho, A.; Cruz, A.; Gallardo, E. Determination of ketamine and its major metabolite, norketamine, in urine and plasma samples using microextraction by packed sorbent and gas chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1004, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.; Soares, S.; Gonçalves, J.; Rosado, T.; Fernández, N.; Rodilla, J.M.; Passarinha, L.A.; Barroso, M.; Gallardo, E. Stability of Cocaine, Opiates, and Metabolites in Dried Saliva Spots. Molecules 2022, 27, 641. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.K.K.; Johansen, S.S. Determination of olanzapine in whole blood using simple protein precipitation and liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2009, 33, 212–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stimpfl, T.; Muller, K.; Gergov, M.; Lebeau, M.; Polettini, A.; Sporkert, F.; Weinmann, W. Recommendations on Sample Collection. Available online: https://www.tiaft.org/data/uploads/documents/tiaft-sta-recommendations-on-sample-collection.pdf (accessed on 9 February 2023).


| Conditions | Chlorpromazine | Levomepromazine | Cyamemazine | Clozapine | Haloperidol | Quetiapine |
|---|---|---|---|---|---|---|
| A | 0.055 | 1.003 | 0.036 | 0.043 | 0.140 | 0.059 |
| B | 0.094 * | 1.784 * | 0.053 * | 0.060 * | 0.086 | 0.084 |
| C | 0.074 | 1.312 | 0.047 | 0.058 | 0.171 * | 0.110 * |
| D | 0.052 | 1.092 | 0.039 | 0.035 | 0.160 | 0.079 |
| Days | Chlorpromazine | Levomepromazine | Cyamemazine | Clozapine | Haloperidol | Quetiapine | |
|---|---|---|---|---|---|---|---|
| Without preservative | 1 | 0.078 | 1.687 | 0.043 | 0.061 | 0.101 | 0.095 |
| 7 | 0.086 | 1.610 | 0.056 | 0.043 | 0.050 | 0.051 | |
| With preservative | 1 | 0.075 | 1.427 | 0.059 | 0.108 | 0.197 | 0.312 |
| 7 | 0.073 | 1.490 | 0.067 | 0.120 | 0.183 | 0.289 |
| Analytes | Days | Concentration (ng/mL) | S.D. | CV (%) | Relative Loss (%) |
|---|---|---|---|---|---|
| Chlorpromazine | 1 | 197.2 | 19.7 | 10 | |
| 3 | 199.8 | 40.0 | 20 | 0 | |
| 7 | 191.9 | 13.4 | 7 | −4 | |
| 14 | 186.6 | 26.1 | 14 | −6 | |
| 21 | 118.3 | 17.7 | 15 | −41 | |
| 28 | 131.4 | 21.0 | 16 | −34 | |
| 44 | 84.1 | 15.1 | 18 | −57 | |
| 117 | 86.8 | 6.1 | 7 | −56 | |
| 146 | 86.8 | 15.6 | 18 | −56 | |
| Levomepromazine | 1 | 206.4 | 26.8 | 13 | |
| 3 | 226.9 | 18.2 | 8 | 10 | |
| 7 | 215.5 | 19.4 | 9 | 4 | |
| 14 | 172.4 | 20.7 | 12 | −16 | |
| 21 | 165.9 | 23.2 | 14 | −20 | |
| 28 | 171.7 | 22.3 | 13 | −17 | |
| 44 | 166.3 | 33.3 | 20 | −19 | |
| 117 | 130.5 | 7.8 | 6 | −37 | |
| 146 | 136.5 | 10.9 | 8 | −34 | |
| Cyamemazine | 1 | 199.3 | 21.9 | 11 | |
| 3 | 206.1 | 20.6 | 10 | 4 | |
| 7 | 226.4 | 18.1 | 8 | 13 | |
| 14 | 179.1 | 14.3 | 8 | −9 | |
| 21 | 165.5 | 8.3 | 5 | −16 | |
| 28 | 185.8 | 35.3 | 19 | −6 | |
| 44 | 165.5 | 26.5 | 16 | −17 | |
| 117 | 168.9 | 11.8 | 7 | −15 | |
| 146 | 162.2 | 17.8 | 11 | −18 | |
| Clozapine | 1 | 203.3 | 8.1 | 4 | |
| 3 | 207.0 | 20.7 | 10 | 2 | |
| 7 | 225.8 | 9.0 | 4 | 11 | |
| 14 | 178.8 | 26.8 | 15 | −12 | |
| 21 | 169.4 | 28.8 | 17 | −17 | |
| 28 | 167.5 | 28.5 | 17 | −18 | |
| 44 | 97.9 | 16.6 | 17 | −52 | |
| 117 | 67.8 | 12.2 | 18 | −67 | |
| 146 | 80.9 | 8.1 | 10 | −61 | |
| Haloperidol | 1 | 42.8 | 4.7 | 11 | |
| 3 | 35.9 | 6.5 | 18 | −16 | |
| 7 | 39.8 | 4.8 | 12 | −7 | |
| 14 | 40.0 | 2.0 | 5 | −6 | |
| 21 | 34.6 | 1.4 | 4 | −19 | |
| 28 | 34.1 | 2.4 | 7 | −20 | |
| 44 | 26.5 | 3.7 | 14 | −38 | |
| 117 | 29.6 | 5.0 | 17 | −31 | |
| 146 | 22.2 | 1.6 | 7 | −48 | |
| Quetiapine | 1 | 212.0 | 17.0 | 8 | |
| 3 | 200.4 | 38.1 | 19 | −6 | |
| 7 | 196.3 | 31.4 | 16 | −7 | |
| 14 | 174.6 | 24.4 | 14 | −18 | |
| 21 | 114.1 | 19.4 | 17 | −46 | |
| 28 | 124.3 | 18.7 | 15 | −41 | |
| 44 | 84.2 | 15.2 | 18 | −60 | |
| 117 | 89.7 | 17.0 | 19 | −58 | |
| 146 | 85.6 | 16.3 | 19 | −60 |
| Analyte | Retention Time | Transitions (m/z) | Collision Energy (eV) | Dwell Time (µs) |
|---|---|---|---|---|
| Promazine * | 11.12 | 283.1–238.2 | 15 | 50 |
| Chlorpromazine | 11.90 | 317.1–233.1 317.1–272.1 | 15 20 | 50 50 |
| Levomepromazine | 11.97 | 227.7–185.1 184.5–141.1 | 20 15 | 50 50 |
| Cyamemazine | 12.36 | 321.9–278.4 321.9–100.0 | 10 10 | 50 50 |
| Clozapine | 14.24 | 325.6–243.0 325.6 -270.3 | 10 20 | 50 50 |
| Haloperidol | 14.91 | 297.9–297.3 297.9–73.3 | 5 20 | 50 50 |
| Quetiapine | 18.90 | 209.3 -139.0 209.3–183.3 | 20 15 | 50 50 |
| Run Order | Temperature | Light | Preservative Concentration | Time |
|---|---|---|---|---|
| 1 | 4 °C | Presence | Low | 1 Day |
| 2 | 4 °C | Presence | Low | 7 Days |
| 3 | 4 °C | Presence | High | 7 Days |
| 4 | 4 °C | Absence | Low | 1 Day |
| 5 | 25 °C | Presence | High | 7 Days |
| 6 | 25 °C | Absence | Low | 7 Days |
| 7 | 25 °C | Absence | High | 7 Days |
| 8 | 25 °C | Absence | Low | 1 Day |
| 9 | 4 °C | Absence | High | 7 Days |
| 10 | 25 °C | Absence | High | 1 Day |
| 11 | 25 °C | Presence | Low | 7 Days |
| 12 | 4 °C | Absence | Low | 7 Days |
| 13 | 25 °C | Presence | High | 1 Day |
| 14 | 4 °C | Presence | High | 1 Day |
| 15 | 25 °C | Presence | Low | 1 Day |
| 16 | 4 °C | Absence | High | 1 Day |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gameiro, C.; Gonçalves, J.; Soares, S.; Rosado, T.; Araujo, A.R.T.S.; Passarinha, L.A.; Barroso, M.; Gallardo, E. Evaluation of Antipsychotic Drugs’ Stability in Oral Fluid Samples. Molecules 2023, 28, 2030. https://doi.org/10.3390/molecules28052030
Gameiro C, Gonçalves J, Soares S, Rosado T, Araujo ARTS, Passarinha LA, Barroso M, Gallardo E. Evaluation of Antipsychotic Drugs’ Stability in Oral Fluid Samples. Molecules. 2023; 28(5):2030. https://doi.org/10.3390/molecules28052030
Chicago/Turabian StyleGameiro, Carina, Joana Gonçalves, Sofia Soares, Tiago Rosado, André R. T. S. Araujo, Luís A. Passarinha, Mário Barroso, and Eugenia Gallardo. 2023. "Evaluation of Antipsychotic Drugs’ Stability in Oral Fluid Samples" Molecules 28, no. 5: 2030. https://doi.org/10.3390/molecules28052030
APA StyleGameiro, C., Gonçalves, J., Soares, S., Rosado, T., Araujo, A. R. T. S., Passarinha, L. A., Barroso, M., & Gallardo, E. (2023). Evaluation of Antipsychotic Drugs’ Stability in Oral Fluid Samples. Molecules, 28(5), 2030. https://doi.org/10.3390/molecules28052030










