Enhancing the Antimicrobial Effect of Ozone with Mentha piperita Essential Oil
Abstract
:1. Introduction
2. Results
2.1. The Obtaining and Characterisation of EOs
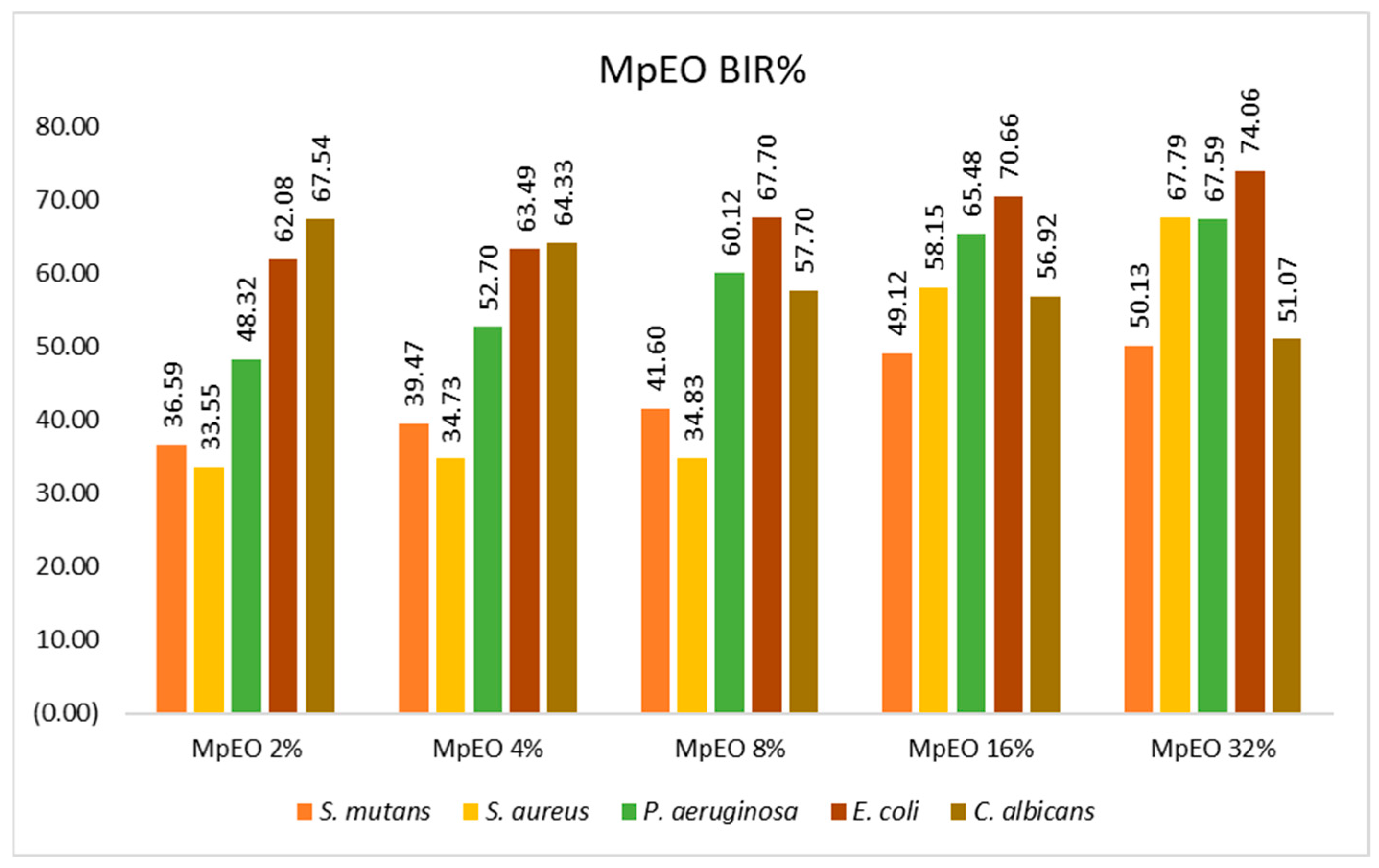
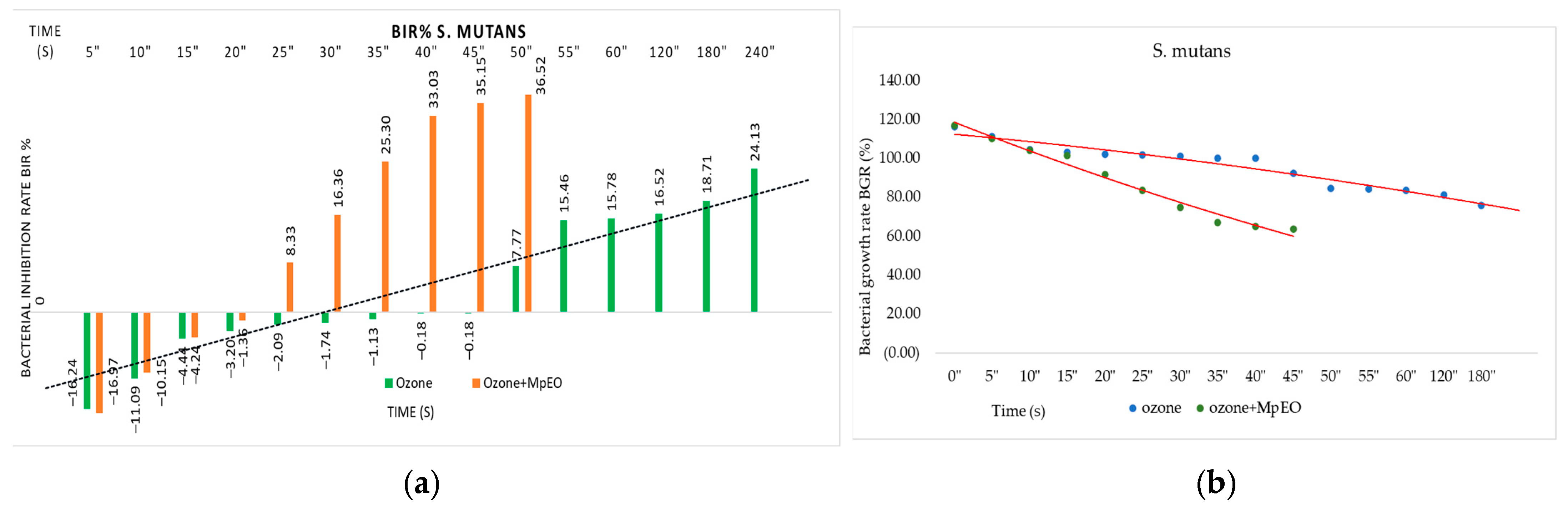
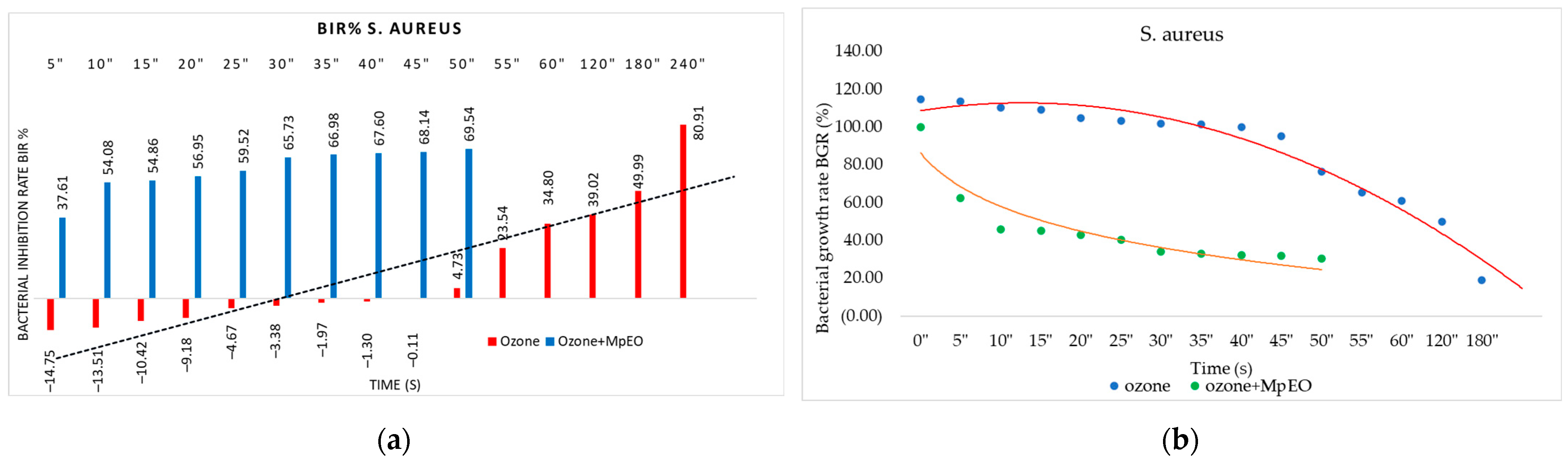
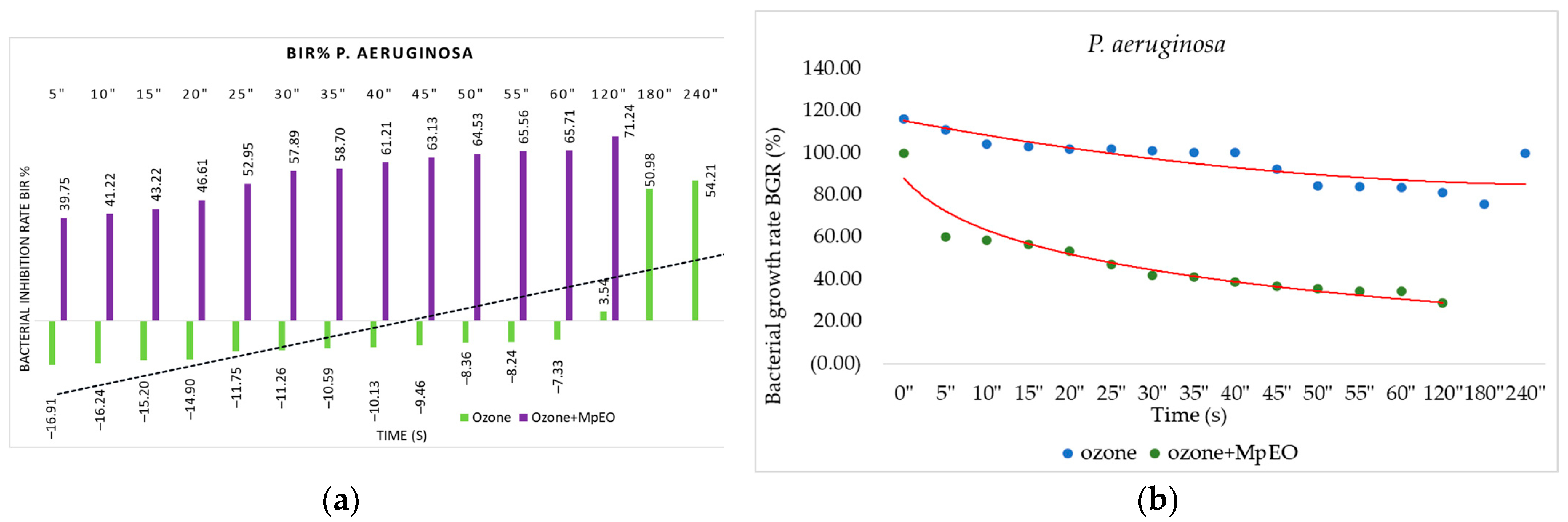
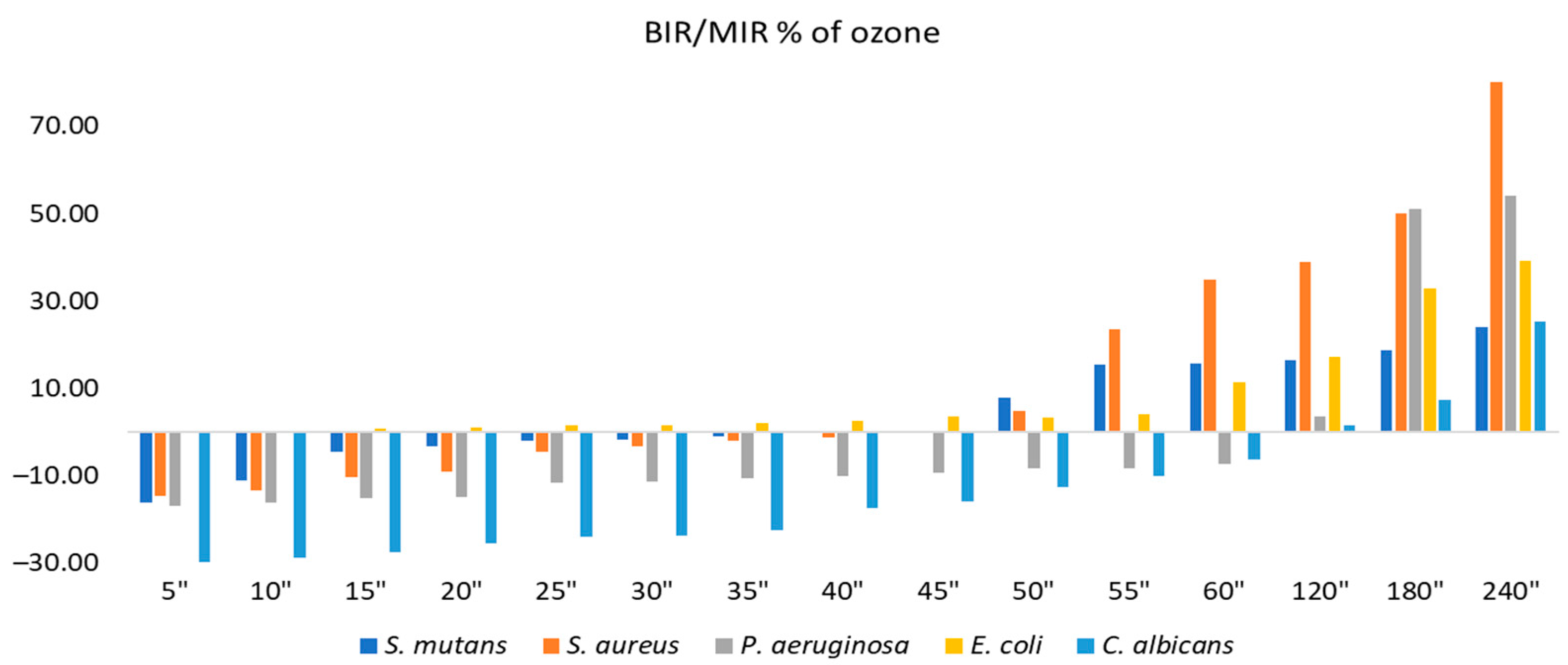
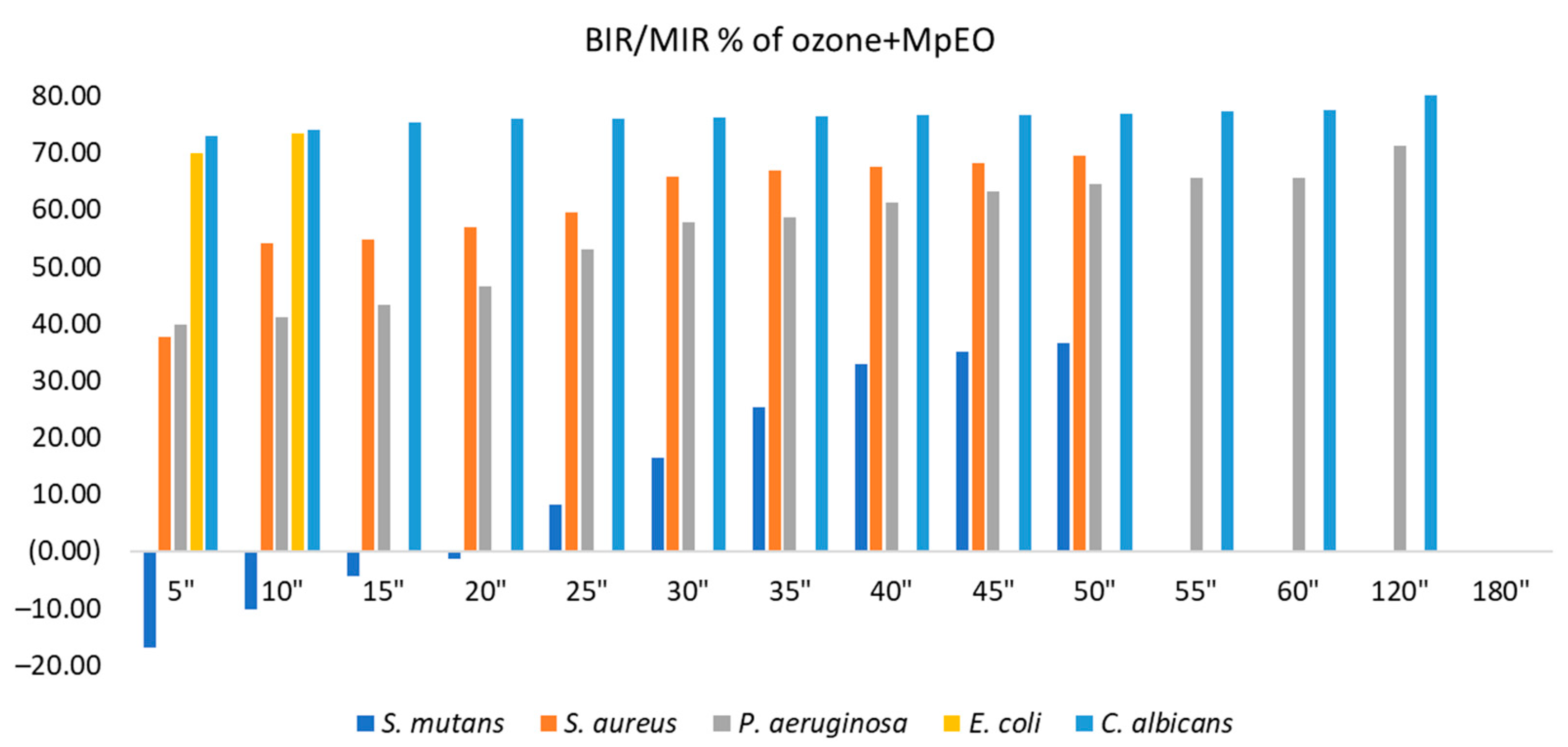
2.2. Evaluation of MpEO Antimicrobial Activity and Ozone Potential as Single and Enhanced with MpEO Antimicrobial Agent
3. Discussion
3.1. The Obtaining and Characterisation of EOs
3.2. The Ozone Potential as Single and Enhanced with MpEO Antimicrobial Agent
4. Materials and Methods
4.1. The Obtaining and Characterisation of EOs
4.2. Microbiological Assay
4.2.1. Microbial Strains
4.2.2. Microbial Culture Preparation and Essential Oil Efficacy Assessment
4.2.3. Ozonation Procedure
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elsayad, I.I. Chemical analysis and surface morphology of enamel following ozone application with different concentrations and exposure times. J. Adv. Res. 2011, 2, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Viebahn-Haensler, R.; Fernández, O.L. Ozone in Medicine. The Low-Dose Ozone Concept and Its Basic Biochemical Mechanisms of Action in Chronic Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 7890. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications for Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef] [PubMed]
- Kreulen, C.; Spijker, A.V.; Rodriguez, J.; Bronkhorst, E.; Creugers, N.; Bartlett, D. Systematic Review of the Prevalence of Tooth Wear in Children and Adolescents. Caries Res. 2010, 44, 151–159. [Google Scholar] [CrossRef]
- Wang, X.; Liao, D.; Ji, Q.-M.; Yang, Y.-H.; Li, M.-C.; Yi, X.-Y.; Li, C.; Chen, Y.; Tao, H.-B.; Zhai, W.-H. Analysis of Bactericidal Effect of Three Medical Ozonation Dosage Forms on Multidrug-Resistant Bacteria from Burn Patients. Infect. Drug Resist. 2022, 15, 1637–1643. [Google Scholar] [CrossRef]
- Gholamipourfard, K.; Salehi, M.; Banchio, E. Mentha piperita phytochemicals in agriculture, food industry and medicine: Features and applications. S. Afr. J. Bot. 2021, 141, 183–195. [Google Scholar] [CrossRef]
- Lazăr, R.N.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Pătruică, S. The Effect of the Use of Essential Oils in the Feed of Bee Families on Honey Chemical Composition and Antimicrobial Activity. Appl. Sci. 2022, 12, 1094. [Google Scholar] [CrossRef]
- Ashrafi, B.; Rashidipour, M.; Marzban, A.; Soroush, S.; Azadpour, M.; Delfani, S.; Ramak, P. Mentha piperita essential oils loaded in a chitosan nanogel with inhibitory effect on biofilm formation against S. mutans on the dental surface. Carbohydr. Polym. 2019, 212, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Prot. 2003, 22, 39–44. [Google Scholar] [CrossRef]
- Singh, R.; Shushni, M.A.; Belkheir, A. Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef] [Green Version]
- van Vuuren, S.; Suliman, S.; Viljoen, A. The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials. Lett. Appl. Microbiol. 2009, 48, 440–446. [Google Scholar] [CrossRef]
- Shalayel, M.H.F.; Asaad, A.M.; Qureshi, M.A.; Elhussein, A.B. Anti-bacterial activity of peppermint (Mentha piperita) extracts against some emerging multi-drug resistant human bacterial pathogens. J. Herb. Med. 2017, 7, 27–30. [Google Scholar] [CrossRef]
- Mane, M.B.; Bhandari, V.M.; Ranade, V.V. Safe water and technology initiative for water disinfection: Application of natural plant derived materials. J. Water Process. Eng. 2021, 43, 102280. [Google Scholar] [CrossRef]
- Mane, M.B.; Bhandari, V.M.; Balapure, K.; Ranade, V.V. Destroying antimicrobial resistant bacteria (AMR) and difficult, opportunistic pathogen using cavitation and natural oils/plant extract. Ultrason. Sonochem. 2020, 69, 105272. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, S.; Falcón, L.; Maqueira, Y. Therapeutic efficacy of topical OLEOZON® in patients suffering from onychomycosis: OLEOZON® against Onychomycosis. Mycoses 2011, 54, e272–e277. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Zanardi, I.; Lim, Y.; Belmonte, G.; Miracco, C.; Sticozzi, C.; Bocci, V.; Travagli, V. Ozonated oils as functional dermatological matrices: Effects on the wound healing process using SKH1 mice. Int. J. Pharm. 2013, 458, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Travagli, V.; Zanardi, I.; Valacchi, G.; Bocci, V. Ozone and Ozonated Oils in Skin Diseases: A Review. Mediat. Inflamm. 2010, 2010, 610418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagayoshi, M.; Fukuizumi, T.; Kitamura, C.; Yano, J.; Terashita, M.; Nishihara, T. Efficacy of ozone on survival and permeability of oral microorganisms. Oral Microbiol. Immunol. 2004, 19, 240–246. [Google Scholar] [CrossRef]
- Ghobashy, S.; El-Tokhey, H. In vivo study of the effectiveness of ozonized olive oil gel on inhibiting enamel demineralization during orthodontic treatment. Egypt. Orthod. J. 2012, 41, 93–113. [Google Scholar] [CrossRef] [Green Version]
- El Hadary, A.A.; Yassin, H.H.; Mekhemer, S.T.; Holmes, J.C.; Grootveld, M. Evaluation of the Effect of Ozonated Plant Oils on the Quality of Osseointegration of Dental Implants Under the Influence of Cyclosporin A: An In Vivo Study. J. Oral Implant. 2011, 37, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.; Arora, N.; Puri, G.; Aravinda, K.; Dixit, A.; Jatti, D. Efficacy of ozonized olive oil in the management of oral lesions and conditions: A clinical trial. Contemp. Clin. Dent. 2016, 7, 51. [Google Scholar] [CrossRef]
- Pietrocola, G.; Ceci, M.; Preda, F.; Poggio, C.; Colombo, M. Evaluation of the antibacterial activity of a new ozonized olive oil against oral and periodontal pathogens. J. Clin. Exp. Dent. 2018, 10, e1103–e1108. [Google Scholar] [CrossRef]
- Ugazio, E.; Tullio, V.; Binello, A.; Tagliapietra, S.; Dosio, F. Ozonated Oils as Antimicrobial Systems in Topical Applications. Their Characterization, Current Applications, and Advances in Improved Delivery Techniques. Molecules 2020, 25, 334. [Google Scholar] [CrossRef] [Green Version]
- Sechi, L.; Lezcano, I.; Nunez, N.; Espim, M.; Dupre, I.; Pinna, A.; Molicotti, P.; Fadda, G.; Zanetti, S. Antibacterial activity of ozonized sunflower oil (Oleozon). J. Appl. Microbiol. 2001, 90, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Kazi, M.; Chatzopoulou, P.; Christos, L. Effect of Ozonation on the Essential Oil Composition of Dried Aromatic Plants. In Proceedings of the 8th International Conference on Information and Communication Technologies in Agriculture, Food and Environment, Chania, Greece, 21–24 September 2017. [Google Scholar]
- Karlsson, M.F.; Birgersson, G.; Prado, A.M.C.; Bosa, F.; Bengtsson, M.; Witzgall, P. Plant Odor Analysis of Potato: Response of Guatemalan Moth to Above- and Belowground Potato Volatiles. J. Agric. Food Chem. 2009, 57, 5903–5909. [Google Scholar] [CrossRef]
- Sumalan, R.M.; Kuganov, R.; Obistioiu, D.; Popescu, I.; Radulov, I.; Alexa, E.; Negrea, M.; Salimzoda, A.F.; Sumalan, R.L.; Cocan, I. Assessment of Mint, Basil, and Lavender Essential Oil Vapor-Phase in Antifungal Protection and Lemon Fruit Quality. Molecules 2020, 25, 1831. [Google Scholar] [CrossRef] [PubMed]
- Muntean, D.; Licker, M.; Alexa, E.; Popescu, I.; Jianu, C.; Buda, V.; Dehelean, C.A.; Ghiulai, R.; Horhat, F.; Horhat, D.; et al. Evaluation of essential oil obtained from Mentha × piperita L. against multidrug-resistant strains. Infect. Drug Resist. 2019, 12, 2905–2914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Liang, C.; Fang, H.; Qi, X.; Li, W.; Shang, Q. Variation of trichome morphology and essential oil composition of seven Mentha species. Biochem. Syst. Ecol. 2018, 79, 30–36. [Google Scholar] [CrossRef]
- Desam, N.R.; Al-Rajab, A.J.; Sharma, M.; Mylabathula, M.M.; Gowkanapalli, R.R.; Albratty, M. Chemical constituents, in vitro antibacterial and antifungal activity of Mentha × Piperita L. (peppermint) essential oils. J. King Saud Univ.-Sci. 2019, 31, 528–533. [Google Scholar] [CrossRef]
- Tomescu, A.; Sumalan, R.-M.; Pop, G.; Alexa, E.; Poiana, M.-A.; Copolovici, D.-M.; Sorina, C.; Stroe, M.; Negrea, M.; Galuscan, A. Chemical Composition and Protective Antifugal Activity of Mentha piperita L. and Salvia Officinalis L. Essential Oils Against Fusarium Graminearum Spp. Rev. Chim. 2015, 66, 1027–1030. [Google Scholar]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Tosi, G.; Massi, P.; Pistelli, L.; Mancianti, F. In Vitro Antimicrobial Activity of Essential Oils against Salmonella enterica Serotypes Enteritidis and Typhimurium Strains Isolated from Poultry. Molecules 2019, 24, 900. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Wang, Y.; Wu, K.; Yu, G.; Liu, C.; Su, C.; Yi, F. Study on the Effect of Mentha × piperita L. Essential Oil on Electroencephalography upon Stimulation with Different Visual Effects. Molecules 2022, 27, 4059. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Najar, B.; Bertelloni, F.; Pistelli, L.; Mancianti, F.; Nardoni, S. Chemical Composition and In Vitro Antimicrobial Efficacy of Sixteen Essential Oils against Escherichia coli and Aspergillus fumigatus Isolated from Poultry. Veter.-Sci. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalemba, D.; Synowiec, A. Agrobiological Interactions of Essential Oils of Two Menthol Mints: Mentha piperita and Mentha arvensis. Molecules 2019, 25, 59. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, G.; Ricevuti, G.; Galoforo, A.; Franzini, M. Microbiological aspects of ozone: Bactericidal activity and antibiotic/antimicrobial resistance in bacterial strains treated with ozone. Ozone Ther. 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, S.R.; Amaechi, B.T. Ozone: A paradigm shift in dental therapy. J. Glob. Oral Health 2019, 2, 68–77. [Google Scholar] [CrossRef]
- Prebeg, D.; Katunarić, M.; Budimir, A.; Pavelić, B.; Šegović, S.; Anić, I. Antimicrobial Effect of Ozone Made by KP Syringe of High-Frequency Ozone Generator. Acta Stomatol. Croat. 2016, 50, 134–142. [Google Scholar] [CrossRef]
- Wilczyńska-Borawska, M.; Leszczyńska, K.; Nowosielski, C.; Stokowska, W. Ozone in Dentistry: Microbiological Effects of Gas Action Depending on the Method and the Time of Application Using the Ozonytron Device. Experimental Study. Ann. Acad. Med. Stetin. 2011, 57, 99–103. [Google Scholar]
- Baysan, A.; Whiley, R.; Lynch, E. Antimicrobial Effect of a Novel Ozone–Generating Device on Micro–Organisms Associated with Primary Root Carious Lesions in vitro. Caries Res. 2000, 34, 498–501. [Google Scholar] [CrossRef]
- Eick, S.; Tigan, M.; Sculean, A. Effect of ozone on periodontopathogenic species—An in vitro study. Clin. Oral Investig. 2012, 16, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Polydorou, O.; Halili, A.; Wittmer, A.; Pelz, K.; Hahn, P. The antibacterial effect of gas ozone after 2 months of in vitro evaluation. Clin. Oral Investig. 2012, 16, 545–550. [Google Scholar] [CrossRef]
- Johansson, E.; Claesson, R.; van Dijken, J. Antibacterial effect of ozone on cariogenic bacterial species. J. Dent. 2009, 37, 449–453. [Google Scholar] [CrossRef]
- Huth, K.C.; Quirling, M.; Maier, S.; Kamereck, K.; AlKhayer, M.; Paschos, E.; Welsch, U.; Miethke, T.; Brand, K.; Hickel, R. Effectiveness of ozone against endodontopathogenic microorganisms in a root canal biofilm model. Int. Endod. J. 2009, 42, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Hauser-Gerspach, I.; Pfäffli-Savtchenko, V.; Dähnhardt, J.E.; Meyer, J.; Lussi, A. Comparison of the immediate effects of gaseous ozone and chlorhexidine gel on bacteria in cavitated carious lesions in children in vivo. Clin. Oral Investig. 2009, 13, 287–291. [Google Scholar] [CrossRef]
- Makeeva, I.M.; Turkina, A.Y.; Margaryan, E.G.; Paramonov, Y.O.; Polyakova, M.A. Assessment of antibacterial efficacy of ozone therapy in treatment of caries at the white spot stage. Stomatologiya 2017, 96, 7–10. [Google Scholar] [CrossRef]
- Song, M.; Zeng, Q.; Xiang, Y.; Gao, L.; Huang, J.; Huang, J.; Wu, K.; Lu, J. The antibacterial effect of topical ozone on the treatment of MRSA skin infection. Mol. Med. Rep. 2017, 17, 2449–2455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M. Ozone: An Emerging Prospect in Dentistry. Indian J. Dent. Sci. 2012, 4, 47–50. [Google Scholar]
- Montevecchi, M.; Dorigo, A.; Cricca, M.; Checchi, L. Comparison of the Antibacterial Activity of an Ozonated Oil with Chlorhexidine Digluconate and Povidone-Iodine. A Disk Diffusion Test. N. Microbiol. 2013, 36, 289–302. [Google Scholar]
- Epelle, E.I.; Emmerson, A.; Nekrasova, M.; Macfarlane, A.; Cusack, M.; Burns, A.; Mackay, W.; Yaseen, M. Microbial Inactivation: Gaseous or Aqueous Ozonation? Ind. Eng. Chem. Res. 2022, 61, 9600–9610. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.G. Keine Resistenzbildung Der Mikroflora, Keine Allergischen Reaktionen. Zahnarzt Woche 2004, 19, 24. [Google Scholar]
- Grandi, G.; Cavallo, R.; Zanotto, E.; Cipriani, R.; Panico, C.; Protti, R.; Scapagnini, G.; Davinelli, S.; Costagliola, C. In vitro antimicrobial activity of ozonated oil in liposome eyedrop against multidrug-resistant bacteria. Open Med. 2022, 17, 1057–1063. [Google Scholar] [CrossRef]
- Saravani, S.; Ghaffari, M.; Valizadeh, M.; Ali-Malayeri, F.; Biabangard, A. Antimicrobial Activity of Mentha piperita, Rosmarinus officinalis, and Withania somnifera Prepared by Ultrasound against Escherichia coli Isolated from Poultry Stool. Gene Cell Tissue 2021, 9, e109104. [Google Scholar] [CrossRef]
- Heydari, M.; Zanfardino, A.; Taleei, A.; Bushehri, A.A.S.; Hadian, J.; Maresca, V.; Sorbo, S.; Di Napoli, M.; Varcamonti, M.; Basile, A.; et al. Effect of Heat Stress on Yield, Monoterpene Content and Antibacterial Activity of Essential Oils of Mentha × piperita var. Mitcham and Mentha arvensis var piperascens. Molecules 2018, 23, 1903. [Google Scholar] [CrossRef] [Green Version]
- Camele, I.; Gruľová, D.; Elshafie, H. Chemical Composition and Antimicrobial Properties of Mentha × piperita cv. ‘Kristinka’ Essential Oil. Plants 2021, 10, 1567. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [Green Version]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Hulea, A.; Obiștioiu, D.; Cocan, I.; Alexa, E.; Negrea, M.; Neacșu, A.-G.; Hulea, C.; Pascu, C.; Costinar, L.; Iancu, I.; et al. Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential. Antibiotics 2022, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Obistioiu, D.; Cocan, I.; Tîrziu, E.; Herman, V.; Negrea, M.; Cucerzan, A.; Neacsu, A.-G.; Cozma, A.; Nichita, I.; Hulea, A.; et al. Phytochemical Profile and Microbiological Activity of Some Plants Belonging to the Fabaceae Family. Antibiotics 2021, 10, 662. [Google Scholar] [CrossRef]
- CLSI Standard M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Noites, R.; Pina-Vaz, C.; Rocha, R.; Carvalho, M.F.; Gonçalves, A.; Pina-Vaz, I. Synergistic Antimicrobial Action of Chlorhexidine and Ozone in Endodontic Treatment. BioMed. Res. Int. 2014, 2014, 592423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baysan, A.; Lynch, E. Effect of ozone on the oral microbiota and clinical severity of primary root caries. Am. J. Dent. 2004, 17, 56–60. [Google Scholar]


| Compounds | Type | LRI a | % of Total Compounds |
|---|---|---|---|
| Linalool | MO | 1533 | 0.752 |
| α-pinene | MH | 1021 | 1.476 |
| β-pinene | MH | 1106 | 1.179 |
| Sabinene | MH | 1135 | 0.508 |
| Limonene | MH | 1196 | 3.137 |
| β-trans-ocimene | MH | 1199 | 0.233 |
| γ-terpinene | MH | 1202 | 0.399 |
| Eucalyptol | MO | 1204 | 5.639 |
| p-cymene | MH | 1284 | 0.454 |
| Menthofurane | MO | 1474 | 2.960 |
| Linalol acetate | MO | 1541 | 0.558 |
| Menthyl-acetate iso | MO | 1546 | 0.375 |
| Menthyl acetateone racemic | MO | 1548 | 10.322 |
| p-menthan-3-one | MO | 1552 | 23.643 |
| Menthol, acetate, iso- | MO | 1562 | 1.882 |
| Isomenthone | MO | 1582 | 4.046 |
| Terpinen-4-ol | MO | 1593 | 1.191 |
| Menthol | MO | 1634 | 32.648 |
| Germacrene D | SH | 1708 | 5.410 |
| γ-Elemene | SH | 1717 | 0.244 |
| p-menth-1-en-8-ol | MO | 1724 | 0.433 |
| Pulegone | MO | 1730 | 1.636 |
| Piperitone | MO | 1750 | 0.521 |
| Caryophyllene oxide | SO | 1889 | 0.355 |
| Total of compounds | 100.000 | ||
| Monoterpene hydrocarbonates | MH | 7.386 | |
| Monoterpene oxygenate | MO | 86.606 | |
| Sesquiterpene hydrocarbonates | SH | 5.653 | |
| Sesquiterpene oxygenates | SO | 0.355 |
| MIC Values of Ozone | MIC Values of Ozone+MpEO | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. mutans | S. aureus | P. aeruginosa | E. coli | C. albicans | S. mutans | S. aureus | P. aeruginosa | E. coli | C. albicans | ||
| 5″ | 5″ | 5″ | 5″ | 5″ | 5″ | 5″ | 5″ | 5″ | 5″ | ||
| 10″ | 10″ | 10″ | 10″ | 10″ | 10″ | 10″ | 10″ | 10″ | 10″ | ||
| 15″ | 15″ | 15″ | 15″ | 15″ | 15″ | 15″ | 15″ | 15″ | 15″ | ||
| 20″ | 20″ | 20″ | 20″ | 20″ | 20″ | 20″ | 20″ | 20″ | 20″ | ||
| 25″ | 25″ | 25″ | 25″ | 25″ | 25″ | 25″ | 25″ | 25″ | 25″ | ||
| 30″ | 30″ | 30″ | 30″ | 30″ | 30″ | 30″ | 30″ | 30″ | 30″ | ||
| 35″ | 35″ | 35″ | 35″ | 35″ | 35″ | 35″ | 35″ | 35″ | 35″ | ||
| 40″ | 40″ | 40″ | 40″ | 40″ | 40″ | 40″ | 40″ | 40″ | 40″ | ||
| 45″ | 45″ | 45″ | 45″ | 45″ | 45″ | 45″ | 45″ | 45″ | 45″ | ||
| 50″ | 50″ | 50″ | 50″ | 50″ | 50″ | 50″ | 50″ | 50″ | 50″ | ||
| 55″ | 55″ | 55″ | 55″ | 55″ | 55″ | 55″ | 55″ | 55″ | 55″ | ||
| 60″ | 60″ | 60″ | 60″ | 60″ | 60″ | 60″ | 60″ | 60″ | 60″ | ||
| 120″ | 120″ | 120″ | 120″ | 120″ | 120″ | 120″ | 120″ | 120″ | 120″ | ||
| 180″ | 180″ | 180″ | 180″ | 180″ | 180″ | 180″ | 180″ | 180″ | 180″ | ||
| 240″ | 240″ | 240″ | 240″ | 240″ | 240″ | 240″ | 240″ | 240″ | 240″ | ||
| S. mutans | S. aureus | P. aeruginosa | E. coli | C. albicans | |||||||
| MpEO% | 2 | 2 | 2 | 2 | 2 | ||||||
| MpEO% | 4 | 4 | 4 | 4 | 4 | ||||||
| MpEO% | 8 | 8 | 8 | 8 | 8 | ||||||
| MpEO% | 16 | 16 | 16 | 16 | 16 | ||||||
| MpEO% | 32 | 32 | 32 | 32 | 32 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floare, A.-D.; Dumitrescu, R.; Alexa, V.T.; Balean, O.; Szuhanek, C.; Obistioiu, D.; Cocan, I.; Neacsu, A.-G.; Popescu, I.; Fratila, A.D.; et al. Enhancing the Antimicrobial Effect of Ozone with Mentha piperita Essential Oil. Molecules 2023, 28, 2032. https://doi.org/10.3390/molecules28052032
Floare A-D, Dumitrescu R, Alexa VT, Balean O, Szuhanek C, Obistioiu D, Cocan I, Neacsu A-G, Popescu I, Fratila AD, et al. Enhancing the Antimicrobial Effect of Ozone with Mentha piperita Essential Oil. Molecules. 2023; 28(5):2032. https://doi.org/10.3390/molecules28052032
Chicago/Turabian StyleFloare, Alin-Daniel, Ramona Dumitrescu, Vlad Tiberiu Alexa, Octavia Balean, Camelia Szuhanek, Diana Obistioiu, Ileana Cocan, Alina-Georgeta Neacsu, Iuliana Popescu, Aurora Doris Fratila, and et al. 2023. "Enhancing the Antimicrobial Effect of Ozone with Mentha piperita Essential Oil" Molecules 28, no. 5: 2032. https://doi.org/10.3390/molecules28052032
APA StyleFloare, A.-D., Dumitrescu, R., Alexa, V. T., Balean, O., Szuhanek, C., Obistioiu, D., Cocan, I., Neacsu, A.-G., Popescu, I., Fratila, A. D., & Galuscan, A. (2023). Enhancing the Antimicrobial Effect of Ozone with Mentha piperita Essential Oil. Molecules, 28(5), 2032. https://doi.org/10.3390/molecules28052032











