Hematite: A Good Catalyst for the Thermal Decomposition of Energetic Materials and the Application in Nano-Thermite
Abstract
:1. Introduction
2. Preparation of Hematite with Different Morphology and Their Catalytic Effect on EMs
2.1. Preparation of Polyhedral Hematite and the Effect on EMs
2.2. Preparation of Granular Hematite and the Effect on EMs
2.3. Preparation of Rod-Shaped Hematite and the Effect on EMs
2.4. Preparation of Hematite with Other Morphologies and the Effect on EMs
3. The Preparation of Ferrite and the Application on the EMs
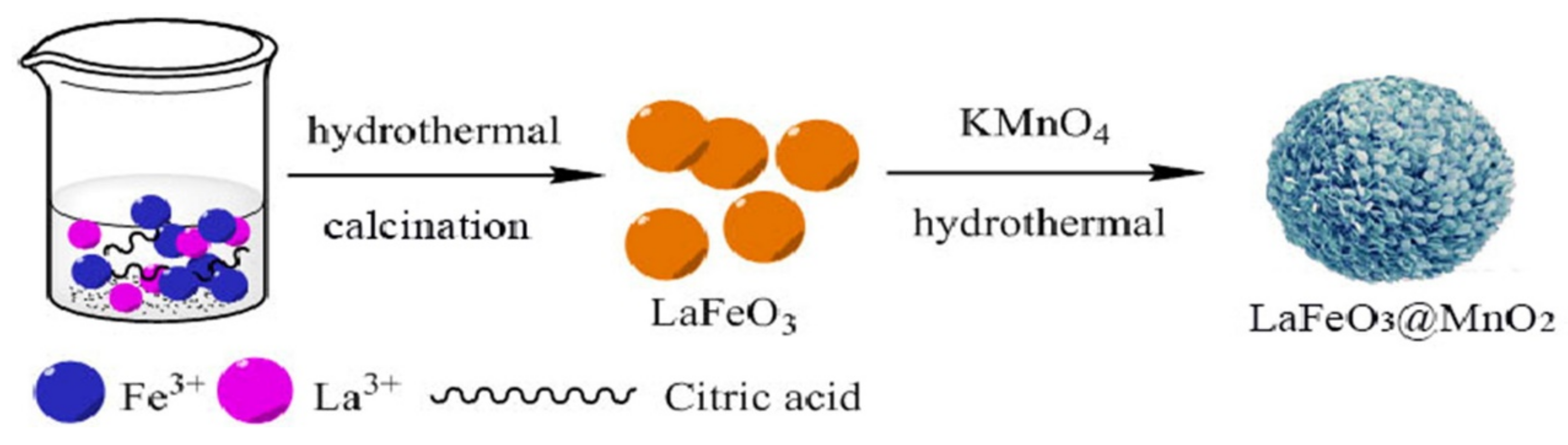
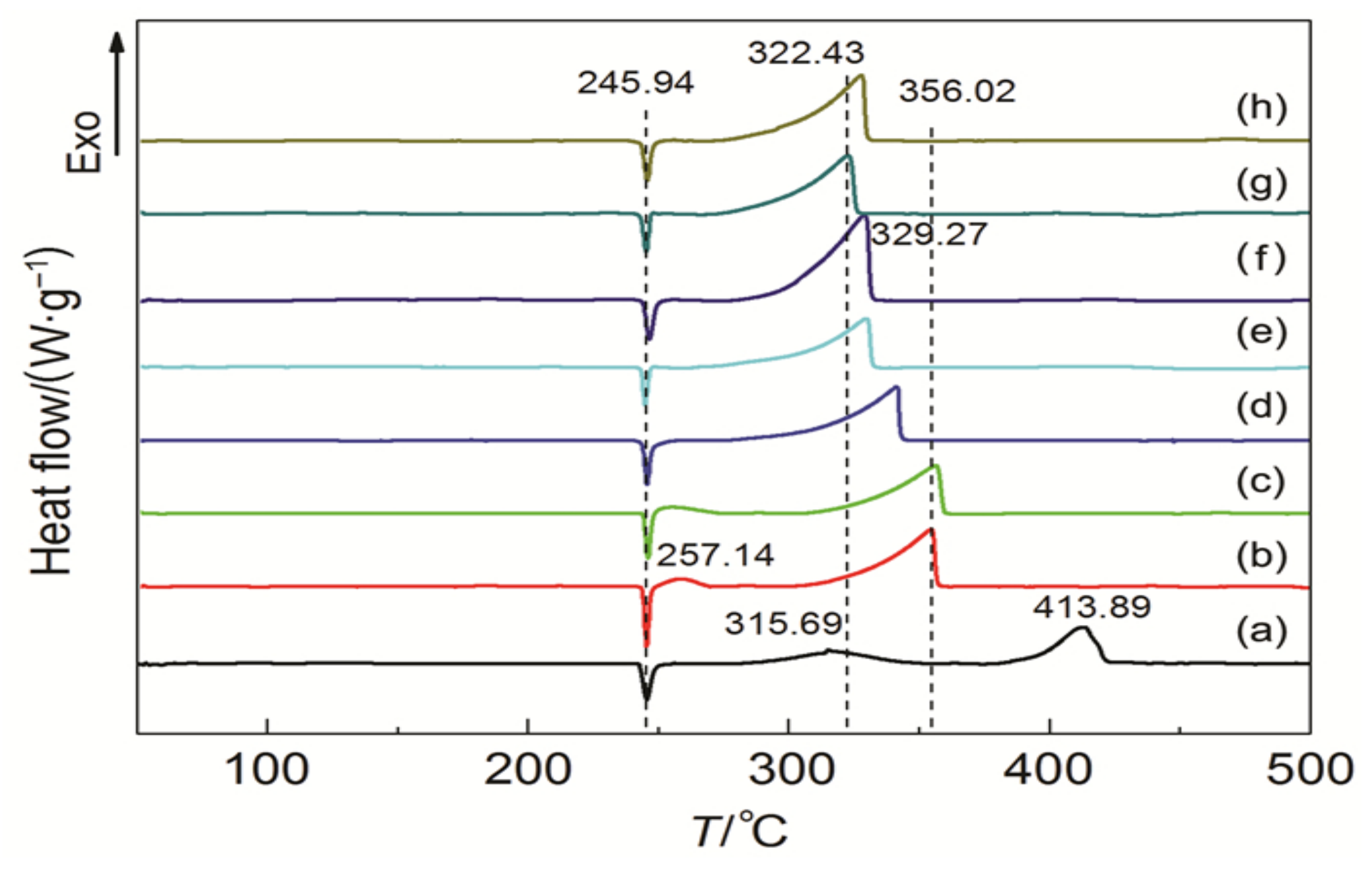
3.1. Perovskite Ferrite
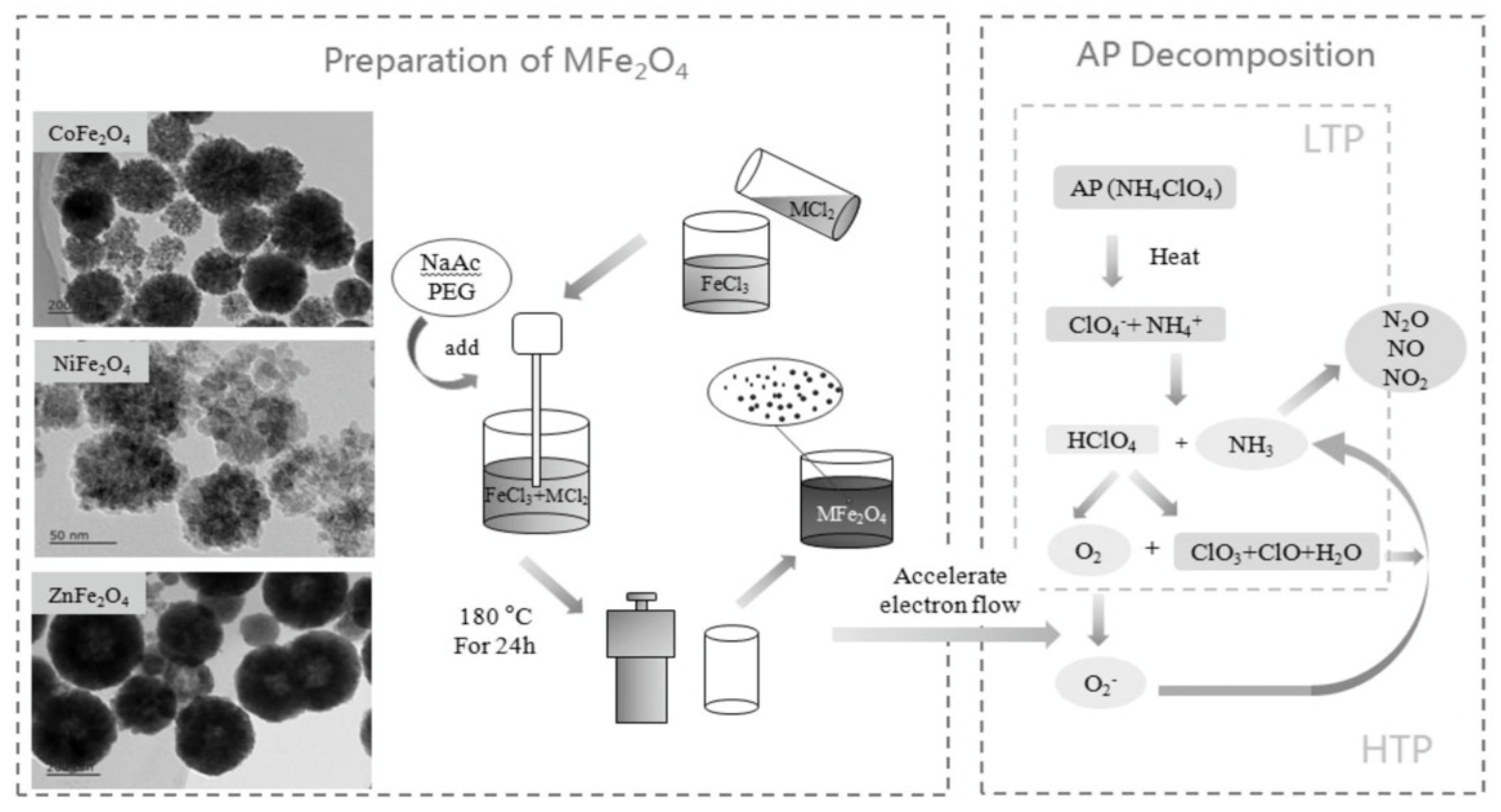
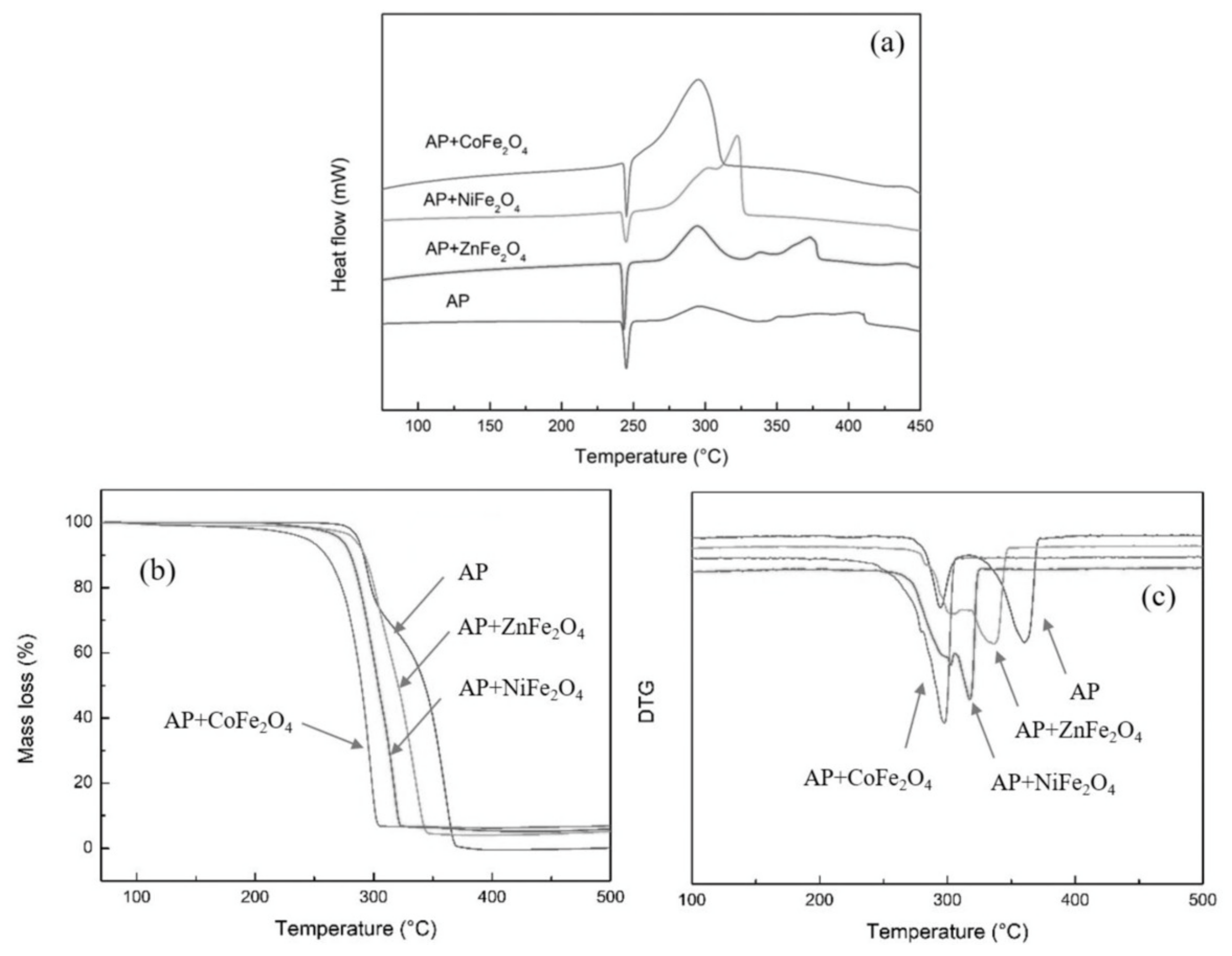
3.2. Spinel Ferrite
| Materials | Samples | Preparation Method | Mass Ratios | Tp | ΔH | E | Refs. |
|---|---|---|---|---|---|---|---|
| ABO3 | AP | solvothermal and post-heat treatment | 0 | 403.7 | - | 139.1 | [59] |
| LaFeO3/AP | 20% | 343.2 | - | 126.5 | |||
| LaFeO3@MnO2/AP | 20% | 436.4 | - | 110.9 | |||
| CL-20 | 0 | 252.1 | - | - | |||
| LaFeO3/CL-20 | 20% | 247.4 | - | - | |||
| LaFeO3@MnO2/CL-20 | 20% | 245.9 | - | - | |||
| HMX | 0 | 283.0 | - | - | |||
| LaFeO3/HMX | 20% | 279.9 | - | - | |||
| LaFeO3@MnO2/HMX | 20% | 273.4 | - | - | |||
| XY2O4 | AP | colloidal crystal template | 0 | 413.8 | 488.2 | - | [73] |
| Nanoporous | CoFe2O4/AP | 1% | 354.5 | 950.5 | - | ||
| Nanoporous | CoFe2O4/AP | 2% | 356.0 | 1120.8 | - | ||
| Nanoporous | CoFe2O4/AP | 3% | 341.0 | 997.2 | - | ||
| Nanoporous | CoFe2O4/AP | 4% | 328.7 | 1109.6 | - | ||
| Nanoporous | CoFe2O4/AP | 5% | 329.3 | 1114.5 | 74.0 | ||
| Nanosphere | CoFe2O4/AP | 5% | 348.2 | - | 94.6 | ||
| Nanoporous | CoFe2O4/AP | 6% | 322.4 | 971.1 | - | ||
| Nanoporous | CoFe2O4/AP | 7% | 327.5 | 1113.2 | - | ||
| XY2O4 | AP | solvothermal | 0 | 404.3 | - | 162.3 | [79] |
| NiFe2O4/AP | 10% | 322.3 | - | - | |||
| ZnFe2O4/AP | 10% | 373.4 | - | - | |||
| CoFe2O4/AP | 10% | 295.3 | - | 124.9 | |||
| Ce doped NiFe2O4 | sol-gel combustion synthesis | 0 | 432.7 | 857.0 | - | [82] | |
| NiFe2O4 | NiFe2O4/AP | 5% | 413.2 | 1144.0 | - | ||
| NiFe2Ce0.03O4 | NiFe2Ce0.03O4/AP | 5% | 407.9 | 1147.0 | - | ||
| NiFe2Ce0.06O4 | NiFe2Ce0.06O4/AP | 5% | 406.8 | 1190.0 | - | ||
| NiFe2Ce0.09O4 | NiFe2Ce0.09O4/AP | 5% | 374.9 | 1259.0 | - |
4. The Preparation of Carbon Composites and Their Application on the EMs
4.1. Hematite/Graphene Composites
4.2. Ferrite/Graphene Composites
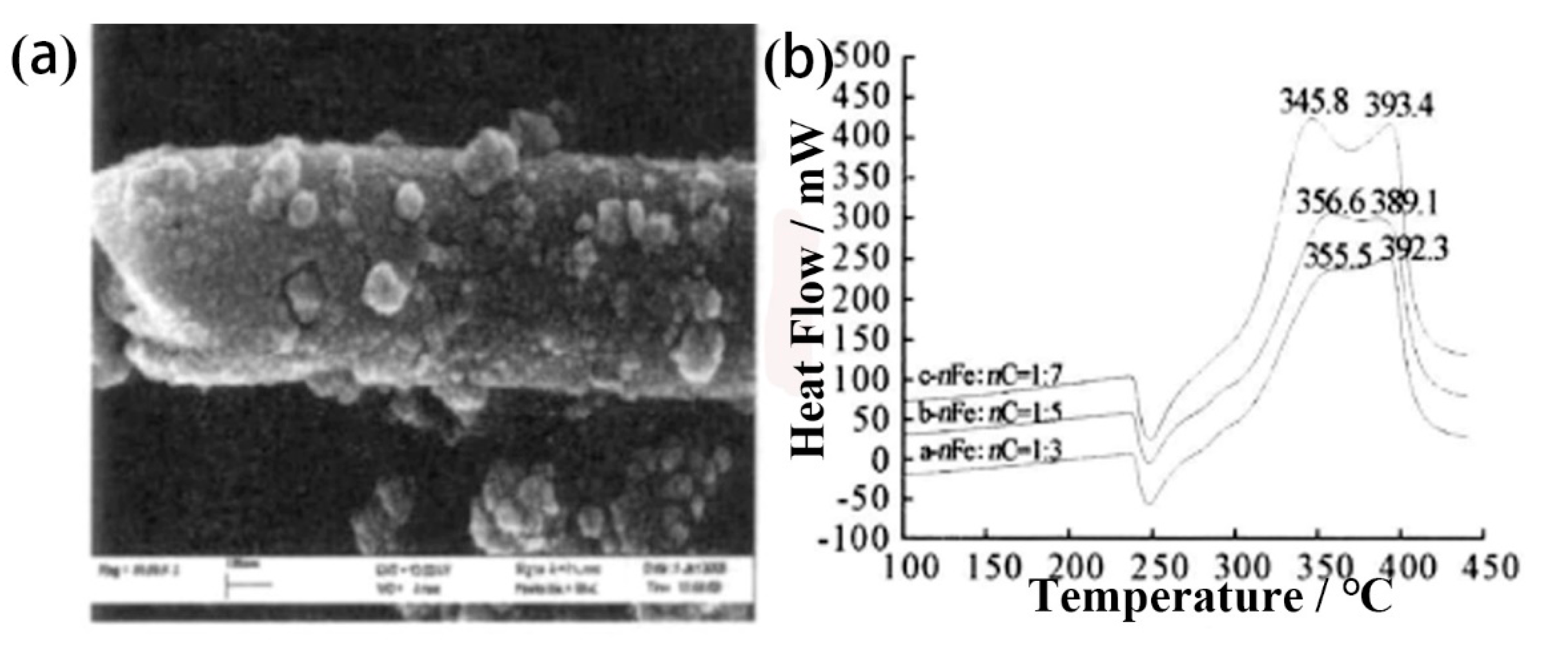
4.3. Carbon Nanotubes
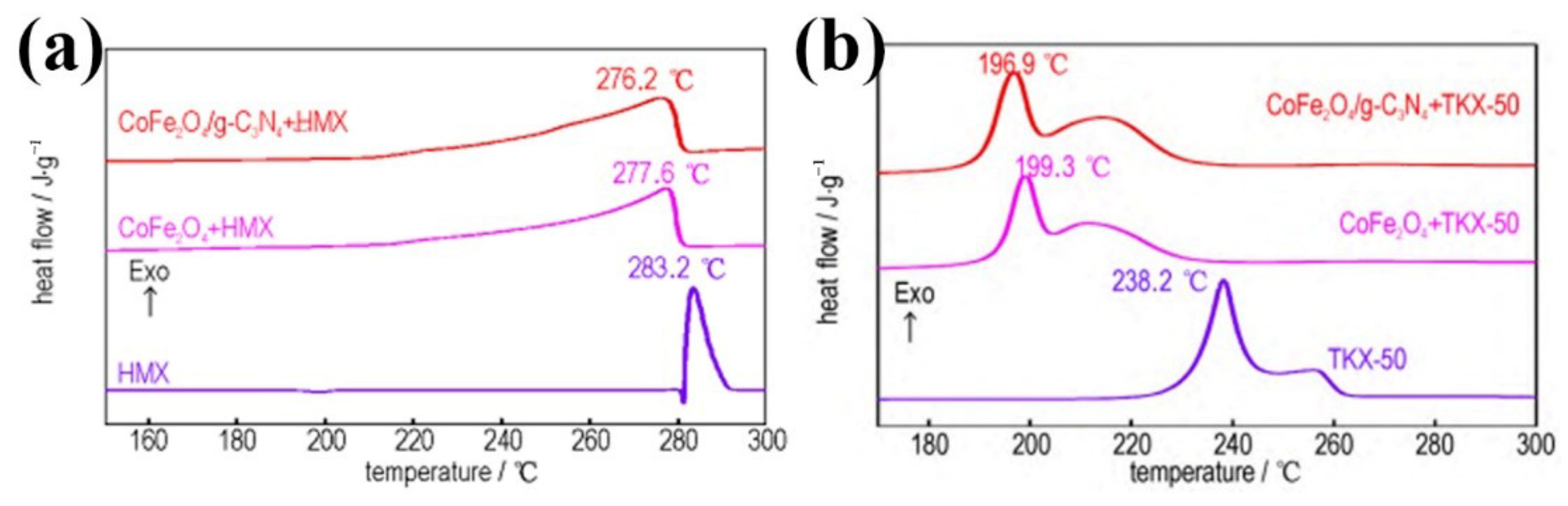
4.4. Graphitic Carbon Nitride
5. The Nano-Thermites and Their Application
5.1. Influence of Fuel Additives on Nano-Thermite
5.2. Influence of Oxidant and Structure on Nano-Thermite
5.3. Nano-Thermites with Different Morphologies and Their Catalytic Effects on EMs
6. Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Li, F.; Ni, B.B.; Xu, J.; Fu, Z.P.; Lu, Y.L. Enhanced visible photocatalytic activity of hybrid Pt/alpha-Fe2O3 nanorods. RSC Adv. 2012, 2, 10057–10063. [Google Scholar] [CrossRef]
- Eggleston, C.M. Toward new uses for hematite. Science 2008, 320, 184–185. [Google Scholar] [CrossRef]
- Sakurai, S.; Namai, A.; Hashimoto, K.; Ohkoshi, S. First Observation of Phase Transformation of All Four Fe2O3 Phases (γ → ε → β → α-Phase). J. Am. Chem. Soc. 2009, 131, 18299–18303. [Google Scholar] [CrossRef]
- Ando, M.; Kadono, K.; Haruta, M.; Sakaguchi, T.; Miya, M. Large third-order optical nonlinearities in transition-metal oxides. Nature 1995, 374, 625–627. [Google Scholar] [CrossRef]
- Sun, B.; Horvat, J.; Kim, H.S.; Kim, W.S.; Ahn, J.; Wang, G.X. Synthesis of Mesoporous alpha-Fe2O3 Nanostructures for Highly Sensitive Gas Sensors and High Capacity Anode Materials in Lithium Ion Batteries. J. Phys. Chem. C 2010, 114, 18753–18761. [Google Scholar] [CrossRef]
- Reddy, M.V.; Yu, T.; Sow, C.H.; Shen, Z.X.; Lim, C.T.; Rao, G.V.S.; Chowdari, B.V.R. α-Fe2O3 nanoflakes as an anode material for Li-ion batteries. Adv. Funct. Mater. 2007, 17, 2792–2799. [Google Scholar] [CrossRef]
- Zhong, L.S.; Hu, J.S.; Liang, H.P.; Cao, A.M.; Song, W.G.; Wan, L.J. Self-assembled 3D flowerlike iron oxide nanostructures and their application in water treatment. Adv. Mater. 2006, 18, 2426–2431. [Google Scholar] [CrossRef]
- Zhao, N.N.; He, C.C.; Liu, J.B.; Gong, H.J.; An, T.; Xu, H.X.; Zhao, F.Q.; Hu, R.Z.; Ma, H.X.; Zhang, J.Z. Dependence of catalytic properties of Al/Fe2O3 thermites on morphology of Fe2O3 particles in combustion reactions. J. Solid State Chem. 2014, 219, 67–73. [Google Scholar] [CrossRef]
- Ling, Y.C.; Wang, G.M.; Reddy, J.; Wang, C.C.; Zhang, J.Z.; Li, Y. The Influence of Oxygen Content on the Thermal Activation of Hematite Nanowires. Angew. Chem. Int. Ed. Engl. 2012, 51, 4074–4079. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.N. Research on the Preparation, Properties and Application of Micro-Nano Metal Oxides and Thermite in Composite Propellants. Ph.D. Thesis, Northwest University, Xi’an, China, 2015. [Google Scholar]
- Larcher, D.; Bonnin, D.; Cortes, R.; Rivals, I.; Personnaz, L.; Tarascon, J.M. Combined XRD, EXAFS, and Mossbauer studies of the reduction by lithium of alpha-Fe2O3 with various particle sizes. J. Electrochem. Soc. 2003, 150, A1643–A1650. [Google Scholar] [CrossRef]
- Xue, D.S.; Gao, C.X.; Liu, Q.F.; Zhang, L.Y. Preparation and characterization of haematite nanowire arrays. J. Phys. Condens. Matter 2003, 15, 1455–1459. [Google Scholar] [CrossRef]
- Jia, C.J.; Sun, L.D.; Yan, Z.G.; You, L.P.; Luo, F.; Han, X.D.; Pang, Y.C.; Zhang, Z.; Yan, C.H. Iron oxide nanotubes—Single-crystalline iron oxide nanotubes. Angew. Chem. Int. Ed. 2005, 44, 4328–4333. [Google Scholar] [CrossRef]
- Jia, C.J.; Sun, L.D.; Luo, F.; Han, X.D.; Heyderman, L.J.; Yan, Z.G.; Yan, C.H.; Zheng, K.; Zhang, Z.; Takano, M.; et al. Large-Scale Synthesis of Single-Crystalline Iron Oxide Magnetic Nanorings. J. Am. Chem. Soc. 2008, 130, 16968–16977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltschka, M.; Klaui, M.; Ruediger, U.; Kasama, T.; Cervera-Gontard, L.; Dunin-Borkowski, R.E.; Luo, F.; Heyderman, L.J.; Jia, C.J.; Sun, L.D.; et al. Correlation between magnetic spin structure and the three-dimensional geometry in chemically synthesized nanoscale magnetite rings. Appl. Phys. Lett. 2008, 92, 222508. [Google Scholar] [CrossRef] [Green Version]
- Uhm, Y.R.; Kim, W.W.; Rhee, C.K. A study of synthesis and phase transition of nanofibrous Fe2O3 derived from hydrolysis of Fe nanopowders. Scr. Mater. 2004, 50, 561–564. [Google Scholar] [CrossRef]
- Li, L.L.; Chu, Y.; Liu, Y.; Dong, L.H. Template-free synthesis and photocatalytic properties of novel, Fe2O3 hollow spheres. J. Phys. Chem. C 2007, 111, 2123–2127. [Google Scholar] [CrossRef]
- Wen, X.G.; Wang, S.H.; Ding, Y.; Wang, Z.L.; Yang, S.H. Controlled growth of large-area, uniform, vertically aligned arrays of alpha-Fe2O3 nanobelts and nanowires. J. Phys. Chem. B 2005, 109, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.Y.; Tang, K.B.; Li, T.W.; Liang, Z.H.; Wang, D.; Wang, Y.K.; Qi, Y.X.; Zhou, W.W. Facile route for the fabrication of porous hematite nanoflowers: Its synthesis, growth mechanism, application in the lithium ion battery, and magnetic and photocatalytic properties. J. Phys. Chem. C 2008, 112, 4836–4843. [Google Scholar] [CrossRef]
- Gotic, M.; Drazic, G.; Music, S. Hydrothermal synthesis of alpha-Fe2O3 nanorings with the help of divalent metal cations, Mn2+, Cu2+, Zn2+ and Ni2+. J. Mol. Struct. 2011, 993, 167–176. [Google Scholar] [CrossRef]
- Lv, B.L.; Xu, Y.; Wu, D.; Sun, Y.H. Single-crystal alpha-Fe2O3 hexagonal nanorings: Stepwise influence of different anionic ligands (F− and SCN− anions). Chem. Commun. 2011, 47, 967–969. [Google Scholar] [CrossRef]
- Lv, B.L.; Xu, Y.; Wu, D.; Sun, Y.H. Modification Effect of SCN− Ions on the Morphology of alpha-Fe2O3 Nanoparticles. Chem. Lett. 2008, 37, 1152–1153. [Google Scholar] [CrossRef]
- Zhang, X.L.; Sui, C.H.; Gong, J.; Su, Z.M.; Qu, L.Y. Preparation and formation mechanism of different alpha-Fe2O3 morphologies from snowflake to paired microplates, dumbbell, and spindle microstructures. J. Phys. Chem. C 2007, 111, 9049–9054. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.-Y.; Sun, S.-G.; Ding, Y.; Wang, Z.L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.H.; Kim, M.; Lee, Y.W.; Choi, W.; Oh, W.T.; Park, Q.H.; Han, S.W. Polyhedral Au Nanocrystals Exclusively Bound by {110} Facets: The Rhombic Dodecahedron. J. Am. Chem. Soc. 2009, 131, 1672–1673. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Kuang, Q.; Jiang, Z.Y.; Xie, Z.X.; Huang, R.B.; Zheng, L.S. Synthesis of Trisoctahedral Gold Nanocrystals with Exposed High-Index Facets by a Facile Chemical Method. Angew. Chem. Int. Ed. 2008, 47, 8901–8904. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R.R. Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters. Science 2007, 315, 220–222. [Google Scholar] [CrossRef] [Green Version]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Markovic, N.M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, R.; El-Sayed, M.A. Shape-dependent catalytic activity of platinum nanoparticles in colloidal solution. Nano Lett. 2004, 4, 1343–1348. [Google Scholar] [CrossRef]
- Vidal-Iglesias, F.J.; Solla-Gullon, J.; Rodriguez, P.; Herrero, E.; Montiel, V.; Feliu, J.M.; Aldaz, A. Shape-dependent electrocatalysis: Ammonia oxidation on platinum nanoparticles with preferential (100) surfaces. Electrochem. Commun. 2004, 6, 1080–1084. [Google Scholar] [CrossRef]
- Yin, J.Z. Synthesis of Functional Nanostructures Assisted by Cellulose-based Polymeric Surfactants. Ph.D. Thesis, Nanjing University, NanKing, China, 2011. [Google Scholar]
- Ma, H.X.; Zhao, N.N.; Zhao, F.Q. Nano-Metal Oxides and Super-Thermite; Science Press: Beijing, China, 2022. [Google Scholar]
- Jiao, H.; Yang, H. Thermal decomposition synthesis of 3D urchin-like alpha-Fe2O3 superstructures. Mater. Sci. Eng. B Adv. Funct. Solid State Mater. 2009, 156, 68–72. [Google Scholar]
- Lv, B.L.; Liu, Z.Y.; Tian, H.; Xu, Y.; Wu, D.; Sun, Y.H. Single-Crystalline Dodecahedral and Octodecahedral alpha-Fe2O3 Particles Synthesized by a Fluoride Anion-Assisted Hydrothermal Method. Adv. Funct. Mater. 2010, 20, 3987–3996. [Google Scholar] [CrossRef]
- Lv, B.L.; Zhou, H.; Wu, D.; Xu, Y. Single-crystalline dodecahedral alpha-Fe2O3 particles with nanometer size: Synthesis and characterization. J. Nanopart. Res. 2014, 16, 2799. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Zhang, L. Selective preparation of nanorods and micro-octahedrons of Fe2O3 and their catalytic performances for thermal decomposition of ammonium perchlorate. Powder Technol. 2008, 185, 176–180. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, H.; Zhuang, J.; Wang, X. Morphology-Controlled Synthesis of Hematite Nanocrystals and Their Facet Effects on Gas-Sensing Properties. Inorg. Chem. 2011, 50, 10143–10151. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zhao, N.N.; Liu, J.B.; Zhang, T.; Ma, H.X. Catalytic Effect of Two Shapes of Nano-Fe2O3 on Hexanitrohexaazaisowurtzitane Using a Non-Isothermal Decomposition Kinetic Method. Propellants Explos. Pyrotech. 2016, 41, 936–941. [Google Scholar] [CrossRef]
- Benhammada, A.; Trache, D.; Kesraoui, M.; Tarchoun, A.F.; Chelouche, S.; Mezroua, A. Synthesis and characterization of alpha-Fe2O3 nanoparticles from different precursors and their catalytic effect on the thermal decomposition of nitrocellulose. Thermochim. Acta 2020, 686, 178570. [Google Scholar] [CrossRef]
- Zhao, N.N.; Li, J.C.; Gong, H.J.; An, T.; Zhao, F.Q.; Yang, A.W.; Hu, R.Z.; Ma, H.X. Effects of alpha-Fe2O3 nanoparticles on the thermal behavior and non-isothermal decomposition kinetics of nitrocellulose. J. Anal. Appl. Pyrolysis 2016, 120, 165–173. [Google Scholar] [CrossRef]
- Elbasuney, S.; Yehia, M.; Hamed, A.; Ismael, S.; Mokhtar, M.; Elsaka, E.; Gobara, M.; Saleh, A.; El-Sayyad, G.S. Ferric oxide colloid: Novel nanocatalyst for heterocyclic nitramines. J. Mater. Sci. Mater. Electron. 2021, 32, 4185–4195. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, G.; Ou, P.; Han, G.-R. Preparation and catalytic property of single crystal multiporous alpha-Fe2O3 nanorods. J. Inorg. Mater. 2008, 23, 459–463. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Cao, S.B.; Zhang, L.L.; Xiang, G.; Zeng, X.F.; Chu, G.W.; Chen, J.F. Quantitatively evaluating activity and number of catalytic sites on metal oxide for ammonium perchlorate decomposition. AICHE J. 2022, 68, 17582. [Google Scholar] [CrossRef]
- Shi, S.H.; Xu, G. Uniform hematite hexagonal nanodisks with dominant (001) facets: Hydrothermal synthesis and catalytic performance for the decomposition of ammonium perchlorate. Appl. Mech. Mater. 2014, 651–653, 161–164. [Google Scholar] [CrossRef]
- Sharma, J.K.; Srivastava, P.; Akhtar, M.S.; Singh, G.; Ameen, S. alpha-Fe2O3 hexagonal cones synthesized from the leaf extract of Azadirachta indica and its thermal catalytic activity. New J. Chem. 2015, 39, 7105–7111. [Google Scholar] [CrossRef]
- Cao, S.B.; Han, X.G.; Zhang, L.L.; Wang, J.X.; Luo, Y.; Zou, H.K.; Chen, J.F. Facile and scalable preparation of alpha-Fe2O3 nanoparticle by high-gravity reactive precipitation method for catalysis of solid propellants combustion. Powder Technol. 2019, 353, 444–449. [Google Scholar] [CrossRef]
- Li, C.C.; Zhang, T.; An, J.; Zeng, J.; Ma, H.X. Research progress of three-dimensional ordered macroporous perovskite metal oxides as highly efficient combustion catalysts. Chem. Ind. Eng. Prog. 2021, 40, 3181–3190. [Google Scholar]
- Wu, Q.Z.; Li, Y.G. Progress of applications of three-dimensionally ordered macroporous materials. Chem. Ind. Eng. Prog. 2008, 27, 358–363. [Google Scholar]
- Tanaka, H.; Misono, M. Advances in designing perovskite catalysts. Curr. Opin. Solid State Mater. Sci. 2001, 5, 381–387. [Google Scholar] [CrossRef]
- Crespin, M.; Hall, W.K. The surface chemistry of some perovskite oxides. J. Catal. 1981, 69, 359–370. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.D.; Luo, W. Perovskite-type lanthanum ferrite based photocatalysts: Preparation, properties, and applications. J. Energy Chem. 2022, 66, 314–338. [Google Scholar] [CrossRef]
- Shi, J.W.; Guo, L.J. ABO3-based photocatalysts for water splitting. Prog. Nat. Sci. Mater. Int. 2012, 22, 592–615. [Google Scholar] [CrossRef] [Green Version]
- Thirumalairajan, S.; Girija, K.; Ganesh, I.; Mangalaraj, D.; Viswanathan, C.; Balamurugan, A.; Ponpandian, N. Controlled synthesis of perovskite LaFeO3 microsphere composed of nanoparticles via self-assembly process and their associated photocatalytic activity. Chem. Eng. J. 2012, 209, 420–428. [Google Scholar] [CrossRef]
- Shen, S.T.; Weng, H.S. Comparative Study of Catalytic Reduction of Nitric Oxide with Carbon Monoxide over the La1−xSrxBO3 (B=Mn, Fe, Co, Ni) Catalysts. Ind. Eng. Chem. Res. 1998, 37, 2654–2661. [Google Scholar] [CrossRef]
- Belessi, V.C.; Trikalitis, P.N.; Ladavos, A.K.; Bakas, T.V.; Pomonis, P.J. Structure and catalytic activity of La1−xFeO3 system (x = 0.00, 0.05, 0.10, 0.15, 0.20, 0.25, 0.35) for the NO+CO reaction. Appl. Catal. A Gen. 1999, 177, 53–68. [Google Scholar] [CrossRef]
- Li, J.; Si, F. Study on Modification Technology of the Combustion Property of the NEPE Propellant. Energetic Mater. 2002, 10, 4–9. [Google Scholar]
- Wei, Z.X.; Xu, Y.Q.; Liu, H.Y.; Hu, C.W. Preparation and catalytic activities of LaFeO3 and Fe2O3 for HMX thermal decomposition. J. Hazard. Mater. 2009, 165, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Yang, X.J.; Lu, L.D.; Wang, X. Experimental study on preparation of LaMO3 (M = Fe, Co, Ni) nanocrystals and their catalytic activity. Thermochim. Acta 2006, 443, 225–230. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, Y.; Li, C.C.; Li, Y.Y.; Li, J.C.; Zhao, F.Q.; Ma, H.X. The effect of LaFeO3@MnO2 on the thermal behavior of energetic compounds: An efficient catalyst with core-shell structure. Adv. Powder Technol. 2020, 31, 4510–4516. [Google Scholar] [CrossRef]
- Chikazumi, S. Physics of Ferromagnetism (International Series of Monographs on Physics); Oxford University Press Inc.: New York, NY, USA, 2009. [Google Scholar]
- Goldman, A. Modern Ferrite Technology; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses; Wiley-VCH Verlag Gmbh &. Co. KgaA: Weinheim, Germany, 2003. [Google Scholar]
- Long, N.V.; Yang, Y.; Teranishi, T.; Thi, C.M.; Cao, Y.Q.; Nogami, M. Synthesis and magnetism of hierarchical iron oxide particles. Mater. Des. 2015, 86, 797–808. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Abbas, M.; Islam, M.N.; Rao, B.P.; Aitah, K.E.A.; Kim, C. Facile approach for synthesis of high moment Fe/ferrite and FeCo/ferrite core/shell nanostructures. Mater. Lett. 2015, 139, 161–164. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Long, N.V.; Yang, Y.; Yuasa, M.; Minh, T.C.; Cao, Y.; Nann, T.; Nogami, M. Controlled synthesis and characterization of iron oxide nanostructures with potential applications for gas sensors and the environment. RSC Adv. 2014, 4, 6383–6390. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, X.; Mao, C.; Du, J. Preparation and microwave-absorbing properties of NiFe2O4-polystyrene composites. Phys. B Condens. Matter 2009, 404, 69–72. [Google Scholar] [CrossRef]
- Rodenas, L.A.G.; Blesa, M.A.; Morando, P.J. Reactivity of metal oxides: Thermal and photochemical dissolution of MO and MFe2O4 (M = Ni, Co, Zn). J. Solid State Chem. 2008, 181, 2350–2358. [Google Scholar] [CrossRef]
- Zu, Y.; Zhang, Y.; Xu, K.; Zhao, F.Q. A graphene oxide-MgWO4 nanocomposite as an efficient catalyst for the thermal decomposition of RDX, HMX. RSC Adv. 2016, 6, 31046–31052. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, X. Magnetically Separable ZnFe2O4-Graphene Catalyst and its High Photocatalytic Performance under Visible Light Irradiation. Ind. Eng. Chem. Res. 2011, 50, 7210–7218. [Google Scholar] [CrossRef]
- Zu, Y.; Zhao, Y.; Xu, K.; Tong, Y.; Zhao, F. Preparation and comparison of catalytic performance for nano MgFe2O4, GO-loaded MgFe2O4 and GO-coated MgFe2O4 nanocomposites. Ceram. Int. 2016, 42, 18844–18850. [Google Scholar] [CrossRef]
- Xiong, W.H.; Zhang, W.C.; Yu, C.P.; Shen, R.Q.; Cheng, J.; Ye, J.H.; Qin, Z.C. Preparation of Nanoporous CoFe2O4 and Its Catalytic Performance during the Thermal Decomposition of Ammonium Perchlorate. Acta Phys. Chim. Sin. 2016, 32, 2093–2100. [Google Scholar] [CrossRef]
- Han, A.; Liao, J.; Ye, M.; Li, Y.; Peng, X. Preparation of Nano-MnFe2O4 and Its Catalytic Performance of Thermal Decomposition of Ammonium Perchlorate. Chin. J. Chem. Eng. 2011, 19, 1047–1051. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, G.; Wang, Z.; Zhu, Y.; Yan, Z.; Wang, Y. Porous cobaltate: Structure, active sites, thermocatalytic properties for ammonium perchlorate decomposition. J. Alloys Compd. 2022, 908, 164624. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Zeng, J.; Zhang, T.; Li, J.; Guo, Z.; Qin, Z.; Yi, J.; Zhao, F.; Ma, H. Enhanced catalytic activity and ignition characteristics of three- dimensional ordered macroporous FeCo2O4 through controlled synthesis. Adv. Powder Technol. 2022, 33, 103799. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Yang, P.; Hu, B. Preparation of nanometer CuFe2O4 by auto-combustion and its catalytic activity on the thermal decomposition of ammonium perchlorate. Mater. Lett. 2008, 62, 4056–4058. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R. Potential Applications of Magnetic β-AgVO3/ZnFe2O4 Nanocomposites in Dyes, Photocatalytic Degradation, and Catalytic Thermal Decomposition of Ammonium Perchlorate. Ind. Eng. Chem. Res. 2017, 56, 623–634. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, F.; Yang, Y.; An, T.; Qu, W.; Li, H.; Zhang, J.; Li, N. Catalytic Activity of Ferrates (NiFe2O4, ZnFe2O4 and CoFe2O4) on the Thermal Decomposition of Ammonium Perchlorate. Propellants Explos. Pyrotech. 2020, 45, 463–471. [Google Scholar] [CrossRef]
- Liu, D.; Lu, Y.; Tan, H.-Q.; Chen, W.L.; Zhang, Z.M.; Li, Y.G.; Wang, E.B. Polyoxometalate-based purely inorganic porous frameworks with selective adsorption and oxidative catalysis functionalities. Chem. Commun. 2013, 49, 3673–3675. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.; Zhang, Y.; Chen, H. Preparation of Ce doped NiFe2O4 nanoparticles and its catalysis on thermal decomposition of AP. J. Solid Rocket Technol. 2012, 35, 504–507. [Google Scholar]
- Huang, R.C.; Chiu, W.J.; Lai, I.P.J.; Huang, C.C. Multivalent Aptamer/Gold Nanoparticle-Modified Graphene Oxide for Mass Spectrometry-Based Tumor Tissue Imaging. Sci. Rep. 2015, 5, 10292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Stoller, M.D.; Park, S.J.; Zhu, Y.W.; An, J.H.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. Electrochemically Exfoliated Graphene and Graphene Oxide for Energy Storage and Electrochemistry Applications. Chem. A Eur. J. 2016, 22, 153–159. [Google Scholar] [CrossRef]
- Lin, T.W.; Wu, H.Y.; Tasi, T.T.; Lai, Y.H.; Shen, H.H. Surface-enhanced Raman spectroscopy for DNA detection by the self-assembly of Ag nanoparticles onto Ag nanoparticle-graphene oxide nanocomposites. Phys. Chem. Chem. Phys. 2015, 17, 18443–18448. [Google Scholar] [CrossRef]
- Haghighi, N.; Hallaj, R.; Salimi, A. Immobilization of glucose oxidase onto a novel platform based on modified TiO2 and graphene oxide, direct electrochemistry, catalytic and photocatalytic activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.C.; Yu, R.; Zhou, Z.H.; Liu, H.W.; Jiang, R.L. Encapsulation of a Core-Shell Porous Fe3O4@Carbon Material with Reduced Graphene Oxide for Li+ Battery Anodes with Long Cyclability. Langmuir 2021, 37, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Wang, Y.; Lu, L.G.; Zhang, B.; Wang, C.X.; He, B.; Wei, R.; Xu, D.D.; Hao, Q.L.; Liu, B. Hierarchically Hollow and Porous NiO/NiCo2O4 Nanoprisms Encapsulated in Graphene Oxide for Lithium Storage. Langmuir 2020, 36, 9668–9674. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.S.; Yu, H.; Wang, H.J.; Peng, F. Controllable synthesis and catalytic performance of graphene-supported metal oxide nanoparticles. Chin. J. Catal. 2014, 35, 952–959. [Google Scholar] [CrossRef]
- Ding, X.; Huang, Z.H.; Shen, W.C.; Kang, F.Y. Preparation and electrochemical performance of a CuO/graphene composite. New Carbon Mater. 2013, 28, 172–177. [Google Scholar] [CrossRef]
- Danish, M.; Athar, M.S.; Ahmad, I.; Warshagha, M.Z.A.; Rasool, Z.; Muneer, M. Highly efficient and stable Fe2O3/g-C3N4/GO nanocomposite with Z-scheme electron transfer pathway: Role of photocatalytic activity and adsorption isotherm of organic pollutants in wastewater. Appl. Surf. Sci. 2022, 604, 154604. [Google Scholar] [CrossRef]
- Kumar, R.; del Pino, A.P.; Sahoo, S.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A.; Joanni, E. Laser processing of graphene and related materials for energy storage: State of the art and future prospects. Prog. Energy Combust. Sci. 2022, 91, 100981. [Google Scholar] [CrossRef]
- Sharif, H.M.A.; Asif, M.B.; Wang, Y.; Hou, Y.-N.; Yang, B.; Xiao, X.; Li, C. Spontaneous intra-electron transfer within rGO@Fe2O3-MnO catalyst promotes long-term NOx reduction at ambient conditions. J. Hazard. Mater. 2023, 441, 129951. [Google Scholar] [CrossRef]
- Li, Z.M.; Wang, Y.; Zhang, Y.Q.; Liu, L.; Zhang, S.J. CL-20 hosted in graphene foam as a high energy material with low sensitivity. RSC Adv. 2015, 5, 98925–98928. [Google Scholar] [CrossRef]
- Smeu, M.; Zahid, F.; Ji, W.; Guo, H.; Jaidann, M.; Abou-Rachid, H. Energetic Molecules Encapsulated Inside Carbon Nanotubes and between Graphene Layers: DFT Calculations. J. Phys. Chem. C 2011, 115, 10985–10989. [Google Scholar] [CrossRef]
- Qin, M.; Zheng, X.; Tang, W.; Zhang, Y.; Shi, Q.; Qiu, S. Preparation and catalytic performance of Fe2O3/graphene composite. J. Solid Rocket Technol. 2017, 40, 601–604. [Google Scholar]
- Zhang, T.; Li, C.C.; Wang, W.; Guo, Z.Q.; Pang, A.M.; Ma, H.X. Construction of Three-Dimensional Hematite/Graphene with Effective Catalytic Activity for the Thermal Decomposition of CL-20. Acta Phys. Chim. Sin. 2020, 36, 1905048. [Google Scholar]
- Pei, J.Y.; Zhao, H.Y.; Yang, F.; Yan, D. Graphene Oxide/Fe2O3 Nanocomposite as an Efficient Catalyst for Thermal Decomposition of Ammonium Perchlorate via the Vacuum-Freeze-Drying Method. Langmuir 2021, 37, 6132–6138. [Google Scholar] [CrossRef] [PubMed]
- Fertassi, M.A.; Liu, Q.; Li, R.Z.; Liu, P.G.; Liu, J.Y.; Chen, R.R.; Liu, L.H.; Wang, J. Ex situ synthesis of G/alpha-Fe2O3 nanocomposite and its catalytic effect on the thermal decomposition of ammonium perchlorate. Bull. Mater. Sci. 2017, 40, 691–698. [Google Scholar] [CrossRef] [Green Version]
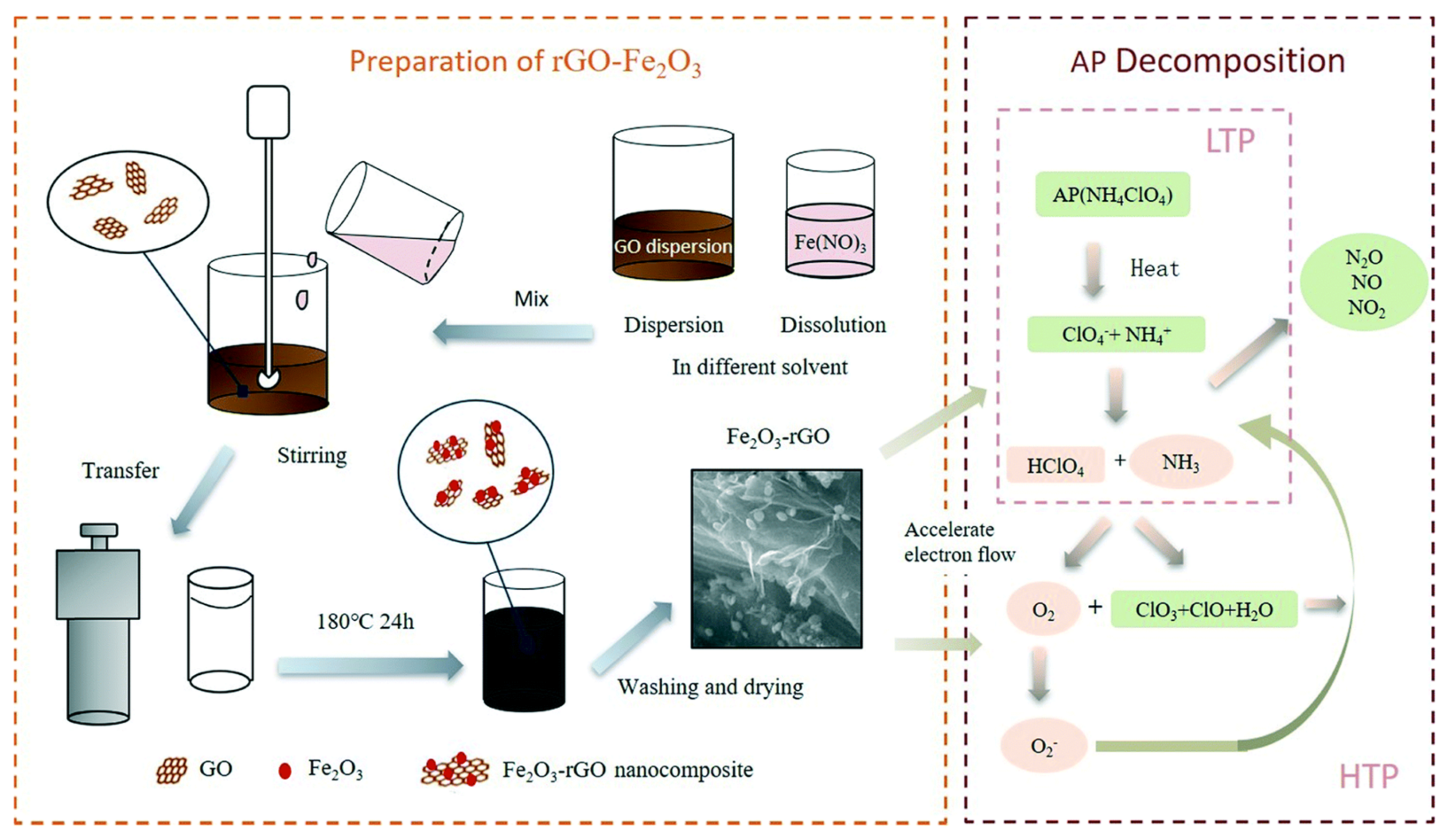
- Zhang, M.; Zhao, F.Q.; Yang, Y.J.; Zhang, J.K.; Li, N.; Gao, H.X. Effect of rGO-Fe2O3 nanocomposites fabricated in different solvents on the thermal decomposition properties of ammonium perchlorate. Crystengcomm 2018, 20, 7010–7019. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascon, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Villar-Rodil, S.; Paredes, J.I.; Martinez-Alonso, A.; Tascon, J.M.D. Preparation of graphene dispersions and graphene-polymer composites in organic media. J. Mater. Chem. 2009, 19, 3591–3593. [Google Scholar] [CrossRef]
- Yan, N.; Qin, L.J.; Li, J.G.; Zhao, F.Q.; Feng, H. Atomic layer deposition of iron oxide on reduced graphene oxide and its catalytic activity in the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2018, 451, 155–161. [Google Scholar] [CrossRef]
- Elbasuney, S.; El-Sayyad, G.S.; Yehia, M.; Aal, S.K.A. Facile synthesis of RGO-Fe2O3 nanocomposite: A novel catalyzing agent for composite propellants. J. Mater. Sci. Mater. Electron. 2020, 31, 20805–20815. [Google Scholar] [CrossRef]
- Xu, X.; Wang, T.; Nie, K.; Jiang, X.; Jia, R. Preparation of BiFeO3/rGO Nanocomposites and Its Catalytic Performance for the Thermal Decomposition of Ammonium Perchlorate. Chin. J. Explos. Propellants 2021, 44, 181–188. [Google Scholar]
- Guo, Z.Q.; Qian, X.; Zhang, T.; Wang, Y.W.; Tao, B.W.; Li, L.; Ma, H.X.; Gu, J. Application of BiFeO3/GO composite combustion catalyst in HTPB propellant. J. Solid Rocket Technol. 2022, 45, 61–66. [Google Scholar]
- Chen, T.; Du, P.; Jiang, W.; Liu, J.; Hao, G.; Gao, H.; Xiao, L.; Ke, X.; Zhao, F.; Xuan, C. A facile one-pot solvothermal synthesis of CoFe2O4/RGO and its excellent catalytic activity on thermal decomposition of ammonium perchlorate. RSC Adv. 2016, 6, 83838–83847. [Google Scholar] [CrossRef]
- Chen, T.; Jiang, W. A Facile Solvothermal Synthesis of NiFe2O4/RGO and Its Enhanced Catalytic Activity on Thermal Decomposition of Ammonium Perchlorate. In Proceedings of the 2017 IEEE 17th International Conference on Nanotechnology (IEEE-NANO), Pittsburgh, PA, USA, 25–28 July 2017; pp. 607–610. [Google Scholar]
- Wang, W.; Guo, S.; Zhang, D.; Yang, Z. One-pot hydrothermal synthesis of reduced graphene oxide/zinc ferrite nanohybrids and its catalytic activity on the thermal decomposition of ammonium perchlorate. J. Saudi Chem. Soc. 2019, 23, 133–140. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Li, W.Z.; Liang, C.H.; Qiu, J.S.; Zhou, W.J.; Han, H.M.; Wei, Z.B.; Sun, G.Q.; Xin, Q. Carbon nanotubes as support for cathode catalyst of a direct methanol fuel cell. Carbon 2002, 40, 791–794. [Google Scholar] [CrossRef]
- Wang, J.N.; Li, X.D. Preparation of Ferric Oxide/Carbon Nanotubes Composite Nano-Particles and Catalysis on Burning Rate of Ammonium Perchlorate. Chin. J. Explos. Propellants 2006, 29, 44–47. [Google Scholar]
- Jiang, W.; Liu, J.X.; Liu, Y.; Cui, P.; Li, F.S. Preparation of Fe2O3/CNTs composites and its catalysis on thermal decomposition of AP. J. Solid Rocket. Technol. 2008, 31, 65–68. [Google Scholar]
- Cui, Q.Z.; Jiao, J.Q.; Zhao, W.D. Preparation of Fe2O3/CNTs and Its Catalytic Mechanism on Thermal Decomposition of RDX. Chin. J. Explos. Propellants 2009, 32, 68–71. [Google Scholar]
- Groenewolt, M.; Antonietti, M. Synthesis of g-C3N4 nanoparticles in mesoporous silica host matrices. Adv. Mater. 2005, 17, 1789–1792. [Google Scholar] [CrossRef]
- Ghane, N.; Sadrnezhaad, S.K.; Hosseini, H.S.M. Combustion synthesis of g-C3N4/Fe2O3 nanocomposite for superior photoelectrochemical catalytic performance. Appl. Surf. Sci. 2020, 534, 147563. [Google Scholar] [CrossRef]
- Yan, S.D.; Shi, Y.; Tao, Y.F.; Zhang, H. Enhanced persulfate-mediated photocatalytic oxidation of bisphenol A using bioelectricity and a g-C3N4/Fe2O3 heterojunction. Chem. Eng. J. 2019, 359, 933–943. [Google Scholar] [CrossRef]
- Lian, X.; Xue, W.; Dong, S.; Liu, E.; Li, H.; Xu, K. Construction of S-scheme Bi2WO6/g-C3N4 heterostructure nanosheets with enhanced visible-light photocatalytic degradation for ammonium dinitramide. J. Hazard. Mater. 2021, 412, 125217. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; He, Y.; Peng, R.F. Graphitic carbon nitride (g-C3N4) as a metal-free catalyst for thermal decomposition of ammonium perchlorate. RSC Adv. 2015, 5, 24507–24512. [Google Scholar] [CrossRef]
- Wang, R.H.; Song, W.K.; Guo, C.P.; Gong, W.; Wang, D.J. Characterization of g-C3N4/Fe2O3 Composite Catalyst and Its Catalytic Effect on Ammonium Perchlorate. J. Propuls. Technol. 2022, 43, 373–378. [Google Scholar]
- Wan, C.; Wang, C.; Chen, S.H.; Xu, K.Z. Catalytic Decomposition Properties of CoFe2O4/g-C3N4 on HMX and TKX-50. Chin. J. Energetic Mater. 2022, 30, 703–709. [Google Scholar]
- Wang, J.; Lian, X.; Chen, S.; Li, H.; Xu, K. Effect of Bi2WO6/g-C3N4 composite on the combustion and catalytic decomposition of energetic materials: An efficient catalyst with g-C3N4 carrier. J. Colloid Interface Sci. 2022, 610, 842–853. [Google Scholar] [CrossRef]
- An, T.; Zhao, F.Q.; Pei, Q.; Xiao, L.B.; Xu, S.Y.; Gao, H.X.; Xing, X.L. Preparation, Characterization and Combustion Catalytic Activity of Nanopartical Super Thermites. Chin. J. Inorg. Chem. 2011, 27, 231–238. [Google Scholar]
- An, T.; Zhao, F.Q.; Yi, J.H.; Fan, X.Z.; Gao, H.X.; Hao, H.X.; Wang, X.H.; Hu, R.Z.; Pei, Q. Preparation, Characterization, Decomposition Mechanism and Non-Isothermal Decomposition Reaction Kinetics of the Super Thermite Al/CuO Precursor. Acta Phys. Chim. Sin. 2011, 27, 281–288. [Google Scholar]
- Xu, H.; Li, X.; Zhao, F.; Pang, W.; Jia, S.; Mo, H. Review on Application of Nano-metal Powders in Explosive and Propellants. Energetic Mater. 2011, 19, 232–239. [Google Scholar]
- Pang, W.Q.; Fan, X.Z.; Lv, K. Physicochemical properties of boron powder and its application in fuel-rich solid propellants. Aerodyn. Missile J. 2009, 10, 53–58. [Google Scholar]
- Pei, J.; Zhao, F.; Song, X.; Zheng, W. Research Progress in the Application of Lightweight Carbon Materials and Their Composites in Solid Rocket Propellants. Chin. J. Explos. Propellants 2014, 37, 1–6. [Google Scholar]
- Puszynski, J.A.; Bulian, C.J.; Swiatkiewicz, J.J. Processing and ignition characteristics of aluminum-bismuth trioxide nanothermite system. J. Propuls. Power 2007, 23, 698–706. [Google Scholar] [CrossRef]
- Qi, Q.F.; Zhang, X.H.; Yan, Q.L.; Song, Z.W.; Liu, P.; Li, J.Z. Research progress on the application of nano-metals and their composites in solid propellants. Chem. Propellants Polym. Mater. 2012, 10, 66–72. [Google Scholar]
- Bhattacharya, S.; Gao, Y.F.; Apperson, S.; Subramaniam, S.; Shende, R.; Gangopadhyay, S.; Talantsev, E. A novel on-chip diagnostic method to measure burn rates of energetic materials. J. Energetic Mater. 2006, 24, 1–15. [Google Scholar] [CrossRef]
- Nie, H.Q.; Chan, H.Y.; Pisharath, S.; Hng, H.H. Combustion characteristic and aging behavior of bimetal thermite powders. Def. Technol. 2021, 17, 755–762. [Google Scholar] [CrossRef]
- Shen, L.H.; Li, G.P.; Luo, Y.J.; Gao, K.; Ge, Z. Preparation and characterization of Al/B/Fe2O3 nanothermites. Sci. China Chem. 2014, 57, 797–802. [Google Scholar] [CrossRef]
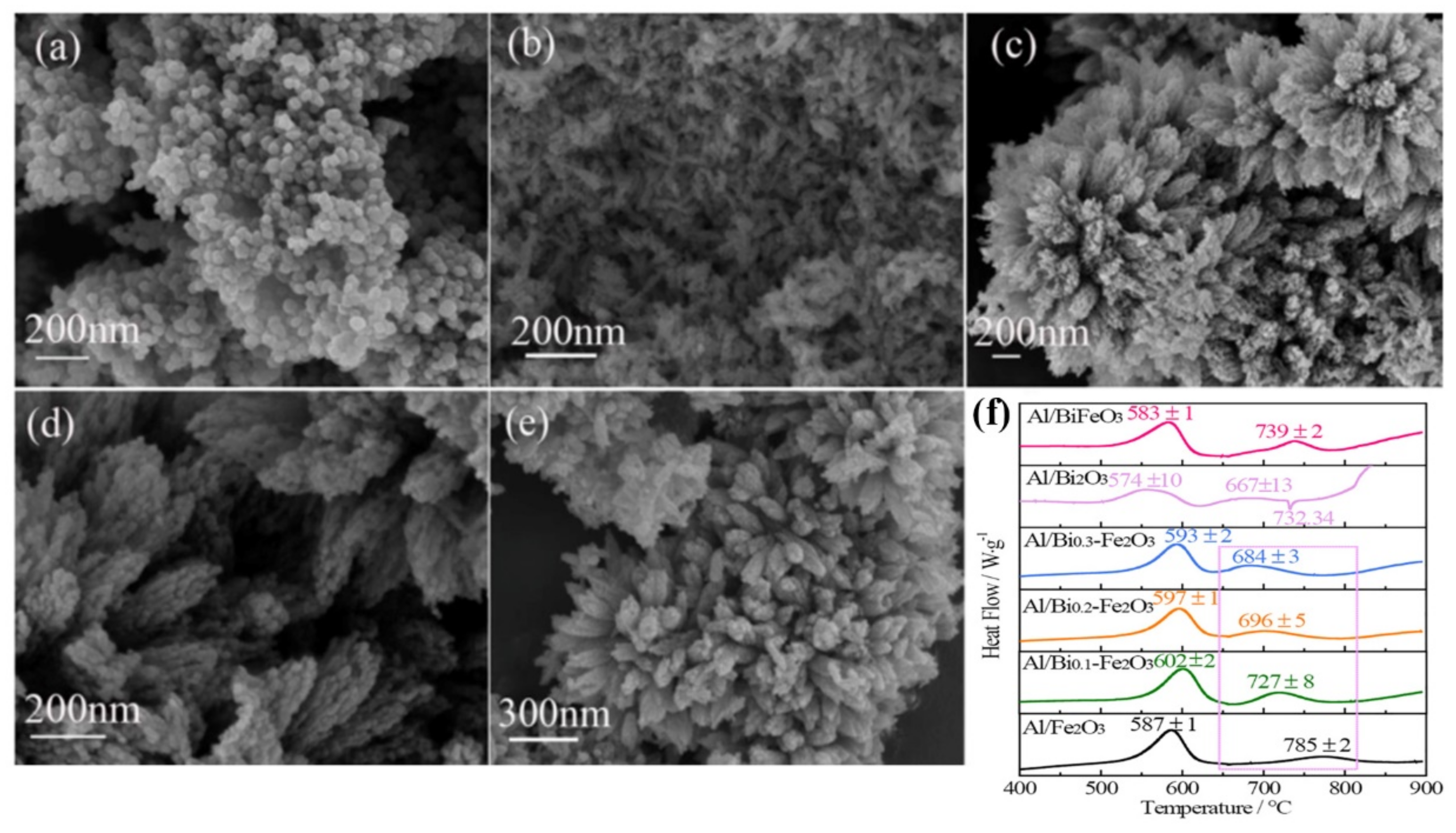
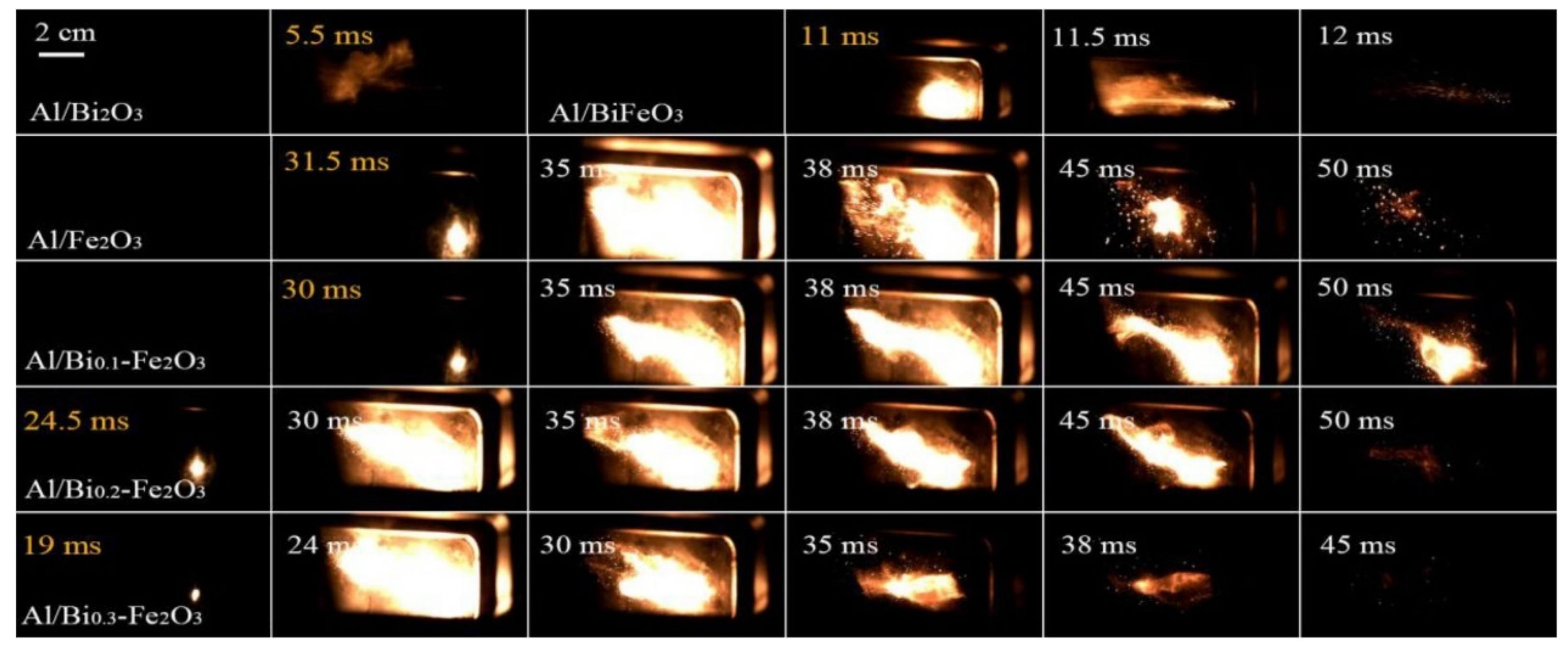
- Zhang, T.; Li, J.C.; Qin, Z.; Li, C.C.; Gao, H.X.; Zhao, F.Q.; Ma, H.X. Doping hematite with bismuth to enhance its catalytic and oxidizing properties. Chem. Eng. J. 2021, 421, 129436. [Google Scholar] [CrossRef]
- Yan, N.; Qin, L.J.; Hao, H.X.; Hui, L.F.; Zhao, F.Q.; Feng, H. Iron oxide/aluminum/graphene energetic nanocomposites synthesized by atomic layer deposition: Enhanced energy release and reduced electrostatic ignition hazard. Appl. Surf. Sci. 2017, 408, 51–59. [Google Scholar] [CrossRef]
- Malchi, J.Y.; Foley, T.J.; Yetter, R.A. Electrostatically Self-Assembled Nanocomposite Reactive Microspheres. Acs Appl. Mater. Interfaces 2009, 1, 2420–2423. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, J.D.; Cervantes, O.G.; Gash, A.E.; Munir, Z.A. Tantalum-tungsten oxide thermite composites prepared by sol-gel synthesis and spark plasma sintering. Combust. Flame 2010, 157, 1566–1571. [Google Scholar] [CrossRef]
- Ke, X.; Zhou, X.; Hao, G.Z.; Xiao, L.; Liu, J.; Jiang, W. Rapid fabrication of superhydrophobic Al/Fe2O3 nanothermite film with excellent energy-release characteristics and long-term storage stability. Appl. Surf. Sci. 2017, 407, 137–144. [Google Scholar] [CrossRef]
- He, W.; Tao, B.W.; Yang, Z.J.; Yang, G.C.; Guo, X.; Liu, P.J.; Yan, Q.L. Mussel-inspired polydopamine-directed crystal growth of core-shell n-Al@PDA@CuO metastable intermixed composites. Chem. Eng. J. 2019, 369, 1093–1101. [Google Scholar] [CrossRef]
- He, W.; Liu, P.J.; He, G.Q.; Gozin, M.; Yan, Q.L. Highly Reactive Metastable Intermixed Composites (MICs): Preparation and Characterization. Adv. Mater. 2018, 30, 1706293. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.P.; Qiao, Z.Q.; Wang, J.; Yang, G.C.; Chen, J.; Li, Z.Q.; Liao, X.; Wang, H.Y.; Zachariah, M.R. Reaction mechanism of Al-CuO nanothermites with addition of multilayer graphene. Thermochim. Acta 2018, 666, 60–65. [Google Scholar] [CrossRef]
- Wang, A.Q.; Bok, S.; Thiruvengadathan, R.; Gangopadhyay, K.; McFarland, J.A.; Maschmann, M.R.; Gangopadhyay, S. Reactive nanoenergetic graphene aerogel synthesized by one-step chemical reduction. Combust. Flame 2018, 196, 400–406. [Google Scholar] [CrossRef]
- Zhao, W.J.; Ren, H.; Yan, T.; Ou, Y.P.; Jiao, Q.J.; Wang, H.Y.; Kline, D.J.; Zachariah, M.R. Tailoring energy release of nano-Si based thermites via incorporation of Ti nanoparticles. Chem. Eng. J. 2020, 396, 124559. [Google Scholar] [CrossRef]
- Martirosyan, K.S. Nanoenergetic Gas-Generators: Principles and applications. J. Mater. Chem. 2011, 21, 9400–9405. [Google Scholar] [CrossRef]
- Wu, T.; Lahiner, G.; Tenailleau, C.; Reig, B.; Hungria, T.; Esteve, A.; Rossi, C. Unexpected enhanced reactivity of aluminized nanothermites by accelerated aging. Chem. Eng. J. 2021, 418, 129432. [Google Scholar] [CrossRef]
- Zakiyyan, N.; Wang, A.Q.; Thiruvengadathan, R.; Staley, C.; Mathai, J.; Gangopadhyay, K.; Maschmann, M.R.; Gangopadhyay, S. Combustion of aluminum nanoparticles and exfoliated 2D molybdenum trioxide composites. Combust. Flame 2018, 187, 1–10. [Google Scholar] [CrossRef]
- Wang, W.M.; Liu, B.; Xu, K.Z.; Zu, Y.Q.; Song, J.R.; Zhao, F.Q. In-situ preparation of MgFe2O4-GO nanocomposite and its enhanced catalytic reactivity on decomposition of AP and RDX. Ceram. Int. 2018, 44, 19016–19020. [Google Scholar] [CrossRef]
- Yan, Q.L.; Zhao, F.Q.; Kuo, K.K.; Zhang, X.H.; Zeman, S.; DeLuca, L.T. Catalytic effects of nano additives on decomposition and combustion of RDX-, HMX-, and AP-based energetic compositions. Prog. Energy Combust. Sci. 2016, 57, 75–136. [Google Scholar] [CrossRef]
- Wang, W.M.; Li, H.; Zhang, M.; Zhao, F.Q.; Xu, S.Y.; Wang, C.J.; Qin, Z.; An, T.; Xu, K.Z. Effects of oxidizer and architecture on the thermochemical reactivity, laser ignition and combustion properties of nanothermite. Fuel 2022, 314, 123141. [Google Scholar] [CrossRef]
- Shi, L.M.; Zhang, W.C.; Cheng, J.; Yu, C.P.; Shen, R.Q.; Ye, J.H.; Qin, Z.C.; Chao, Y.M. A high energy output and low onset temperature nanothermite based on three-dimensional ordered macroporous nano-NiFe2O4. RSC Adv. 2016, 6, 93330–93334. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.T.; Liu, Y.F.; Huang, H.T.; Zhao, Y.; Song, K.G.; Wang, H.Y.; Xie, W.X.; Cheng, Y.; Fan, X.Z. Preparation and characterization of the Al/Fe2O3/RDX/NC nanocomposites by electrospray. J. Therm. Anal. Calorim. 2019, 137, 1615–1620. [Google Scholar] [CrossRef]
- Gao, K.; Li, G.P.; Luo, Y.J.; Wang, L.; Shen, L.H.; Wang, G. Preparation and characterization of the AP/Al/Fe2O3 ternary nano-thermites. J. Therm. Anal. Calorim. 2014, 118, 43–49. [Google Scholar] [CrossRef]
- Zhao, N.N.; He, C.C.; Liu, J.B.; Ma, H.X.; An, T.; Zhao, F.Q.; Hu, R.Z. Preparation and Characterization of Superthermite Al/Fe2O3 and Its Effect on Thermal Decomposition of Cyclotrimethylene Trinitramine. Acta Phys. Chim. Sin. 2013, 29, 2498–2504. [Google Scholar]
- Zhang, T.; Zhao, N.; Li, J.; Gong, H.; An, T.; Zhao, F.; Ma, H. Thermal behavior of nitrocellulose-based superthermites: Effects of nano-Fe2O3 with three morphologies. RSC Adv. 2017, 7, 23583–23590. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Zhou, X.; Huang, X.Y.; Zheng, D.W.; Mao, Y.F.; Wang, R.H.; Wang, D.J. Combustion/decomposition characteristics of 3D-printed Al/CuO, Al/ Fe2O3, Al/Bi2O3 and Al/PTFE hollow filaments. Mater. Chem. Phys. 2021, 271, 124874. [Google Scholar] [CrossRef]
- Kabra, S.; Gharde, S.; Gore, P.M.; Jain, S.; Khire, V.H.; Kandasubramanian, B. Recent trends in nanothermites: Fabrication, characteristics and applications. Nano Express 2020, 1, 032001. [Google Scholar] [CrossRef]
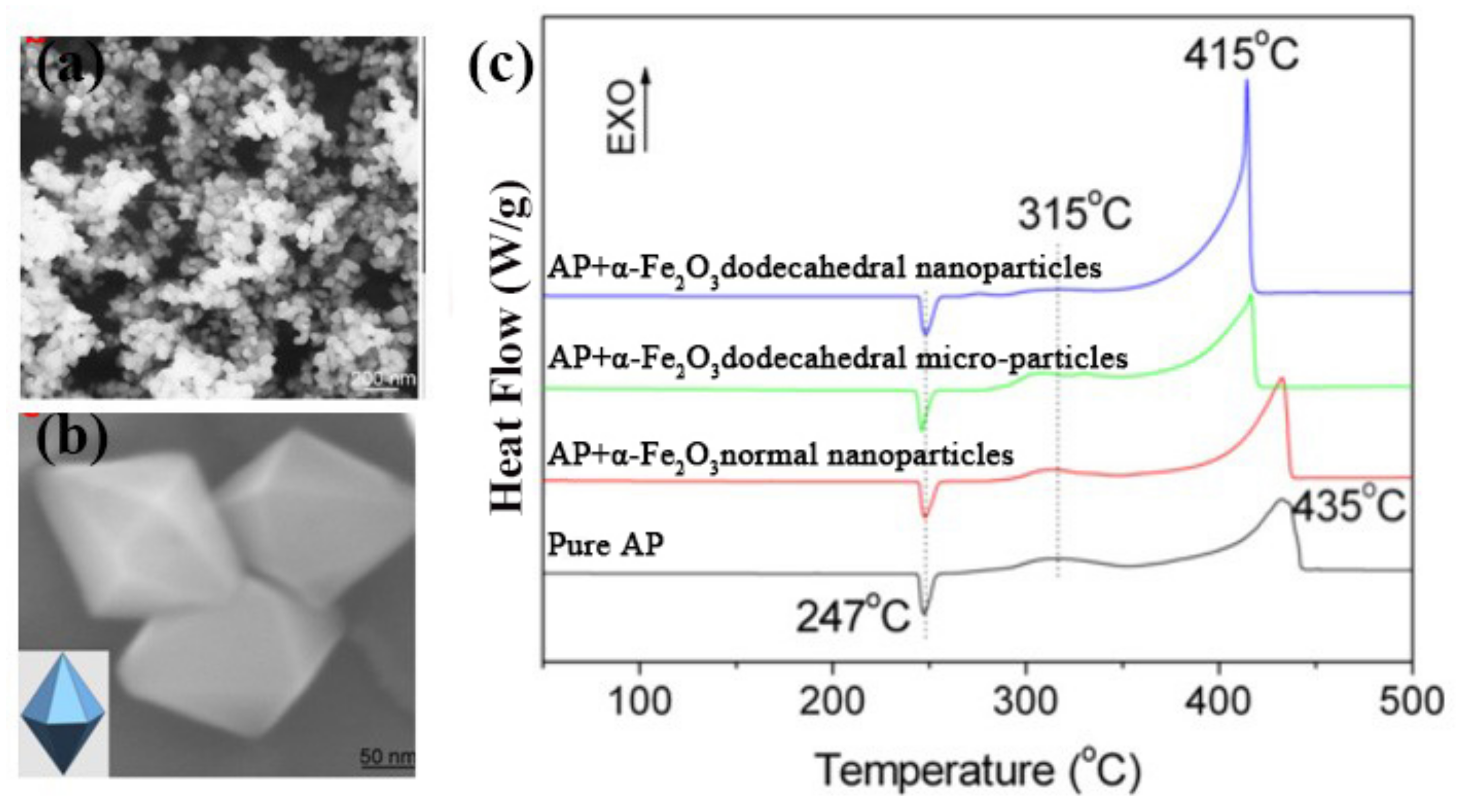
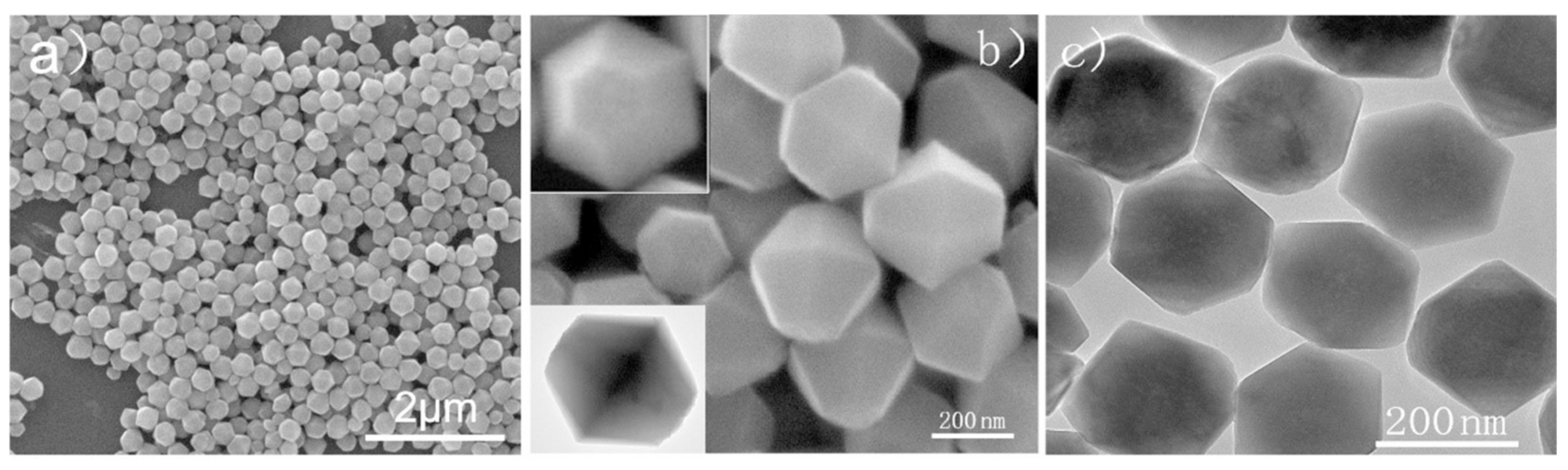
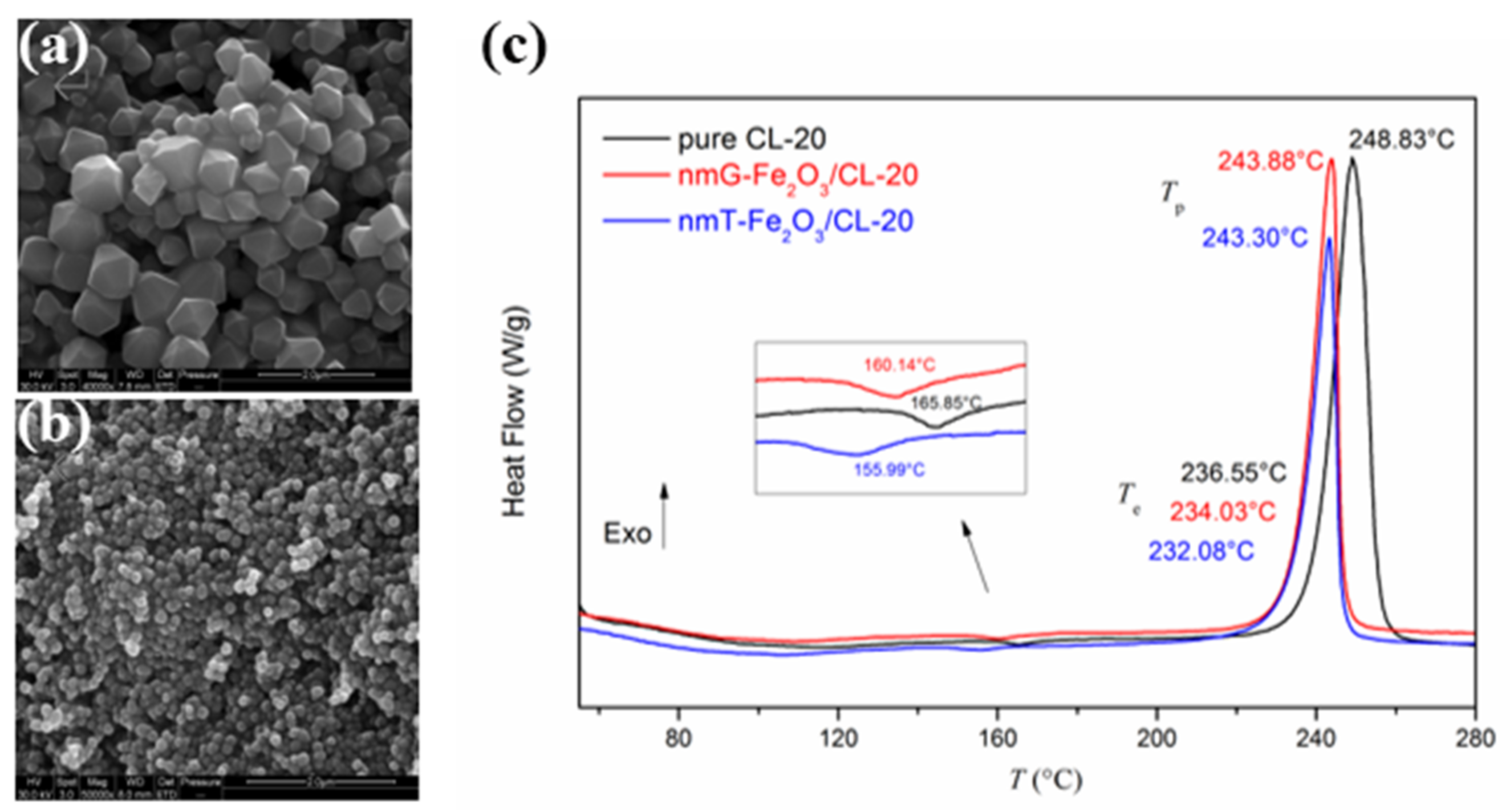

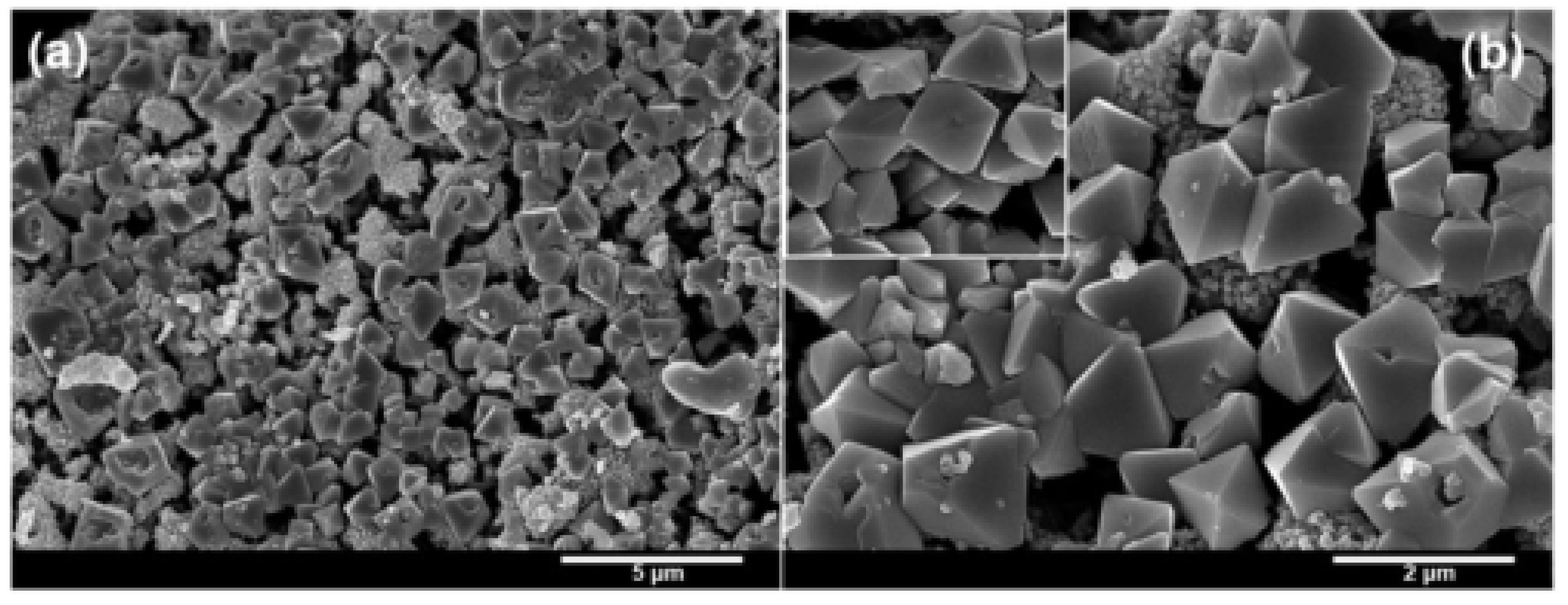







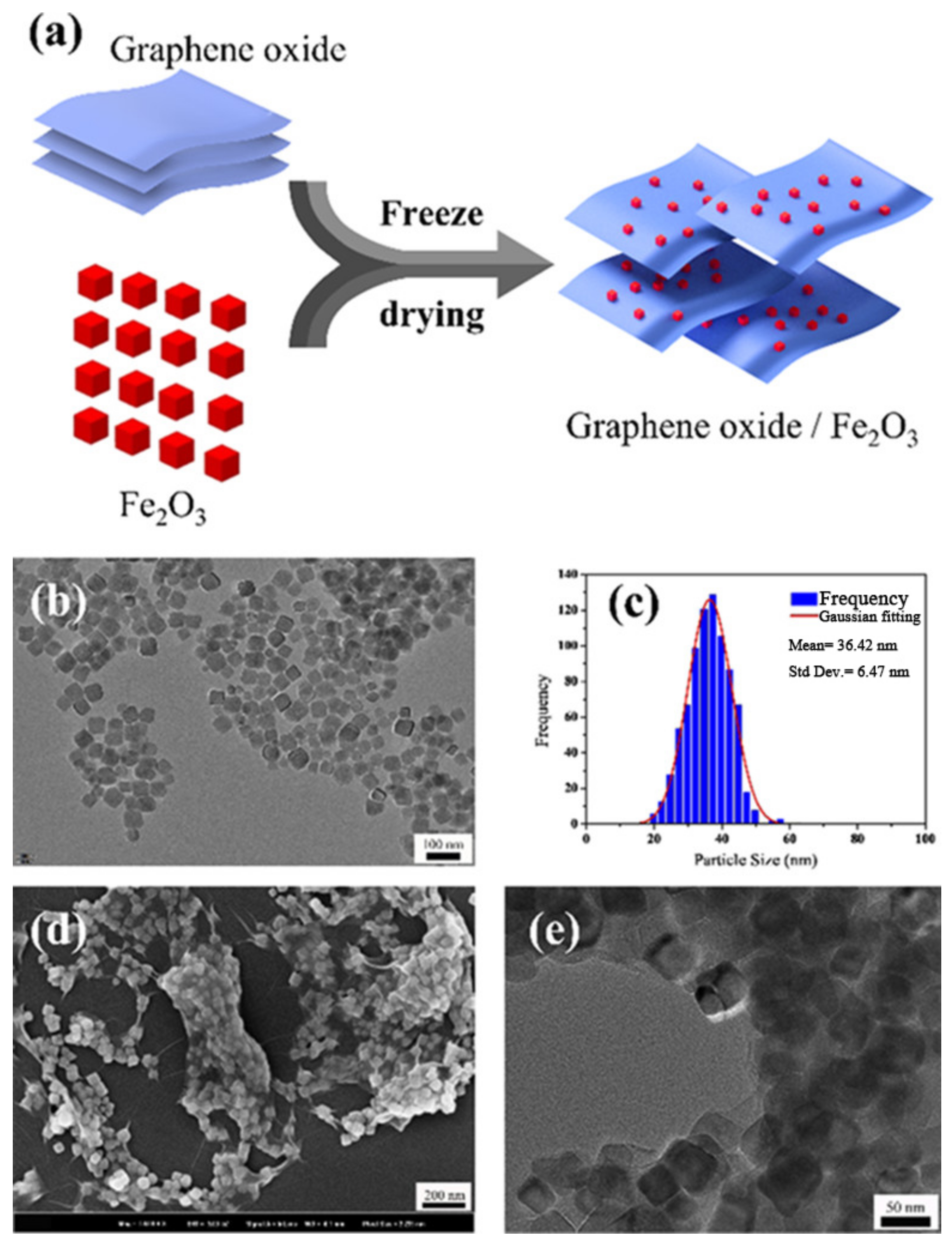
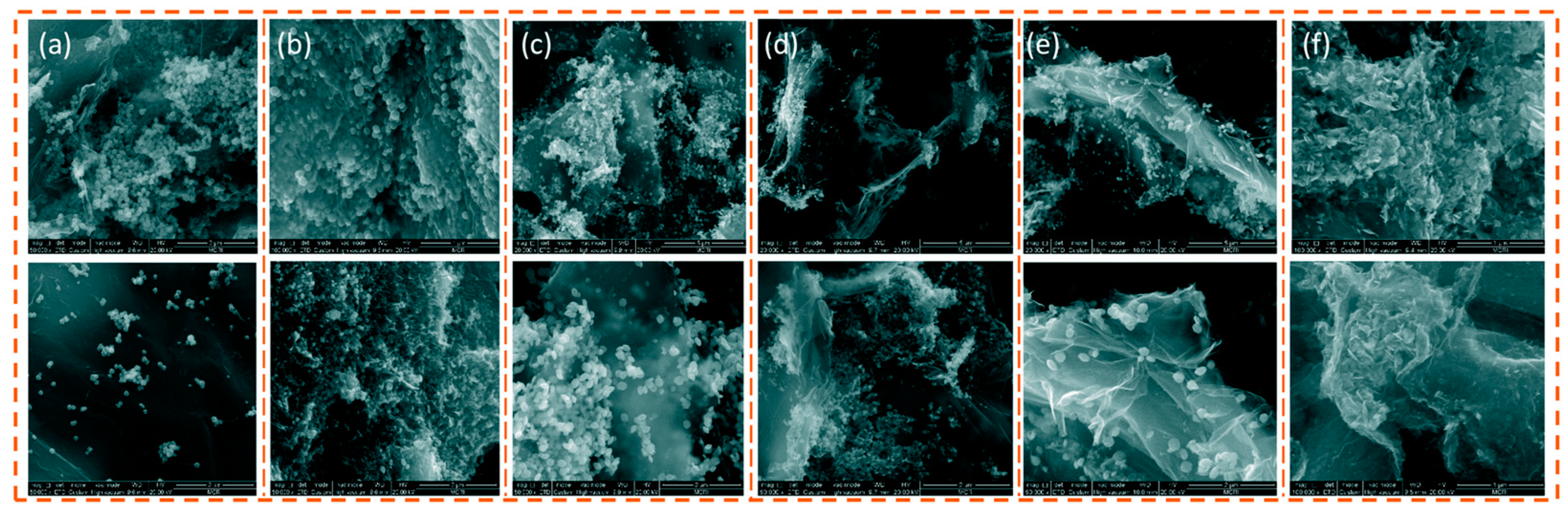

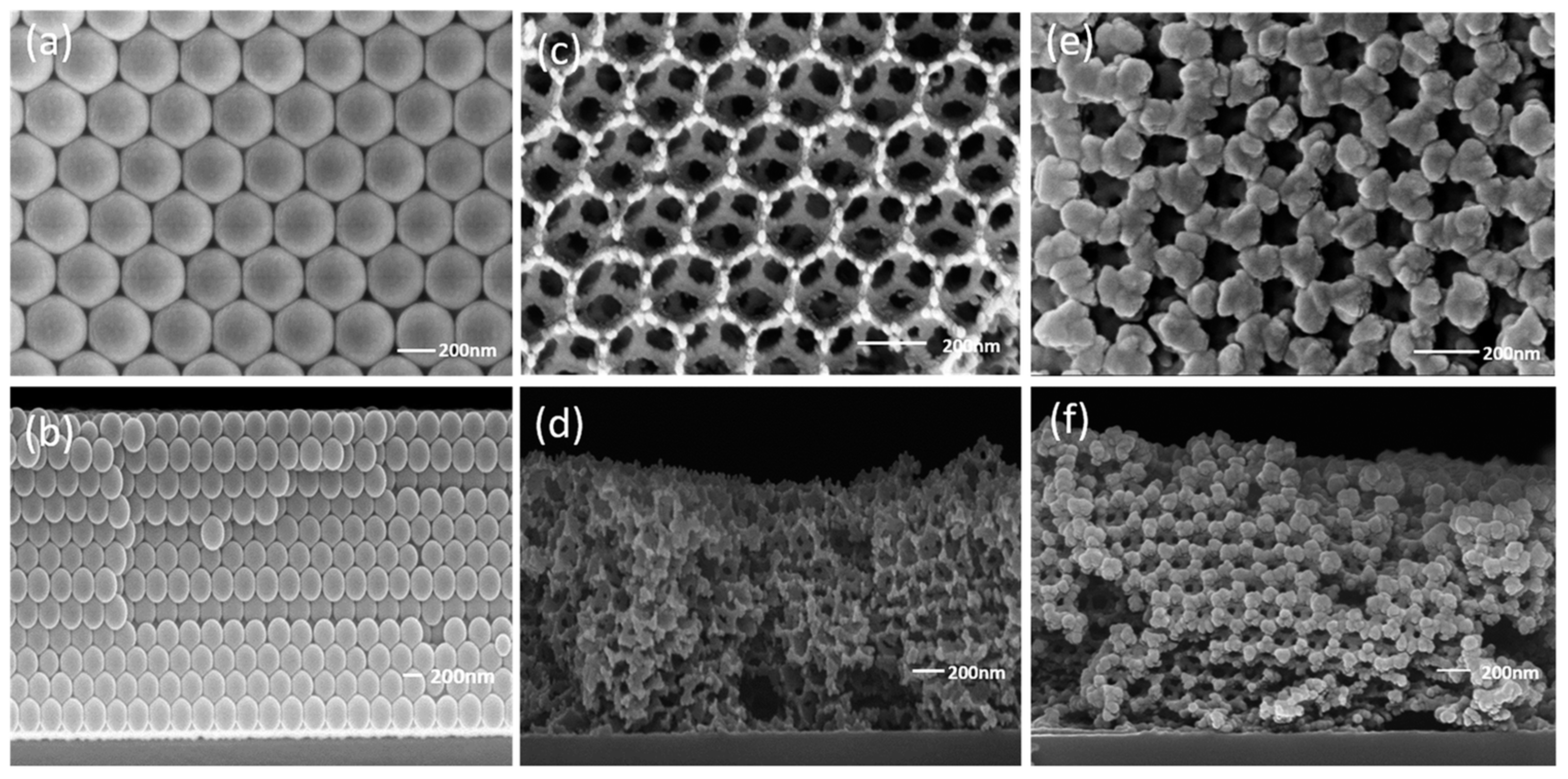
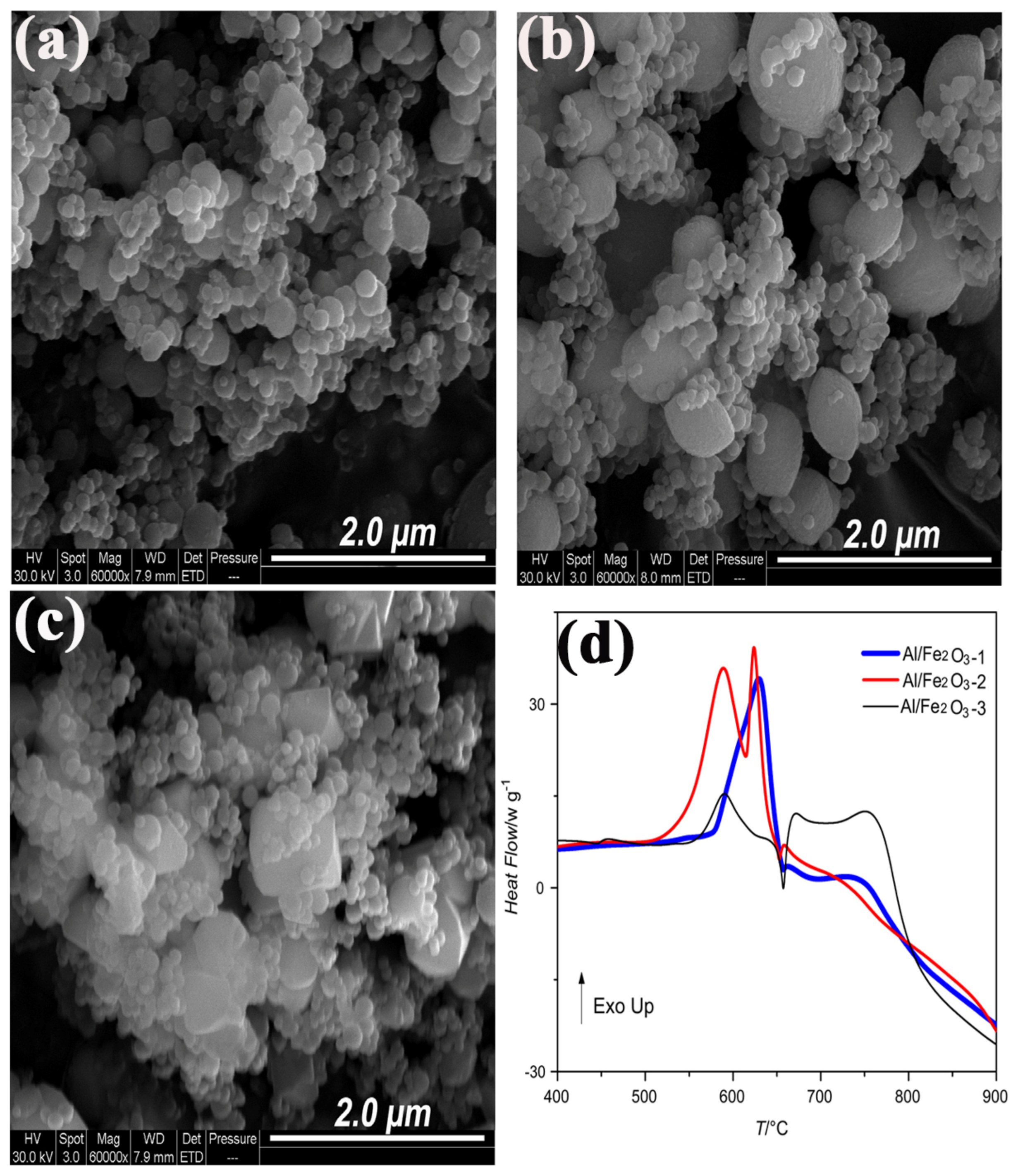
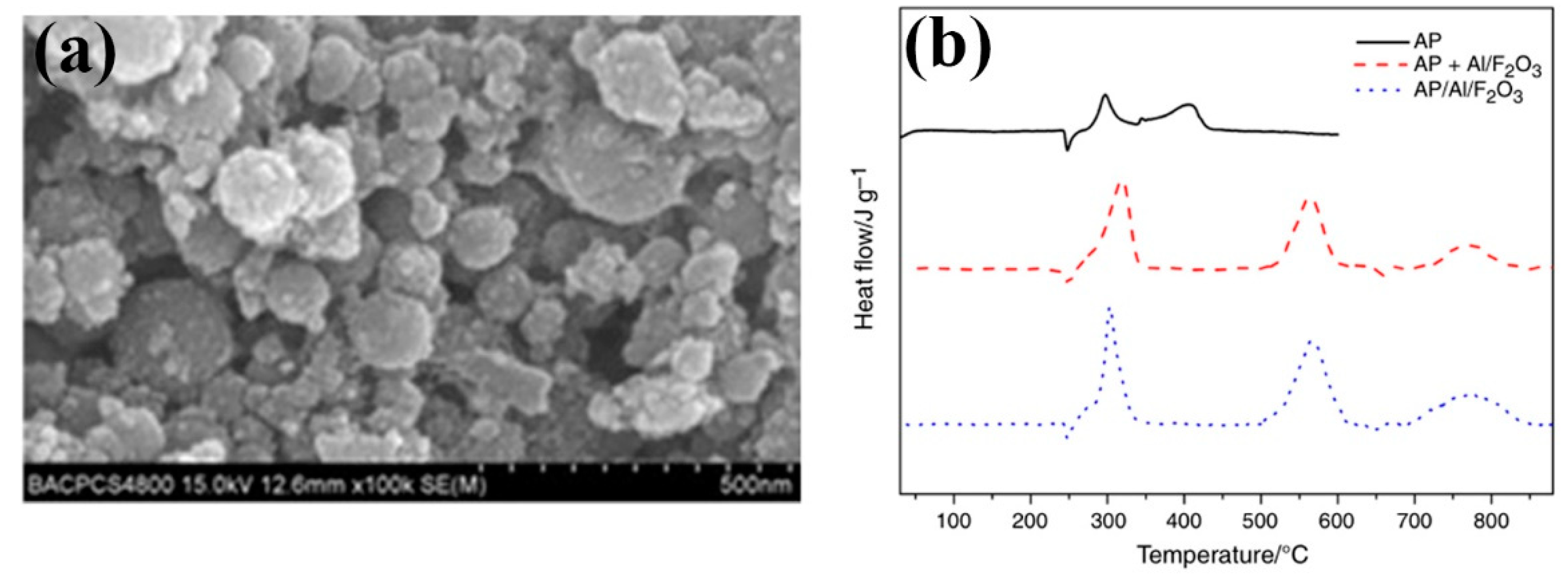



| Morphologies | Samples | Preparation Method | Mass Ratios | Tp | ΔH | E | Refs. |
|---|---|---|---|---|---|---|---|
| AP | assistance of F− | 0 | 435.0 | - | - | [35] | |
| dodecahedral | Fe2O3/AP | 2% | 415.0 | - | - | ||
| AP | one-pot | 0 | 436.4 | - | - | [36] | |
| nanorods | Fe2O3/AP | 1% | 387.5 | - | - | ||
| micro-octahedrons | Fe2O3/AP | 1% | 426.4 | - | - | ||
| CL-20 | hydrothermal | 0 | 248.8 | - | 181.1 | [38] | |
| tetrakaidecahedral | Fe2O3/CL-20 | 33.33% | 243.3 | - | 177.8 | ||
| grainy | Fe2O3/CL-20 | 33.33% | 243.9 | - | 176.1 | ||
| NC | hydrothermal | 0 | 208.6 | - | (17 ± 2) x 10 + 01 | [39] | |
| granular | Fe2O3/NC/Cl | 5% | 209.2 | - | (16 ± 2) x 10 + 01 | ||
| Fe2O3/NC/N | 5% | 209.4 | - | (17 ± 3) x 10 + 01 | |||
| Fe2O3/NC/S | 5% | 209.1 | - | (17 ± 1) x 10 + 01 | |||
| NC | hydrothermal | 0 | 209.6 | - | - | [40] | |
| granular | Fe2O3/NC | 12.6% | 209.9 | - | 189.9 | ||
| HMX | co-precipitation | 0 | 285.0 | - | - | [41] | |
| granular | Fe2O3/HMX | 1% | 272.0 | - | - | ||
| AP | hydrothermal | 0 | 428.5 | - | - | [42] | |
| nano-rods | Fe2O3/AP | 2% | 351.7 | - | - | ||
| AP | hydrothermal | 0 | 434.9 | - | 245.8 ± 32.4 | [43] | |
| rod-like | ro-Fe2O3/AP | 2% | 358.1 | 1517.4 | 133.6 ± 6.3 | ||
| rhombic | rh-Fe2O3/AP | 2% | 381.5 | 1494.8 | 153.0 ± 8.7 | ||
| pseudo-cubic | pc-Fe2O3/AP | 2% | 395.0 | 1469.1 | 161.2 ± 0.5 | ||
| AP | hydrothermal | 0 | 516.6 | - | - | [44] | |
| nano-disks | Fe2O3/AP | 1% | 394.0 | - | - | ||
| irregular particles | Fe2O3/AP | 1% | 419.8 | - | - | ||
| AP | indica (neem) leaf extract. | 0 | 445.0 | - | 160.0 ± 2.0 | [45] | |
| hexagonal cone | Fe2O3/AP | 1% | 370.0 | - | 112.0 ± 5.0 | ||
| AP | high-gravity reactive precipitation | 0 | 437.4 | 860.0 | 218.0 | [46] | |
| rhombohedron | G30 Fe2O3/AP | 2% | 396.7 | 984.0 | 167.1 | ||
| G220 Fe2O3/AP | 2% | 384.0 | 1235.0 | 163.3 |
| Materials | Samples | Preparation Method | Mass Ratios | Tp | ΔH | E | Refs. |
|---|---|---|---|---|---|---|---|
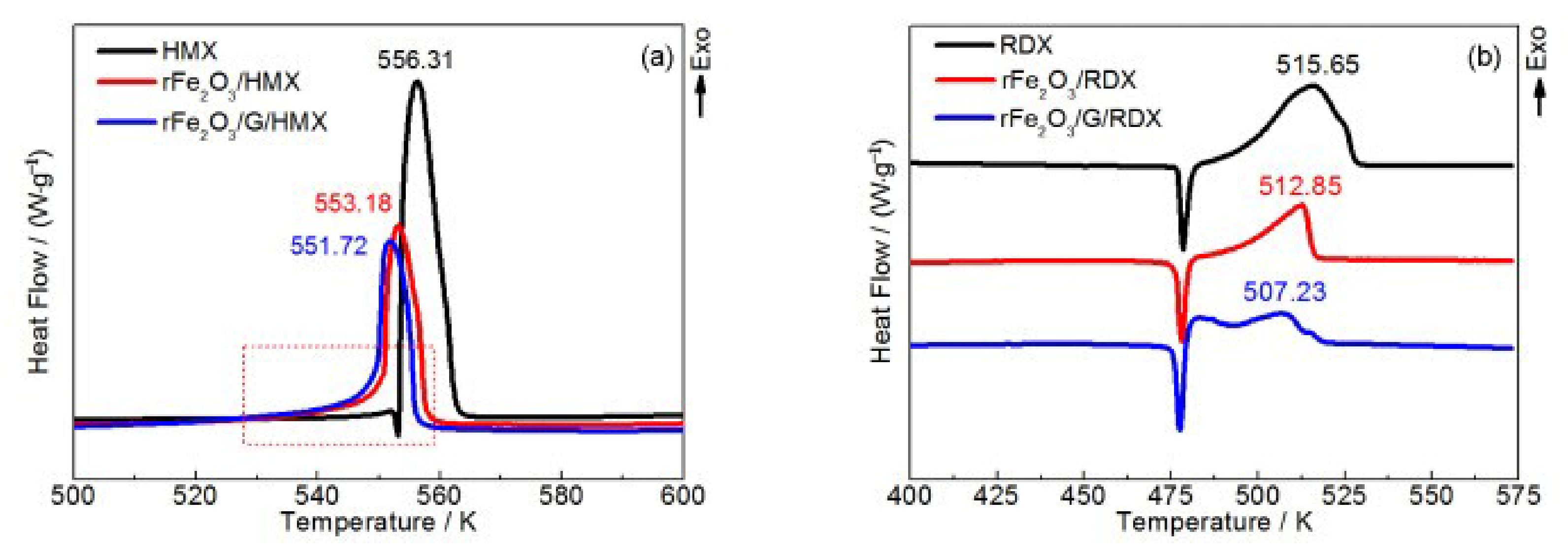
| GO | Fe2O3/GO/HMX | hydrothermal | 1% | 242.6 | - | 139.9 | [97] |
| G | CL-20 | hydrothermal | 0 | 252.1 | 179.2 | 183.4 | [98] |
| pFe2O3/CL-20 | 20% | 246.3 | - | 172.6 | |||
| rFe2O3/CL-20 | 20% | 245.9 | 161.5 | 165.6 | |||
| pFe2O3/G/CL-20 | 20% | 244.4 | - | 159.9 | |||
| rFe2O3/G/CL-20 | 20% | 243.8 | 148.9 | 153.0 | |||
| HMX | 0 | 283.2 | - | - | |||
| rFe2O3/HMX | 20% | 280.0 | - | - | |||
| rFe2O3/G/HMX | 20% | 278.6 | - | - | |||
| RDX | 0 | 242.5 | - | - | |||
| rFe2O3/RDX | 20% | 239.7 | - | - | |||
| rFe2O3/G/RDX | 20% | 234.1 | - | - | |||
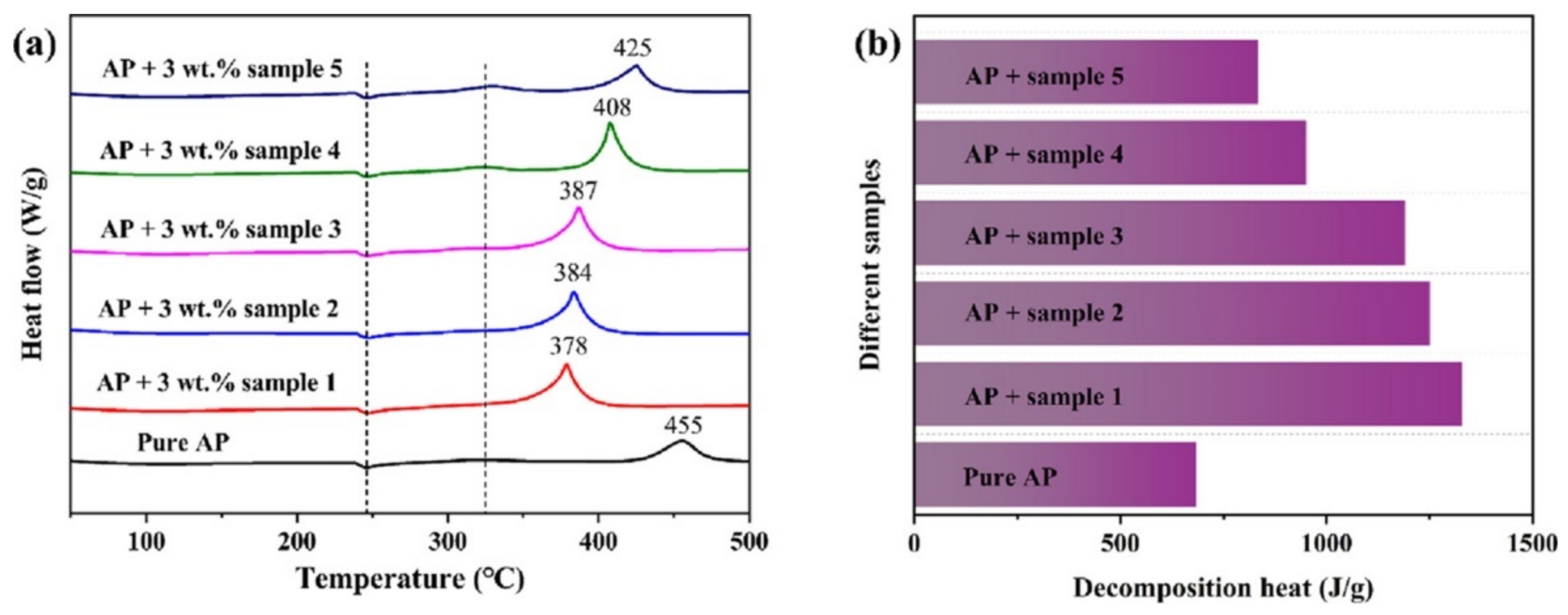
| GO | AP | vacuum-freeze-drying | 0 | 455.0 | - | - | [99] |
| Sample 1 | 10:1 Fe2O3/GO/AP | 3% | 378.0 | - | - | ||
| Sample 2 | 20:3 Fe2O3/GO/AP | 3% | 384.0 | - | - | ||
| Sample 3 | 10:3 Fe2O3/GO/AP | 3% | 387.0 | - | - | ||
| Sample 4 | 1:1 Fe2O3/GO/AP | 3% | 408.0 | - | - | ||
| Sample 5 | 3:1 Fe2O3/GO/AP | 3% | 425.0 | - | - | ||
| GO | AP | hydrothermal | 0 | 432.0 | - | 129.0 | [100] |
| GO/AP | 1.84% | 400.0 | - | 100.1 | |||
| Fe2O3/AP | 1.84% | 397.0 | - | 89.3 | |||
| Fe2O3/G/AP | 1.84% | 380.0 | - | 80.3 | |||
| GO | AP | solvothermal | 0 | 440.8 | - | 290.0 | [101] |
| GO/AP | 5% | 372.5 | - | 216.2 | |||
| H2O | rGO/AP | 5% | 360.8 | - | 128.8 | ||
| H2O | Fe2O3/AP | 5% | 369.4 | - | 170.3 | ||
| DMF | Fe2O3/rGO/AP | 5% | 321.2 | - | 119.8 | ||
| rGO | AP | atomic layer deposition | 0 | 440 ± 5 | - | - | [104] |
| Fe2O3/rGO/AP | 5% | 358 | - | - | |||
| rGO | AP | direct precipitation | 0 | 425.8 | - | - | [105] |
| Fe2O3/rGO/AP | 1% | 413.1 | - | - | |||
| rGO | AP | hydrothermal | 0 | 462.0 | 887.0 | 172.1 | [106] |
| BiFeO3/AP | - | 449.0 | - | 144.5 | |||
| 1% BiFeO3/rGO/AP | - | 427.0 | - | - | |||
| 2% BiFeO3/rGO/AP | - | 396.0 | - | - | |||
| 3% BiFeO3/rGO/AP | - | 354.0 | - | - | |||
| 4% BiFeO3/rGO/AP | - | 295.0 | 2518.0 | 128.4 | |||
| 5% BiFeO3/rGO/AP | - | 308.0 | - | - | |||
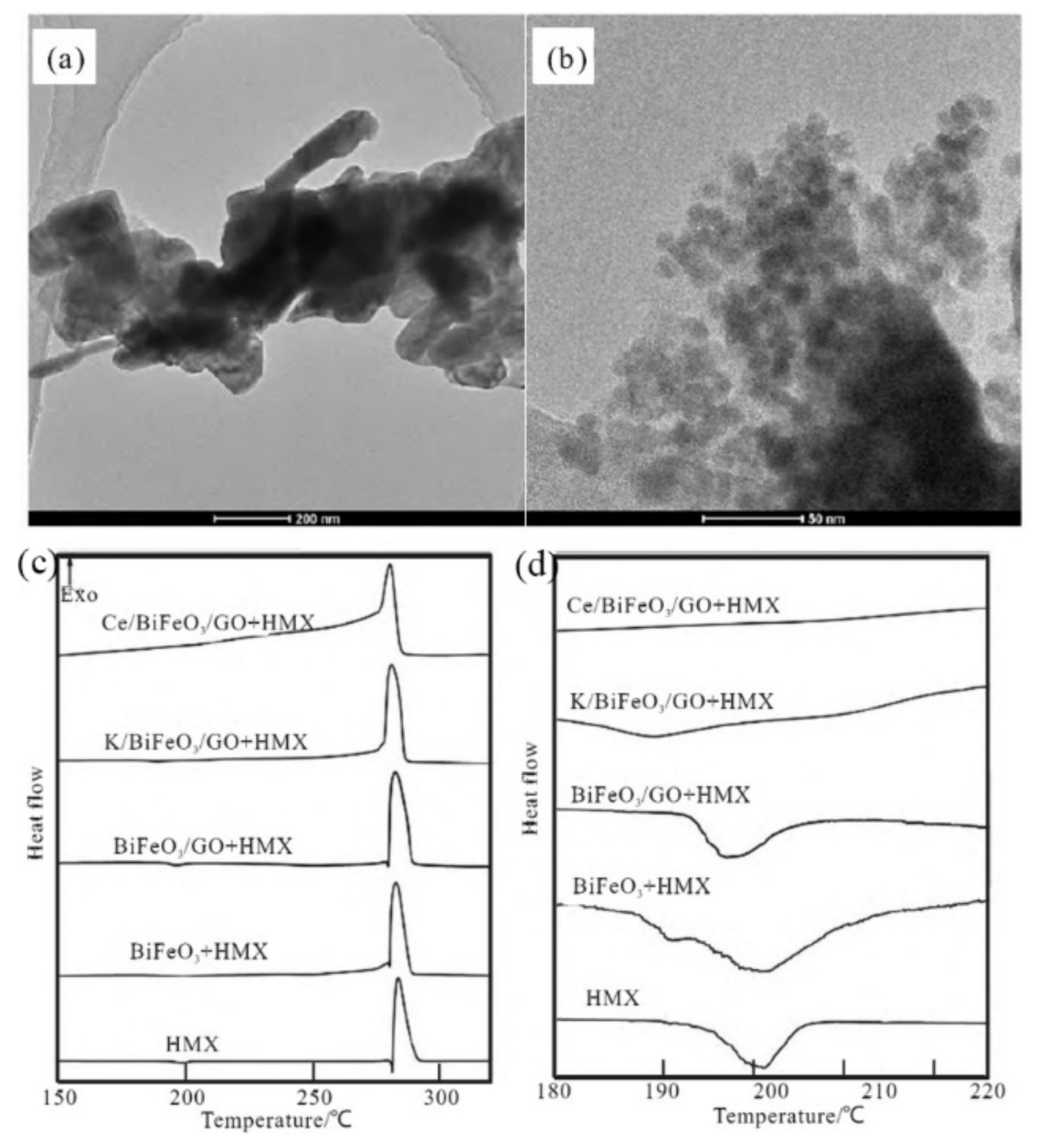
| GO | HMX | hydrothermal | 0 | 283.4 | - | - | [107] |
| BiFeO3/HMX | 20% | 282.7 | - | - | |||
| BiFeO3/GO/HMX | 20% | 282.4 | - | - | |||
| K/BiFeO3/GO/HMX | 20% | 280.9 | - | - | |||
| Ce/BiFeO3/GO/HMX | 20% | 280.5 | - | - | |||
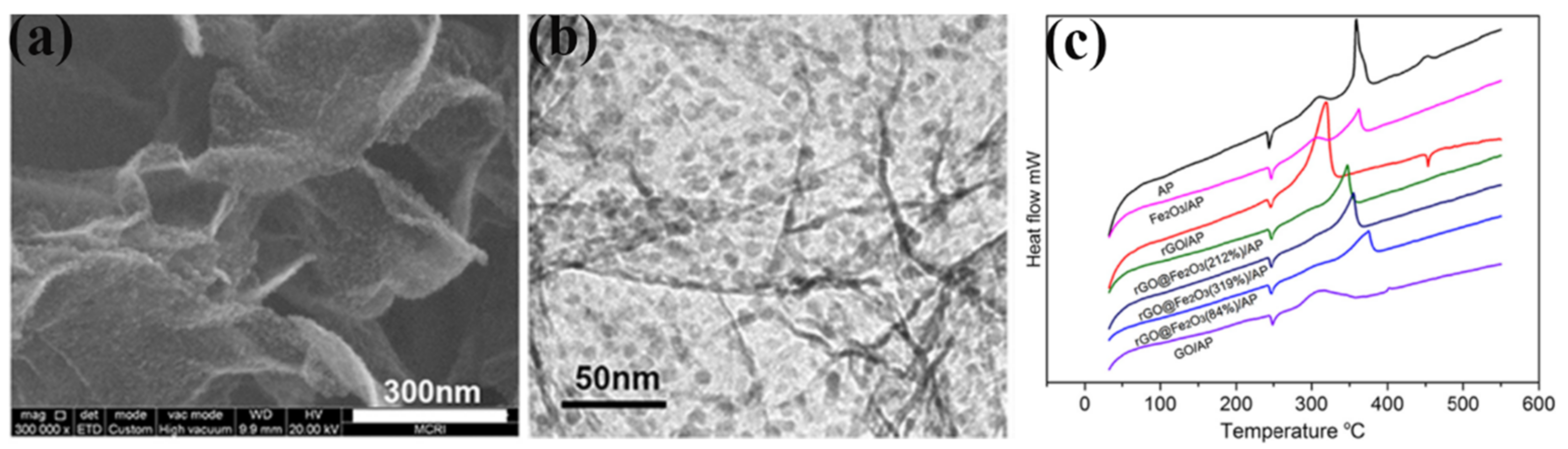
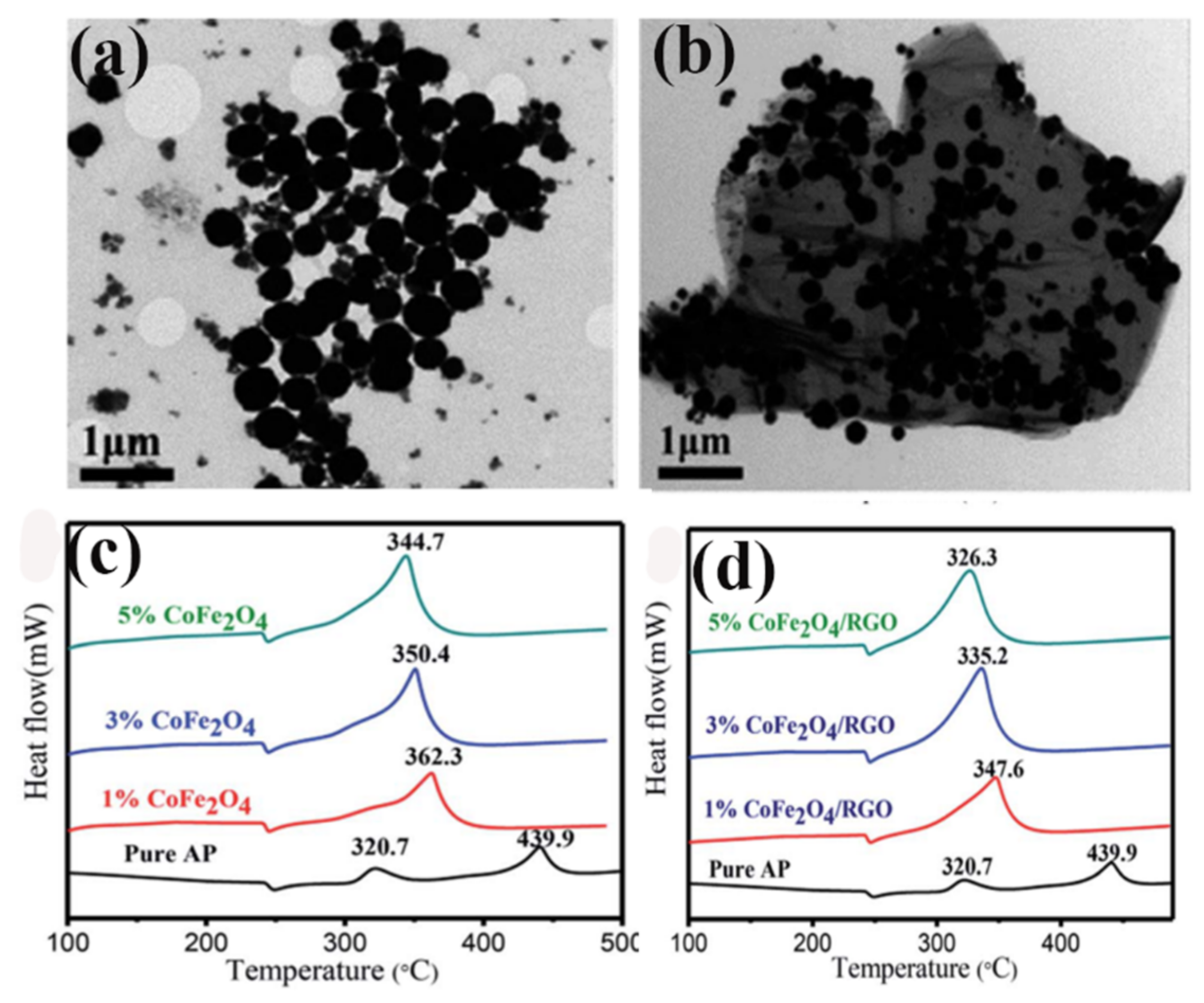
| rGO | AP | one-pot solvothermal | 0 | 439.9 | - | 147.6 | [108] |
| 1% CoFe2O4/AP | - | 362.3 | - | - | |||
| 3% CoFe2O4/AP | - | 350.4 | - | 131.5 | |||
| 5% CoFe2O4/AP | - | 344.7 | - | - | |||
| 1% CoFe2O4/rGO/AP | - | 347.6 | - | - | |||
| 3% CoFe2O4/rGO/AP | - | 335.2 | - | 117.9 | |||
| 5% CoFe2O4/rGO/AP | - | 326.3 | - | - | |||
| rGO | AP | solvothermal | 0 | 439.9 | - | 147.6 | [109] |
| 3% rGO/AP | - | 433.7 | - | - | |||
| 3% NiFe2O4/AP | - | 363.4 | - | - | |||
| 1% NiFe2O4/rGO/AP | - | 359.2 | - | - | |||
| 3% NiFe2O4/rGO/AP | - | 347.3 | - | 128.3 | |||
| 5% NiFe2O4/rGO/AP | - | 336.1 | - | - | |||
| rGO | AP | one-step hydrothermal | 0 | 424.7 | - | 160.3 | [110] |
| ZnFe2O4/AP | 1% | 380.7 | - | - | |||
| ZnFe2O4/AP | 3% | 365.0 | - | - | |||
| ZnFe2O4/AP | 5% | 360.8 | - | 143.7 | |||
| ZnFe2O4/rGO/AP | 1% | 380 | - | - | |||
| ZnFe2O4/rGO/AP | 3% | 367.7 | - | - | |||
| ZnFe2O4/rGO/AP | 5% | 354.6 | - | 127.6 | |||
| CNTs | AP | sol-gel | 0 | 478.1 | - | 178.0 | [114] |
| simple mix Fe2O3/CNTs/AP | 2% | 390.9 | - | 134.0 | |||
| particle composite Fe2O3/CNTs/AP | 2% | 376.0 | - | 118.0 | |||
| CNTs | RDX | sol-gel | 0 | 240.9 | - | 109.2 | [115] |
| Fe2O3/CNTs/RDX | 5% | 226.8 | - | 67.17 | |||
| g-C3N4 | AP | direct copolymerization | 0 | 454.4 | - | 216.0 | [120] |
| g-C3N4/AP | 10% | 384.4 | - | 119.8 | |||
| g-C3N4 | AP | In situ | 0 | 437.4 | - | - | [121] |
| 1% Fe2O3/C3N4/AP | 2% | 376.2 | - | - | |||
| 2% Fe2O3/C3N4/AP | 2% | 368.5 | - | - | |||
| 3% Fe2O3/C3N4/AP | 2% | 358.9 | - | - | |||
| 5% Fe2O3/C3N4/AP | 2% | 348.1 | - | - | |||
| g-C3N4 | HMX | solvothermal | 0 | 283.2 | - | 502.2 | [122] |
| CoFe2O4/HMX | 20% | 277.6 | - | 225.6 | |||
| CoFe2O4/g-C3N4/HMX | 276.2 | - | 161.1 | ||||
| TKX-50 | 238.2 | - | 172.1 | ||||
| CoFe2O4/TKX-50 | 199.3 | - | 159.8 | ||||
| CoFe2O4/g-C3N4/TKX-50 | 196.9 | - | 151.1 |
| Materials | Preparation Method | Application | Texo1 | Texo2 | ΔH | Ref. |
|---|---|---|---|---|---|---|
| Al/B/Fe2O3 | sol-gel synthetic | thermite | 582.0 | 790.0 | 3270.0 | [133] |
| Al/B/Fe2O3 | physical blending | 559.0 | 780.0 | 2610.0 | ||
| Al/Fe2O3 | one-step hydrothermal | solid propellants | 587.0 ± 1.0 | 785.0 ± 2.0 | 3125.0 ± 31.0 | [134] |
| Al/Bi0.1-Fe2O3 | 602.0 ± 2.0 | 727.0 ± 8.0 | 2893.0 ± 16.0 | |||
| Al/Bi0.2-Fe2O3 | 597.0 ± 1.0 | 696.0 ± 5.0 | 2720.0 ± 22.0 | |||
| Al/Bi0.3-Fe2O3 | 593.0 ± 2.0 | 684.0 ± 3.0 | 2349.0 ± 14.0 | |||
| Al/Bi2O3 | 574.0 ± 10.0 | 667.0 ± 13.0 | 852.0 ± 34.0 | |||
| Al/BiFeO3 | 583.0 ± 1.0 | 739.0 ± 2.0 | 1812.0 ± 23.0 | |||
| in Ar Al/CuO | virtual thermal aging | thermite | 528.0 | 691.0 | 3945.0 | |
| in Ar Al/Fe2O3 | 514.0 | 733.0 | 2791.0 | [145] | ||
| in Ar Al/Fe3O4 | 527.0 | 770.0 | 2516.0 | |||
| in Ar Al/Co3O4 | 534.0 | 698.0 | 2457.0 | |||
| in air Al/CuO | 538.0 | 697.0 | 5125.0 | |||
| in air Al/Fe2O3 | 539.0 | 705.0 | 4214.0 | |||
| in air Al/Fe3O4 | 540.0 | 710.0 | 4302.0 | |||
| in air Al/Co3O4 | 536.0 | 702.0 | 3958.0 | |||
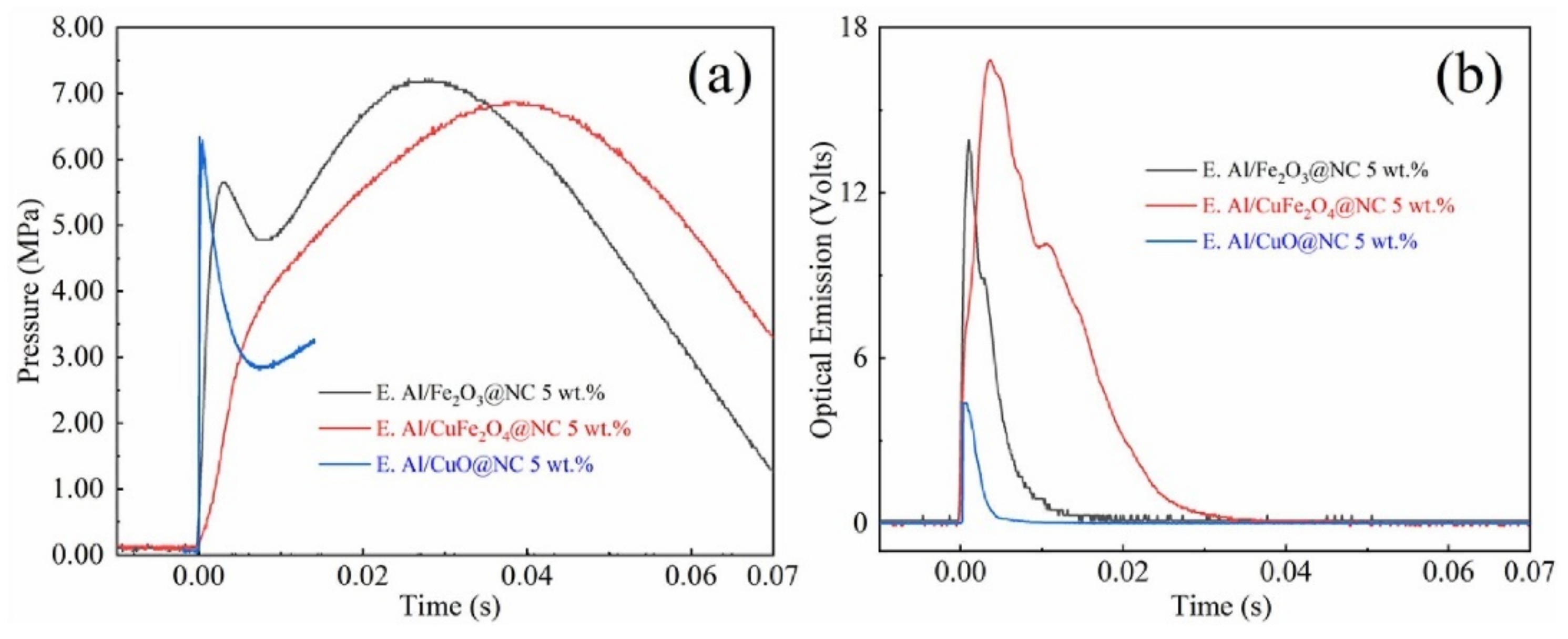
| Al/CuFe2O4@NC | electrospray | 567.8 | - | 2064.6 | [149] | |
| Al/Fe2O3@NC 5wt% | 576.5 | - | 2348.5 | |||
| Al/Cuo@NC 5wt% | 598.8 | - | 1763.9 | |||
| Al/NiFe2O4 | templating | MEMS pyrotechnics | 359.2 | 594.6 | 2921.7 | [150] |
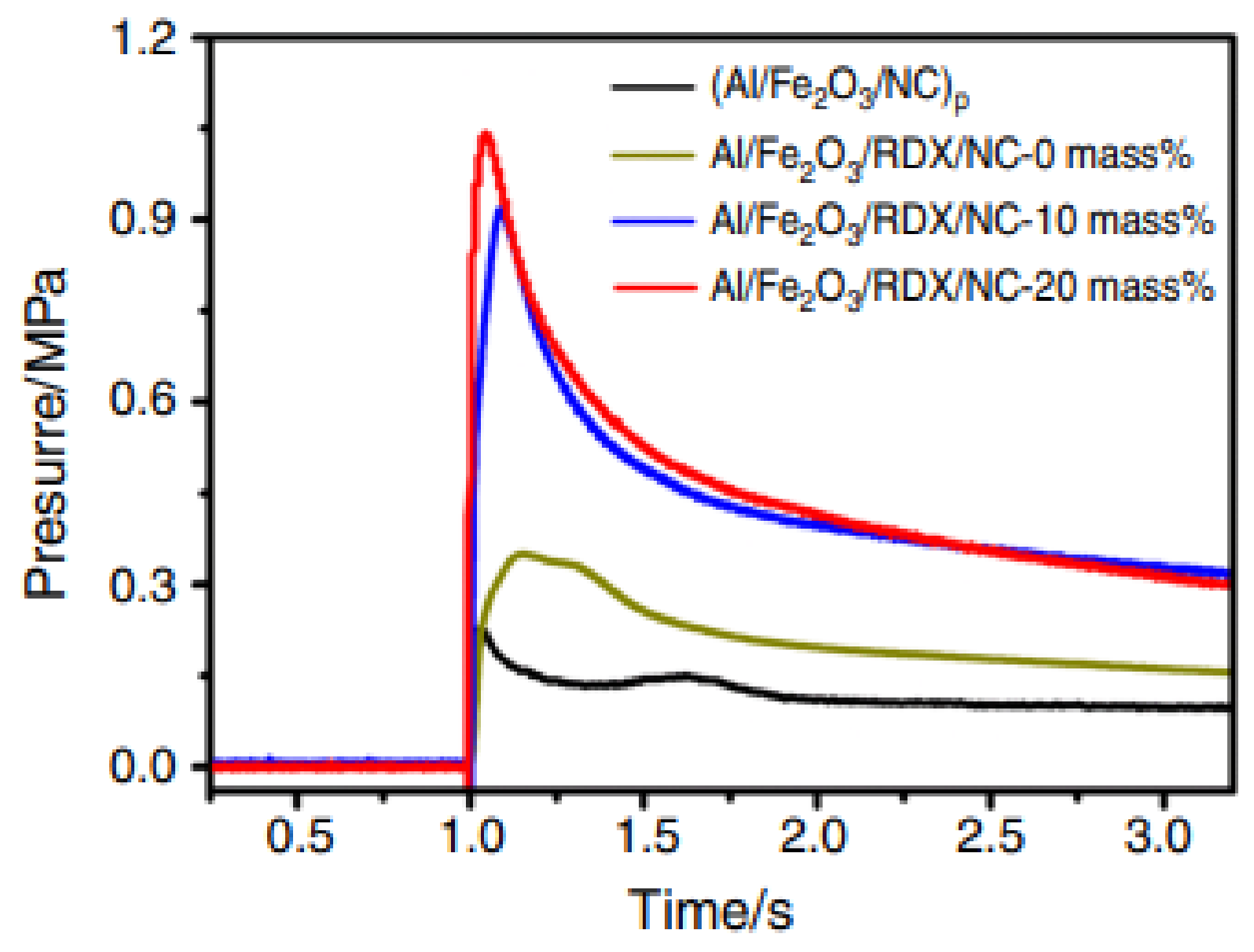
| Al/Fe2O3/NC | electrospray | thermite | 201.4 | 587.8 | - | [151] |
| Al/Fe2O3/RDX/NC-0 | 206.2 | 589.6 | - | |||
| Al/Fe2O3/RDX/NC-10 | 224.7 | 581.8 | - | |||
| Al/Fe2O3/RDX/NC-20 | 227.7 | 584.6 | - | |||
| Al/Fe2O3+AP | physicalmixing | micro-propellants | - | - | 1820.0 | [152] |
| Al/Fe2O3/AP | solvent-anti-solvent | - | - | 1400.0 | ||
| Al/Fe2O3/RDX (1:19) | hydrothermal | super thermites | 241.6 | - | - | [153] |
| Al/Fe2O3/RDX (1:9) | 246.3 | - | - | |||
| Al/Fe2O3/RDX (1:5) | 246.0 | - | - | |||
| Al/Fe2O3/RDX (1:4) | 245.9 | - | - | |||
| Al/Fe2O3/RDX (1:3) | 242.5 | 250.9 | - | |||
| Al/Fe2O3/RDX (1:2) | 239.6 | 247.2 | - | |||
| Al/Fe2O3/RDX (1:1) | 244.5 | - | - | |||
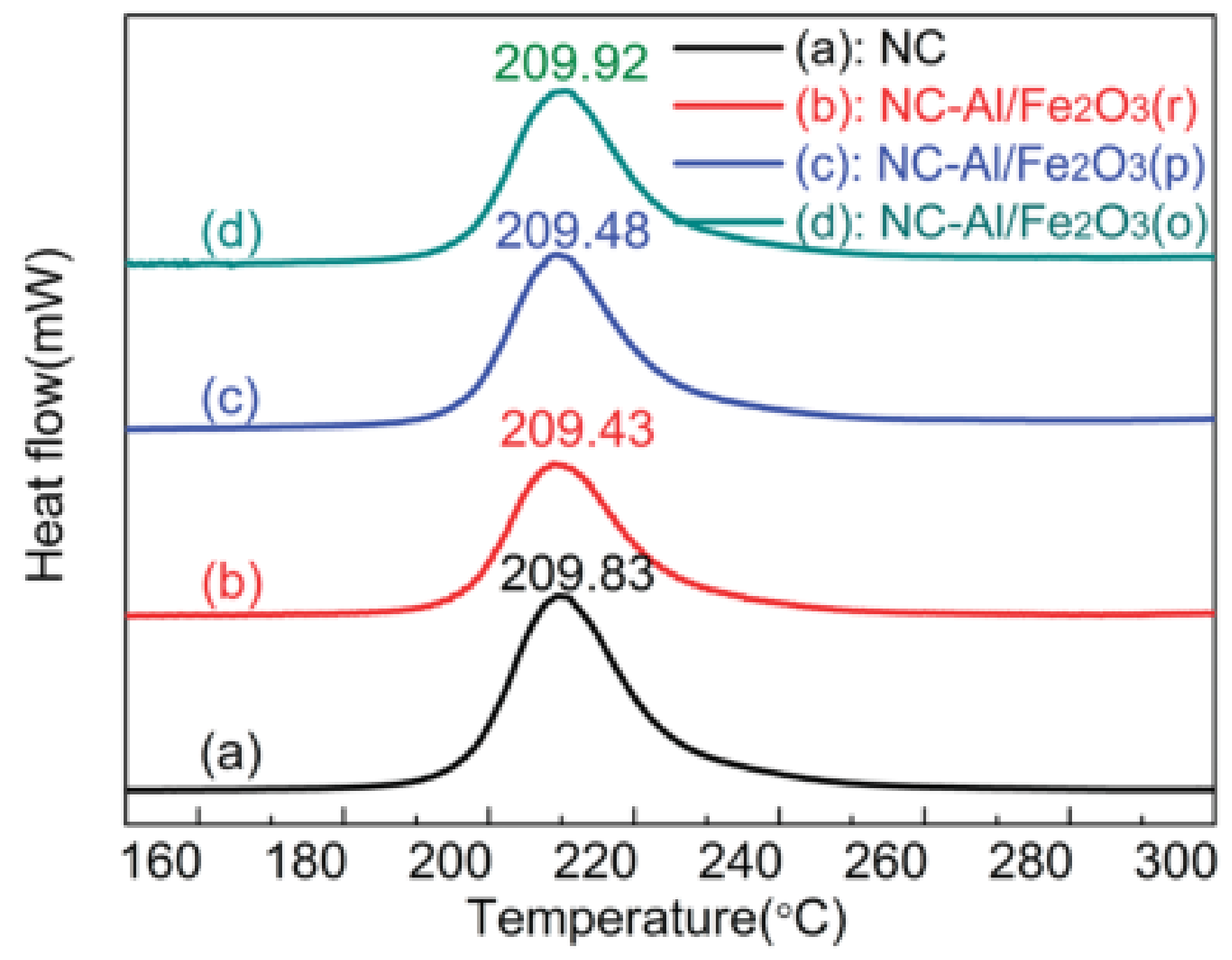
| Al/Fe2O3(p)/NC | hydrothermal | micro-propellants | 209.5 | - | - | [154] |
| Al/Fe2O3(o)/NC | 209.9 | - | - | |||
| Al/Fe2O3(r)/NC | 209.4 | - | - | |||
| Al/CuO/F2311 | 3D printing | 288.0 | - | 1500.0 | ||
| Al/Fe2O3/F2311 | 368.0 | - | 1552.0 | |||
| Al/Bi2O3/F2311 | 410.0 | - | 1071.0 | |||
| Al/PTFE/F2311 | 377.0 | - | 7200.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Dang, J.; Ma, Y.; Ma, H. Hematite: A Good Catalyst for the Thermal Decomposition of Energetic Materials and the Application in Nano-Thermite. Molecules 2023, 28, 2035. https://doi.org/10.3390/molecules28052035
Li Y, Dang J, Ma Y, Ma H. Hematite: A Good Catalyst for the Thermal Decomposition of Energetic Materials and the Application in Nano-Thermite. Molecules. 2023; 28(5):2035. https://doi.org/10.3390/molecules28052035
Chicago/Turabian StyleLi, Yu, Jia Dang, Yuqiang Ma, and Haixia Ma. 2023. "Hematite: A Good Catalyst for the Thermal Decomposition of Energetic Materials and the Application in Nano-Thermite" Molecules 28, no. 5: 2035. https://doi.org/10.3390/molecules28052035
APA StyleLi, Y., Dang, J., Ma, Y., & Ma, H. (2023). Hematite: A Good Catalyst for the Thermal Decomposition of Energetic Materials and the Application in Nano-Thermite. Molecules, 28(5), 2035. https://doi.org/10.3390/molecules28052035





